Promising Bioregulators for Higher Water Productivity and Oil Quality of Chia under Deficit Irrigation in Semiarid Regions
Abstract
1. Introduction
2. Results and Discussion
2.1. Climatic Conditions of the Study Location
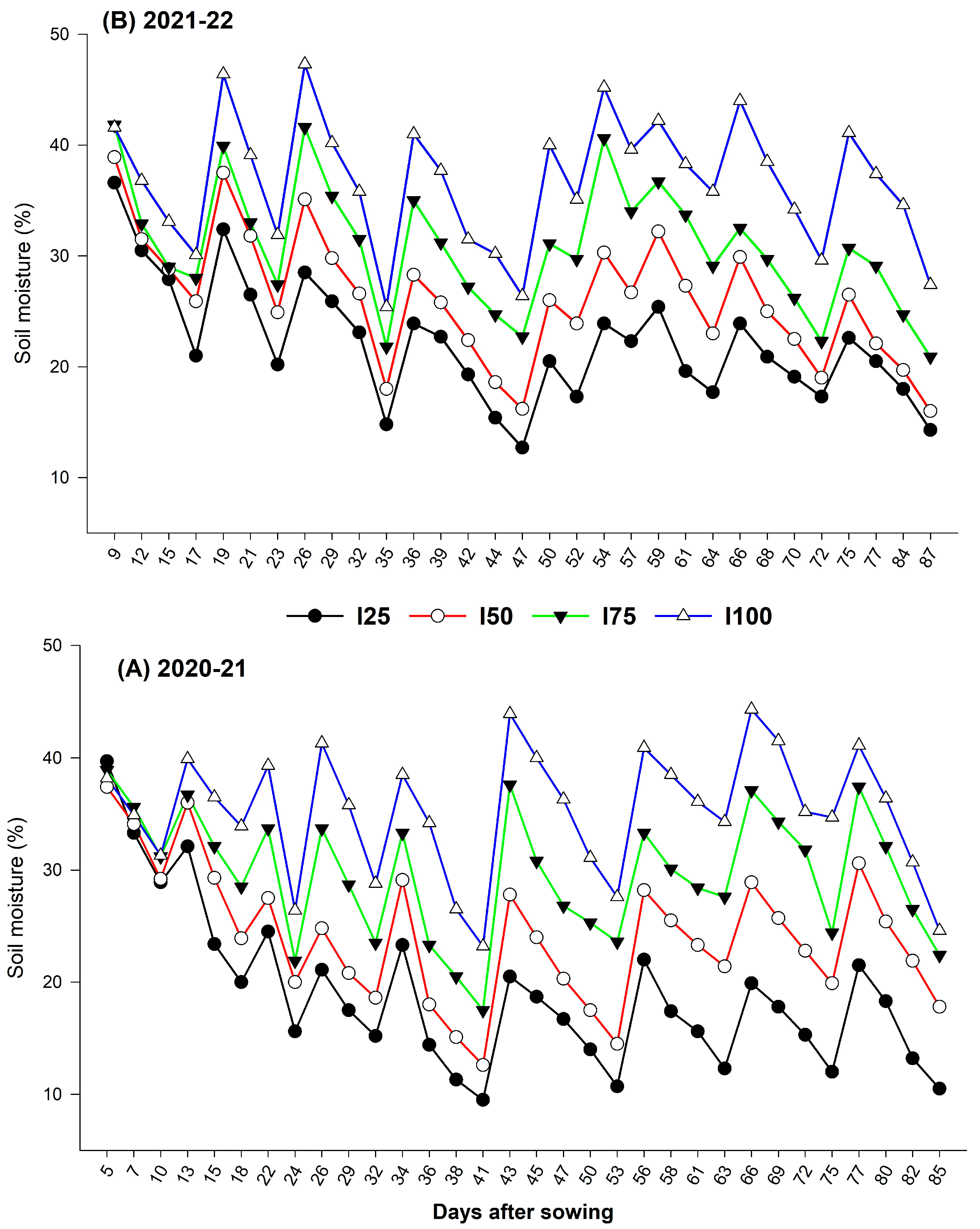
2.2. Effect of Water Deficit on Seed, Oil, and Omega–3 Yield
2.3. Effect of Bioregulators on Seed, Oil, and Omega–3 Yield
2.4. Effect of Deficit Irrigation and BRs on Growth and Physiology of Chia
2.5. Fatty Acid Composition
2.6. Water Productivity for Seed
2.7. Interrelationship between Traits as Influenced by Bioregulators
3. Materials and Methods
3.1. Soil and Weather Conditions of the Location
3.2. Experimental Details and Crop Management
3.3. Irrigation and Bioregulators Application
3.4. Growth and Physiological Parameters
3.5. Yield and Yield Attributes of Chia
3.6. Water Productivity (WP)
3.7. Extraction of Chia Seed Oil
3.8. Fatty Acid Methyl Esters (FAME) Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, B.P.; Anunciaçao, P.C.; Matyelka, J.C.D.S.; Della Lucia, C.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Chemical composition of Brazilian chia seeds grown in different places. Food Chem. 2017, 221, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Migliavacca, R.A.; Silva, T.R.B.; Vasconcelos, A.L.S.; Mourão Filho, W.; Baptistella, J.L.C. Chia’s cultivation in Brazil: Future and perspectives. J. Agron. Crop Sci. 2014, 3, 161–179. [Google Scholar]
- Felisberto, M.H.F.; Galvão, M.T.E.L.; Picone, C.S.F.; Cunha, R.L.; Pollonio, M.A.R. Effect of prebiotic ingredients on the rheological properties and microstructure of reduced-sodium and low-fat meat emulsions. LWT Food Sci. Technol. 2015, 60, 148–155. [Google Scholar] [CrossRef]
- Pathak, P.O.; Nagarsenker, M.S.; Barhate, C.R.; Padhye, S.G.; Dhawan, V.V.; Bhattacharyya, D.; Viswanathan, C.L.; Steiniger, F.; Fahr, A. Cholesterol anchored arabinogalactan for asialoglycoprotein receptor targeting: Synthesis, characterization, and proof of concept of hepatospecific delivery. Carbohydr. Res. 2015, 408, 33–43. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.; Gomaa, A.; Subirade, M.; Rios-Ade, O.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Ayerza, H.R.; Coates, W. Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispanica L.). Ind. Crops Prod. 2011, 34, 1366–1371. [Google Scholar] [CrossRef]
- Ayerza, R. Seed composition of two chia (Salvia hispanica L.) genotypes which differ in seed colour. Emir. J. Food Agric. 2013, 25, 495–500. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Romani, V.P.; Filipini, G.S.; Martins, V.G. Chia seeds to develop new biodegradable polymers for food packaging: Properties and biodegradability. Polym. Eng. Sci. 2020, 60, 1–10. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Wu, P.T.; Zhao, X.N.; Cheng, X.F. Water—saving mechanisms of intercropping system in improving cropland water use efficiency. Chin. J. Appl. Ecol. 2012, 23, 1400–1406. [Google Scholar]
- Kadasiddappa, M.M.; Rao, V.P.; Reddy, K.Y.; Ramulu, V.; Devi, M.U.; Reddy, S.N. Effect of Irrigation (Drip/Surface) on Sunflower Growth, Seed and Oil Yield, Nutrient Uptake and Water Use Efficiency—A Review. Agric. Rev. 2017, 38, 152–158. [Google Scholar] [CrossRef]
- Acevedo, M.; Pixley, K.; Zinyengere, N.; Meng, S.; Tufan, H.; Cichy, K.; Bizikova, L.; Isaacs, K.; Ghezzi–Kopel, K.; Porciello, J. A scoping review of adoption of climate–resilient crops by small-scale producers in low and middle–income countries. Nat. Plants 2020, 6, 1231–1241. [Google Scholar] [CrossRef]
