Characterization and Therapeutic Applications of Biosynthesized Silver Nanoparticles Using Cassia auriculate Flower Extract
Abstract
1. Introduction
2. Results and Discussion
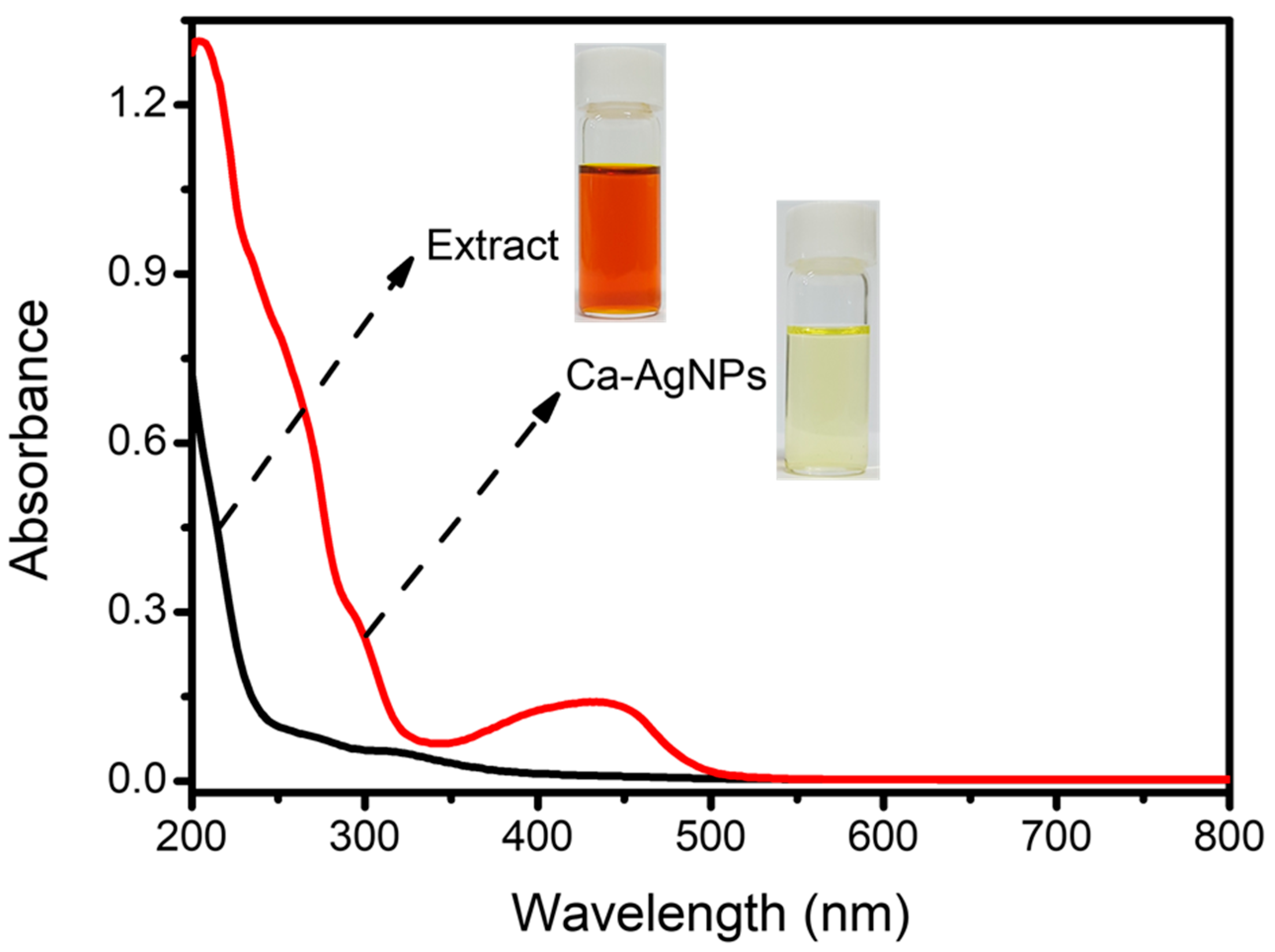
2.1. UV-Vis Spectral Study of Ca-AgNPs
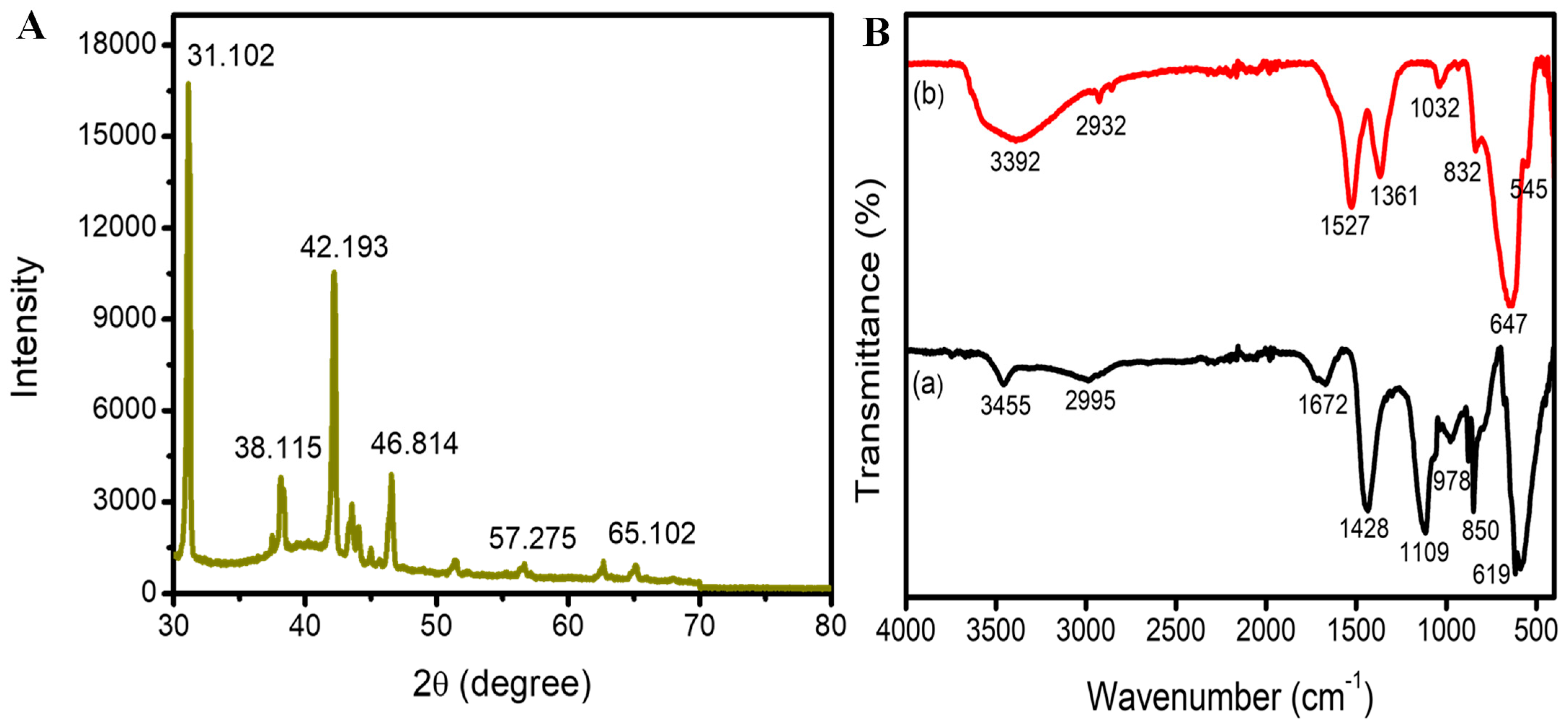
2.2. FTIR Analysis of Synthesized Ca-AgNPs
2.3. XRD Analysis of Synthesized Ca-AgNPs
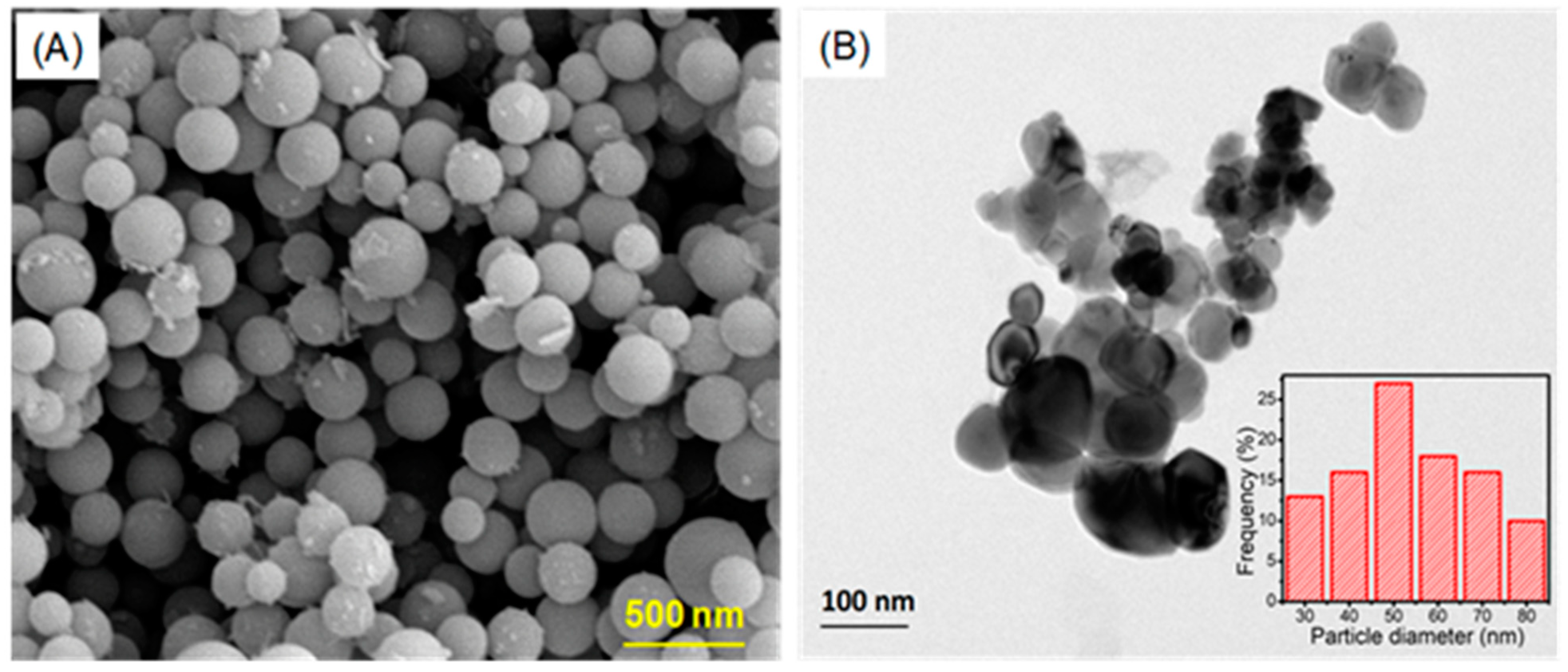
2.4. Morphological Analysis of Synthesized Ca-AgNPs
2.5. Antibacterial Properties
2.6. Antioxidant Properties
2.7. Live/Dead Cells Analysis
2.8. Cytotoxic Analysis
3. Materials and Methods
3.1. Preparation of the Extract
3.2. Synthesis of Silver Nanoparticles
3.3. Characterization of Biosynthesized Silver Nanoparticles
3.3.1. UV–Vis Spectroscopy
3.3.2. Fourier Transform Infrared (FTIR)
3.3.3. X-ray Diffraction (XRD)
3.3.4. Scanning Electron Microscopy (SEM)
3.3.5. Transmission Electron Microscopy (TEM) Analysis
3.4. Antimicrobial Analysis of Ca-AgNPs
3.5. Antioxidant Activity
3.6. Live/Dead Cells Staining Assess
3.7. Assessment of Cells Viability Study by MTT Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef]
- Tanase, C.; Berta, L.; Coman, N.A.; Roșca, I.; Man, A.; Toma, F.; Mocan, A.; Jakab-Farkas, L.; Biró, D.; Mare, A. Investigation of In Vitro Antioxidant and Antibacterial Potential of Silver Nanoparticles Obtained by Biosynthesis Using Beech Bark Extract. Antioxidants 2019, 8, 459. [Google Scholar] [CrossRef]
- Aktepe, N.; Baran, A. Fast and low cost biosynthesis of AgNPs with almond leaves: Medical applications with biocompatible structures: Fast and low cost biosynthesis of AgNPs. Prog. Nutr. 2021, 23, e2021271. [Google Scholar]
- Ahmad, N. Green Synthesis of Silver Nanoparticles Using Extracts of Ananas comosus. Green Sustain. Chem. 2012, 2, 141–147. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Siadati, S.A.; Afzali, M.; Sayyadi, M. Could silver nano-particles control the 2019-nCoV virus? An urgent glance to the past. Chem. Rev. Lett. 2020, 3, 9–11. [Google Scholar]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Ahn, E.Y.; Jin, H.; Park, Y. Assessing the antioxidant, cytotoxic, apoptotic and wound healing properties of silver nanoparticles green-synthesized by plant extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 204–216. [Google Scholar] [CrossRef]
