Sustainable Soil Management: Effects of Clinoptilolite and Organic Compost Soil Application on Eco-Physiology, Quercitin, and Hydroxylated, Methoxylated Anthocyanins on Vitis vinifera
Abstract
1. Introduction
2. Results
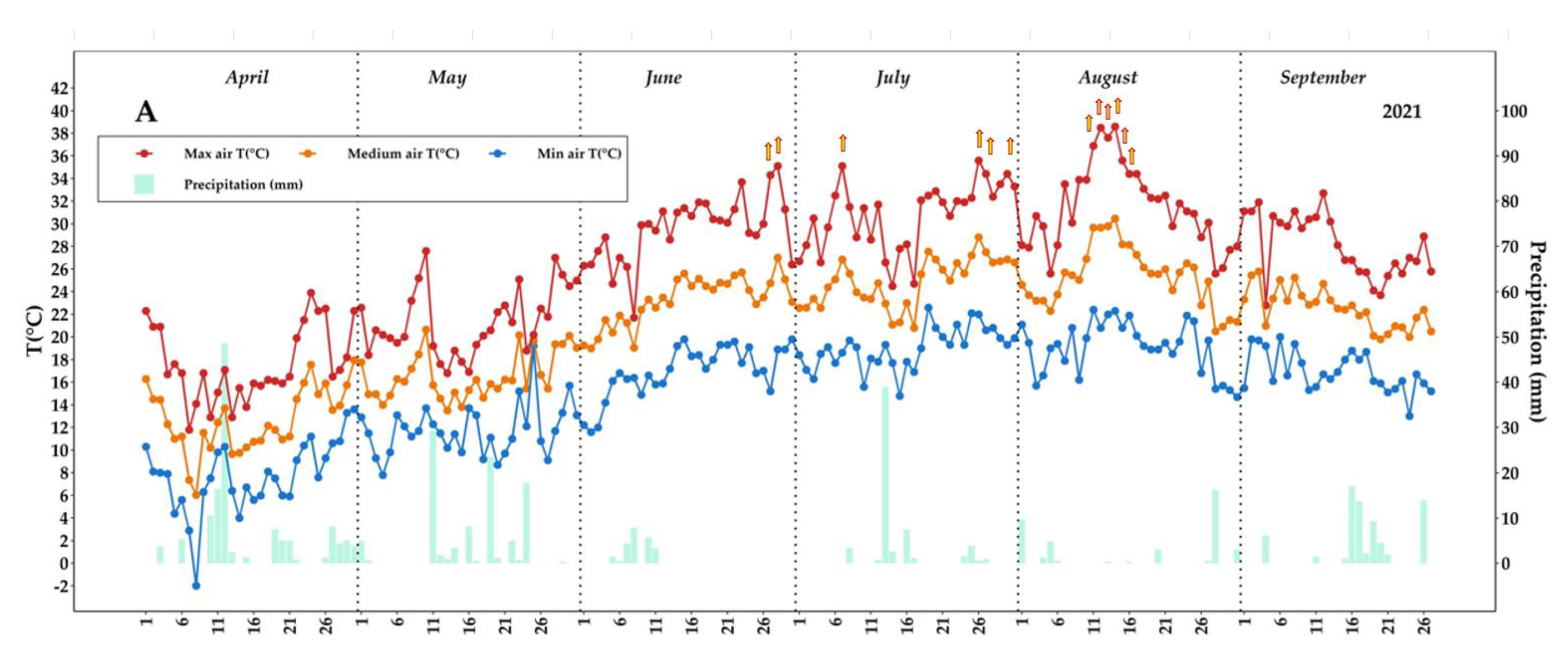
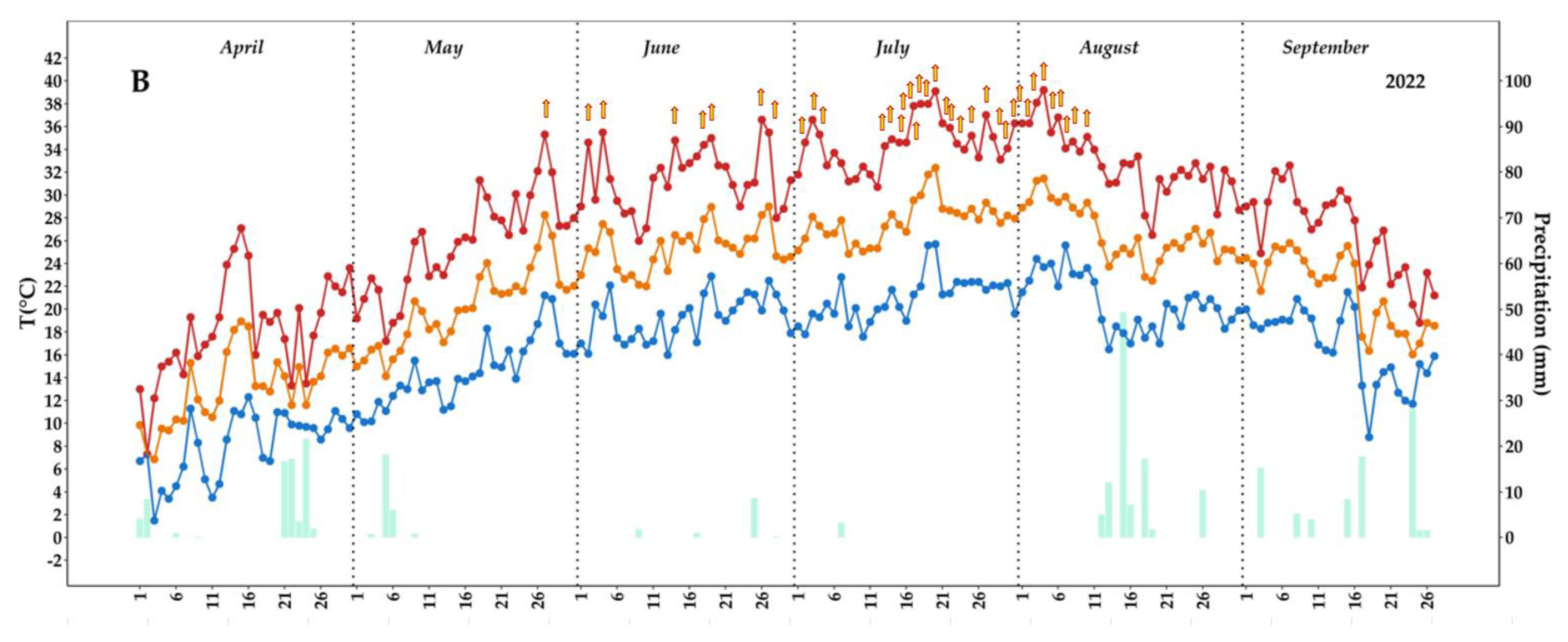
2.1. Weather Parameters
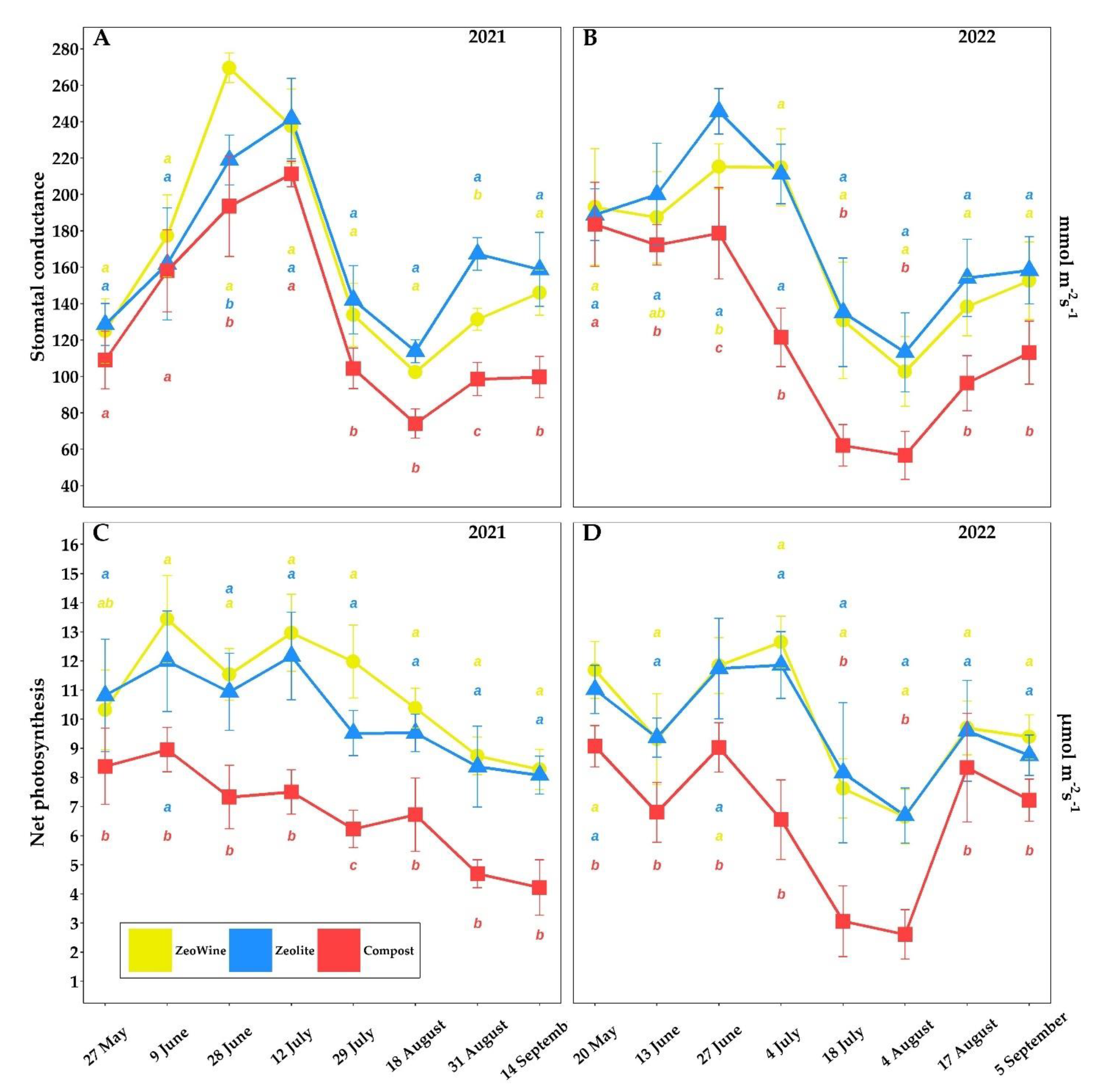
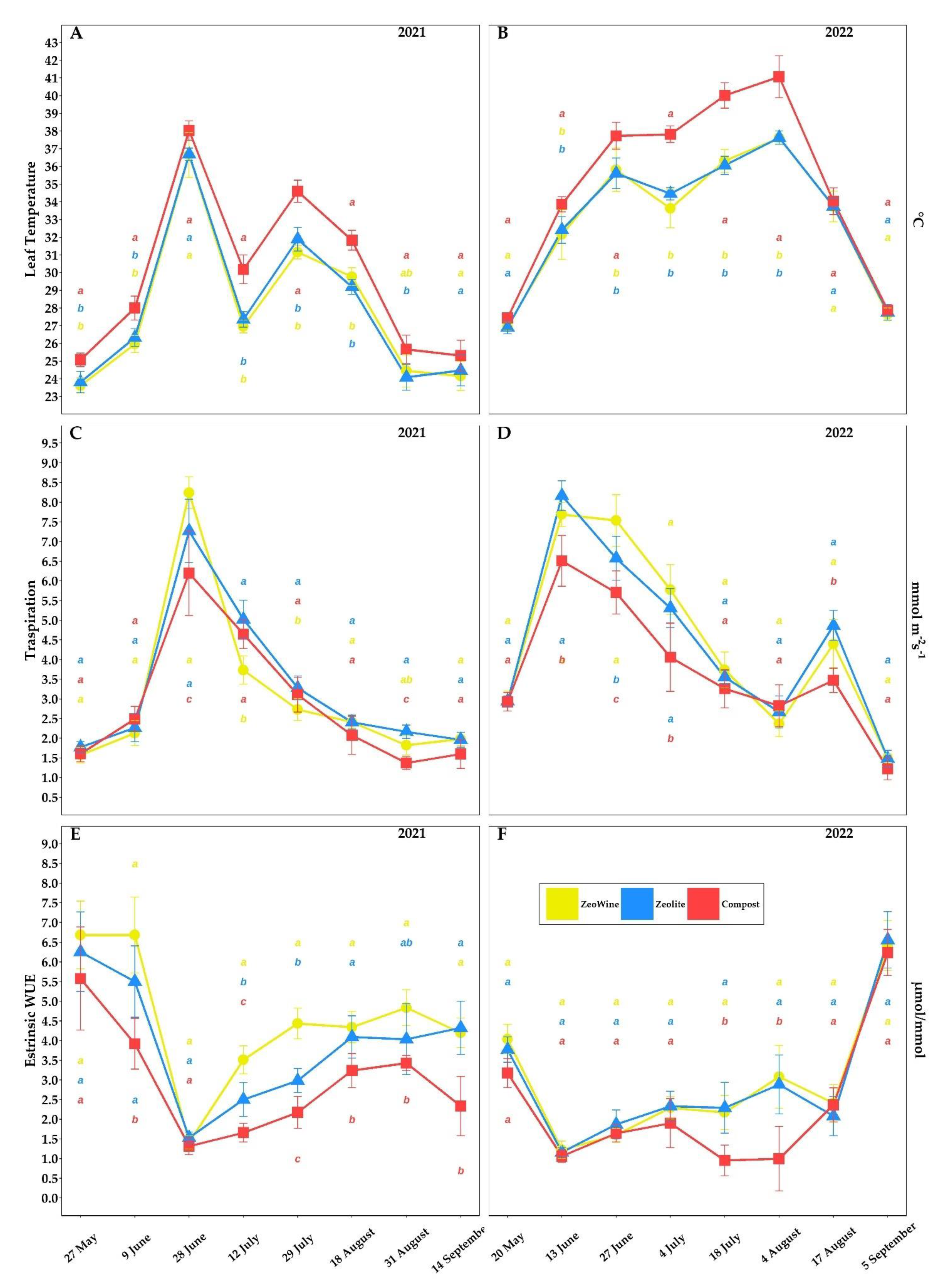
2.2. Ecophysiological Survey (Gaseous Exchange), Midday Stem Water Potential, and Leaf Chlorophyll a Fluorescence
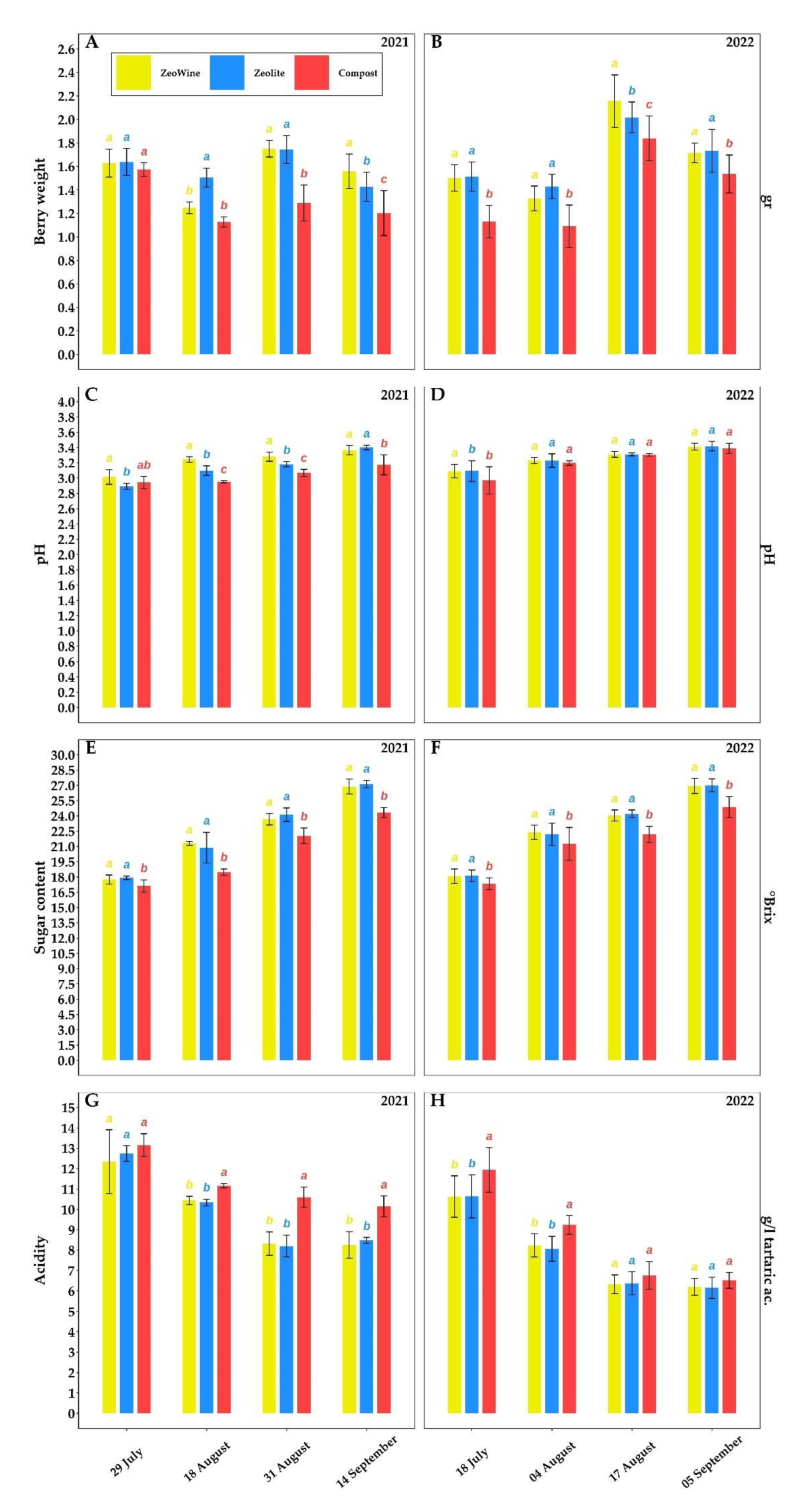
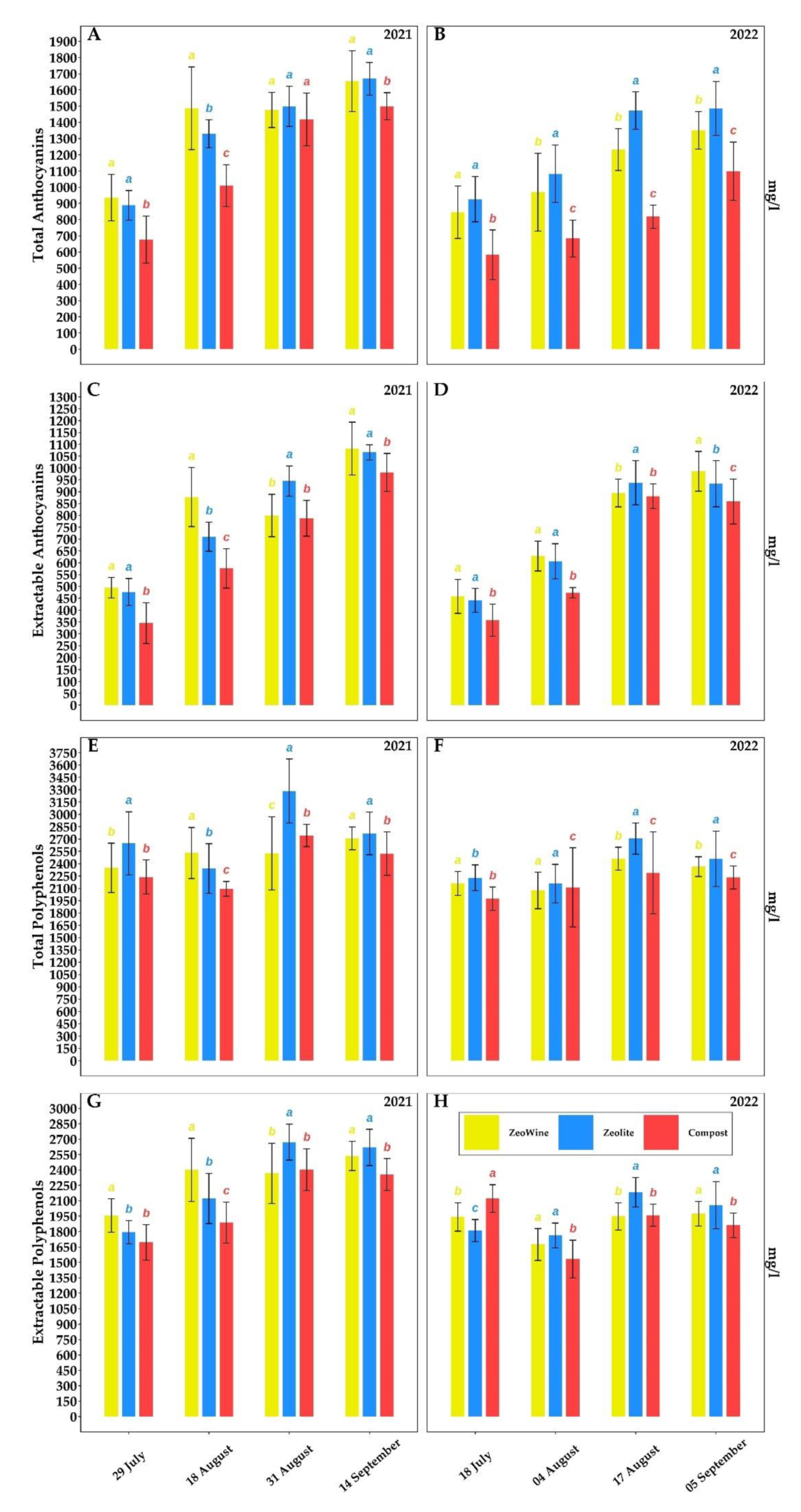
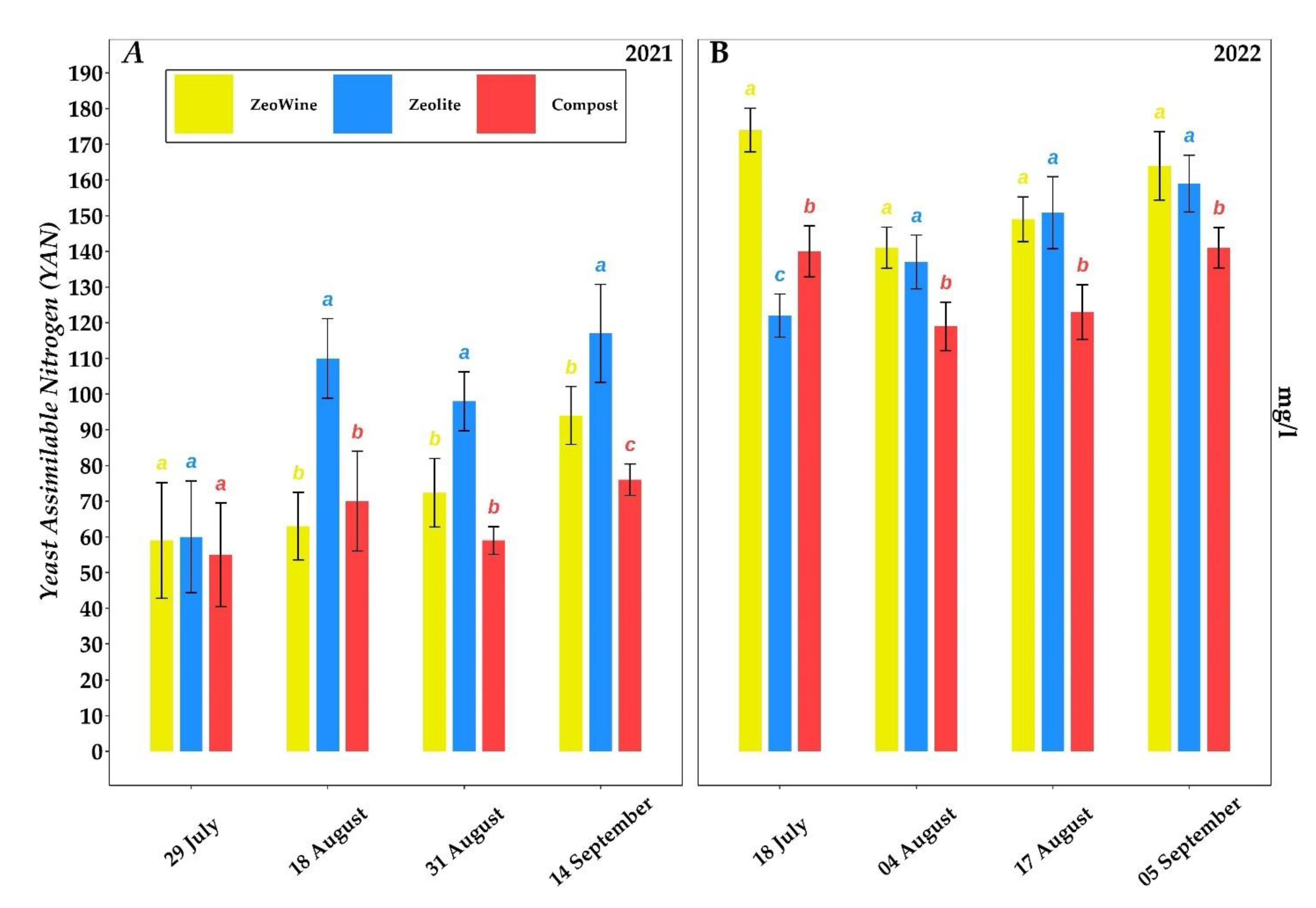
2.3. Berry Quality
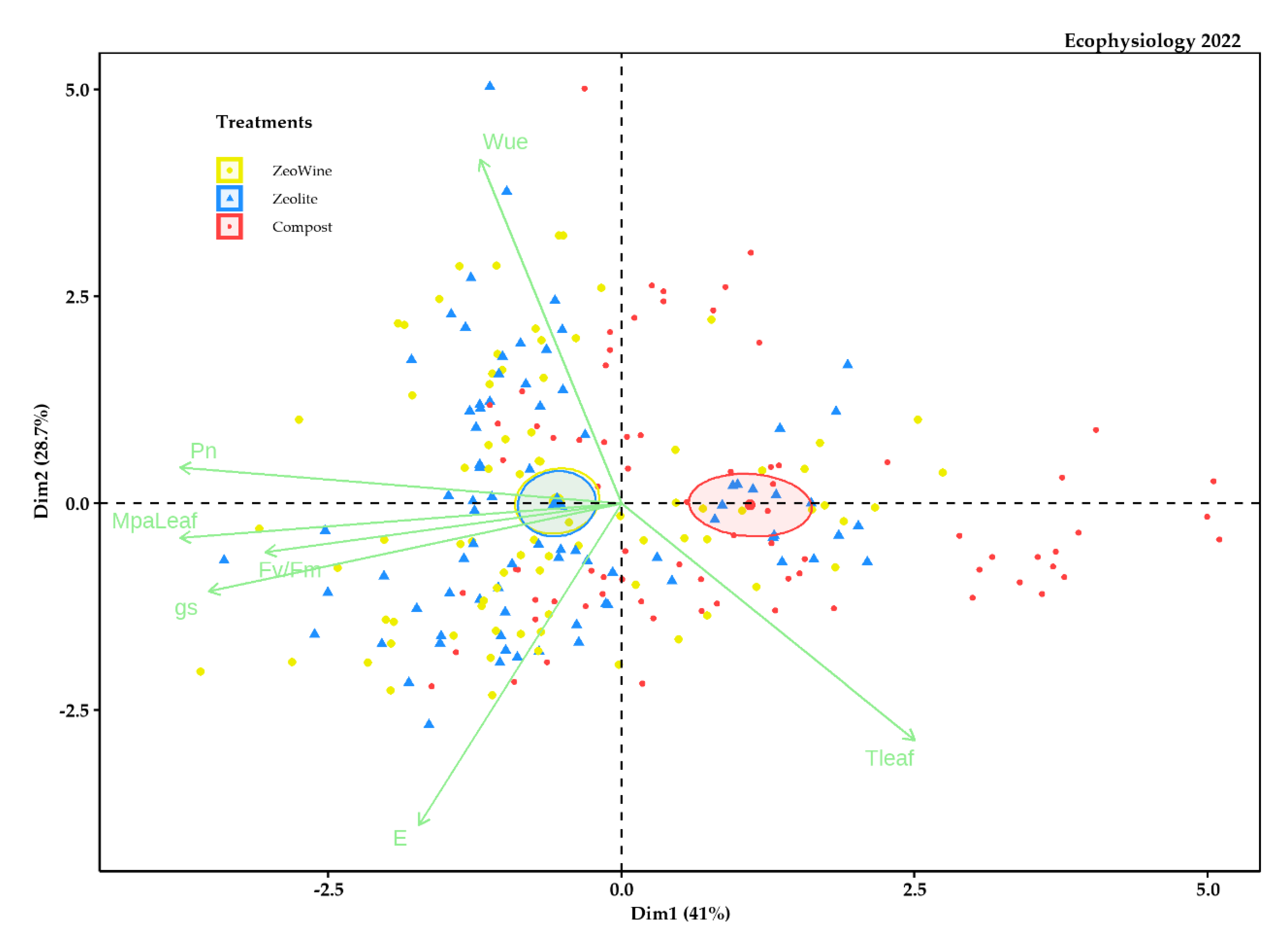
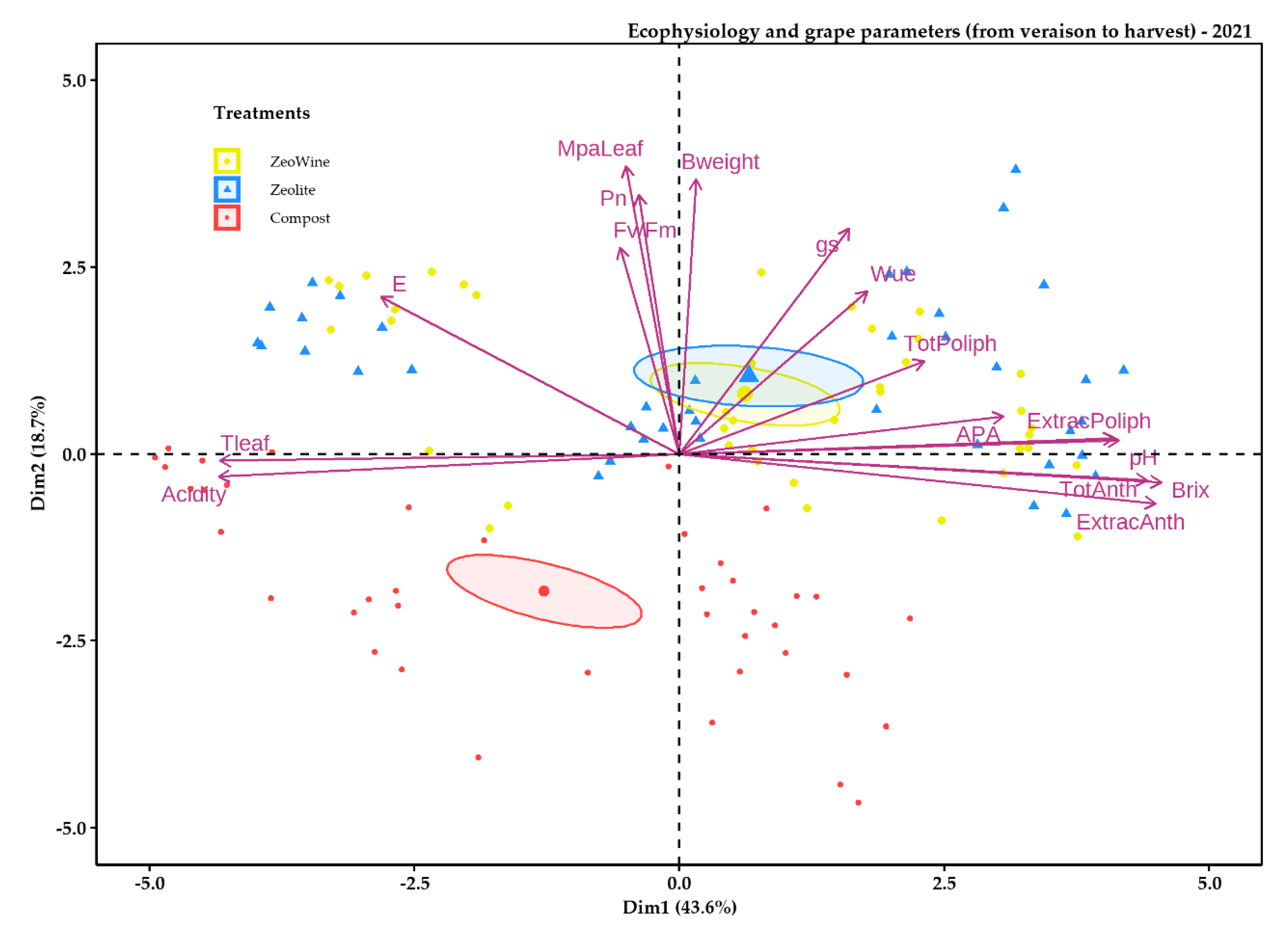
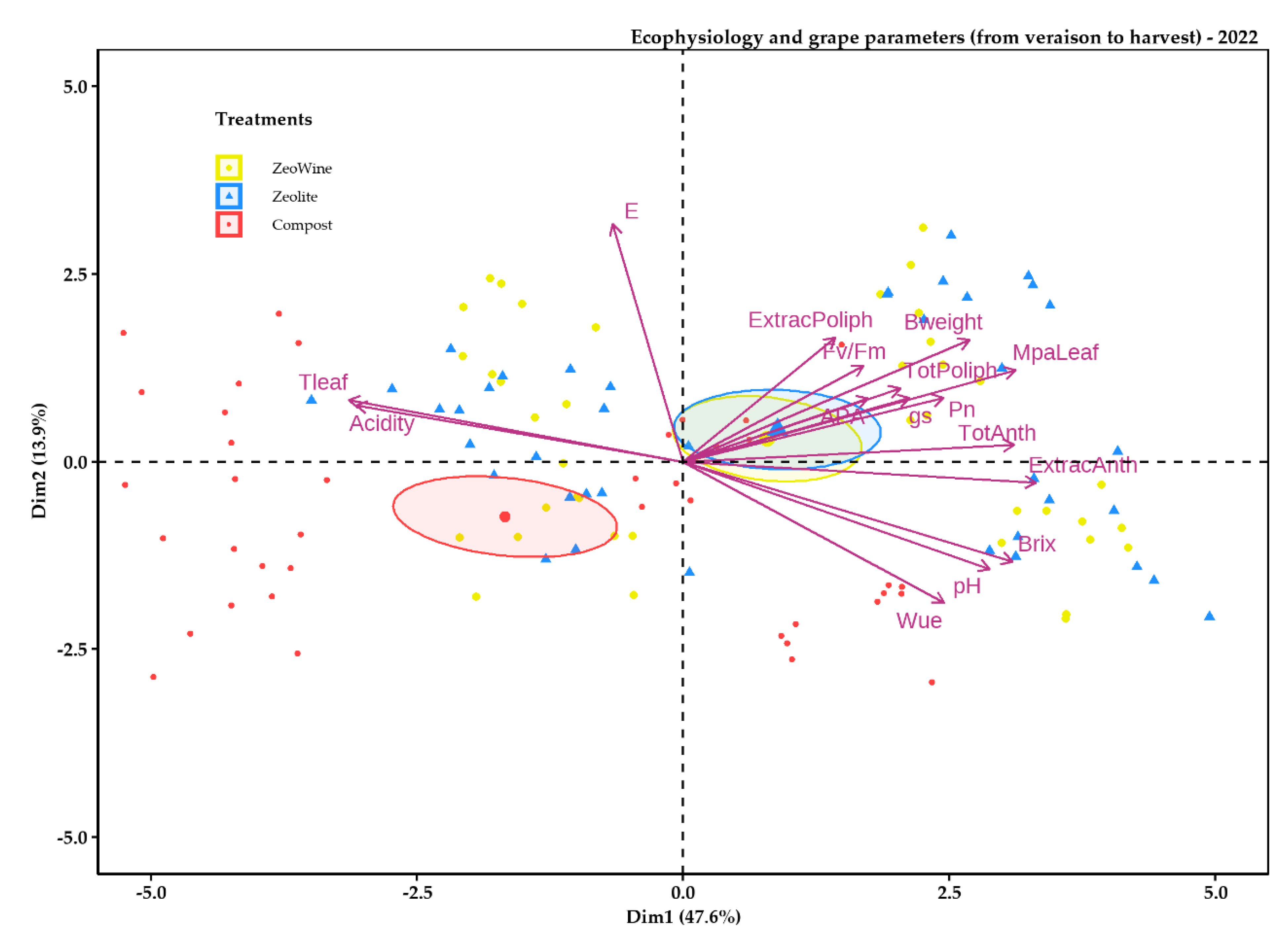
2.4. Principal Component Analysis
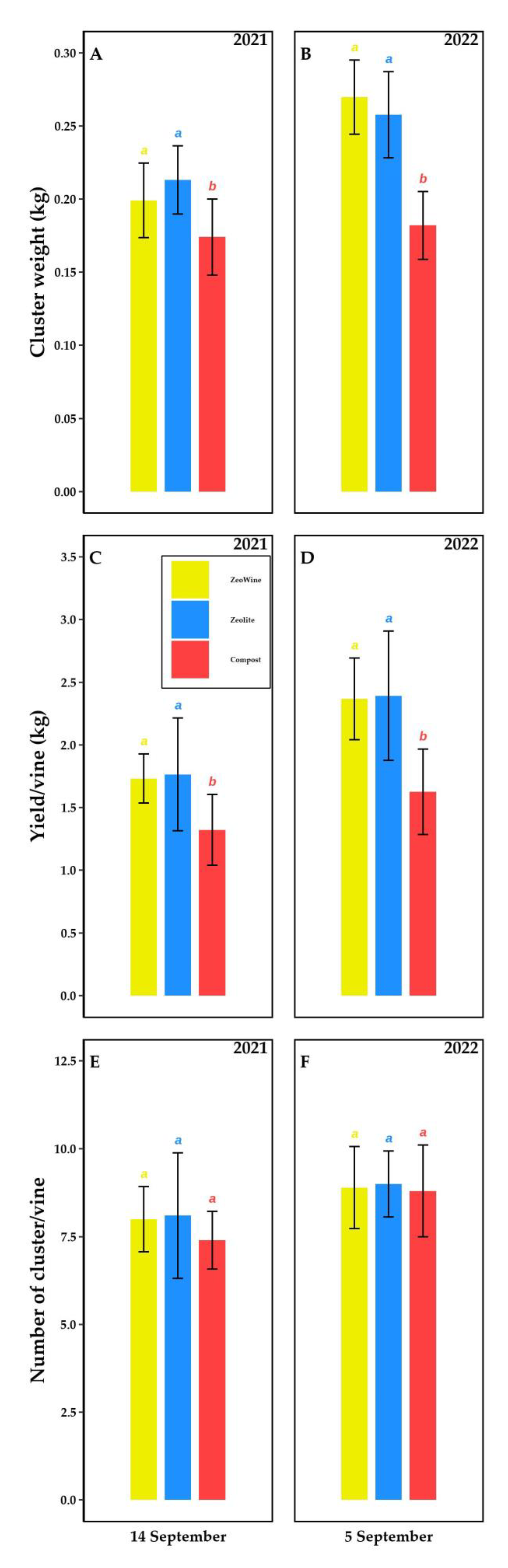
2.5. Production
3. Discussion
4. Materials and Methods
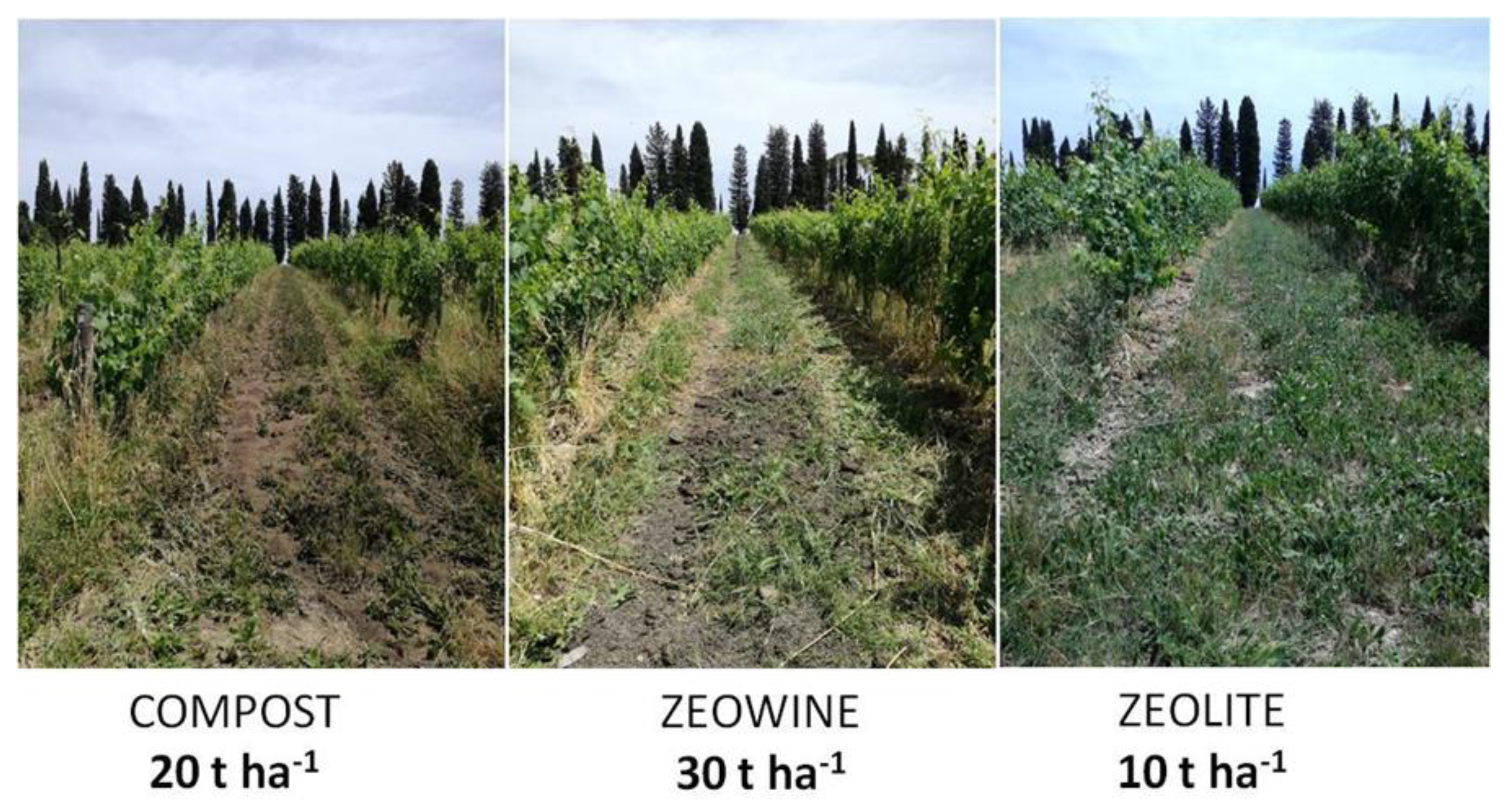
4.1. Experimental Project, Place of Setting, and Composting Process
4.2. Ecophysiological Survey (Gaseous Exchange), Midday Stem Water Potential, and Leaf Chlorophyll a Fluorescence
4.3. Berry Quality
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beheshti, S.; Heydari, J.; Sazvar, Z. Food waste recycling closed loop supply chain optimization through renting waste recycling facilities. Sustain. Cities Soc. 2022, 78, 103644. [Google Scholar] [CrossRef]
- Dwivedi, Y.K.; Hughes, L.; Kar, A.K.; Baabdullah, A.M.; Grover, P.; Abbas, R.; Andreini, D.; Abumoghli, I.; Barlette, Y.; Bunker, D.; et al. Climate change and COP26: Are digital technologies and information management part of the problem or the solution? An editorial reflection and call to action. Int. J. Inf. Manag. 2022, 63, 102456. [Google Scholar] [CrossRef]
- Burg, P.; Vítěz, T.; Turan, J.; Burgová, J. Evaluation of grape pomace composting process. Acta Univ. Agric. Silvic. Mendel. Brun. 2014, 62, 875–881. [Google Scholar] [CrossRef]
- Bian, B.; Hu, X.; Zhang, S.; Lv, C.; Yang, Z.; Yang, W.; Zhang, L. Pilot-scale composting of typical multiple agricultural wastes: Parameter optimization and mechanisms. Bioresour 2019, 287, 121482. [Google Scholar] [CrossRef]
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Grapes. In Integrated Processing Technologies for Food and Agricultural by-Products; Academic Press: Cambridge, MA, USA, 2019; pp. 133–163. [Google Scholar]
- Tamelová, B.; Malaťák, J.; Velebil, J.; Gendek, A.; Aniszewska, M. Energy utilization of torrefied residue from wine production. Materials 2021, 14, 1610. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Pérez, K.; Cassano, A.; Ruby-Figueroa, R. Recovery of anthocyanins and monosaccharides from grape marc extract by nanofiltration membranes. Molecules 2021, 26, 2003. [Google Scholar] [CrossRef]
- Fia, G.; Bucalossi, G.; Gori, C.; Borghini, F.; Zanoni, B. Recovery of bioactive compounds from unripe red grapes (cv. Sangiovese) through a green extraction. Foods 2020, 9, 566. [Google Scholar] [CrossRef]
- Oliveira, M.; Duarte, E. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Fia, G.; Zanoni, B.; Gori, C. A new technique for exploitation of wine lees. Agric. Agric. Sci. Procedia 2016, 8, 748–754. [Google Scholar] [CrossRef]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and future strategies for wine yeast lees valorization. Int. Food Res. J. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Barros, E.S.C.; de Amorim, M.C.C.; Olszevski, N.; Silva, P.T.D.S.E. Composting of winery waste and characteristics of the final compost according to Brazilian legislation. J. Environ. Sci. Heal. Part B 2021, 56, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Felix, M.; Martínez, I.; Sayago, A.; Recamales, M.Á.F. Wine lees: From waste to O/W emulsion stabilizer. Innov. Food Sci. Emerg. Technol. 2021, 74, 102810. [Google Scholar] [CrossRef]
- Mulidzi, A.R. Evaluating Sustainable Use and Management of Winery Solid Wastes through Composting. South Afr. J. Enol. Vitic. 2021, 42, 193–200. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Arlorio, M.; Coïsson, J.D. Spent grape pomace as a still potential by-product. Int. J. Food Sci. Technol. 2015, 50, 2022–2031. [Google Scholar] [CrossRef]
- Soceanu, A.; Dobrinas, S.; Sirbu, A.; Manea, N.; Popescu, V. Economic aspects of waste recovery in the wine industry. A multidisciplinary approach. Sci. Total Environ. 2021, 759, 143543. [Google Scholar] [CrossRef] [PubMed]