- Herman, S.; Marco, G.; Cecilia, B.; Alfonso, V.; Luis, M.; Cristián, V.; Sebastián, P.; Sebastián, A. Effect of water availability on growth, water use efficiency and omega3 (ALA) content in two phenotypes of chia (Salvia hispanica L.) established in the arid Mediterranean zone of Chile. Agric. Water Manag. 2016, 173, 67–75. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D. Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
- De Falco, B.; Fiore, A.; Bochicchio, R.; Amato, M.; Lanzotti, V. Metabolomic analysis by UAE-GC MS and antioxidant activity of Salvia hispanica (L.) seeds grown under different irrigation regimes. Ind. Crops Prod. 2018, 112, 584–592. [Google Scholar] [CrossRef]
- Ozer, H.; Cobana, F.; Sahinb, U.; Orsb, S. Response of black cumin (Nigella sativa L.) to deficit irrigation in a semi–arid region: Growth, yield, quality, and water productivity. Ind. Crops Prod. 2020, 144, 112048. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Minhas, P.S.; Ratnakumar, P.; Choudhary, R.L. Optimising supplemental irrigation for wheat (Triticum aestivum L.) and the impact of plant bio–regulators in a semi–arid region of Deccan Plateau in India. Agric. Water Manag. 2016, 172, 9–17. [Google Scholar] [CrossRef]
- Harisha, C.B.; Asangi, H.A.; Singh, R. Growth, yield, water use efficiency of coriander (Coriandrum sativum) affected by irrigation levels and fertigation. Indian J. Agric. Sci. 2019, 89, 1167–1172. [Google Scholar] [CrossRef]
- Hassanein, R.A.; Amin, A.A.E.; Rashad, E.M.; Ali, A. Effect of thiourea andsalicylic acid on antioxidant defense of wheat plants under drought stress. Int. J. Chem. Tech. Res. 2015, 7, 346–354. [Google Scholar]
- Wakchaure, G.C.; Minhas, P.S.; Meena, K.K.; Kumar, S.; Rane, J. Effect of plant growth regulators and deficit irrigation on canopy traits, yield, water productivity and fruit quality of eggplant (Solanum melongena L.) grown in the water scarce environment. J. Environ. Manag. 2020, 262, 110320. [Google Scholar] [CrossRef]
- Ratnakumar, P.; Khan, M.I.R.; Minhas, P.S.; Farooq, M.A.; Sultana, R.; Per, T.S.; Deokate, P.P.; Khan, N.A.; Singh, Y.; Rane, J. Can plant bio-regulators minimize crop productivity losses caused by drought, salinity and heat stress? An integrated review. J. Appl. Bot. Food Qual. 2016, 89, 113–125. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Sönmez, O. Promotive effect of exogenously applied thiourea on key physiological parameters and oxidative defense mechanism in salt–stressed Zea mays L. plants. Turk. J. Bot. 2015, 39, 786–795. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Ratnakumar, P.; Minhas, P.S.; Suprasanna, P. Plant bio–regulators for sustainable agriculture; integrating redox signaling as a possible unifying mechanism. Adv. Agron. 2016, 137, 237–278. [Google Scholar] [CrossRef]
- Bhunia, S.R.; Verma, I.M.; Sahu, M.P.; Sharma, N.C.; Balai, K. Effect of drip irrigation and bioregulators on yield, economics and water use of fenugreek (Trigonella foenum-graecum). J. Spices Aromat. Crops. 2015, 24, 102–105. [Google Scholar]
- Gimeno, V.; Díaz–López, L.; Simón–Grao, S.; Martínez, V.; Martínez–Nicolás, J.J.; García–Sánchez, F. Foliar potassium nitrate application improves the tolerance of Citrus macrophylla L. seedlings to drought conditions. Plant Physiol. Biochem. 2014, 83, 308–315. [Google Scholar] [CrossRef]
- Shahi, S.; Kumar, R.; Srivastava, M. Response of black gram (Vigna mungo) to potassium under water stress condition. Ann. Plant Soil Res. 2019, 21, 93–97. [Google Scholar]