- Karmous, I.; Pandey, A.; Haj, K.B.; Chaoui, A. Efficiency of the Green Synthesized Nanoparticles as New Tools in Cancer Therapy: Insights on Plant-Based Bioengineered Nanoparticles, Biophysical Properties, and Anticancer Roles. Biol. Trace Elem. Res. 2020, 196, 330–342. [Google Scholar] [CrossRef]
- Yeşilot, Ş.; Aydın Acar, Ç. Silver nanoparticles; a new hope in cancer therapy? East. J. Med. 2019, 24, 111–116. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Hembram, K.C.; Kumar, R.; Kandha, L.; Parhi, P.K.; Kundu, C.N.; Bindhani, B.K. Therapeutic prospective of plant-induced silver nanoparticles: Application as antimicrobial and anticancer agent. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S38–S51. [Google Scholar] [CrossRef] [PubMed]
- Shumail, H.; Khalid, S.; Ahmad, I.; Khan, H.; Amin, S.; Ullah, B. Review on Green Synthesis of Silver Nanoparticles through Plants. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Li, C.; Hu, H.; Zhang, X. Development of silver nanoparticles loaded chitosan-alginate constructs with biomedical potentialities. Int. J. Biol. Macromol. 2017, 105 Pt 1, 393–400. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wei, F.; Ma, Z.; Zhang, H.; Yang, Q.; Yao, B.; Huang, Z.; Li, J.; Zeng, C.; Zhang, Q. Green synthesis of silver nanoparticles using seed extract of Alpinia katsumadai, and their antioxidant, cytotoxicity, and antibacterial activities. RSC Adv. 2017, 7, 39842–39851. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Ahmed, I.; Hassan, S.T.S.; Nawaz, M.Z.; Iqbal, H.M.N. Biogenic Nanoparticle–Chitosan Conjugates with Antimicrobial, Antibiofilm, and Anticancer Potentialities: Development and Characterization. Int. J. Environ. Res. Public Health 2019, 16, 598. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial Effect of Silver Nanoparticles Synthesized Using Murraya koenigii (L.) against Multidrug-Resistant Pathogens. Bioinorg. Chem. Appl. 2019, 2019, 4649506. [Google Scholar] [CrossRef]