- Motti, R.; Bonanomi, G.; de Falco, B. Wild and cultivated plants used in traditional alcoholic beverages in Italy: An ethnobotanical review. Eur. Food Res. Technol. 2022, 248, 1089–1106. [Google Scholar] [CrossRef]
- D’Souza, C.; Marjoribanks, T.; Young, S.; Sullivan Mort, G.; Nanere, M.; John, J.J. Environmental management systems: An alternative marketing strategy for sustainability. J. Strat. Mark. 2019, 27, 417–434. [Google Scholar] [CrossRef]
- Muktiono, E.; Soediantono, D. Literature Review of ISO 14001 Environmental Management System Benefits and Proposed Applications in the Defense Industries. J. Ind. Eng. Manag. 2022, 3, 1–12. [Google Scholar]
- Pinto, R.; Brito, L.M.; Gonçalves, F.; Mourão, I.; Torres, L.; Coutinho, J. Recycling wastes from Douro wine industry by composting. In Proceedings of the III International Symposium on Growing Media, Composting and Substrate Analysis, Milan, Italy, 24–28 June 2019; pp. 285–292. [Google Scholar]
- Ferrari, V.; Taffarel, S.R.; Espinosa-Fuentes, E.; Oliveira, M.L.; Saikia, B.K.; Oliveira, L.F. Chemical evaluation of by-products of the grape industry as potential agricultural fertilizers. J. Clean. Prod. 2019, 208, 297–306. [Google Scholar] [CrossRef]
- Kolbe, A.R.; Aira, M.; Gómez-Brandón, M.; Pérez-Losada, M.; Domínguez, J. Bacterial succession and functional diversity during vermicomposting of the white grape marc Vitis vinifera v. Albariño. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Sgubin, G.; Swingedouw, D.; García de Cortázar-Atauri, I.; Ollat, N.; Van Leeuwen, C. The impact of possible decadal-scale cold waves on viticulture over Europe in a context of global warming. Agronomy 2019, 9, 397. [Google Scholar] [CrossRef]
- Kizildeniz, T.; Irigoyen, J.J.; Pascual, I.; Morales, F. Simulating the impact of climate change (elevated CO2 and temperature, and water deficit) on the growth of red and white Tempranillo grapevine in three consecutive growing seasons (2013–2015). Agric. Water Manag. 2018, 202, 220–230. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Wang, W.; Ran, H.; Song, T.; Guo, Y. Evaluation of the crop water stress index as an indicator for the diagnosis of grapevine water deficiency in greenhouses. Horticulturae 2020, 6, 86. [Google Scholar] [CrossRef]
- Singh, B.; Usha, K. Effect of macro and micro-nutrient spray on fruit yield and quality of grape (Vitis vinifera L.) cv. Perlette. In Proceedings of the International Symposium on Foliar Nutrition of Perennial Fruit Plants, Meran, Italy, 11–15 September 2001; pp. 197–202. [Google Scholar]
- Ramos, M.C.; Jones, G.V.; Martínez-Casasnovas, J.A. Structure and trends in climate parameters affecting winegrape production in northeast Spain. Clim. Res. 2008, 38, 1–15. [Google Scholar] [CrossRef]
- Tomasi, D.; Jones, G.V.; Giust, M.; Lovat, L.; Gaiotti, F. Grapevine phenology and climate change: Relationships and trends in the Veneto region of Italy for 1964–2009. Am. J. Enol. Vitic. 2011, 62, 329–339. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Darriet, P. The impact of climate change on viticulture and wine quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- Andreoli, V.; Cassardo, C.; La Iacona, T.; Spanna, F. Description and preliminary simulations with the Italian vineyard integrated numerical model for estimating physiological values (IVINE). Agronomy 2019, 9, 94. [Google Scholar] [CrossRef]
- Abeysinghe, S.K.; Greer, D.H.; Rogiers, S.Y. The effect of light intensity and temperature on berry growth and sugar accumulation in Vitis vinifera ‘Shiraz’under vineyard conditions. Vitis 2019, 58, 7–16. [Google Scholar]
- Wen, P.-F.; Chen, J.-Y.; Wan, S.-B.; Kong, W.-F.; Zhang, P.; Wang, W.; Zhan, J.-C.; Pan, Q.-H.; Huang, W.-D. Salicylic acid activates phenylalanine ammonia-lyase in grape berry in response to high temperature stress. Plant Growth Regul. 2008, 55, 1–10. [Google Scholar] [CrossRef]
- Astaneh, R.K.; Bolandnazar, S.; Nahandi, F.Z.; Oustan, S. Effect of selenium application on phenylalanine ammonia-lyase (PAL) activity, phenol leakage and total phenolic content in garlic (Allium sativum L.) under NaCl stress. Inf. Process. Agric. 2018, 5, 339–344. [Google Scholar] [CrossRef]
- Ramos, M.C.; Martínez-Casasnovas, J.A. Soil water balance in rainfed vineyards of the Penedès region (Northeastern Spain) affected by rainfall characteristics and land levelling: Influence on grape yield. Plant Soil 2010, 333, 375–389. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M. Does water stress exacerbate the impacts of heat stress on berry development of Vitis vinifera cv. Semillon vines grown in controlled environment conditions? N. Z. J. Crop Hortic. Sci. 2021, 1–16. [Google Scholar] [CrossRef]
