Ocbil Theory as a Potential Unifying Framework for Investigating Narrow Endemism in Mediterranean Climate Regions
Abstract
1. Introduction
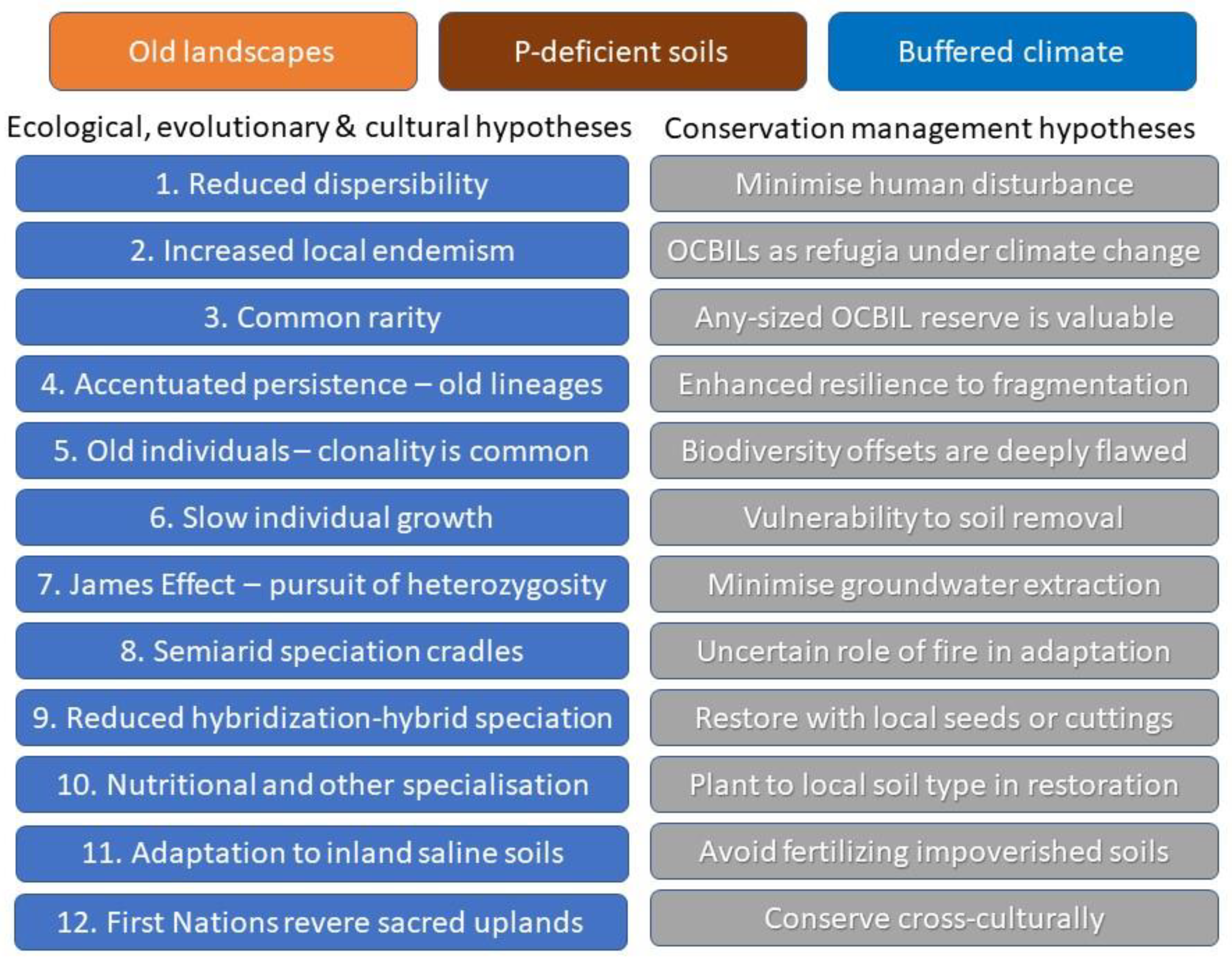
2. Hypotheses of OCBIL Theory
3. Mediterranean Climate Regions and OCBILs
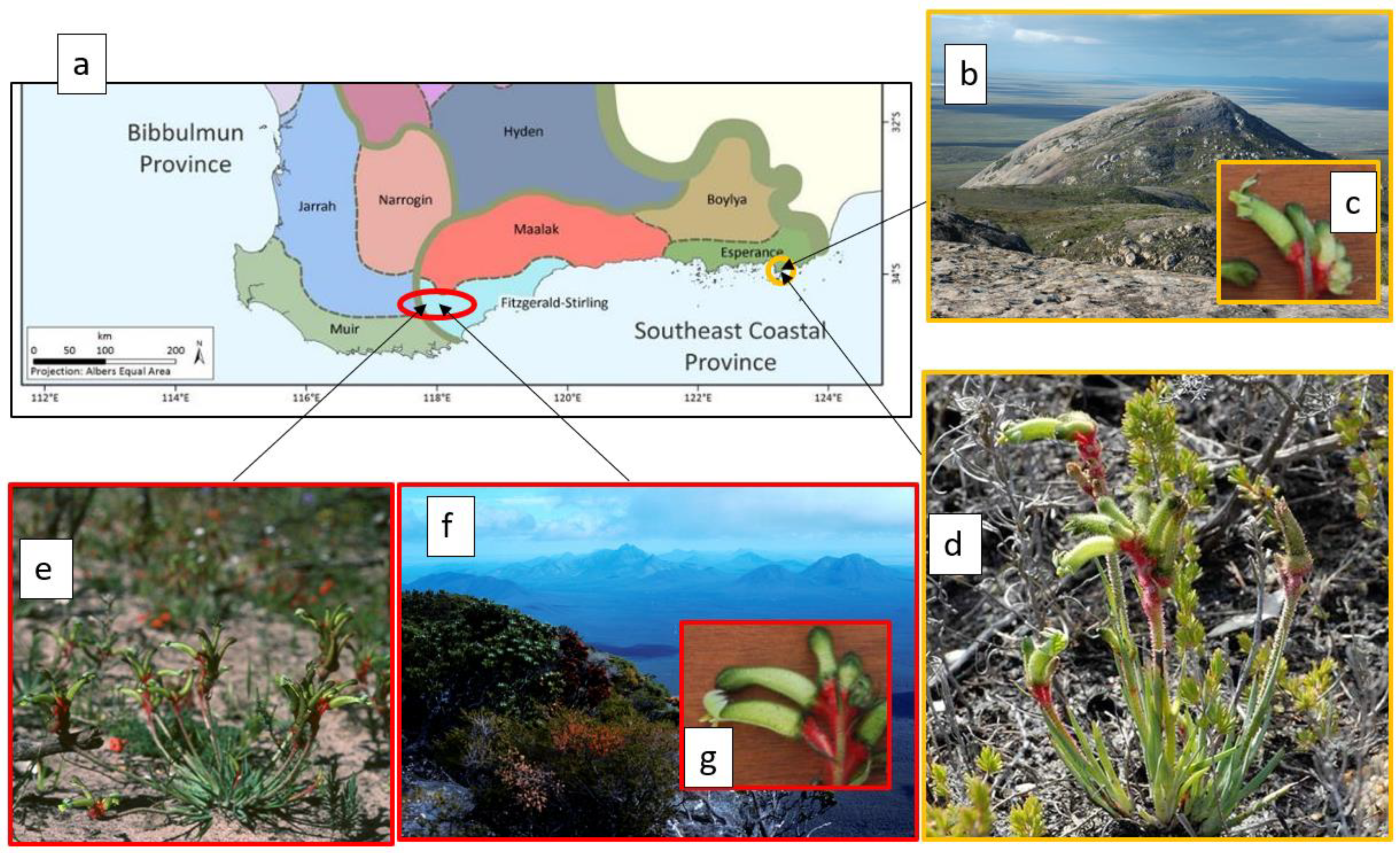
4. Narrow Endemism, OCBILs, and the Southwest Australian Floristic Region
5. Narrow Endemism, OCBILs, and the Greater Cape Floristic Region
6. Narrow Endemism, OCBILs, and the Mediterranean Floristic Region
7. Conservation Management Predictions of OCBIL Theory
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hopper, S.D.; Gioia, P. The Southwest Australian Floristic Region: Evolution and conservation of a global hotspot of biodiversity. Ann. Rev. Ecol. Evol. Syst. 2004, 35, 623–650. [Google Scholar] [CrossRef]
- Gioia, P.; Hopper, S.D. A new phytogeographic map for the Southwest Australian Floristic Region after an exceptional decade of collection and discovery. Bot. J. Linn. Soc. 2017, 184, 1–15. [Google Scholar] [CrossRef]
- Hopper, S.D. OCBIL theory: Towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically-buffered, infertile landscapes. Plant Soil 2009, 322, 49–86. [Google Scholar] [CrossRef]
- Hopper, S.D. Natural hybridization in the context of Ocbil theory. S. Afr. J. Bot. 2018, 118, 284–289. [Google Scholar] [CrossRef]
- Hopper, S.D.; Silveira, F.A.O.; Fiedler, P.L. Biodiversity hotspots and Ocbil theory. (Marschner review). Plant Soil 2016, 403, 167–216. [Google Scholar] [CrossRef]
- Hopper, S.D.; Lambers, H.; Fiedler, P.L.; Silviera, F.A.O. Ocbil theory examined: Reassessing evolution, ecology, and conservation in the world’s ancient, climatically-buffered and infertile landscapes. Biol. J. Linn. Soc. 2021, 133, 266–296. [Google Scholar] [CrossRef]
- Born, J.; Linder, H.P.; Desmet, P. The greater cape floristic region. J. Biogeogr. 2007, 34, 147–162. [Google Scholar] [CrossRef]
- Rull, V.; Vegas-Vilarrubia, T.; Huber, O.; Senaris, C. (Eds.) Biodiversity of Pantepui. The pristine “Lost World” of the Neotropical Guiana Highlands; Academic Press: London, UK, 2019. [Google Scholar]
