Abstract
Alopecia and gray hair are common hair abnormalities affecting physical appearance and causing psychological problems. Chemical treatments partially restore hair disorders but have distressing side effects. Bioactive plant compounds constitute promising sources of potential medicinal substances instead of chemical agents, producing high side effects. In this study, we focused on the waste of local rice cultivars: Bue Bang 3 CMU (BB3CMU) and Bue Bang 4 CMU (BB4CMU) from the north of Thailand. The rice bran oil (RBO), defatted rice bran extract (DFRB), and rice husk (H) were determined for in vitro hair revitalization in melanin production, nitric oxide (NO) secretion, and steroid 5α-reductase inhibition. The results indicated that BB4CMU-RBO with high contents of iron, zinc, and free fatty acids showed a comparable induction of melanin production on melanocytes (130.18 ± 9.13% of control) to the standard drug theophylline with no significant difference (p > 0.05). This promising melanin induction could be related to activating the NO secretion pathway, with the NO secretion level at 1.43 ± 0.05 µM. In addition, BB4CMU-RBO illustrated a significant inhibitory effect on both steroid 5α-reductase genes (SRD5A) type 1 and type 2, which relates to its primary source of tocopherols. Hence, rice bran oil from the Thai rice variety BB4CMU could be applied as a promising hair revitalizing candidate, from natural resources, to help promote hair growth and re-pigmentation effects.
1. Introduction
Plant-based substances have been recently developed for current global trends in the cosmeceutical industry, depending on customer behaviors and demands [1]. Furthermore, active compounds from plant resources, especially agricultural by-products, are environmentally-friendly with health benefits [2,3]. Modifying agricultural residues into value-added products is a sustainable development method for effectively using natural resources while reducing harmful environmental impacts [4]. It also complies with the Sustainable Development Goals (SDGs), such as SDG 3: Good health and well-being and SDG 12: Responsible consumption and production.
Oryza sativa L. (rice) is a cereal grain used for worldwide consumption. Polished rice is derived from whole grain rice and is used to prepare many kinds of food [5]. Rice bran and rice husk are major by-products generated from the rice milling process. Many studies have shown that high-value components in rice waste can be used in a variety of applications, including construction materials [6], nutritional supplements [7], and skin care products [8,9]. Similarly, we previously reported on both rice bran and rice husk extracts from the Thai rice variety, Bue Bang 3 CMU (BB3CMU), which possesses hair growth properties with high tocopherol contents [10]. Our current phytochemical studies of northern Thai rice cultivars demonstrated that oryzanol and tocopherol were profoundly enriched in BB3CMU and Bue Bang 4 CMU (BB4CMU) rice brans [11]. Taken together, both rice varieties may constitute potential sources for hair care applications. In our previous work, the extracts from rice by-products were separated into three distinct parts: non-polar rice bran oil extracts, rice bran residue extracts, and rice husk extracts. We further elucidated the phytochemical quantification of oryzanols, tocopherols, free fatty acids, and polyphenols [12].
The global market size of hair care was around USD 75.06 billion in 2020. This market is predicted to be worth USD 112.97 billion by 2028 [13]. Furthermore, hair loss treatment products and hair dyes accounted for 30–35% of the total market share among hair care products [14]. The demand for hair loss treatment products and hair colorants has rapidly increased because hair baldness and hair graying are recognized signs of aging. Visible hair problems mainly cause low self-confidence and affect general well-being as well as mental health [15,16]. Androgenetic alopecia (AGA) comprises chronic hair miniaturization affecting both male and female hair patterns. Higher levels of serum sex hormone, 5α-reductase, and androgen receptors in hair follicles are associated with premature baldness. The pathogenesis of AGA can be related to cystic acne, hirsutism, or virilization among females [17,18]. Therapeutic agents, such as topical minoxidil and antiandrogens are approved for AGA treatment [19]. The clinical evidence implicated adverse events with long-term medication. Itching, redness, and hypertrichosis on body areas are the common side effects of topical minoxidil. Moreover, sexual dysfunction, metabolic syndromes, and depression are related to an alteration in androgens [20,21].
Hair graying is visualized as a loss of pigment in the hair shaft. Melanin pigments play a crucial role in hair photoprotection, especially black eumelanin. Melanin production is regulated by various proteins, such as tyrosinase and tyrosinase-related proteins [22]. An earlier study found that inherited mutations of melanogenic proteins can cause cutaneous hypopigmentation with canities [23]. Moreover, diet and nutritional status have a considerable impact on premature hair graying. A dietary supplement is partially effective in reversing hypopigmented hair [24,25]. Hair dying is another option for covering gray hair [26]. Additionally, excipient ingredients, such as p-phenylenediamine, preservatives, or surfactants in hair colorants, may cause allergies and cancer [27,28].
Therefore, this study aimed to determine the biological activities of three different rice by-products (BB3CMU and BB4CMU) as botanical therapeutic agents in terms of antioxidant properties, melanin production, nitric oxide production, and any inhibitory effects on steroid 5α-reductase isoenzymes.
2. Results and Discussion
2.1. Extraction Process
Both Thai rice varieties BB3CMU and BB4CMU were successfully registered as new plant varieties through Plant Breeder’s Rights (PBR) under the Department of Agriculture, Thailand, in November 2020 and February 2022, respectively. Both rice cultivars have white non-glutinous grains with brown husks. The distinct morphological characteristics of these varieties are based on stem and leaf features. The stem diameter of BB3CMU is approximately 10.19 mm, and the leaf dimensions are 1.55 cm and 42.34 cm. In contrast, the rice stem diameter of BB4CMU is about 8.05 mm, with a larger leaf size (1.59 cm and 58.90 cm) and a hairy surface on the leaf blade and leaf sheath. According to our previous experiment, the proximate analysis indicated that rice variety BB3CMU possessed the highest crude fat content of 18.68 ± 0.11%, followed by that of BB4CMU at 18.55 ± 0.19%, among eleven rice varieties. Moreover, Wisetkomolmat et al. reported that BB3CMU and BB4CMU showed high contents of crude fiber and crude protein. For the mineral content analysis, both BB3CMU and BB4CMU demonstrated high contents of K (1.98 ± 0.00 and 2.13 ± 0.00 g/100 g sample), Mg (0.63 ± 0.00 and 0.72 ± 0.02 g/100 g sample), Ca (288.93 ± 35.96 and 310.63 ± 13.26 mg/kg sample), Fe (71.62 ± 0.74 and 83.17 ± 1.09 mg/kg sample), Mn (112.88 ± 5.76 and 136.50 ± 0.60 mg/kg sample), Zn (66.07 ± 1.73 and 73.57 ± 3.98 mg/kg sample), and Na (33.10 ± 0.78 and 39.00 ± 0.63 mg/kg sample), respectively [11].
After the screw press process, augmented with dichloromethane extraction, rice bran oils (RBO) from BB3CMU and BB4CMU are dark brown viscous oils with extraction yields of 20.12% and 19.17% w/w based on rice bran materials, respectively. The screw press is a favored oil extraction method without heat treatment [29]. Moreover, dichloromethane is an effective inorganic solvent for plant oil production [30]. Thus, mechanical extraction with an inorganic solvent was conducted to refine the crude oil from rice bran samples. Then, the defatted residues were macerated with an ethanol solution to obtain other semi-polar and polar compounds in defatted rice bran extracts (DFRB), comprising light brown greasy pastes. The extraction yields of both BB3CMU-DFRB and BB4CMU-DFRB were 7.67% and 7.13% w/w of de-oiled rice bran samples, respectively. For rice husk (H) portions, the physical attributes of both BB3CMU-H and BB4CMU-H were non-glossy crude extracts with extraction yields of 2.09% and 2.00% w/w, based on the dry husk mass [12].
2.2. In Vitro Antioxidant Activities
The antioxidant activities of O. sativa by-product extracts were demonstrated by the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) assay, the 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical (ABTS) assay, and metal chelation. DPPH is a stable radical and can be scavenged by antioxidants in lipophilic samples, whereas the ABTS assay is used to examine both lipophilic and hydrophilic compounds [31]. Metal chelation is used to determine the chelating process with an ion-substrate complex in the system, such as ferrous-ferrozine formation [32]. The antioxidant capacity obtained from these methods correlated to biomolecules in medicinal plants, e.g., flavonoids and phenolic compounds [33,34].
The results of the present study showed that rice husk extracts possessed antioxidant properties (Table 1), which were higher than those of rice bran oil and rice bran extracts. In vitro scavenging activities using the DPPH and ABTS methods were observed for BB3CMU-H (334.70 ± 4.64 and 196.13 ± 10.09 mg TE/g extract), and BB4CMU-H (198.04 ± 2.75 and 164.10 ± 8.45 mg TE/g extract), respectively. The highest chelating activity determined using iron chelation was found for BB3CMU-H with 283.22 ± 55.35 mg EDTAE/g extract. The antioxidant effects of both BB3CMU-H and BB4CMU-H agreed with those of the high phenolic content in the rice husk portion. The bioactive compositions of the rice husk extracts were performed using the high-performance liquid chromatography (HPLC) analysis, as reported in our previous work. The rice husk extracts of O. sativa cv. BB3CMU and BB4CMU were found to be high in polyphenols, such as phytic acid, p-coumaric acid, and kaempferol [12]. In biochemical systems, phytic acid with a strong chelating activity can prevent the formation of hydroxyl radicals [35]. The previous studies showed that p-coumaric acid was involved in the promotion of hair growth by reducing oxidative stress [36,37]. Additionally, numerous reports revealed that kaempferol had a potent scavenging ability and regulated inflammatory responses [10,38].