- Berhe, D.T. Post-Veraison Water Stress and Pruning Level on Merlot Grapevine (Vitis vinifera L.): Effects on Berry Development and Composition. J. Agron. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Caruso, G.; Palai, G.; Gucci, R.; D’Onofrio, C. The effect of regulated deficit irrigation on growth, yield, and berry quality of grapevines (cv. Sangiovese) grafted on rootstocks with different resistance to water deficit. Irrig. Sci. 2022, 1–15. [Google Scholar] [CrossRef]
- Alatzas, A.; Theocharis, S.; Miliordos, D.-E.; Leontaridou, K.; Kanellis, A.K.; Kotseridis, Y.; Hatzopoulos, P.; Koundouras, S. The effect of water deficit on two Greek Vitis vinifera L. cultivars: Physiology, Grape Composition and Gene Expression during berry development. Plants 2021, 10, 1947. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Pérez, D.; Risco, D.; Yeves, A.; Castel, J.R. Yield components and grape composition responses to seasonal water deficits in Tempranillo grapevines. Irrig. Sci. 2012, 30, 339–349. [Google Scholar] [CrossRef]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.-M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 1–33. [Google Scholar] [CrossRef]
- Gambuti, A.; Picariello, L.; Rinaldi, A.; Forino, M.; Blaiotta, G.; Moine, V.; Moio, L. New insights into the formation of precipitates of quercetin in Sangiovese wines. J. Food Sci. Technol. 2020, 57, 2602–2611. [Google Scholar] [CrossRef]
- Haselgrove, L.; Botting, D.; Van Heeswijck, R.; Høj, P.B.; Dry, P.R.; Ford, C.; Land, P.G.I. Canopy microclimate and berry composition: The effect of bunch exposure on the phenolic composition of Vitis vinifera L cv. Shiraz grape berries. Aust. J. Grape Wine Res. 2000, 6, 141–149. [Google Scholar] [CrossRef]
- Ibrahim-Saeedi, F.; Sepaskhah, A.R. Effects of a bentonite–water mixture on soil-saturated hydraulic conductivity. Arch. Agron. Soil Sci. 2013, 59, 377–392. [Google Scholar] [CrossRef]
- de Campos Bernardi, A.C.; Oliviera, P.P.A.; de Melo Monte, M.B.; Souza-Barros, F. Brazilian sedimentary zeolite use in agriculture. Microporous Mesoporous Mater. 2013, 167, 16–21. [Google Scholar] [CrossRef]
- Srilai, S.; Tanwongwal, W.; Onpecth, K.; Wongkitikun, T.; Panomsuwan, G.; Fuji, M.; Eiad-Ua, A. Influence of crystallization time for synthesis of zeolite A and zeolite X from natural kaolin. In Key Engineering Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2019; Volume 824, pp. 231–235. [Google Scholar]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of zeolites for sustainable agriculture: A review on water and nutrient retention. Water Air Soil Pollut. 2017, 228, 1–34. [Google Scholar] [CrossRef]
- Zijun, Z.; Effeney, G.; Millar, G.J.; Stephen, M. Synthesis and cation exchange capacity of zeolite W from ultra-fine natural zeolite waste. Environ. Technol. Innov. 2021, 23, 101595. [Google Scholar] [CrossRef]
- Aslani, P.; Davari, M.; Mahmoodi, M.A.; Hosseinpanahi, F.; Khaleghpanah, N. Effect of zeolite and nitrogen on some basic soil properties and wheat yield in potato-wheat rotation. J. Soil Sci. Agric. Eng. (Sci. J. Agr.) 2021, 44. [Google Scholar] [CrossRef]
- Méndez Argüello, B.; Vera Reyes, I.; Cárdenas Flores, A.; Santos Villarreal, G.; Ibarra Jiménez, L.; Lira Saldivar, R.H. Water holding capacity of substrates containing zeolite and its effect on growth, biomass production and chlorophyll content of Solanum lycopersicum Mill. Nova Sci. 2018, 10, 45–60. [Google Scholar] [CrossRef]
- Wu, Q.; Chi, D.; Xia, G.; Chen, T.; Sun, Y.; Song, Y. Effects of zeolite on drought resistance and water–nitrogen use efficiency in paddy rice. J. Irrig. Drain. Eng. 2019, 145, 04019024. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Alghamdi, A.G. Effect of the particle size of clinoptilolite zeolite on water content and soil water storage in a loamy sand soil. Water 2021, 13, 607. [Google Scholar] [CrossRef]
- Szatanik-Kloc, A.; Szerement, J.; Adamczuk, A.; Józefaciuk, G. Effect of low zeolite doses on plants and soil physicochemical properties. Materials 2021, 14, 2617. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; Martinez de Toda, F. Strategies in vineyard establishment to face global warming in viticulture: A mini review. J. Sci. Food Agric. 2021, 101, 1261–1269. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; de Toda, F.M. Current viticultural techniques to mitigate the effects of global warming on grape and wine quality: A comprehensive review. Food Res. Int. 2021, 139, 109946. [Google Scholar] [CrossRef]