- Silveira, F.A.O.; Dayrell, R.L.C.; Fiorini, C.F.; Negreiros, D.; Borba, E.L. Diversification in ancient and nutrient poor neotropical ecosystems: How geological and climatic buffering shaped plant diversity in some of the world’s neglected hotspots. In Neotropical Diversification: Patterns and Processes; Rull, V., Carnaval, A.C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 329–368. [Google Scholar]
- Mucina, L.; Wardell-Johnson, G. Landscape age and soil fertility, climatic stability, and fire regime predictability: Beyond the OCBIL framework. Plant Soil 2011, 341, 1–23. [Google Scholar] [CrossRef]
- Silveira, F.A.O.; Fiedler, P.L.; Hopper, S.D. OCBIL theory: A new science for old ecosystems. Biol. J. Linn. Soc. 2021, 133, 251–265. [Google Scholar] [CrossRef]
- Lullfitz, A.; Pettersen, C.; Reynolds, R.; Eades, A.; Dean, A.; Knapp, L.; Woods, E.; Woods, T.; Eades, E.; Yorkshire-Selby, G.; et al. The Noongar of south-western Australia: A case study of long-term biodiversity conservation in a matrix of old and young landscapes. Biol. J. Linn. Soc. 2021, 133, 432–448. [Google Scholar] [CrossRef]
- Lullfitz, A.; Knapp, L.; Cummings, S.; Hopper, S.D. First Nations’ interactions with underground storage organs in southwestern Australia, a Mediterranean climate Global Biodiversity Hotspot. Plant Soil 2022, 476, 589–625. [Google Scholar] [CrossRef]
- Cowling, R.M.; Potts, A.J.; Bradshaw, P.; Colville, J.; Arianoutsou, M.; Ferrier, S.; Forest, F.; Fyllas, N.M.; Hopper, S.D.; Ojeda, F.; et al. Variation in plant diversity in Mediterranean climate ecosystems: The role of climatic and topographical stability. J. Biogeogr. 2015, 42, 552–564. [Google Scholar] [CrossRef]
- Hopper, S.D.; Van Leeuwen, S.; Brown, A.P.; Patrick, S.J. Western Australia’s Endangered Flora; Department of Conservation and Land Management: Perth, Australia, 1990. [Google Scholar]