- Aslam, A.; Khan, S.; Ibrar, D.; Irshad, S.; Bakhsh, A.; Gardezi, S.T.R.; Ali, M.; Hasnain, Z.; Al–Hashimi, A.; Noor, M.A.; et al. Defensive impact of foliar applied potassium nitrate on growth linked with improved physiological and antioxidative activities in sunflower (Helianthus annuus L.) hybrids grown under salinity stress. Agronomy 2021, 11, 2076. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fathudinova, R.A.; Fathudinova, D.R. Changes in hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Askari, E.; Ehsanzadeh, P. Drought stress mitigation by foliar application of salicylic acid and their interactive effects on physiological characteristics of fennel (Foeniculum vulgare Mill.) genotypes. Acta Physiol. Plant 2015, 37, 4. [Google Scholar] [CrossRef]
- Hesami, S.; Nabizadeh, E.; Rahimi, A.; Rokhzadi, A. Effects of salicylic acid levels and irrigation intervals on growth and yield of coriander (Coriandrum sativum) in field conditions. Environ. Exp. Biol. 2012, 10, 113–116. [Google Scholar]
- Farjam, S.; Siosemardeh, A.; Kazemi–Arbat, H.; Yarnia, M.; Rokhzadi, A. Response of chickpea (Cicer arietinum L.) to exogenous salicylic acid and ascorbic acid under vegetative and reproductive drought stress condition. J. Appl. Bot. Food Qual. 2014, 87, 80–86. [Google Scholar] [CrossRef]
- Abdallah, M.M.S.; El–Bassiouny, H.M.S.; AbouSeeda, M.A. Potential role of kaolin or potassium sulfate as anti-transpirant on improving physiological, biochemical aspects and yield of wheat plants under different watering regimes. Bull. Natl. Res. Cent. 2019, 43, 134. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Rahemi, M.; Karimi, S.; Yazdani, N.; Tajdini, Z.; Sarikhani, S.; Vahdati, K. Role of kaolin on drought tolerance and nut quality of Persian walnut. J. Saudi Soc. Agric. Sci. 2021, 20, 409–416. [Google Scholar] [CrossRef]
- Faghih, S.; Zamani, Z.; Fatahi, R.; Liaghat, A. Effects of deficit irrigation and kaolin application on vegetative growth and fruit traits of two early ripening apple cultivars. Biol. Res. 2019, 52, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Gunes, A.; Pilbeam, D.J.; Inal, A.; Coban, S. Influence of silicon on sunflower cultivars under drought stress, I: Growth, antioxidant mechanisms, and lipid peroxidation. Commun. Soil Sci. Plant Anal. 2008, 39, 1885–1903. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, Y.; Duan, L.; Li, Z.; Eneji, A.E.; Li, J. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet–B radiation. J. Plant Physiol. 2010, 167, 1248–1252. [Google Scholar] [CrossRef]
- Helaly, M.N.; El–Hoseiny, H.; El–Sheery, N.I.; Rastogi, A.; Kalaji, H.M. Regulation and physiological role of silicon in alleviating drought stress of mango. Plant Physiol. Biochem. 2017, 118, 31–44. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Minhas, P.S.; Meena, K.K.; Singh, N.P.; Hegade, P.M.; Sorty, A.M. Growth, bulb yield, water productivity and quality of onion (Allium cepa L.) as affected by deficit irrigation regimes and exogenous application of plant bio–regulators. Agric. Water Manag. 2018, 199, 1–10. [Google Scholar] [CrossRef]
- Ayerza, R. Oil content and fatty acid composition of chia (Salvia hispanica L.) from five north–western locations in Argentina. J. Am. Oil Chem. Soc. 1995, 72, 1079–1081. [Google Scholar] [CrossRef]
- Baginsky, C.; Arenas, J.; Escobar, H.; Garrido, M.; Valero, N.; Tello, D.; Pizarro, L.; Valenzuela, A.; Morales, L.; Silva, H. Growth and yield of chia (Salvia hispanica L.) in the Mediterranean and desert climates of Chile. Chilean J. Agric. Res. 2016, 76, 255–264. [Google Scholar] [CrossRef]