Table 1.
Antioxidant potential of Oryza sativa L. cv. BB3CMU and BB4CMU extracts.
2.3. Cell Viability
The cytotoxic effects of rice extracts were performed on RAW 264.7 (murine macro-phage), B16F10 (mouse melanoma), and DU-145 (human prostate cancer) cells. The maximum non-cytotoxic concentration (>80% cell viability) [39] of each extract was considered as the treatment concentration for subsequent experiments. The viability of RAW 264.7 cells was not altered over the concentration range of 0.0001 to 0.10 mg/mL of rice bran oils and rice husk extracts for 24 h. However, defatted rice bran extracts illustrated a higher toxicity than rice bran oil and husk fractions. The defatted rice bran extracts at 0.0001 mg/mL revealed no cytotoxicity on macrophage cells. Therefore, 0.10 mg/mL of both rice bran oils and rice husk extracts and 0.0001 mg/mL of the defatted rice bran extracts were selected for RAW 264.7 cell exposure. Moreover, all extracts at a concentration of 0.01 mg/mL showed non-cytotoxic effects on melanoma cells after 48 h of incubation. Thus, the melanin content assay would be tested with 0.01 mg/mL of all rice fractions. On prostate cancer cells, no cytotoxic effect was noticed after exposure to 0.50 mg/mL of the defatted rice bran extracts, 0.25 mg/mL of the rice bran oils, and 0.10 mg/mL of the rice husks after 24 h of incubation. These concentrations were used to further evaluate using DU-145 cells. Moreover, rice extracts were evaluated for cytotoxicity in human fibroblasts and dermal papilla cells from human hair follicles (HFDPC). The non-cytotoxic effects of extracts may not affect scalp cells. The rice extracts at 0.25 mg/mL did not show any toxicity to human fibroblasts or HFDPC (>80% cell viability of control). It could be assured that rice extracts at high concentrations might be suitable for human scalp application.
2.4. Effects of Oryza sativa L. Extracts on Melanin Production in Melanoma Cells
Melanin pigments in hair shafts are synthesized by the melanocytes in the hair bulb, interacting with the melanin precursors in the bulb keratinocytes [40]. Hair pigmentation is modulated by melanogenic regulatory factors, such as tyrosinase, tyrosinase-related proteins, melanocyte-stimulating hormone, and melanocortin receptor. The conversion of L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA) by the copper-dependent tyrosinase enzyme is the rate-limiting process in melanin biosynthesis [41,42,43]. Chelating with copper ions in the active site can inhibit this enzyme, whereas L-DOPA, superoxide anion, and nitric oxide (NO) as electron donors can activate enzyme activity [23,44]. Pigmented hair shafts are produced notably during the late anagen phase then regress and diminish in catagen to the resting stage of the hair cycle [45].
However, the lack of melanogenic melanocytes during the anagen phase is the primary cause of hair graying. The failure of melanogenic activity is a consequence of reactive oxygen species (ROS) damage along with the impairment of the antioxidative defense system due to the effects of UV radiation, smoking, and pollution [46,47]. According to the previous research, the natural antioxidant properties from Eurotium cristatum [48], Melissa officinalis [49], Cannabis sativa [50], and Bixa orellana [51] promoted melanogenesis and can be used as potential preventive and therapeutic agents for skin and hair hypopigmentation. Hence, the phenolic profile of rice husk extracts with scavenging effects may provide protection against ROS.
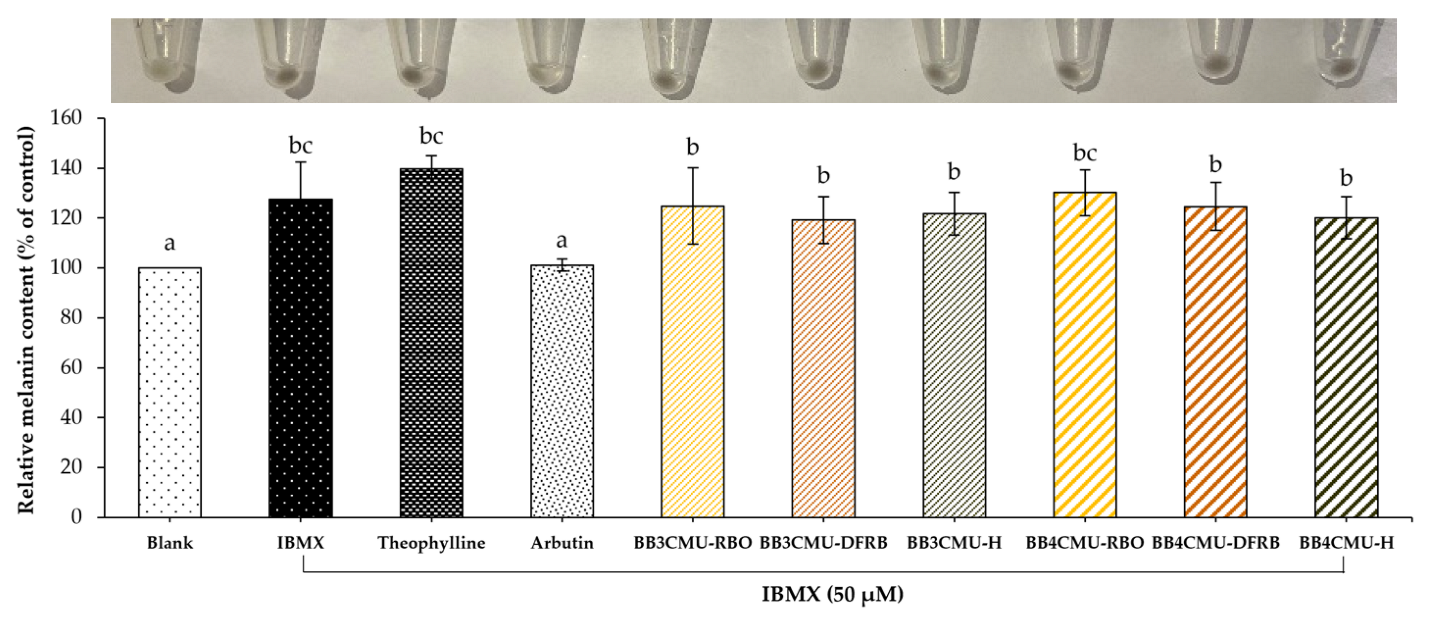
According to the study results, both BB3CMU and BB4CMU extracts could up-regulate melanin synthesis in B16F10 melanoma cells. Notably, the stimulating effects on melanin synthesis of BB4CMU-RBO (130.18 ± 9.13% of control) showed a comparable effect to the standard theophylline (139.73 ± 5.20% of control) with no significant difference at 0.01 mg/mL (Figure 1). Theophylline is used as a melanogenic stimulating agent affecting melanogenesis by elevating cyclic adenine monophosphate (cAMP) via the mitogen-activated protein kinase 1 (MEK 1/2) and the Wnt/β-catenin biological signaling pathways [52,53]. This result suggested that the melanogenesis stimulating ability of BB4CMU-RBO may contribute to iron, zinc, and unsaturated fatty acids. Malnutrition and vitamin deficiencies can cause or contribute to canities. Iron, zinc, copper, calcium, and cobalamin could modulate melanogenesis in hair follicles, leading to reversible graying of hair [47,54,55].
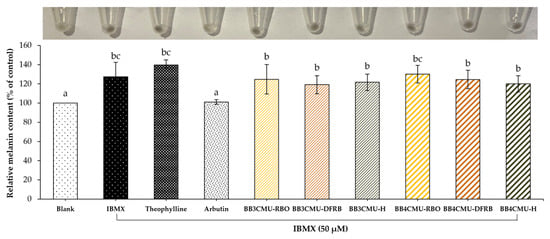
Figure 1.
Effects of Oryza sativa L. cv. BB3CMU and BB4CMU extracts at 0.01 mg/mL on melanin content in B16F10 melanoma cells stimulated with 50 µM 3-isobutyl-1-methylxanthin (IBMX) for 48 h. Theophylline and arbutin (0.01 mg/mL) were used as standard controls. Cells cultured in the medium without treatment refer to the blank. Different letters within each treatment indicate significant differences (p < 0.05).
A previous report illustrated that iron treatment can elevate the number of maturing melanosomes, the pigmentation in melanocytes, and the expression of the melanocyte-inducing transcription factor (MITF) [56]. Interestingly, zinc is recognized as a cofactor of tyrosinase-related protein type 1 [57] and can prevent the cell apoptosis of melanocytes [58]. In addition, previous reports demonstrated that rice bran extracts with high contents of linoleic acid or silicic acid have the potential to restore the melanogenesis process [59,60,61]. Our previous study revealed the nutritional profiles of eleven rice bran varieties from the north of Thailand. The mineral components in BB4CMU surpassed other rice cultivars, especially regarding iron (83.17 ± 1.09 mg/kg rice bran sample) and zinc (73.57 ± 3.98 mg/kg rice bran sample). Moreover, BB4CMU rice bran contained a high concentration of essential unsaturated fatty acids varieties, such as oleic acid, linoleic acid, and α-linolenic acid [11], supporting the melanogenesis ability of BB4CMU-RBO.