- Mickymaray, S. One-step Synthesis of Silver Nanoparticles Using Saudi Arabian Desert Seasonal Plant Sisymbrium irio and Antibacterial Activity Against Multidrug-Resistant Bacterial Strains. Biomolecules 2019, 9, 662. [Google Scholar] [CrossRef]
- Kumari, R.; Saini, A.K.; Kumar, A.; Saini, R.V. Apoptosis induction in lung and prostate cancer cells through silver nanoparticles synthesized from Pinus roxburghii bioactive fraction. J. Biol. Inorg. Chem. 2020, 25, 23–37. [Google Scholar] [CrossRef]
- Baran, M.; Keskin, C.; Atalar, M.; Baran, A. Environmentally Friendly Rapid Synthesis of Gold Nanoparticles from Artemisia absinthium Plant Extract and Application of Antimicrobial Activities. J. Inst. Sci. Technol. 2021, 11, 365–375. [Google Scholar]
- Huang, X.; Wang, R.; Jiao, T.; Zou, G.; Zhan, F.; Yin, J.; Zhang, L.; Zhou, J.; Peng, Q. Facile Preparation of Hierarchical AgNP-Loaded MXene/Fe3O4/Polymer Nanocomposites by Electrospinning with Enhanced Catalytic Performance for Wastewater Treatment. ACS Omega 2019, 4, 1897–1906. [Google Scholar] [CrossRef]
- Bhuvaneswari, R.; Xavier, R.J.; Arumugam, M. Facile synthesis of multifunctional silver nanoparticles using mangrove plant Excoecaria agallocha L. for its antibacterial, antioxidant and cytotoxic effects. J. Parasit. Dis. 2017, 41, 180–187. [Google Scholar] [CrossRef]
- Jeeva, K.; Thiyagarajan, M.; Elangovan, V.; Geetha, N.; Venkatachalam, P. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind. Crops Prod. 2014, 52, 714–720. [Google Scholar] [CrossRef]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Pammi, S.V.N.; Yoon, S.-G. Ultra Small, mono dispersed green synthesized silver nanoparticles using aqueous extract of Sida cordifolia plant and investigation of antibacterial activity. Microb. Pathog. 