- David, R.; Dochain, D.; Mouret, J.R.; Wouwer, A.V.; Sablayrolles, J.M. Modeling of the aromatic profile in wine-making fermentation: The backbone equations. IFAC Proc. Vol. 2011, 44, 10597–10602. [Google Scholar] [CrossRef]
- Ortega, P.; Sánchez, E.; Gil, E.; Matamoros, V. Use of cover crops in vineyards to prevent groundwater pollution by copper and organic fungicides. Soil column studies. Chemosphere 2022, 303, 134975. [Google Scholar] [CrossRef]
- Loffredo, E. Recent advances on innovative materials from biowaste recycling for the removal of environmental estrogens from water and soil. Materials 2022, 15, 1894. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, N.; Gaszynski, C.; Ikumi, D. A review of winery wastewater treatment: A focus on UASB biotechnology optimisation and recovery strategies. J. Environ. 2022, 10, 108172. [Google Scholar] [CrossRef]
- Suter, B.; Triolo, R.; Pernet, D.; Dai, Z.; Van Leeuwen, C. Modeling stem water potential by separating the effects of soil water availability and climatic conditions on water status in grapevine (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1485. [Google Scholar] [CrossRef]
- Saberali, S.F.; Shirmohamadi-Aliakbarkhani, Z. Quantifying seed germination response of melon (Cucumis melo L.) to temperature and water potential: Thermal time, hydrotime and hydrothermal time models. South Afr. J. Bot. 2020, 130, 240–249. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Mokhtassi-Bidgoli, A.; Modarres-Sanavy, S.A.M.; Mohammadi, H.; Nicola, S. Effects of zeolite and water stress on growth, yield and chemical compositions of Aloe vera L. Agric. Water Manag. 2017, 181, 66–72. [Google Scholar] [CrossRef]
- Jarosz, R.; Szerement, J.; Gondek, K.; Mierzwa-Hersztek, M. The use of zeolites as an addition to fertilisers–A review. Catena 2022, 213, 106125. [Google Scholar] [CrossRef]
- Sangeetha, C.; Baskar, P. Zeolite and its potential uses in agriculture: A critical review. Agric. Rev. 2016, 37, 101–108. [Google Scholar] [CrossRef]
- Ozbahce, A.; Tari, A.F.; Gönülal, E.; Simsekli, N.; Padem, H. The effect of zeolite applications on yield components and nutrient uptake of common bean under water stress. Arch. Agron. Soil Sci. 2015, 61, 615–626. [Google Scholar] [CrossRef]
- Ahmadi Azar, F.; Hasanloo, T.; Feizi, V. Water stress and mineral zeolite application on growth and some physiological characteristics of Mallow (Malva sylvestris). J. Plant Res. (Iran. J. Biol.) 2015, 28, 459–474. [Google Scholar]
- Christmann, A.; Weiler, E.W.; Steudle, E.; Grill, E. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007, 52, 167–174. [Google Scholar] [CrossRef]
- Scholasch, T.; Rienth, M. Review of water deficit mediated changes in vine and berry physiology; Consequences for the optimization of irrigation strategies. OENO One 2019, 53, 3. [Google Scholar] [CrossRef]
- Soar, C.J.; Speirs, J.; Maffei, S.M.; Penrose, A.B.; McCarthy, M.G.; Loveys, B.R. Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: Apparent links with ABA physiology and gene expression in leaf tissue. Aust. J. Grape Wine Res. 2006, 12, 2–12. [Google Scholar] [CrossRef]
- Scholasch, T.; Mazens, M.; Lebon, E.; Misosn, L.; Pellegrino, A.; Lecoeur, J. Combined effect of soil and air moisture deficits on vine transpiration cv. Cabernet-Sauvignon. In Proceedings of the 16th International GiESCO Symposium, Davis, CA, USA, 12–15 July 2009; pp. 12–15. [Google Scholar]
- Balbontín, C.; Campos, I.; Franck, N.; Calera, A. Analyzing the effect of environmental conditions on vineyard eco-systemic water use efficiency under semi-arid field conditions. In Proceedings of the VIII International Symposium on Irrigation of Horticultural Crops, Lleida, Spain, 8 June 2015; pp. 91–96. [Google Scholar]
- Greer, D.H.; Weedon, M.M. The impact of high temperatures on Vitis vinifera cv. Semillon grapevine performance and berry ripening. Front 2013, 4, 491. [Google Scholar] [CrossRef]
- Lanari, V.; Palliotti, A.; Sabbatini, P.; Howell, G.S.; Silvestroni, O. Optimizing deficit irrigation strategies to manage vine performance and fruit composition of field-grown ‘Sangiovese’ (Vitis vinifera L.) grapevines. Sci. Hortic. 2014, 179, 239–247. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M. Interactions between light and growing season temperatures on, growth and development and gas exchange of Semillon (Vitis vinifera L.) vines grown in an irrigated vineyard. Plant Physiol. Biochem. 2012, 54, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, U.; Batushansky, A.; Degu, A.; Rachmilevitch, S.; Fait, A. Metabolic and physiological responses of shiraz and cabernet sauvignon (Vitis vinifera L.) to near optimal temperatures of 25 and 35 C. Int. J. Mol. Sci. 2015, 16, 24276–24294. [Google Scholar] [CrossRef]