2.5. Effects of Oryza sativa L. Extracts on Nitric Oxide Production in Macrophages
Nitric oxide (NO) is synthesized from the amino acid L-arginine and oxygen molecules by nitric oxide synthases (NOS). The NOS enzyme was observed in keratinocytes as well as melanocytes. NO plays an important part in the physiological system as a key signaling molecule. The role of NO may involve immune system responses, vascular function, and skin homeostatic regulation [62,63,64]. Neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS) are the three isoforms of NOS [65]. The iNOS usually produces NO after lipopolysaccharide (LPS) stimulation. In the previous experiment, iNOS was expressed on the scalp epidermis, whereas nNOS and eNOS were mostly observed on epidermal melanocytes and dermal keratinocytes [66].
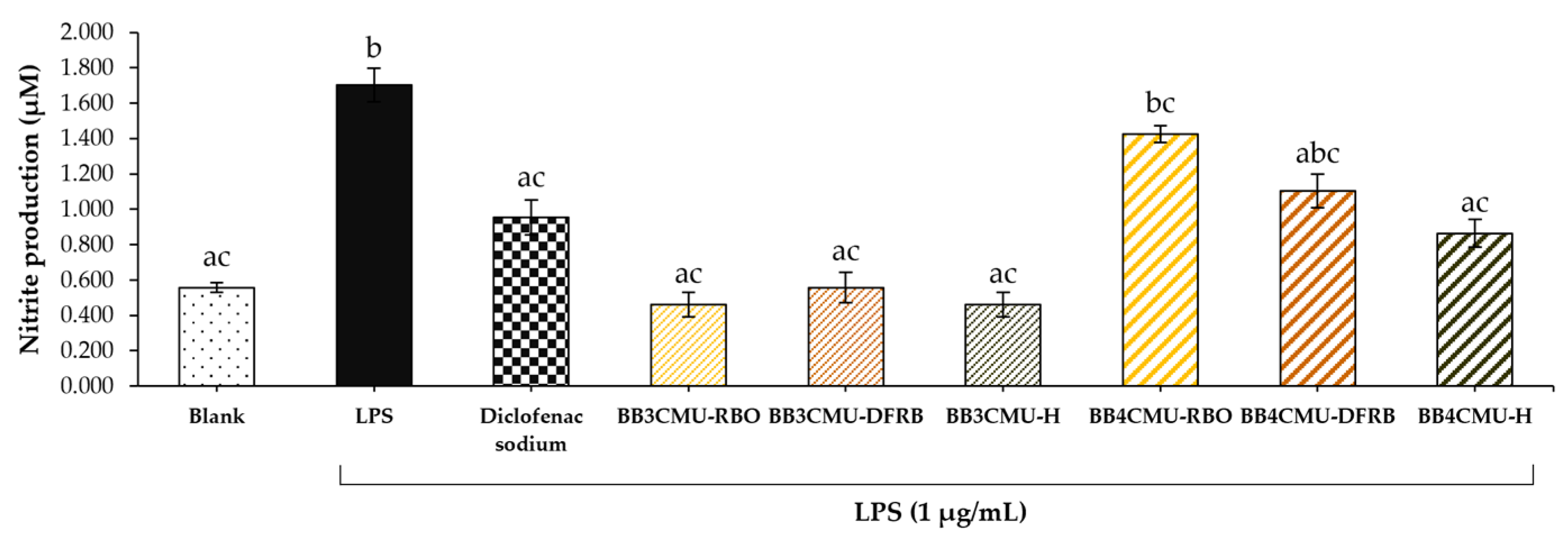
The measured nitrite content indicated NO production in macrophage cells [67]. As shown in Figure 2, BB4CMU-RBO displayed the greatest nitrite production (1.43 ± 0.05 µM), followed by BB4CMU-DFRB (1.10 ± 0.10 µM), which were comparable to the LPS treatment (1.70 ± 0.10 µM). Previous studies suggested that melanin synthesis could be enhanced by NO production [62,68]. After ultraviolet (UV) exposure, the intracellular level of NO is remarkably increased and then activates melanogenesis through the cyclic guanosine monophosphate (cGMP) signaling pathway [69,70]. It has been established that NO regulates soluble guanylate cyclase, which turns guanosine triphosphate (GTP) to cGMP and further elevates protein kinase G (PKG), resulting in increased expressions of pigmentation genes and melanin production [71,72,73]. Some evidence suggested that NO is associated with the complex effects in melanogenesis, including the morphological appearance of dendritic melanocytes, facilitation of melanosome aggregation, and increasing the melanin synthesis activity of melanocytes [74].
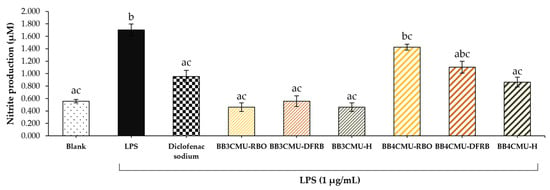
Figure 2.
Effects of Oryza sativa L. cv. BB3CMU and BB4CMU extracts (RBO, 0.10 mg/mL; DFRB, 0.0001 mg/mL; H, 0.10 mg/mL) on nitrite content in RAW 264.7 macrophages induced with 1 µg/mL lipopolysaccharide (LPS) for 24 h. Diclofenac sodium (0.10 mg/mL) was used as standard control. Cells cultured in the medium without treatment refer to the blank. Different letters within each treatment indicate significant differences (p < 0.05).
The previous articles presented that saturated fatty acids (palmitic acid and steric acid) and unsaturated fatty acids, e.g., oleic acid and linoleic acid, could induce NO production in macrophage cells, resulting in macrophage differentiation [75,76]. M1-like macrophages (antigen-presenting cells) are involved in releasing pro-inflammatory cytokines and T-cell function, which can be activated by saturated fatty acids. On the other hand, unsaturated fatty acids could influence the M2-like phenotype (alternatively activated macrophages), leading to tissue repair and anti-inflammatory cytokine secretion [75,77]. In this study, BB4CMU-RBO was rich in free fatty acids, consisting of palmitic acid, steric acid, oleic acid, and linoleic acid [11], corresponding to an increase in melanin content via NO induction. Remarkably, palmitic acid could increase tyrosinase-related proteins, resulting in inducing melanin production [78].
2.6. Effects of Oryza sativa L. Extracts on Gene Expression of Steroid 5α-Reductase Isoenzymes
The different stages of the hair cycle include the growth (anagen), transition (catagen), resting (telogen), and shedding (exogen) stages. Normally, a new hair matrix is formed during the anagen then regresses and undergoes apoptosis in the telogen, and the active hair follicle would regrow after the shedding step [79,80]. The hair-growth cycle is regulated by multiple signaling pathways, including the Wnt/β-catenin, sonic hedgehog (Shh), notch, angiogenesis, and transforming growth factor (TGF)-β signaling pathways. Hair development is thought to be influenced by Wnt, Shh, and vascular growth factors, whereas inflammatory cytokines, TGF-β and 5α-reductase dominate the negative aspects of hair regeneration [81,82]. In particular, the androgen-dependent pathway is prone to associate with disease-induced hair loss [83] and genetic variant-mediated alopecia [84,85].
The pathophysiology of hair loss may be complicated by complex processes. The most common hair loss disorder is AGA, which is consistent with genetic factors and androgen excess. Testosterone, the active androgen in circulation is converted to the most potent androgen, dihydrotestosterone (DHT) by 5α-reductase [21,86]. This enzyme can be divided in multiple isoforms, such as 5α-reductase type 1 (SRD5A1), type 2 (SRD5A2), and type 3 (SRD5A3). SRD5A2, located on the dermal papilla, is the target enzyme for treating hair baldness. Finasteride, a selective SRD5A2 inhibitor, and dutasteride, an inhibitor of both SRD5A1 and SRD5A2, are approved drugs for AGA treatment. However, the SRD5A2 isoenzyme is predominantly distributed in various organs, including the prostate, testes, and liver [87]. Thus, undesirable side effects markedly appear after drug administration, such as sexual dysfunction, testicular pain, and mental illness [88]. Natural substances, attenuating the action of SRD5A isoenzymes, seem highly probable agents for hair loss treatment with fewer adverse effects [89,90].