- Hergert, G.W.; Margheim, J.F.; Pavlista, A.D.; Martin, D.L.; Isbell, T.A.; Supalla, R.J. Irrigation response and water productivity of deficit to fully irrigated spring camelina. Agric. Water Manag. 2016, 177, 46–53. [Google Scholar] [CrossRef]
- Pavlista, A.D.; Isbell, T.A.; Baltensperger, D.D.; Hergert, G.W. Planting date and development of spring–seeded irrigated canola, brown mustard and camelina. Ind. Crops Prod. 2011, 33, 451–456. [Google Scholar] [CrossRef]
- Santos, R.F.; Bassegio, D.; Silva, M.A. Yield and production components of safflower genotypes affected by irrigation at phenological stages. Agric. Water Manag. 2017, 186, 66–74. [Google Scholar] [CrossRef]
- Pandey, M.; Srivastava, A.K.; D’Souza, S.F.; Penna, S. Thiourea, a ROS scavenger, regulates source to sink relationship to enhance crop yield and oil content in Brassica juncea (L.). PLoS ONE 2013, 8, e73921. [Google Scholar] [CrossRef]
- Neupane, D.; Juan, K.Q.; Mclennon, E.; Davison, J.; Lawry, T. Camelina production parameters response to different irrigation regimes. Ind. Crop. Prod. 2020, 148, 112286. [Google Scholar] [CrossRef]
- Ghamarnia, H.; Khosravy, H.; Sepehri, S. Yield and water use efficiency of (Nigella sativa L.) under different irrigation treatments in a semi arid region in the West of Iran. J. Med. Plants Res. 2010, 4, 1612–1616. [Google Scholar] [CrossRef]
- Ebrahimian, E.; Seyyedi, S.M.; Bybordi, A.; Damalas, C.A. Seed yield and oil quality of sunflower, safflower, and sesame under different levels of irrigation water availability. Agric. Water Manag. 2019, 218, 149–157. [Google Scholar] [CrossRef]
- Morsy, A.R.; Mohamed, A.M.; Abo–Marzoka, E.A.; Megahed, M.A.H. Effect of Water Deficit on Growth, Yield and Quality of Soybean Seed. J. Plant Prod. Mansoura Univ. 2018, 9, 709–716. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Saleem, M.F.; Khan, I.H. Amelioration in growth and physiological efficiency of sunflower (Helianthus annuus L.) under drought by potassium application. Commun. Soil Sci. Plant Anal. 2018, 49, 2291–2300. [Google Scholar] [CrossRef]
- Vekaria, G.B.; Talpada, M.M.; Sutaria, G.S.; Akbari, K.N. Effect of foliar nutrition of potassium nitrate on the growth and yield of greengram (Vigna Rabiata L.). Legum. Res. 2013, 36, 162–164. [Google Scholar]
- Singh, A.; Singh, A.K.; Aswin, C. Effect of hydrogel and thiourea on yield, quality and nutrient uptake of Indian mustard under moisture stress condition. Res. Crop. 2017, 18, 42–48. [Google Scholar] [CrossRef]
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Silva, H.; Arriagada, C.; Campos–Saez, S.; Baginsky, C.; Castellaro–Galdames, G.; Morales–Salinas, L. Effect of sowing date and water availability on growth of plants of chia (Salvia hispanica L.) established in Chile. PLoS ONE 2018, 13, e0203116. [Google Scholar] [CrossRef]
- Furtado, G.F.; Xavier, D.A.; Andrade, E.M.G.; de Lima, G.S.; Chaves, L.H.G.; de Vasconcelos, A.C.F.; Wanderley, J.A.C. Growth and physiological responses of sunflower grown under levels of water replacement and potassium fertilization. Afr. J. Agric. Res. 2016, 11, 1273–1281. [Google Scholar] [CrossRef]
- Shoukat, M.R.; Leghari, S.J.; Ahmad, N.; Virk, A.L.; Haider, F.U.; Rehmani, M.I.A.; Laraib, I. Effects of foliar applied thiourea on maize physiology, growth and yield (Zea mays L.) under shaded conditions. J. Plant Nutr. 2022, 45, 1312–1321. [Google Scholar] [CrossRef]