2018, 124, 63–69. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Sivagurunathan, P.; Saratale, G.D.; Dung, T.N.B.; Kannapiran, E. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol. Rep. (Amst) 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Kanagala, P.; Gaddam, S.A.; Gunji, P.; Kotakadi, V.S.; Kalla, C.; Vijaya, T.; Divi, V.; Ramana, S.; Gopal, D.V.R.S. Synthesis of Bio-Inspired Silver Nanoparticles by Ripe and Unripe Fruit Extract of Tinospora cordifolia and Its Antioxidant, Antibacterial and Catalytic Studies Citation. Nano Biomed. Eng. 2020, 12, 214–226. [Google Scholar] [CrossRef]
- Homayouni-Tabrizi, M.; Karimi, E.; Namvar, F.; Soltani, M.; Pouresmaeil, V. Silver–palm pollen nanocomposite exhibits antiproliferative, antioxidant, and proapoptotic properties on MCF-7 breast cancer cells. Res. Chem. Intermed. 2018, 44, 6537–6548. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N.; Bastia, A.K.; Mohanta, T.K. Antimicrobial, Antioxidant and Cytotoxic Activity of Silver Nanoparticles Synthesized by Leaf Extract of Erythrina suberosa (Roxb.). Front. Mol. Biosci. 2017, 4, 14. [Google Scholar] [CrossRef]
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crops Prod. 2013, 46, 132–137. [Google Scholar] [CrossRef]
- Divya, M.; Vaseeharan, B.; Chen, J.; Biruntha, M.; Silva, L.; Durán-Lara, E.; Shreema, K.; Ranjan, S.; Dasgupta, N. Biological Compound Capping of Silver Nanoparticle with the Seed Extracts of Blackcumin (Nigella sativa): A Potential Antibacterial, Antidiabetic, Anti-inflammatory, and Antioxidant. J. Inorg. Organomet. Polym. Mater. 2021, 31, 624–635. [Google Scholar]
- Palithya, S.; Gaddam, S.; Kotakadi, V.S.; Penchalaneni, J.; Golla, N.; Krishna, S.; Naidu, C. Green synthesis of silver nanoparticles using flower extracts of Aerva lanata and their biomedical applications. Part. Sci. Technol. 2022, 40, 84–96. [Google Scholar] [CrossRef]
- Barathi, S.; Meng, Y.; Yu, Z.; Ni, S.-Q.; Meng, F. Roles of nitrite in mediating the composition and metacommunity of multispecies biofilms. J. Water Process Eng. 2021, 40, 101764. [Google Scholar] [CrossRef]