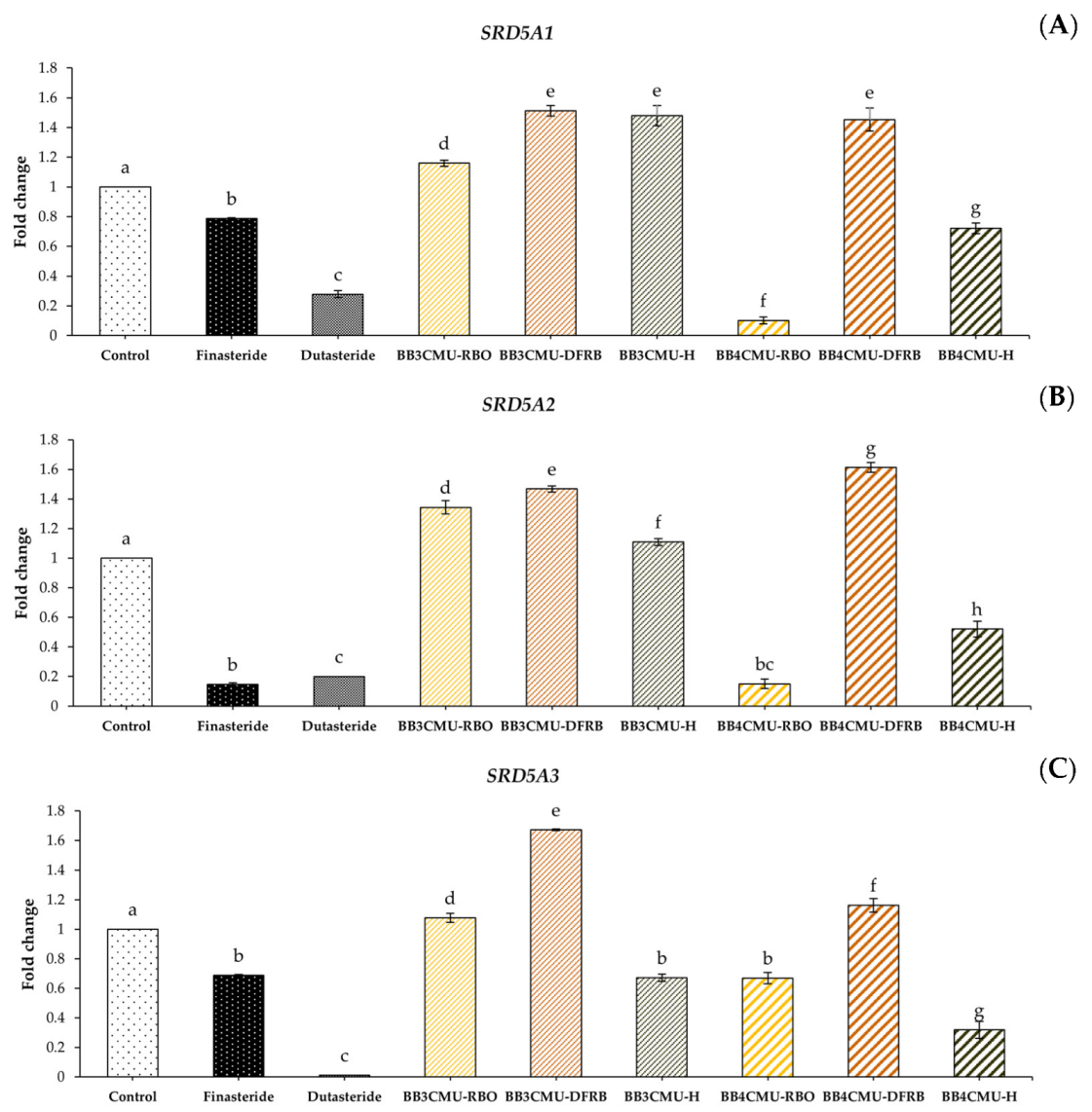
In this study, BB4CMU-RBO resulted in a significant down-regulation in the gene expression of both SRD5A1 and SRD5A2 among all the other extracts. Interestingly, BB4CMU-RBO treatment demonstrated the gene inhibition of SRD5A2 when compared with the standard drugs: finasteride and dutasteride (Figure 3). The highest SRD5A2 gene inhibition activity of BB4CMU-RBO may be attributed to our previous study presenting the highest amount of β-tocopherol (1.16 ± 0.01 mg/100 g crude fat), followed by γ-tocopherol (6.78 ± 0.04 mg/100 g crude fat), and α-tocopherol (15.84 ± 0.03 mg/100 g crude fat), consecutively [11]. Moreover, we also reported the binding affinity of bioactive compounds in rice bran extract using molecular docking, in which β-tocopherol, γ-tocopherol, and α-tocopherol can possess a specific binding to SRD5A2 [91]. Therefore, SRD5A2 inhibition by the BB4CMU-RBO extract may be attributed to the structural features of tocopherols. Our data agreed with the recent studies of Argania spinosa [92] and Prunus mira [93], which displayed hair growth promotion related to the contents of tocopherols. Our findings demonstrated that BB4CMU-RBO, with its main tocopherol compounds, essential fatty acids, and mineral contents presented properties with a high potential for regenerating hair.
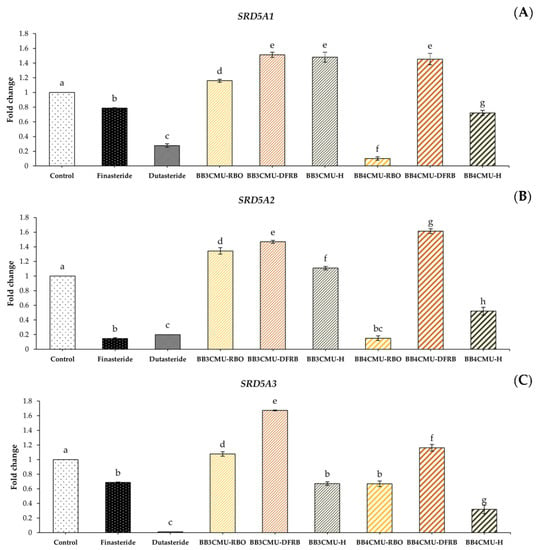
Figure 3.
Effects of Oryza sativa L. cv. BB3CMU and BB4CMU extracts (RBO, 0.25 mg/mL; DFRB, 0.50 mg/mL; H, 0.10 mg/mL) on mRNA expression of (A) SRD5A1; (B) SRD5A2; (C) SRD5A3 in DU-145 prostate cancer cells after 24 h of treatment. Finasteride and dutasteride (0.10 mg/mL) were used as standard controls. Different letters within each treatment indicate significant differences (p < 0.05).
3. Materials and Methods
3.1. Chemicals and Reagents
The materials 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (ethylbenzthiazoline-6-sulfonic acid (ABTS), 3-(2-yyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4′′-disulfonic acid sodium salt (ferrozine), iron (II) chloride tetrahydrate (FeCl2 · 4H2O), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ethylenediaminetetraacetic acid (EDTA), sulforhodamine B (SRB), D-glucose monohydrate, diclofenac sodium, lipopolysaccharide (LPS), arbutin, theophylline were purchased from Sigma Aldrich (St. Louis, MO, USA). Roswell Park Memorial Institute 1640 medium (RPMI-1640), Dulbecco’s Modified Eagle Medium (DMEM), Eagle’s Minimal Essential Medium (MEM), fetal bovine serum (FBS), 0.5% Trypsin-EDTA (10X), and 3-isobutyl-1-methylxanthine (IBMX) were obtained from Gibco Life Technologies (Grand Island, NY, USA). Penicillin/streptomycin solution (100×) was obtained from Capricorn Scientific GmbH (Ebsdorfergrund, Germany). The Griess reagent kit was acquired from Invitrogen, Thermo Fisher Scientific (Waltham, MA, USA). Finasteride and dutasteride were purchased from Wuhan W&Z Biotech (Wuhan, China). RedSafe™ dye was obtained from iNtRON Biotechnology (Gyeonggido, Korea). All other chemical substances were of analytical grade.