- Moran, G.F.; Hopper, S.D. Genetic diversity and the insular population structure of the rare granite rock species, Eucalyptus caesia Benth. Aust. J. Bot. 1983, 31, 161–172. [Google Scholar] [CrossRef]
- Byrne, M.; Hopper, S.D. Granite outcrops as ancient islands in old landscapes: Evidence from the phylogeography and population genetics of Eucalyptus caesia (Myrtaceae) in Western Australia. Biol. J. Linn. Soc. 2008, 93, 177–188. [Google Scholar] [CrossRef]
- Bezemer, N.; Krauss, S.L.; Roberts, D.G.; Hopper, S.D. Conservation of old individual trees and small populations is integral to maintain species’ genetic diversity of a historically fragmented woody perennial. Mol. Ecol. 2019, 28, 3339–3357. [Google Scholar] [CrossRef]
- Hopper, S.D. Out of the Ocbils—New hypotheses for the evolution, ecology and conservation of the eucalypts. Biol. J. Linn. Soc. 2021, 133, 342–372. [Google Scholar] [CrossRef]
- Robins, T.P.; Binks, R.M.; Byrne, M.; Hopper, S.D. Contrasting patterns of population divergence on young and old landscapes in Banksia seminuda (Proteaceae), with evidence for recognition of subspecies. Biol. J. Linn. Soc. 2021, 133, 449–463. [Google Scholar] [CrossRef]
- Heibl, C.; Renner, S.S. Distribution models and a dated phylogeny for Chilean Oxalis species reveal occupation of new habitats by different lineages, not rapid adaptive radiation. Syst. Biol. 2012, 61, 823–834. [Google Scholar] [CrossRef]
- Givnish, T.J.; Zuluaga, A.; Spalink, D.; Soto Gomez, M.; Lam, V.K.Y.; Saarela, J.M.; Sass, C.; Iles, W.J.D.; de Sousa, D.J.L.; Leebens-Mack, J.; et al. Monocot plastid phylogenomics, timeline, net rates of species diversification, the power of multi-gene analyses, and a functional model for the origin of monocots. Am. J. Bot. 2018, 105, 1888–1910. [Google Scholar] [CrossRef]
- Goldblatt, P. Cytology and systematics of the Moraea fugax complex (Iridaceae). Ann. Mo. Bot. Garden 1986, 73, 140–157. [Google Scholar] [CrossRef]
- Young, A.J.; Desmet, P.G. The distribution of the dwarf succulent genus Conophytum N.E.Br. (Aizoaceae) in southern Africa. Bothalia Afr. Biodivers. Conserv. 2016, 46, 1–13. [Google Scholar] [CrossRef]