- Nkrumah, P.; Amadu, A.M.; Ayeh, K.O. Influence of salicylic acid and potassium nitrate on plant height and flowering time of groundnut (Arachis hypogaea L.) under varying salinity and drought–induced stresses. Ghana J. Sci. 2021, 62, 26–36. [Google Scholar] [CrossRef]
- Akladious, S.A. Influence of thiourea application on some physiological and molecular criteria of sunflower (Helianthus annuus L.) plants under conditions of heat stress. Protoplasma 2014, 251, 625–638. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Minhas, P.S.; Kumar, S.; Khapte, P.S.; Meena, K.K.; Rane, J.; Pathak, H. Quantification of water stress impacts on canopy traits, yield, quality and water productivity of onion (Allium cepa L.) cultivars in a shallow basaltic soil of water scarce zone. Agric. Water Manag. 2021, 249, 106824. [Google Scholar] [CrossRef]
- Halli, H.M.; Angadi, S.; Kumar, A.; Govindasamy, P.; Madar, R.; Baskar, V.D.C.; Elansary, H.O.; Tamam, N.; Abdelbacki, A.M.; Abdelmohsen, S.A. Assessment of planting method and deficit irrigation impacts on physio-morphology, grain yield and water use efficiency of maize (Zea Mays L.) on vertisols of semi-arid tropics. Plants 2021, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.F.C.; Goncalves, A.C.A.; Da Silva, C.A., Jr.; Nanni, M.R.; Facco, C.U.; Cenzar, E.; Da Silva, A.A. NDVI Responses to water stress in different phenological stages in culture bean. J. Agron. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Taghvaeian, S.; Comas, L.; DeJonge, K.C.; Trout, T.J. Conventional and simplified canopy temperature indices predict water stress in sunflower. Agric. Water Manag. 2014, 144, 69–80. [Google Scholar] [CrossRef]
- Ashfaq, A.; Hussain, N.; Athar, M. Role of potassium fertilizers in plant growth, crop yield and quality fiber production of cotton. Fuuast. J. Biol. 2015, 5, 27–35. [Google Scholar]
- Flagella, Z.; Rotunno, T.; Tarantino, E.; Di Caterina, R.; De Caro, A. Changes in seed yield and oil fatty acid composition of high oleic sunflower (Helianthus annuus L.) hybrids in relation to the sowing date and water regime. Eur. J. Agron. 2002, 17, 221–230. [Google Scholar] [CrossRef]
- Joshan, Y.; Sani, B.; Jabbari, H.; Mozafari, H.; Moaveni, P. Effect of drought stress on oil content and fatty acids composition of some safflower genotypes. Plant Soil Environ. 2019, 65, 563–567. [Google Scholar] [CrossRef]
- Bellaloui, N.; Hu, Y.; Mengistu, A.; Kassem, M.A.; Abel, C.A. Effects of foliar boron application on seed composition, cell wall boron, and seed δ15N and δ13C isotopes in water–stressed soybean plants. Front. Plant Sci. 2013, 4, 270. [Google Scholar] [CrossRef]
- Amirkhiz, K.F.; Dehaghi, M.A.; Sanavy, S.A.M.M.; Rezazadeh, A. Evaluation of changes in fatty acid profile, grain, and oil yield of Carthamus tinctorius L. in response to foliar application of polyamine compounds under deficit irrigation conditions. Ind. Crops Prod. 2021, 161, 113231. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Samanci, B.; Ozkaynak, E. Effect of Planting Date on Seed Yield, Oil Content and Fatty Acid Composition of Safflower (Carthamus tinctorius) Cultivars Grown in the Mediterranean Region of Turkey. J. Agron. Crop Sci. 2003, 189, 359–360. [Google Scholar] [CrossRef]