3.2. Plant Materials and Preparation of Sample Extraction
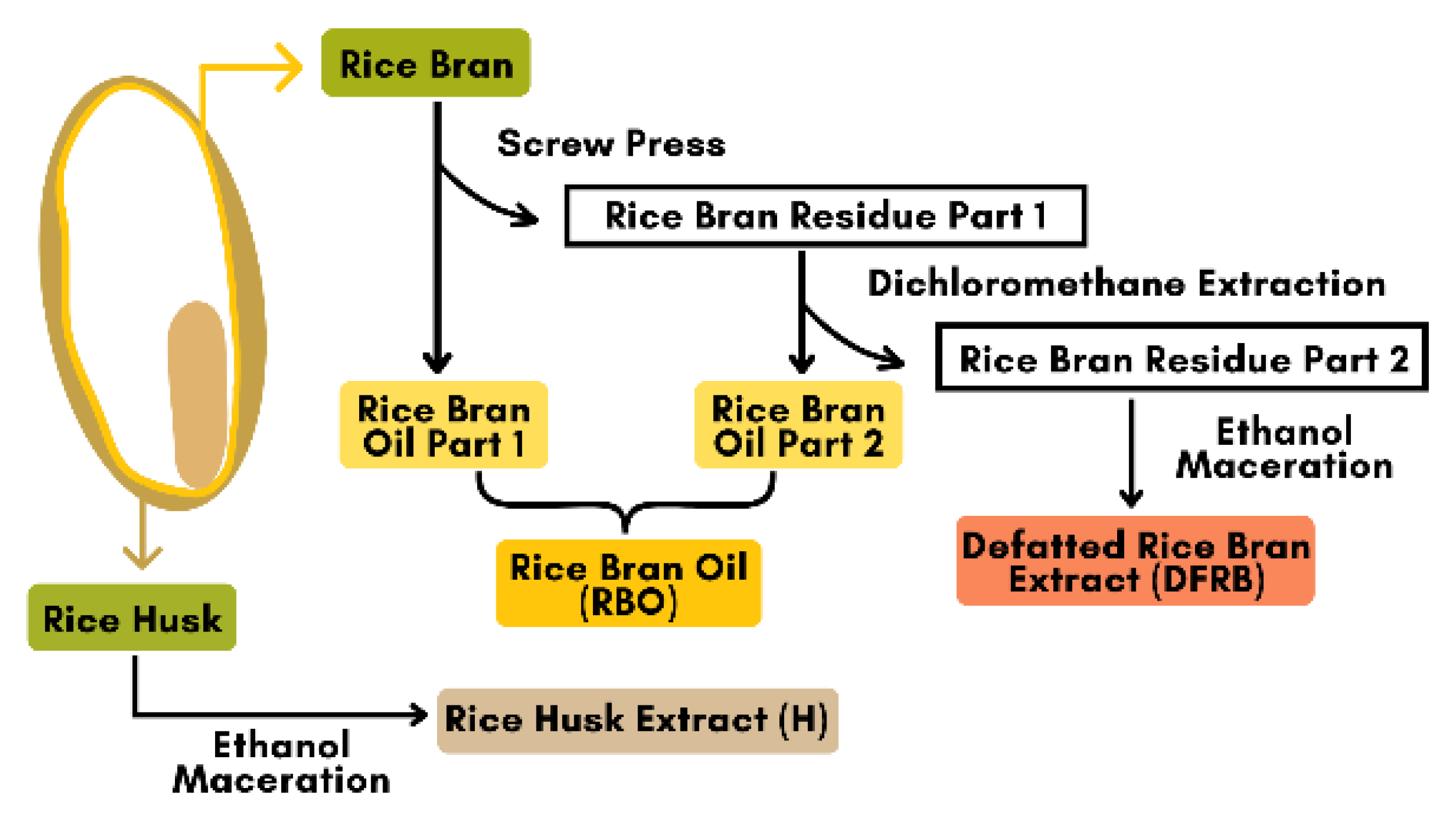
Oryza sativa cv. Bue Bang 3 CMU (BB3CMU) and Bue Bang 4 CMU (BB4CMU) were obtained from the Lanna Rice Research Center, Chiang Mai University, Chiang Mai, Thailand. Both rice cultivars were grown under the same environmental conditions for six months, between June and December 2021. The rice extracts were provided by the Pharmaceutical and Natural Products Research and Development Unit (PNPRDU), Chiang Mai University, Chiang Mai, Thailand. The sample extraction followed the scheme illustrated in Figure 4. Briefly, the rice husk was first removed from the rice seed, and then the rice bran was separated from the white rice grain. Rice bran (1000 g) was fed into the mechanical screw press to obtain the rice bran oil part 1 and its residue. Then, the rice bran residue part 1 (100 g) was further extracted using the dichloromethane extraction (2 L) for 72 h to obtain the rice bran oil part 2 and the rice bran residue part 2. The rice bran oil (RBO) in this study was a mixture of the rice bran oil parts 1 and 2. After that, the rice bran residue part 2 (100 g) was macerated with 95% ethanol (2 L) for 72 h and then filtered and concentrated by a rotary evaporator to obtain the defatted rice bran extract (DFRB). Moreover, the dried husk (100 g) was mixed with 95% ethanol (6 L) and followed with a 48-h maceration to obtain the rice husk extract (H). All stages of the extraction methods were performed at room temperature. The rice samples were labeled as follows: BB3CMU-RBO, BB3CMU-DFRB, BB3CMU-H, BB4CMU-RBO, BB4CMU-DFRB, and BB4CMU-H. Consequently, all extracts were kept at a temperature of 4 °C until used.
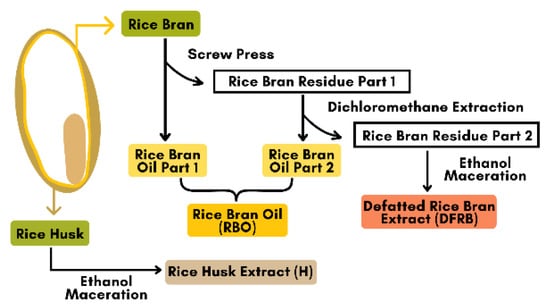
Figure 4.
Scheme of rice by-products extraction from Oryza sativa L. cv. Bue Bang 3 CMU (BB3CMU) and Bue Bang 4 CMU (BB4CMU).
3.3. Antioxidant Activity Assays
The antioxidant potential of the samples was performed using the DPPH radical scavenging assay, the ABTS radical scavenging assay, and the iron chelating assay, as previously described [94]. Briefly, each sample was diluted in the range of 0.01–10 mg/mL. For the DPPH assay, 50 µL of the sample solution was mixed with 50 µL of DPPH working solution, then incubated in a dark at room temperature for 30 min. The absorbance of each well was determined at 517 nm using the EZ2000 microplate reader (Biochrome Ltd., Cambridge, UK). For the ABTS assay, 25 µL of the sample solution was reacted with 200 µL of the ABTS working solution, then incubated at room temperature for 10 min. The absorbance was measured at 734 nm. Trolox was used as the standard scavenging agent for the DPPH and ABTS assays. For metal chelation, 100 µL of the sample solution was reacted with 50 µL of 2 mM ferrous chloride solution and 50 µL of 5 mM ferrozine substrate. After incubating at room temperature for 30 min, the absorbance was measured at 562 nm. EDTA was used as the standard chelating agent. Blanks for every sample without free radical or heavy metal solutions were also conducted for each measured absorbance. The results were obtained from the line slope of each sample, which was divided by the line slope of the standard and expressed as mg of Trolox equivalent/g of extract and mg of EDTA equivalent/g of extract [95].
3.4. Cell Cultures
Mouse skin melanoma cells (B16F10; JCRB no. JCRB0202) and human fibroblast cells (JCRB no. JCRB1006.4F) were purchased from the JCRB Cell Bank (Osaka, Japan). Murine RAW 264.7 macrophage cells and human prostate cancer cells (DU-145) were purchased from the American Type Culture Collection (Rockville, MD, USA). Primary human follicle dermal papilla cells (HFDPC) were obtained from Promo Cell GmbH (Heidelberg, Germany). The B16F10 cells were grown in a MEM culture medium supplemented with 10% FBS and 1% penicillin/streptomycin. The RAW 264.7 cells were grown in a DMEM culture medium supplemented with 4500 mg/L D-glucose, 10% FBS, and 1% penicillin/streptomycin. The DU-145 cells were grown in RPMI-1640 culture medium supplemented with 10% FBS and 1% penicillin/streptomycin. The fibroblast cells were cultured in a DMEM culture medium supplemented with 10% FBS, and 1% penicillin/streptomycin. The HFDPC cells were cultured in a Growth Medium Kit supplemented with 10% FBS and 1% antibiotic-antimycotic 100× solution. All cell lines were maintained under a humidified atmosphere containing 5% CO2 at a temperature of 37 °C.
3.5. Cytotoxicity Assay
The cell viability of B16F10, RAW 264.7, DU-145, fibroblast, and HFDPC cells for determining non-toxic concentrations (above 80% cell viability) was evaluated through the colorimetric SRB assay [10]. The cells were seeded into 96-well plates at a density of 1 × 104 cells/well. After 24 h, the growth medium was replaced with fresh serum-free medium containing various concentrations (0.0001–1 mg/mL) of rice extracts and standard controls on RAW 264.7, DU-145, and HFDPC cells for 24 h and on B16F10 and fibroblast cells for 48 h. Following incubation, 50% trichloroacetic acid was added to fix cells for 1 h at a temperature of 4 °C. The cells were rinsed with deionized water and air-dried. After that, cells were stained using a 0.04% SRB solution for 30 min at room temperature, and then the unbound dye was discarded by washing cells with 1% acetic acid and air-drying. The bound dye was dissolved with a 10 mM Tris base. The absorbance was measured at 515 nm using a microplate reader. The cell viability of the treated cells was compared with untreated cells as the percentage of cell viability of control.
3.6. Melanin Content Assay
This assay was examined on murine melanoma cells by a slight modification method [96,97]. Briefly, the B16F10 melanoma cells (2.5 × 105 cells/well) were seeded in 6-well plates. After 24 h of incubation, the cells were treated with serum-free medium containing 50 µM IBMX and the rice samples or the standard controls (theophylline and arbutin) at a concentration of 0.01 mg/mL for an additional 48 h. Then, the cell pellets were harvested using 0.25% trypsin-EDTA and centrifuged at 10,000× g for 10 min. The intracellular melanin was dissolved in 1 N NaOH containing 10% DMSO at a temperature of 80 °C for 30 min. The amount of melanin was measured using an absorbance of 405 nm. The results were expressed as the relative percentage of melanin content of the control (untreated cells).