- Soltani, L.; Darbemamieh, M. Biosynthesis of Silver Nanoparticles Using Hydroethanolic Extract of Cucurbita pepo L. Fruit and Their Anti-proliferative and Apoptotic Activity Against Breast Cancer Cell Line (MCF-7). Mcijournal 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Li, C.; Iqbal, H.M.N. Biomedical Potentialities of Taraxacum officinale-based Nanoparticles Biosynthesized Using Methanolic Leaf Extract. Curr. Pharm. Biotechnol. 2017, 18, 1116–1123. [Google Scholar] [CrossRef]
- Prabula, S.S.; Hentry, C.; Rose, B.L.; Parvathiraja, C.; Mani, A.; Wabaidur, S.M.; Eldesoky, G.E.; Islam, M.A. Synthesis of Silver Nanoparticles by Using Cassia auriculata Flower Extract and Their Photocatalytic Behavior. Chem. Eng. Technol. 2022, 45, 1919–1925. [Google Scholar] [CrossRef]
- Sudhakar, C.; Poonkothai, M.; Selvankumar, T.; Selvam, K. Facile synthesis of iron oxide nanoparticles using Cassia auriculata flower extract and accessing their photocatalytic degradation and larvicidal effect. J. Mater. Sci. Mater. Electron. 2022, 33, 11434–11445. [Google Scholar] [CrossRef]
- Jain, N.; Jain, P.; Rajput, D.; Patil, U.K. Green synthesized plant-based silver nanoparticles: Therapeutic prospective for anticancer and antiviral activity. Micro Nano Syst. Lett. 2021, 9, 5. [Google Scholar] [CrossRef]
- Chen, X.; Jensen, L. Understanding the shape effect on the plasmonic response of small ligand coated nanoparticles. J. Opt. 2016, 18, 074009. [Google Scholar] [CrossRef]
- Silva, L.P.; Pereira, T.M.; Bonatto, C.C. Frontiers and perspectives in the green synthesis of silver nanoparticles. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–164. [Google Scholar]
- Nagarajan, S.; Kalaivani, G.; Poongothai, E.; Arul, M.; Natarajan, H.J.I. Characterization of silver nanoparticles synthesized from Catharanthus roseus (Vinca rosea) plant leaf extract and their antibacterial activity. Int. J. Res. Anal. Rev. 2019, 6, 680–685. [Google Scholar]