- Bybordi, A. Influence of zeolite, selenium and silicon upon some agronomic and physiologic characteristics of canola grown under salinity. Commun. Soil Sci. Plant Anal. 2016, 47, 832–850. [Google Scholar] [CrossRef]
- De Smedt, C.; Steppe, K.; Spanoghe, P. Beneficial effects of zeolites on plant photosynthesis. Adv. Mater. Sci. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine responses to heat stress and global warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef] [PubMed]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
- Bueno, A.; Alfarhan, A.; Arand, K.; Burghardt, M.; Deininger, A.-C.; Hedrich, R.; Leide, J.; Seufert, P.; Staiger, S.; Riederer, M. Effects of temperature on the cuticular transpiration barrier of two desert plants with water-spender and water-saver strategies. J. Exp. Bot. 2019, 70, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Sadura, I.; Libik-Konieczny, M.; Jurczyk, B.; Gruszka, D.; Janeczko, A. Plasma membrane ATPase and the aquaporin HvPIP1 in barley brassinosteroid mutants acclimated to high and low temperature. J. Plant Physiol. 2020, 244, 153090. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Huang, G.; Peng, S.; Li, Y. Temperature responses of photosynthesis and leaf hydraulic conductance in rice and wheat. Plant Cell Environ. 2020, 43, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Bukhari, S.; Mustafa, A.; Ditta, A.; Alamri, S.; El-Esawi, M.; Rafique, M.; Ashraf, S.; Siddiqui, M. Mitigation of nickel toxicity and growth promotion in sesame through the application of a bacterial endophyte and zeolite in nickel contaminated soil. Int. J. Environ. Res. Public Health 2020, 17, 8859. [Google Scholar] [CrossRef]
- Ohira, S.; Morita, N.; Suh, H.J.; Jung, J.; Yamamoto, Y. Quality control of photosystem II under light stress–turnover of aggregates of the D1 protein in vivo. Photosynth. Res. 2005, 84, 29–33. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Frioni, T.; VanderWeide, J.; Palliotti, A.; Tombesi, S.; Poni, S.; Sabbatini, P. Foliar vs. soil application of Ascophyllum nodosum extracts to improve grapevine water stress tolerance. Sci. Hortic. 2021, 277, 109807. [Google Scholar] [CrossRef]
- Zufferey, V.; Murisier, F.; Vivin, P.; Belcher, S.; Lorenzini, F.; Spring, J.L.; Viret, O. Carbohydrate reserves in grapevine (Vitis vinifera L.‘Chasselas’): The influence of the leaf to fruit ratio. Vitis 2012, 51, 103–110. [Google Scholar]
- Wang, Z.P.; Deloire, A.; Carbonneau, A.; Federspiel, B.; Lopez, F. An in vivo experimental system to study sugar phloem unloading in ripening grape berries during water deficiency stress. Ann. Bot. 2003, 92, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Murcia, G.; Pontin, M.; Reinoso, H.; Baraldi, R.; Bertazza, G.; Gómez-Talquenca, S.; Bottini, R.; Piccoli, P.N. ABA and GA3 increase carbon allocation in different organs of grapevine plants by inducing accumulation of non-structural carbohydrates in leaves, enhancement of phloem area and expression of sugar transporters. Physiol. Plant. 2016, 156, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.C.; Harbertson, J.F.; Heymann, H. A full factorial study on the effect of tannins, acidity, and ethanol on the temporal perception of taste and mouthfeel in red wine. Food Qual. Prefer. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Matthews, M.A.; Anderson, M.M. Fruit ripening in Vitis vinifera L.: Responses to seasonal water deficits. Am. J. Enol. Vitic. 1988, 39, 313–320. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Seguin, G. Incidences de l’alimentation en eau de la vigne, appréciée par l’état hydrique du feuillage, sur le développement de l’appareil végétatif et la maturation du raisin (Vitis vinifera Variété Cabernet Franc, Saint-Emilion 1990). J. Int. Sci. Vigne Vin 1994, 28, 81–110. [Google Scholar]
- Zenoni, S.; Ferrarini, A.; Giacomelli, E.; Xumerle, L.; Fasoli, M.; Malerba, G.; Bellin, D.; Pezzotti, M.; Delledonne, M. Characterization of transcriptional complexity during berry development in Vitis vinifera using RNA-Seq. Plant Physiol. 2010, 152, 1787–1795. [Google Scholar] [CrossRef]