3.7. Intracellular Nitric Oxide Production
The nitric oxide production in macrophage cells was expressed as the amount of accumulated nitrite in the cell supernatant. This method was modified from a previously published method [98,99]. Briefly, the RAW 264.7 cells were plated at a density of 1 × 105 cells/well in 96-well plates for 24 h. Then, the cells were induced by 1 µg/mL LPS for 24 h after pre-treatment with the rice extracts (RBO, 0.10 mg/mL; DFRB, 0.0001 mg/mL; and H, 0.10 mg/mL) or the standard diclofenac sodium (0.10 mg/mL). The supernatant was collected to react with the Griess reagent according to the manufacturer’s recommendations. The nitrite content of each sample was calculated using the standard curve of sodium nitrite.
3.8. RNA Extraction and Semi-Quantitative Reverse Transcription Polymerase Chain Reaction
The modulation of the SRD5A gene expression was observed in prostate tumor cells as previously described [10]. The DU-145 cells were used as an in vitro cell model to determine the expression of 5α-reductase for anti-hair loss applications [100,101,102] because the action of 5α-reductase was obviously evaluated in prostate cells [103]. Total RNA was isolated from the DU-145 cells treated with samples (RBO, 0.25 mg/mL; DFRB, 0.50 mg/mL; and H, 0.10 mg/mL) or SRD5A enzyme inhibitors (dutasteride and finasteride at a concentration of 0.10 mg/mL) for 24 h. The RNA extraction process was performed using the E.Z.N.A.® Total RNA Kit I (Omega Bio-Tek, Norcross, GA, USA) following the manufacturer’s instructions. The RNA concentrations were determined using the Qubit™ 4 fluorometer and Qubit™ RNA HS Assay Kit (Invitrogen, Carlsbad, CA, USA). Complementary DNA (cDNA) was synthesized using the RT-PCR Quick Master Mix (Toyobo, Osaka, Japan) as the template and the following primers for amplification: SRD5A1 (F: 5′-AGCCATTGTGCAGTGTATGC-3′ and R: 5′-AGCCTCCCCTTGGTATTTTG-3′), SRD5A2 (F: 5′-TGAATACCCTGATGGGTGG-3′ and R: 5′-CAAGCCACCTTGTGGAATC-3′), SRD5A3 (F: 5′-TCCTTCTTTGCCCAAACATC-3′ and R: 5′-TCCTTCTTT-GCCCAAACATC-3′), and GAPDH (F: 5′-GGAAGGTGAAGGTCGGAGTC-3′ and R: 5′-CTCAGCCTTGACGGTGCCATG-3′). GAPDH was used to normalize the expression of the target genes. The PCR product was separated through electrophoresis on 1% agarose gel and stained using RedSafe™ dye. The DNA bands were visualized on a gel documentation system (Gel Doc™ EZ, Bio-Rad, Hercules, CA, USA) and Image Lab™ Software (Bio-Rad, Hercules, CA, USA).
3.9. Statistical Analysis
All experiments were performed in triplicate and reported as means ± standard deviation (SD). All data were analyzed using the one-way analysis of variance (ANOVA) test along with the LSD’s post hoc test using SPSS 23.0 Software (SPSS Inc., Chicago, IL, USA). The significant difference was defined as a p-value < 0.05.
4. Conclusions
Rice bran and rice husk are the most common agricultural waste with deteriorated environmental effects. Our previous studies illustrated the abundant constituents of rice by-products extracts from numerous Thai rice varieties. Consecutively, in this study, BB3CMU and BB4CMU were selected for biological testing using cell-based assays to identify a potential for hair nourishing effects and for restoring hair. Impressively, rice bran oil from BB4CMU showed ameliorable effects on NO-induced melanin production, which was attributed to the mineral content and free fatty acid profiles. According to the tocopherols-enrich rice bran oil fraction, the BB4CMU-RBO extract showed promising SRD5A2 down-regulation. Possibly, BB4CMU-RBO is to be regarded as a re-pigmenting agent in graying hair and an effective treatment for androgenetic hair loss. Furthermore, BB4CMU-RBO could be used as a natural alternative treatment and a substitution for conventional drugs that possess strong side effects. Overall, the results in this study illustrated the pharmaceutical potential of plant resources for restoring hair in terms of hair growth and hair re-pigmentation.
Author Contributions
Conceptualization, W.R.; methodology, P.L. and W.R.; validation, P.L. and K.S.; formal analysis, A.M.; investigation, P.L., C.A. and A.M.; resources, C.P.-u.-t., S.J., S.Y. and W.R.; data curation, P.L.; writing—original draft preparation, P.L., C.K. and W.R.; writing—review and editing, W.R., P.L., C.K., K.S., P.R., P.J., K.J., Y.P., F.J.B., S.R.S., R.C. and K.B.; supervision, W.R.; project administration, W.R.; funding acquisition, W.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research project is supported by the CMU Presidential Scholarship: funding number 2564-017, Fundamental Fund 2022 of Chiang Mai University, and partially supported from Chiang Mai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors appreciate the fund received from the CMU Presidential Scholarship, Fundamental Fund 2022 of Chiang Mai University and research facilities from the Faculty of Pharmacy, Chiang Mai University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amberg, N.; Fogarassy, C. Green consumer behavior in the cosmetics market. Resources 2019, 8, 137. [Google Scholar] [CrossRef]
- Faria Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Cosma, P. Hair care cosmetics: From traditional shampoo to solid clay and herbal shampoo, a review. Cosmetics 2019, 6, 13. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Urena, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Hossain, S.S.; Mathur, L.; Roy, P. Rice husk/rice husk ash as an alternative source of silica in ceramics: A review. J. Asian Ceram. Soc. 2018, 6, 299–313. [Google Scholar] [CrossRef]
- Spaggiari, M.; Dall’Asta, C.; Galaverna, G.; del Castillo Bilbao, M.D. Rice bran by-product: From valorization strategies to nutritional perspectives. Foods 2021, 10, 85. [Google Scholar] [CrossRef]
- Manosroi, A.; Ruksiriwanich, W.; Manosroi, W.; Abe, M.; Manosroi, J. In vivo hair growth promotion activity of gel containing niosomes loaded with the Oryza sativa bran fraction (OSF3). Adv. Sci. Lett. 2012, 16, 222–228. [Google Scholar] [CrossRef]
- Manosroi, A.; Ruksiriwanich, W.; Abe, M.; Sakai, H.; Aburai, K.; Manosroi, W.; Manosroi, J. Physico-chemical properties of cationic niosomes loaded with fraction of rice (Oryza sativa) bran extract. J. Nanosci. Nanotechnol. 2012, 12, 7339–7345. [Google Scholar] [CrossRef]
- Khantham, C.; Linsaenkart, P.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Prom U Thai, C. Antioxidation, anti-inflammation, and regulation of SRD5A gene expression of Oryza sativa cv. Bue Bang 3 CMU husk and bran extracts as androgenetic alopecia molecular treatment substances. Plants 2022, 11, 330. [Google Scholar] [CrossRef]
- Wisetkomolmat, J.; Arjin, C.; Satsook, A.; Seel-Audom, M.; Ruksiriwanich, W.; Prom-U-Thai, C.; Sringarm, K. Comparative analysis of nutritional components and phytochemical attributes of selected Thai rice bran. Front. Nutr. 2022, 9, 1–12. [Google Scholar]
- Wisetkomolmat, J.; Arjin, C.; Hongsibsong, S.; Ruksiriwanich, W.; Niwat, C.; Tiyayon, P.; Jamjod, S.; Yamuangmorn, S.; Prom U Thai, C.; Sringarm, K. Antioxidants contents and polyphenols characteristic of selected northern Thai rice husks: The relation with seed attributes. Rice Sci. 2023, 30, 9. [Google Scholar] [CrossRef]
- Hair Care Market Size, Trends, Analysis. Available online: https://www.fortunebusinessinsights.com/hair-care-market-102555 (accessed on 17 August 2022).
- Hair care Market—Growth, Trends, COVID-19 Impact, and Forecasts (2022–2027). Available online: https://www.mordorintelligence.com/industry-reports/hair-care-market-industry (accessed on 17 August 2022).