- Oliva, M.L.; Shannon, J.G.; Sleper, D.A.; Ellersieck, M.R.; Cardinal, A.J.; Paris, R.L.; Lee, J.D. Stability of fatty acid profile in soybean genotypes with modified seed oil composition. Crop Sci. 2006, 6, 2069–2075. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al–Mahmud, J.; Masayuki Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Amiri–Darban, N.; Nourmohammadi, G.; Rad, A.H.S.; JavadMirhadi, S.M.; Heravan, I.M. Potassium sulfate and ammonium sulfate affect quality and quantity of camelina oil grown with different irrigation regimes. Ind. Crops Prod. 2020, 148, 112308. [Google Scholar] [CrossRef]
- Bellaloui, N.; Smith, J.R.; Gillen, A.M.; Ray, J.D. Effects of maturity, genotypic background, and temperature on seed mineral composition in nearisogenic soybean lines in the early soybean production system. Crop Sci. 2011, 51, 1161–1171. [Google Scholar] [CrossRef]
- Tezara, W.; Mitchell, V.; Driscoll, S.P.; Lawlor, D.W. Effects of water deficit and its interaction with CO2 supply on the biochemistry and physiology of photosynthesis in sunflower. J. Exp. Bot. 2002, 53, 1781–1791. [Google Scholar] [CrossRef]
- Dhaker, R.C.; Dubey, R.K.; Tiwari, R.C.; Dubey, S.K. Water use, yield and economics of fenugreek (Trigonella foenum–graecum L.) under varying IW–CPE ratios and fertilizer levels in South West Rajasthan. Legume Res. 2016, 40, 726–730. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y.; Saifullah; Ahmad, M. Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agric. Scand. Soil Plant Sci. 2011, 61, 291–304. [Google Scholar] [CrossRef]
- Minhas, P.S.; Bal, S.K.; Kumar, S.P.; Singh, Y.; Wakchaure, G.C.; Ghadge, S.V.; Nangare, D.D.; Taware, P.B. Turning Basaltic Terrain into Model Research Farm: Chronicle Description; NIASM Tech. Bull-8; ICAR—National Institute of Abiotic Stress Management: Pune, India, 2015; pp. 9–10. [Google Scholar]
- Rajagopal, V.; Choudhary, R.L.; Kumar, N.; Krishnani, K.K.; Singh, Y.; Bal, S.K.; Minhas, P.S.; Singh, N.P. Soil Health Status of NIASM Southern Farm Land; NIASM Technical Bulletin-15; ICAR—National Institute of Abiotic Stress Management: Pune, India, 2018; pp. 51–52. [Google Scholar]
- Pask, A.J.D.; Pietragalla, J.; Mullan, D.M.; Reynolds, M.P. Physiological Breeding II: A Field Guide to Wheat Phenotyping; CIMMYT: Mexico City, Mexico, 2012; pp. 32–39. [Google Scholar]
- Bandyopadhyay, K.K.; Pradhan, S.; Sahoo, R.N.; Singh, R.; Gupta, V.K. Characterization of water stress and prediction of yield of wheat using spectral indices under varied water and irrigation management practices. Agric. Water Manag. 2014, 146, 115–123. [Google Scholar] [CrossRef]
- Howell, T.A. Irrigation Engineering, Evapotranspiration; Encyclopedia of Agricultural Science; Arntzem, C., Ritter, E., Eds.; Academic Press: New York, NY, USA, 1994; pp. 591–600. [Google Scholar]
- AOCS. American Oil Chemist’s Society Official Method Ba 3–38. Sampling and analysis of oilseed by–products. In Official Methods and Recommended Practices of the AOCS, 7th ed.; Firestone, D., Ed.; AOAC International: Urbana, IL, USA, 2017. [Google Scholar]
- AOCS. American Oil Chemists’ Society Official Method Ce 2–66: Preparation of Methyl Esters of Fatty Acids. In Official Methods and Recommended Practices of the AOCS, 7th ed.; Firestone, D., Ed.; AOAC International: Urbana, IL, USA, 2017. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley and Sons Ltd.: New York, NY, USA, 1984; pp. 97–101. [Google Scholar]





| Month | Tmax (°C) | Tmin (°C) | Mean Relative Humidity (%) | Rainfall (mm) | Cumulative Pan Evaporation (mm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2020–21 | 2021–22 | 2020–21 | 2021–22 | 2020–21 | 2021–22 | 2020–21 | 2021–22 | 2020–21 | 2021–22 | |
| November | 30.9 | 30.4 | 17.4 | 18.8 | 63.0 | 68.2 | 0.0 | 14.6 | 141.3 | 123.7 |