- Chorti, E.; Guidoni, S.; Ferrandino, A.; Novello, V. Effect of different cluster sunlight exposure levels on ripening and anthocyanin accumulation in Nebbiolo grapes. Am. J. Enol. Vitic. 2010, 61, 23–30. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Royo, J.B. Water status, leaf area and fruit load influence on berry weight and sugar accumulation of cv. ‘Tempranillo’under semiarid conditions. Sci. Hortic. 2006, 109, 60–65. [Google Scholar] [CrossRef]
- Bybordi, A.; Ebrahimian, E. Growth, yield and quality components of canola fertilized with urea and zeolite. Commun. Soil Sci. Plant Anal. 2013, 44, 2896–2915. [Google Scholar] [CrossRef]
- Aghaalikhani, M.; Gholamhoseini, M.; Dolatabadian, A.; Khodaei-Joghan, A.; Sadat Asilan, K. Zeolite influences on nitrate leaching, nitrogen-use efficiency, yield, and yield components of canola in sandy soil. Arch. Agron. Soil Sci. 2012, 58, 1149–1169. [Google Scholar] [CrossRef]
- Gül, A.; Eroğul, D.; Ongun, A.R. Comparison of the use of zeolite and perlite as substrate for crisp-head lettuce. Sci. Hortic. 2005, 106, 464–471. [Google Scholar] [CrossRef]
- Leung, S.; Barrington, S.; Wan, Y.; Zhao, X.; El-Husseini, B. Zeolite (clinoptilolite) as feed additive to reduce manure mineral content. Bioresour. Technol. 2007, 98, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Nazir, R.; Jamil, N.; Alam, S.; Shah, R.; Khan, M.N.; Saleem, M. Synthesis and characterization of zeolite based nano–composite: An environment friendly slow release fertilizer. Microporous Mesoporous Mater. 2016, 232, 174–183. [Google Scholar] [CrossRef]
- Dai, Z.W.; Ollat, N.; Gomès, E.; Decroocq, S.; Tandonnet, J.-P.; Bordenave, L.; Pieri, P.; Hilbert, G.; Kappel, C.; van Leeuwen, C.; et al. Ecophysiological, genetic, and molecular causes of variation in grape berry weight and composition: A review. Am. J. Enol. Vitic. 2011, 62, 413–425. [Google Scholar] [CrossRef]
- Nisbet, M.A.; Martinson, T.E.; Mansfield, A.K. Accumulation and prediction of yeast assimilable nitrogen in New York winegrape cultivars. Am. J. Enol. Vitic. 2014, 65, 325–332. [Google Scholar] [CrossRef]
- Hannam, K.D.; Neilsen, G.H.; Forge, T.; Neilsen, D. The concentration of yeast assimilable nitrogen in Merlot grape juice is increased by N fertilization and reduced irrigation. Can. J. Plant Sci. 2013, 93, 37–45. [Google Scholar] [CrossRef]
- Neilsen, G.; Dickson, M.S.; Rosen, P.F.; Guo, X.; Navrotsky, A.; Woodfield, B.F. Heat capacity and thermodynamic functions of partially dehydrated cation-exchanged (Na+, Cs+, Cd2+, Li+, and NH4+) RHO zeolites. J. Chem. Thermodyn. 2022, 164, 106620. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, Z.H.; Stewart, B.A. Responses of crop plants to ammonium and nitrate N. Adv. Agron. 2013, 118, 205–397. [Google Scholar]
- González-Sanjosé, M.L.; Diez, C.J.F.C. Relationship between anthocyanins and sugars during the ripening of grape berries. Food Chem. 1992, 43, 193–197. [Google Scholar] [CrossRef]
- Yamane, T.; Jeong, S.T.; Goto-Yamamoto, N.; Koshita, Y.; Kobayashi, S. Effects of temperature on anthocyanin biosynthesis in grape berry skins. Am. J. Enol. Vitic. 2006, 57, 54–59. [Google Scholar] [CrossRef]
- Mori, K.; Sugaya, S.; Gemma, H. 205. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Mangani, S.; Buscioni, G.; Collina, L.; Bocci, E.; Vincenzini, M. Effects of microbial populations on anthocyanin profile of Sangiovese wines produced in Tuscany, Italy. Am. J. Enol. Vitic. 2011, 62, 487–494. [Google Scholar] [CrossRef]
- Bobeica, N.; Poni, S.; Hilbert, G.; Renaud, C.; Gomès, E.; Delrot, S.; Dai, Z. Differential responses of sugar, organic acids and anthocyanins to source-sink modulation in Cabernet Sauvignon and Sangiovese grapevines. Front 2015, 6, 382. [Google Scholar] [CrossRef] [PubMed]
- de Rosas, I.; Deis, L.; Baldo, Y.; Cavagnaro, J.B.; Cavagnaro, P.F. High temperature alters anthocyanin concentration and composition in grape berries of Malbec, Merlot, and Pinot Noir in a cultivar-dependent manner. Plants 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Zarrouk, O.; Brunetti, C.; Egipto, R.; Pinheiro, C.; Genebra, T.; Gori, A.; Lopes, C.M.; Tattini, M.; Chaves, M.M. Grape ripening is regulated by deficit irrigation/elevated temperatures according to cluster position in the canopy. Front. Plant Sci. 2016, 7, 1640. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Azuma, A.; Ban, Y.; Sato, A.; Kono, A.; Shiraishi, M.; Yakushiji, H.; Kobayashi, S. MYB diplotypes at the color locus affect the ratios of tri/di-hydroxylated and methylated/non-methylated anthocyanins in grape berry skin. Tree Genet. Genomes 2015, 11, 1–13. [Google Scholar] [CrossRef]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry temperature and solar radiation alter acylation, proportion, and concentration of anthocyanin in Merlot grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Di Gaspero, G. Transcriptional control of anthocyanin biosynthetic genes in extreme phenotypes for berry pigmentation of naturally occurring grapevines. BMC Plant Biol. 2007, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Bento, A.; Baraldi, I.; Malheiro, R. Selection of grapevine leaf varieties for culinary process based on phytochemical composition and antioxidant properties. Food Chem. 2016, 212, 291–295. [Google Scholar] [CrossRef]
- Bontpart, T.; Marlin, T.; Vialet, S.; Guiraud, J.-L.; Pinasseau, L.; Meudec, E.; Sommerer, N.; Cheynier, V.; Terrier, N. Two shikimate dehydrogenases, VvSDH3 and VvSDH4, are involved in gallic acid biosynthesis in grapevine. J. Exp. Bot. 2016, 67, 3537–3550. [Google Scholar] [CrossRef] [PubMed]
- Zadernowski, R.; Naczk, M.; Nesterowicz, J. Phenolic acid profiles in some small berries. J. Agric. Food Chem. 2005, 53, 2118–2124. [Google Scholar] [CrossRef] [PubMed]
- Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Martínez-Abaigar, J.; Núñez-Olivera, E. Phenolic characteristics acquired by berry skins of Vitis vinifera cv. Tempranillo in response to close-to-ambient solar ultraviolet radiation are mostly reflected in the resulting wines. J. Sci. Food Agric. 2020, 100, 401–409. [Google Scholar] [CrossRef]
- Xi, Z.M.; Zhang, Z.W.; Cheng, Y.F.; Hua, L.I. The effect of vineyard cover crop on main monomeric phenols of grape berry and wine in Vitis vinifera L. cv. Cabernet Sauvignon. Agric. Sci. China 2010, 9, 440–448. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, R.; He, F.; Zhou, P.P.; Duan, C.Q. Copigmentation of malvidin-3-O-glucoside with five hydroxybenzoic acids in red wine model solutions: Experimental and theoretical investigations. Food Chem. 2015, 170, 226–233. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Stefanovic, D.; Nikolic, N.; Kostic, L.; Todic, S.; Nikolic, M. Early Leaf Removal Increases Berry and Wine Phenolics in Cabernet Sauvignon Grown in Eastern Serbia. Agronomy 2021, 11, 238. [Google Scholar] [CrossRef]
- Simonetti, G.; Buiarelli, F.; Bernardini, F.; Di Filippo, P.; Riccardi, C.; Pomata, D. Profile of free and conjugated quercetin content in different Italian wines. Food Chem. 2022, 382, 132377. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, V.; Pizzinato, D.; Sparrow, C.; Pagni, D.; Cascella, F.; Carapelli, C.; Vincenzi, S. Prevention of quercetin precipitation in red wines: A promising enzymatic solution. OENO One 2022, 56, 41–51. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Amin, M.S.M.; Anuar, A.R.; Saberioon, M.M. Water stress and natural zeolite impacts on phisiomorphological characteristics of moldavian balm (Dracocephalum moldavica l.). Aust. J. Basic Appl. Sci. 2010, 4, 5184–5190. [Google Scholar]
- AL-Busaidi, A.; Yamamoto, T.; Tanigawa, T.; Rahman, H.A. Use of zeolite to alleviate water stress on subsurface drip irrigated barley under hot environments. Irrig. Drain. 2011, 60, 473–480. [Google Scholar] [CrossRef]
- Najafinezhad, H.; Tahmasebi Sarvestani, Z.; Modarres Sanavy, S.A.M.; Naghavi, H. Evaluation of yield and some physiological changes in corn and sorghum under irrigation regimes and application of barley residue, zeolite and superabsorbent polymer. Arch. Agron. Soil Sci. 2015, 61, 891–906. [Google Scholar] [CrossRef]
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam 2004, 12, 183–189. [Google Scholar]
- Rehakova, M.; Čuvanová, S.; Dzivak, M.; Rimár, J.; Gaval’Ová, Z. Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Curr. Opin. Solid State Mater. Sci. 2004, 8, 397–404. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Kim, J.S. A new method for conservation of nitrogen in aerobic composting processes. Bioresour. Technol. 2001, 79, 129–133. [Google Scholar] [CrossRef]
- Cadena, E.; Colón, J.; Artola, A.; Sánchez, A.; Font, X. Environmental impact of two aerobic composting technologies using life cycle assessment. Int. J. Life Cycle Assess. 2009, 14, 401–410. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; Chen, Z.; Wen, Q.; Wang, Y. Maturity and security assessment of pilot-scale aerobic co-composting of penicillin fermentation dregs (PFDs) with sewage sludge. Bioresour. Technol. 2016, 204, 185–191. [Google Scholar] [CrossRef]