- O’Sullivan, J.D.; Nicu, C.; Picard, M.; Chéret, J.; Bedogni, B.; Tobin, D.J.; Paus, R. The biology of human hair greying. Biol. Rev. 2021, 96, 107–128. [Google Scholar] [CrossRef]
- Hunt, N.; McHale, S. The psychological impact of alopecia. Br. Med. J. 2005, 331, 951–953. [Google Scholar] [CrossRef]
- Price, V.H. Androgenetic alopecia in women. J. Investig. Dermatol. Symp. Proc. 2003, 8, 24–27. [Google Scholar] [CrossRef]
- Piraccini, B.; Alessandrini, A. Androgenetic alopecia. G. Ital. Dermatol. Venereol. 2014, 149, 15–24. [Google Scholar] [PubMed]
- Kelly, Y.; Blanco, A.; Tosti, A. Androgenetic alopecia: An update of treatment options. Drugs 2016, 76, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, D.; Sinclair, R. Male androgenetic alopecia. Expert Opin. Pharmacother. 2010, 11, 1295–1304. [Google Scholar] [CrossRef]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Fernandez Flores, A.; Saeb Lima, M.; Cassarino, D.S. Histopathology of aging of the hair follicle. J. Cutan. Pathol. 2019, 46, 508–519. [Google Scholar] [CrossRef]
- Videira, I.F.d.S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef]
- Acer, E.; Kaya Erdoğan, H.; İğrek, A.; Parlak, H.; Saraçoğlu, Z.N.; Bilgin, M. Relationship between diet, atopy, family history, and premature hair graying. J. Cosmet. Dermatol. 2019, 18, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.G.; Marchitto, M.C.; Ly, B.C.K.; Chien, A.L. Evaluation of physiological, psychological, and lifestyle factors associated with premature hair graying. Int. J. Trichol. 2019, 11, 153. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, R.; Bala, I. Therapeutics of premature hair graying: A long journey ahead. J. Cosmet. Dermatol. 2019, 18, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Boonchai, W.; Winayanuwattikun, W.; Limphoka, P.; Sukakul, T. Contact allergy to hair cosmetic allergens in Thailand. Contact Derm. 2019, 81, 426–431. [Google Scholar] [CrossRef]
- Durán, B.E.; Romero-Pérez, D.; Salvador, J.S. Allergic contact dermatitis due to paraphenylenediamine: An update. Actas. Dermosifiliogr. 2018, 109, 602–609. [Google Scholar]
- Wongwaiwech, D.; Weerawatanakorn, M.; Tharatha, S.; Ho, C.-T. Comparative study on amount of nutraceuticals in by-products from solvent and cold pressing methods of rice bran oil processing. J. Food Drug Anal. 2019, 27, 71–82. [Google Scholar] [CrossRef]
- Watson, J.; Lu, J.; de Souza, R.; Si, B.; Zhang, Y.; Liu, Z. Effects of the extraction solvents in hydrothermal liquefaction processes: Biocrude oil quality and energy conversion efficiency. Energy 2019, 167, 189–197. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Szabo, K.; Diaconeasa, Z.; Cătoi, A.-F.; Vodnar, D.C. Screening of ten tomato varieties processing waste for bioactive components and their related antioxidant and antimicrobial activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef]
- Suleria, H.A.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Bloot, A.P.M.; Kalschne, D.L.; Amaral, J.A.S.; Baraldi, I.J.; Canan, C. A review of phytic acid sources, obtention, and applications. Food Rev. Int. 2021, 1–20. [Google Scholar] [CrossRef]
- Serruya, R.; Maor, Y. Hair growth-promotion effects at the cellular level and antioxidant activity of the plant-based extract Phyllotex™. Heliyon 2021, 7, e07888. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Sringarm, K.; Ferrer, E. Phytochemical constitution, anti-inflammation, anti-androgen, and hair growth-promoting potential of shallot (Allium ascalonicum L.) extract. Plants 2022, 11, 1499. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- López García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell viability and proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Zhou, S.; Zeng, H.; Huang, J.; Lei, L.; Tong, X.; Li, S.; Zhou, Y.; Guo, H.; Khan, M.; Luo, L. Epigenetic regulation of melanogenesis. Ageing Res. Rev. 2021, 69, 101349. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Zippin, J.H.; Ito, S. Chemical and biochemical control of skin pigmentation with special emphasis on mixed melanogenesis. Pigment Cell Melanoma Res. 2021, 34, 730–747. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef]
- Tobin, D.J.; Paus, R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp. Gerontol. 2001, 36, 29–54. [Google Scholar] [CrossRef]
- De Tollenaere, M.; Chapuis, E.; Auriol, P.; Auriol, D.; Scandolera, A.; Reynaud, R. Global repigmentation strategy of grey hair follicles by targeting oxidative stress and stem cells protection. Appl. Sci. 2021, 11, 1533. [Google Scholar] [CrossRef]
- Kumar, A.B.; Shamim, H.; Nagaraju, U. Premature graying of hair: Review with updates. Int. J. Trichol. 2018, 10, 198. [Google Scholar] [CrossRef]
- Zhao, P.; Park, N.H.; Alam, M.B.; Lee, S.H. Fuzhuan brick tea boosts melanogenesis and prevents hair graying through reduction of oxidative stress via NRF2-HO-1 signaling. Antioxidants 2022, 11, 599. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Castillo, J.; Micol, V. Lemon balm extract (Melissa officinalis, L.) promotes melanogenesis and prevents UVB-induced oxidative stress and DNA damage in a skin cell model. J. Dermatol. Sci. 2016, 84, 169–177. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Kietthanakorn, B.O.; Ruksiriwanich, W.; Chaikul, P.; Boonpisuttinant, K.; Sainakham, M.; Manosroi, W.; Tangjai, T.; Manosroi, J. Pharmaceutical and cosmeceutical biological activities of hemp (Cannabis sativa L. var. sativa) leaf and seed extracts. Chiang Mai J. Sci. 2019, 46, 180–195. [Google Scholar]
- Rojo de la Vega, M.; Zhang, D.D.; Wondrak, G.T. Topical bixin confers NRF2-dependent protection against photodamage and hair graying in mouse skin. Front. Pharmacol. 2018, 9, 287. [Google Scholar] [CrossRef]
- Huang, H.C.; Yen, H.; Lu, J.Y.; Chang, T.M.; Hii, C.H. Theophylline enhances melanogenesis in B16F10 murine melanoma cells through the activation of the MEK 1/2, and Wnt/β-catenin signaling pathways. Food Chem. Toxicol. 2020, 137, 111165. [Google Scholar] [CrossRef]
- Chaikul, P.; Kanlayavattanakul, M.; Somkumnerd, J.; Lourith, N. Phyllanthus emblica L. (amla) branch: A safe and effective ingredient against skin aging. J. Tradit. Complement Med. 2021, 11, 390–399. [Google Scholar] [CrossRef]
- Yale, K.; Juhasz, M.; Mesinkovska, N.A. Medication-induced repigmentation of gray hair: A systematic review. Skin Appendage Disord. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246. [Google Scholar] [CrossRef]
- Mahendiratta, S.; Sarma, P.; Kaur, H.; Kaur, S.; Kaur, H.; Bansal, S.; Prasad, D.; Prajapat, M.; Upadhay, S.; Kumar, S. Premature graying of hair: Risk factors, co-morbid conditions, pharmacotherapy and reversal—A systematic review and meta-analysis. Dermatol. Ther. 2020, 33, e13990. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Ito, S. Skin pigmentation: Is the control of melanogenesis a target within reach? Int. J. Mol. Sci. 2018, 19, 4040. [Google Scholar] [CrossRef]
- Mirnezami, M.; Rahimi, H. Serum zinc level in vitiligo: A case-control study. Indian J. Dermatol. 2018, 63, 227. [Google Scholar]
- Manosroi, A.; Chaikul, P.; Chankhampan, C.; Ruksiriwanich, W.; Manosroi, W.; Manosroi, J. 5α-reductase inhibition and melanogenesis induction of the selected Thai plant extracts. Chiang Mai J. Sci. 2018, 45, 220–236. [Google Scholar]
- Jang, H.J.; Seo, Y.K. Pigmentation effect of rice bran extracted minerals comprising soluble silicic acids. Evid. Based Complement Alternat. Med. 2016, 2016, 3137486. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lim, H.M.; Lee, E.C.; Seo, Y.K. Pigmentation effect of rice bran extract in hair follicle-like tissue and organ culture models. Tissue Eng. Regen. Med. 2020, 17, 15–23. [Google Scholar] [CrossRef]
- Man, M.Q.; Wakefield, J.S.; Mauro, T.M.; Elias, P.M. Role of nitric oxide in regulating epidermal permeability barrier function. Exp. Dermatol. 2022, 31, 290–298. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric oxide in macrophage immunometabolism: Hiding in plain sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef]
- Frank, S.; Kämpfer, H.; Wetzler, C.; Pfeilschifter, J. Nitric oxide drives skin repair: Novel functions of an established mediator. Kidney Int. 2002, 61, 882–888. [Google Scholar] [CrossRef]
- Tobin, D.J.; Hordinsky, M.; Bernard, B.A. Hair pigmentation: A research update. J. Investig. Dermatol. Symp. Proc. 2005, 10, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Sowden, H.; Naseem, K.; Tobin, D. Differential expression of nitric oxide synthases in human scalp epidermal and hair follicle pigmentary units: Implications for regulation of melanogenesis. Br. J. Dermatol. 2005, 153, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Vargas Maya, N.I.; Padilla Vaca, F.; Romero González, O.E.; Rosales Castillo, E.A.S.; Rangel Serrano, Á.; Arias Negrete, S.; Franco, B. Refinement of the Griess method for measuring nitrite in biological samples. J. Microbiol. Methods 2021, 187, 106260. [Google Scholar] [CrossRef]