| December | 29.8 | 28.2 | 14.1 | 14.8 | 62.5 | 71.3 | 0.0 | 5.4 | 128.6 | 83.8 |
| January | 30.7 | 27.8 | 16.7 | 12.8 | 64.9 | 66.1 | 10.6 | 0.0 | 125.3 | 112.5 |
| February | 31.4 | 31.9 | 14.0 | 13.5 | 52.0 | 55.0 | 0.0 | 0.0 | 147.9 | 155.8 |
| Avg./total * | 30.7 | 29.5 | 15.5 | 14.9 | 60.6 | 65.1 | 10.6 * | 20 * | 543.1 * | 475.8 * |
| I/BRs | Seed Yield (kg ha–1) | Oil Yield (kg ha–1) | Omega–3 Yield (kg ha–1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I25 | I50 | I75 | I100 | Mean BRs | I25 | I50 | I75 | I100 | Mean BRs | I25 | I50 | I75 | I100 | Mean BRs | |
| No BRs | 300.1 n | 538.8 j | 666.0 f | 687.3 de | 548.0e | 92.6 m | 169.9 h | 215.3 e | 224.9 d | 171.7 e | 47.4 q | 87.4 l | 112.2 h | 118.6 fg | 91.4 f |
| KO | 308.7 mn | 550.1 ij | 673.6 ef | 700.2 d | 558.2d | 95.6 lm | 173.3 h | 217.2 e | 229.1 d | 178.8 d | 49.1 pq | 89.7 l | 113.5 h | 121.0 f | 93.3 e |
| SA | 331.1 kl | 604.4 g | 718.6 c | 737.7 ab | 597.9 b | 104.8 i–k | 193.4 f | 235.3 a–c | 243.1 ab | 194.2 b | 54.3 m–o | 101.5 j | 124.5 e | 131.0 bc | 102.8 c |
| PS | 318.9 lm | 560.1 hi | 687.7 de | 728.9 a–c | 573.9 c | 100.1 kl | 178.9 g | 223.7 d | 240.5 a–c | 185.8 c | 51.4 no | 93.0 k | 117.3 g | 127.8 d | 97.4 d |
| SB | 323.8 lm | 567.9 h | 688.7 de | 731.4 a–c | 577.9 c | 101.8 jk | 181.8 g | 224.9 d | 241.1 ab | 187.4 c | 52.6 op | 95.0 k | 118.6 fg | 127.8 d | 98.5 d |
| TU | 334.1 kl | 608.3 g | 724.6 bc | 738.3 ab | 601.3 b | 106.2 ij | 196.0 f | 238.3 bc | 244.2 a | 196.2 b | 55.4 mn | 103.7 ij | 127.2 de | 133.8 ab | 105.0 b |
| KN | 344.8 k | 615.0 g | 738.5 a | 742.1 a | 610.1 a | 109.1 i | 198.5 f | 242.2 ab | 245.0 a | 198.7 a | 57.0 m | 104.8 i | 129.7 cd | 134.39 a | 106.5 a |
| Mean I | 323.1 d | 577.8 c | 699.7 b | 723.7 a | 101.5 d | 184.5 c | 228.2 b | 238.3 a | 52.51 d | 96.49 c | 120.47 b | 127.82 a | |||
| Pooled analysis (Year × Irrigation × Bioregulators) | |||||||||||||||
| p value | Y | <0.0001 | Y × I | <0.0001 | Y | <0.0001 | Y × I | <0.0001 | Y | <0.0001 | Y × I | <0.0001 | |||
| I | <0.0001 | Y × BRs | 0.0002 | I | <0.0001 | Y × BRs | <0.0001 | I | <0.0001 | Y × BRs | <0.0001 | ||||
| BRs | <0.0001 | Y × I × BRs | BRs | <0.0001 | Y × I × BRs | 0.0003 | BRs | <0.0001 | Y × I × BRs | 0.0023 | |||||
| Number of Spikes per Plant | Spike Length (cm) | 1000-Seed Weight (g) | Oil Content of Seeds (%) | Harvest Index (%) | |
|---|---|---|---|---|---|
| Irrigation | |||||
| I25 | 18.67 d | 13.98 d | 1.13 d | 31.39 d | 12.35 d |
| I50 | 21.03 c | 16.12 c | 1.16 c | 31.91 c | 19.03 b |
| I75 | 23.49 b | 19.01 b | 1.20 b | 32.60 b | 20.20 a |
| I100 | 24.76 a | 20.17 a | 1.23 a | 32.91 a | 17.21 c |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Bioregulators | |||||
| No BRs | 20.19 d | 15.07 c | 1.17 c | 31.85 d | 17.81 a |
| KO | 20.22 d | 14.62 c | 1.18 a–c | 31.86 d | 17.97 a |
| SA | 22.72 b | 19.42 a | 1.19 a | 32.33 a–c | 17.16 b |
| PS | 21.48 c | 16.48 b | 1.18 a–c | 32.20 c | 17.20 b |
| SB | 21.37 c | 16.57 b | 1.18 bc | 32.26 bc | 17.22 b |
| TU | 23.63 a | 19.39 a | 1.19 ab | 32.49 a | 16.49 c |
| KN | 24.28 a | 19.68 a | 1.19 a | 32.43 ab | 16.54 c |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Year | |||||
| 2020–21 | 21.9 a | 17.11 b | 1.19 a | 31.77 b | 16.91 b |
| 2021–22 | 22.0 a | 17.52 a | 1.17 b | 32.64 a | 17.49 a |
| p value | 0.810 | 0.035 | <0.0001 | <0.0001 | <0.0001 |
| Treatments | Palmitic Acid (%) | Stearic Acid (%) | Oleic Acid (%) | Linoleic Acid (%) | Linolenic Acid (%) | Saturated Fatty Acids (%) | Polyunsaturated Fatty Acids (%) |