- Powell, R.F.; Suarez, L.P.; Magee, A.R.; Boatwright, J.S.; Kapralov, M.V.; Young, A.J. Genome size variation and endopolyploidy in the diverse succulent plant family Aizoaceae. Bot. J. Linn. Soc. 2020, 194, 47–68. [Google Scholar] [CrossRef]
- Krejčíková, J.; Sudová, R.; Lučanová, M.; Trávníček, P.; Urfus, T.; Vít, P.; Weiss-Schneeweiss, H.; Kolano, B.; Oberlander, K.; Dreyer, L.L.; et al. High ploidy diversity and distinct patterns of cytotype distribution in a widespread species of Oxalis in the Greater Cape Floristic Region. Ann. Bot. 2013, 111, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Skeels, A.; Cardillo, M. Environmental niche conservatism explains the accumulation of species richness in Mediterranean-hotspot plant genera. Evolution 2017, 71, 582–594. [Google Scholar] [CrossRef]
- Van Santen, M.; Linder, H.P. The assembly of the Cape flora is consistent with an edaphic rather than climatic filter. Mol. Phylogenet. Evol. 2020, 142, 106645. [Google Scholar] [CrossRef]
- Thompson, J.D. Plant Evolution in the Mediterranean. Insights for Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2020. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopper, S.D. Ocbil Theory as a Potential Unifying Framework for Investigating Narrow Endemism in Mediterranean Climate Regions. Plants 2023, 12, 645. https://doi.org/10.3390/plants12030645
Hopper SD. Ocbil Theory as a Potential Unifying Framework for Investigating Narrow Endemism in Mediterranean Climate Regions. Plants. 2023; 12(3):645. https://doi.org/10.3390/plants12030645
Chicago/Turabian StyleHopper, Stephen D. 2023. "Ocbil Theory as a Potential Unifying Framework for Investigating Narrow Endemism in Mediterranean Climate Regions" Plants 12, no. 3: 645. https://doi.org/10.3390/plants12030645
APA StyleHopper, S. D. (2023). Ocbil Theory as a Potential Unifying Framework for Investigating Narrow Endemism in Mediterranean Climate Regions. Plants, 12(3), 645. https://doi.org/10.3390/plants12030645