- Merecz Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Kucharska, E.; Zajdel, R. The Modulation of melanogenesis in B16 cells upon treatment with plant extracts and isolated plant compounds. Molecules 2022, 27, 4360. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Jin, Z. Paracrine regulation of melanogenesis. Br. J. Dermatol. 2018, 178, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Fisher, D.E. MITF and UV responses in skin: From pigmentation to addiction. Pigment Cell Melanoma Res. 2019, 32, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Poli, A.; Di Cosmo, A.; D’Ischia, M. N-Methyl-D-aspartate receptor stimulation activates tyrosinase and promotes melanin synthesis in the ink gland of the cuttlefish Sepia officinalis through the nitric oxide/cGMP signal transduction pathway: A novel possible role for glutamate as physiologic activator of melanogenesis. J. Biol. Chem. 2000, 275, 16885–16890. [Google Scholar]
- Xue, L.; Chang, L.; Li, Y.; Dong, Y.; He, X. Stimulation of melanin synthesis by UVB is mediated by NO/cGMP/PKG cascade targeting PAK4 in vitro. In Vitro Cell. Dev. Biol. Anim. 2021, 57, 280–289. [Google Scholar] [CrossRef]
- Sasaki, M.; Horikoshi, T.; Uchiwa, H.; Miyachi, Y. Up-regulation of tyrosinase gene by nitric oxide in human melanocytes. Pigment Cell Res. 2000, 13, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Cals Grierson, M.; Ormerod, A. Nitric oxide function in the skin. Nitric. Oxide 2004, 10, 179–193. [Google Scholar] [CrossRef]
- Wu, H.M.; Ni, X.X.; Xu, Q.Y.; Wang, Q.; Li, X.Y.; Hua, J. Regulation of lipid-induced macrophage polarization through modulating peroxisome proliferator-activated receptor-gamma activity affects hepatic lipid metabolism via a Toll-like receptor 4/NF-κB signaling pathway. J. Gastroenterol. Hepatol. 2020, 35, 1998–2008. [Google Scholar] [CrossRef]
- de Lima, T.M.; de Sa Lima, L.; Scavone, C.; Curi, R. Fatty acid control of nitric oxide production by macrophages. FEBS Lett. 2006, 580, 3287–3295. [Google Scholar] [CrossRef]
- Radzikowska, U.; Rinaldi, A.O.; Çelebi Sözener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The influence of dietary fatty acids on immune responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Hakozaki, M.; Uemura, A.; Yamashita, T. Effect of fatty acids on melanogenesis and tumor cell growth in melanoma cells. J. Lipid Res. 2019, 60, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Borrelli, M.R.; Tapking, C.; Popp, D.; Puladi, B.; Ooms, M.; Chelliah, M.P.; Rein, S.; Pförringer, D.; Thor, D. Molecular mechanisms of hair growth and regeneration: Current understanding and novel paradigms. Dermatology 2020, 236, 271–280. [Google Scholar] [CrossRef]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal effects on hair follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Targeting Wnt/β-catenin pathway for developing therapies for hair loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Mechanism of action of herbs and their active constituents used in hair loss treatment. Fitoterapia 2016, 114, 18–25. [Google Scholar] [CrossRef]
- Moradi, F.; Enjezab, B.; Ghadiri-Anari, A. The role of androgens in COVID-19. Diabetes Metab. Synd. Clin. Res. Rev. 2020, 14, 2003–2006. [Google Scholar] [CrossRef] [PubMed]
- Heilmann Heimbach, S.; Hochfeld, L.M.; Henne, S.K.; Nöthen, M.M. Hormonal regulation in male androgenetic alopecia—Sex hormones and beyond: Evidence from recent genetic studies. Exp. Dermatol. 2020, 29, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Goren, A.; Vano-Galvan, S.; Wambier, C.G.; McCoy, J.; Gomez Zubiaur, A.; Moreno Arrones, O.M.; Shapiro, J.; Sinclair, R.D.; Gold, M.H.; Kovacevic, M. A preliminary observation: Male pattern hair loss among hospitalized COVID-19 patients in Spain-A potential clue to the role of androgens in COVID-19 severity. J. Cosmet. Dermatol. 2020, 19, 1545–1547. [Google Scholar] [CrossRef]
- Katzer, T.; Leite Junior, A.; Beck, R.; da Silva, C. Physiopathology and current treatments of androgenetic alopecia: Going beyond androgens and anti-androgens. Dermatol. Ther. 2019, 32, e13059. [Google Scholar] [CrossRef]
- Dhurat, R.; Sharma, A.; Rudnicka, L.; Kroumpouzos, G.; Kassir, M.; Galadari, H.; Wollina, U.; Lotti, T.; Golubovic, M.; Binic, I. 5-Alpha reductase inhibitors in androgenetic alopecia: Shifting paradigms, current concepts, comparative efficacy, and safety. Dermatol. Ther. 2020, 33, e13379. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M. Post-finasteride syndrome: A surmountable challenge for clinicians. Fertil. Steril. 2020, 113, 21–50. [Google Scholar] [CrossRef]
- Dhariwala, M.Y.; Ravikumar, P. An overview of herbal alternatives in androgenetic alopecia. J. Cosmet. Dermatol. 2019, 18, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cao, S.; Yuan, H.; Park, S. Alleviation of androgenetic alopecia with aqueous Paeonia lactiflora and Poria cocos extract intake through suppressing the steroid hormone and inflammatory pathway. Pharmaceuticals 2021, 14, 1128. [Google Scholar] [CrossRef] [PubMed]
- Khantham, C.; Yooin, W.; Sringarm, K.; Sommano, S.R.; Jiranusornkul, S.; Carmona, F.D.; Nimlamool, W.; Jantrawut, P.; Rachtanapun, P.; Ruksiriwanich, W. Effects on steroid 5-alpha reductase gene expression of Thai rice bran extracts and molecular dynamics study on SRD5A2. Biology 2021, 10, 319. [Google Scholar] [CrossRef]
- Bejaoui, M.; Taarji, N.; Saito, M.; Nakajima, M.; Isoda, H. Argan (Argania spinosa) press cake extract enhances cell proliferation and prevents oxidative stress and inflammation of human dermal papilla cells. J. Dermatol. Sci. 2021, 103, 33–40. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, G.; Li, X.; Sun, W.; Liang, Y.; Gan, D.; Liu, G.; Song, W.; Wang, Z. Study on the chemical constituents of nut oil from Prunus mira Koehne and the mechanism of promoting hair growth. J. Ethnopharmacol. 2020, 258, 112831. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Khantham, C.; Linsaenkart, P.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R. In vitro and in vivo regulation of SRD5A mRNA expression of supercritical carbon dioxide extract from Asparagus racemosus Willd. root as anti-sebum and pore-minimizing active ingredients. Molecules 2022, 27, 1535. [Google Scholar] [CrossRef] [PubMed]
- Canabady-Rochelle, L.L.; Harscoat-Schiavo, C.; Kessler, V.; Aymes, A.; Fournier, F.; Girardet, J.-M. Determination of reducing power and metal chelating ability of antioxidant peptides: Revisited methods. Food Chem. 2015, 183, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Fan, X.; Lv, Q.; Zhang, Q.; Xiao, J.; Qian, Y.; Zheng, B.; Gao, H.; Gao, S.; Chen, L. Folium nelumbinis (Lotus leaf) volatile-rich fraction and its mechanisms of action against melanogenesis in B16 cells. Food Chem. 2020, 330, 127030. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.K.; Pham, C.H.; Lee, I.S.; Jung, S.-H.; Jeong, J.H.; Shin, H.-S.; Yoo, H.M. Anti-melanogenesis activity of 6-O-isobutyrylbritannilactone from Inula britannica on B16F10 melanocytes and in vivo zebrafish models. Molecules 2020, 25, 3887. [Google Scholar] [CrossRef] [PubMed]
- Nazir, Y.; Linsaenkart, P.; Khantham, C.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R. High efficiency in vitro wound healing of Dictyophora indusiata extracts via anti-Inflammatory and collagen stimulating (MMP-2 inhibition) mechanisms. J. Fungi 2021, 7, 1100. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Linsaenkart, P.; Chaitep, T.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Režek Jambrak, A.; Nazir, Y.; Yooin, W. Anti-inflammation of bioactive compounds from ethanolic extracts of edible bamboo mushroom (Dictyophora indusiata) as functional health promoting food ingredients. Int. J. Food Sci. Technol. 2022, 57, 110–122. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M.; Chaikul, P. Para rubber seed oil: The safe and efficient bio-material for hair loss treatment. J. Cosmet. Dermatol. 2021, 20, 2160–2167. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Parichatikanond, W.; Junyaprasert, V.B.; Morakul, B. Pumpkin seed oil-loaded niosomes for topical application: 5α-reductase inhibitory, anti-inflammatory, and in vivo anti-hair loss effects. Pharmaceuticals 2022, 15, 930. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Phimolsiripol, Y.; Barba, F.J.; Sringarm, K.; Rachtanapun, P.; Jantanasakulwong, K.; Jantrawut, P.; Chittasupho, C. Guava (Psidium guajava L.) leaf extract as bioactive substances for anti-androgen and antioxidant activities. Plants 2022, 11, 3514. [Google Scholar] [CrossRef]
- Pejčić, T.; Tosti, T.; Džamić, Z.; Gašić, U.; Vuksanović, A.; Dolićanin, Z.; Tešić, Ž. The polyphenols as potential agents in prevention and therapy of prostate diseases. Molecules 2019, 24, 3982. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).