|---|---|---|---|---|---|---|---|
| Irrigation | |||||||
| I25 | 9.92 a | 6.24 a | 9.16 a | 22.90 d | 51.72 d | 16.16 a | 74.63 d |
| I50 | 9.80 a | 5.65 b | 8.88 b | 23.39 c | 52.24 c | 15.44 b | 75.64 c |
| I75 | 9.57 b | 5.01 c | 8.63 c | 23.98 b | 52.77 b | 14.59 c | 76.75 b |
| I100 | 9.00 c | 4.70 d | 8.29 d | 24.36 a | 53.62 a | 13.70 d | 77.98 a |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Bioregulators | |||||||
| No BRs | 10.00 a | 5.76 a | 9.11 a | 23.21 f | 51.89 f | 15.76 a | 75.11 f |
| KO | 9.84 ab | 5.86 a | 8.85 b | 23.33 e | 52.06 e | 15.71 a | 75.39 e |
| SA | 9.36 d | 5.28 c | 8.54 d | 23.97 a | 52.80 b | 14.08 d | 76.77 c |
| PS | 9.64 c | 5.47 b | 8.91 b | 23.71 c | 52.25 d | 15.11 c | 75.96 d |
| SB | 9.75 bc | 5.52 b | 8.69 c | 23.56 d | 52.43 c | 14.64 b | 76.00 d |
| TU | 9.17 d | 5.06 d | 8.55 d | 23.83 b | 53.30 a | 15.28 e | 77.14 b |
| KN | 9.22 d | 4.86 e | 8.52 d | 24.01 a | 53.37 a | 14.24 f | 77.38 a |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Year | |||||||
| 2020–21 | 9.55 a | 5.39 a | 8.73 b | 23.62 b | 52.50 b | 14.95 b | 76.12 b |
| 2021–22 | 9.59 a | 5.41 a | 8.76 a | 23.70 a | 52.67 a | 15.00 a | 76.38 a |
| p value | 0.364 | 0.382 | 0.012 | <0.0001 | <0.0001 | 0.010 | <0.0001 |
| Treatments | Irrigation Water Applied (mm) | Common Irrigation (mm) | Rainfall (mm) | Total Water Supplied (mm) | |||
|---|---|---|---|---|---|---|---|
| 2020–21 | 2021–22 | 2020–21 & 2021–22 | 2020–21 | 2021–22 | 2020–21 | 2021–22 | |
| I25 (25% CPE) | 72.6 | 66.3 | 50 | 10.6 | 20.0 | 133.2 | 136.3 |
| I50 (50% CPE) | 145.2 | 132.7 | 50 | 10.6 | 20.0 | 205.8 | 202.7 |
| I75 (75% CPE) | 217.8 | 199.0 | 50 | 10.6 | 20.0 | 278.5 | 269.0 |
| I100 (100% CPE) | 290.5 | 265.5 | 50 | 10.6 | 20.0 | 351.1 | 335.5 |
| Number of irrigations (scheduled time; DAS) | 8 (5, 13, 24, 33, 41, 54, 64, 76) | 8 (8, 18, 24, 35, 45, 58, 65, 74) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harisha, C.B.; Narayanpur, V.B.; Rane, J.; Ganiger, V.M.; M. Prasanna, S.; Vishwanath, Y.C.; G. Reddi, S.; Halli, H.M.; Boraiah, K.M.; Basavaraj, P.S.; et al. Promising Bioregulators for Higher Water Productivity and Oil Quality of Chia under Deficit Irrigation in Semiarid Regions. Plants 2023, 12, 662. https://doi.org/10.3390/plants12030662
Harisha CB, Narayanpur VB, Rane J, Ganiger VM, M. Prasanna S, Vishwanath YC, G. Reddi S, Halli HM, Boraiah KM, Basavaraj PS, et al. Promising Bioregulators for Higher Water Productivity and Oil Quality of Chia under Deficit Irrigation in Semiarid Regions. Plants. 2023; 12(3):662. https://doi.org/10.3390/plants12030662
Chicago/Turabian StyleHarisha, Chowdasandra Byregowda, Vijaykumar B. Narayanpur, Jagadish Rane, Vasant M. Ganiger, Sugooru M. Prasanna, Yeragenahalli Chandrashekaharappa Vishwanath, Sanjeevraddi G. Reddi, Hanamant M. Halli, Karnar Manjanna Boraiah, Patil Siddanagouda Basavaraj, and et al. 2023. "Promising Bioregulators for Higher Water Productivity and Oil Quality of Chia under Deficit Irrigation in Semiarid Regions" Plants 12, no. 3: 662. https://doi.org/10.3390/plants12030662
APA StyleHarisha, C. B., Narayanpur, V. B., Rane, J., Ganiger, V. M., M. Prasanna, S., Vishwanath, Y. C., G. Reddi, S., Halli, H. M., Boraiah, K. M., Basavaraj, P. S., Mahmoud, E. A., Casini, R., & Elansary, H. O. (2023). Promising Bioregulators for Higher Water Productivity and Oil Quality of Chia under Deficit Irrigation in Semiarid Regions. Plants, 12(3), 662. https://doi.org/10.3390/plants12030662