- Anbu, P.; Gopinath, S.C.B.; Jayanthi, S. Synthesis of gold nanoparticles using Platycodon grandiflorum extract and its antipathogenic activity under optimal conditions. Nanomater. Nanotechnol. 2020, 10, 1847980420961697. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Rodríguez-Vázquez, B.E.; Martínez-Higuera, A.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Navarro, R.E.; López-Esparza, R.; Iñiguez-Palomares, R.A. Synthesis of Gold Nanoparticles Using Mimosa tenuiflora Extract, Assessments of Cytotoxicity, Cellular Uptake, and Catalysis. Nanoscale Res. Lett. 2019, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]





| Zone of Inhibition | |||||
|---|---|---|---|---|---|
| Samples | Conc (µg/mL) | S. epidermidis | P. aeruginosa | V. cholerae | E. coli |
| Extract | 75 | 11 ± 0.5 | 9 ± 0.6 | 12 ± 0.5 | 5 ± 0.4 |
| Ca-AgNPs | 75 | 13 ± 0.2 | 12 ± 0.4 | 16 ± 0.6 | 10 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabapathi, N.; Ramalingam, S.; Aruljothi, K.N.; Lee, J.; Barathi, S. Characterization and Therapeutic Applications of Biosynthesized Silver Nanoparticles Using Cassia auriculate Flower Extract. Plants 2023, 12, 707. https://doi.org/10.3390/plants12040707
Sabapathi N, Ramalingam S, Aruljothi KN, Lee J, Barathi S. Characterization and Therapeutic Applications of Biosynthesized Silver Nanoparticles Using Cassia auriculate Flower Extract. Plants. 2023; 12(4):707. https://doi.org/10.3390/plants12040707
Chicago/Turabian StyleSabapathi, Nadana, Srinivasan Ramalingam, Kandasamy Nagarajan Aruljothi, Jintae Lee, and Selvaraj Barathi. 2023. "Characterization and Therapeutic Applications of Biosynthesized Silver Nanoparticles Using Cassia auriculate Flower Extract" Plants 12, no. 4: 707. https://doi.org/10.3390/plants12040707
APA StyleSabapathi, N., Ramalingam, S., Aruljothi, K. N., Lee, J., & Barathi, S. (2023). Characterization and Therapeutic Applications of Biosynthesized Silver Nanoparticles Using Cassia auriculate Flower Extract. Plants, 12(4), 707. https://doi.org/10.3390/plants12040707