- Hou, N.; Wen, L.; Cao, H.; Liu, K.; An, X.; Li, D.; Wang, H.; Du, X.; Li, C. Role of psychrotrophic bacteria in organic domestic waste composting in cold regions of China. Bioresour. Technol. 2017, 236, 20–28. [Google Scholar] [CrossRef]
- Himanen, M.; Hänninen, K. Effect of commercial mineral-based additives on composting and compost quality. Waste Manag. 2009, 29, 2265–2273. [Google Scholar] [CrossRef]
- Venglovsky, J.; Sasakova, N.; Vargova, M.; Pacajova, Z.; Placha, I.; Petrovsky, M.; Harichova, D. Evolution of temperature and chemical parameters during composting of the pig slurry solid fraction amended with natural zeolite. Bioresour. Technol. 2005, 96, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, E.C.; Salvi, L.S.; Paoli, F.P.; Fucile, M.F.; Masciandaro, G.M.; Manzi, D.M.; Masini, C.M.M.; Mattii, G.B.M. Effects of natural clinoptilolite on physiology, water stress, sugar, and anthocyanin content in Sanforte (Vitis vinifera L.) young vineyard. J. Agric. Sci. 2021, 159, 488–499. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Mattii, G.B. Effects of defoliation at fruit Set on vine physiology and berry composition in cabernet sauvignon grapevines. Plants 2021, 10, 1183. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.; Van Epps, V.; Kim, S.H.; Doty, S.L. Endophytes increased fruit quality with higher soluble sugar production in honeycrisp apple (Malus pumila). Microorganisms 2020, 8, 699. [Google Scholar] [CrossRef]
- Williams, L.E.; Araujo, F.J. Correlations among predawn leaf, midday leaf, and midday stem water potential and their correlations with other measures of soil and plant water status in Vitis vinifera. J. Am. Soc. Hortic. Sci. 2002, 127, 448–454. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Comparison of methods for estimating phenolic maturity in grapes: Correlation between predicted and obtained parameters. Anal. Chim. Acta 2010, 660, 127–133. [Google Scholar] [CrossRef]
- OIV Official Methods for the Analysis of Musts and Wines of the International Organization of Vine and Wine (OIV). Methods of Analysis of Wines and Musts. (OIV-MA-INT-00-2012). Available online: http://www.oiv.int/oiv/info/enmethodesinternationalesvin (accessed on 2 December 2022).
- Sun, J.; Liu, W.; Zhang, M.; Geng, P.; Shan, Y.; Li, G.; Zhao, Y.; Chen, P. The analysis of phenolic compounds in daylily using UHPLC-HRMSn and evaluation of drying processing method by fingerprinting and metabolomic approaches. J. Food Process. Preserv. 2018, 42, e13325. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Jaccard, J.; Becker, M.A.; Wood, G. Pairwise multiple comparison procedures: A review. Psycholog. Bull. 1984, 96, 589. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Pang, Y.; Cai, X.; Lu, J.; Ren, X.; Kong, Q. Phenolics composition and contents, as the key quality parameters of table grapes, may be influenced obviously and differently in response to short-term high temperature. LWT 2021, 149, 111791. [Google Scholar] [CrossRef]
- Esparza, I.; Cimminelli, M.J.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Stability of phenolic compounds in grape stem extracts. Antioxidants 2020, 9, 720. [Google Scholar] [CrossRef] [PubMed]





| 6 August 2021 | 18 August 2021 | 31 August 2021 | 14 September 2021 | u.m. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zeowine | Zeolite | Compost | Zeowine | Zeolite | Compost | Zeowine | Zeolite | Compost | Zeowine | Zeolite | Compost | ||
| Cyanidin-3-glucoside | 12.90 ±4.25 a | 17.30 ±6.18 a | 18.10 ±2.12 a | 20.80 ±1.66 a | 19.20 ±3.52 a | 15.00 ±3.04 a | 17.20 ±3.31 a | 5.50 ±3.47 b | 19.90 ±2.88 a | 14.20 ±5.12 a | 10.20 ±4.21 b | 17.60 ±3.07 a | % |
| Delphinidin-3-glucoside | 13.70 ±1.37 a | 14.90 ±2.14 a | 13.90 ±2.25 a | 15.90 ±2.15 a | 15.30 ±2.47 a | 16.70 ±1.07 a | 15.70 ±4.18 a | 7.00 ±3.52 a | 13.90 ±3.47 a | 14.10 ±5.01 a | 12.90 ±3.92 a | 16.10 ±2.17 a | % |
| Malvidin-3-acetylglucoside | <0.10 ±0.00 a | 1.70 ±0.62 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 b | 7.40 ±3.44 a | 0.80 ±0.12 b | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | % |
| Malvidin-3-cumarylglucoside | <0.10 ±0.00 a | 1.20 ±0.53 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | 0.40 ±0.22 b | 10.60 ±2.74 a | 0.90 ±0.18 b | <0.10 ±0.00 a | 0.60 ±0.19 a | <0.10 ±0.00 a | % |
| Malvidin-3-glucoside | 42.90 ±5.78 a | 35.40 ±6.24 b | 37.60 ±3.56 ab | 32.50 ±4.18 a | 32.30 ±4.36 a | 37.90 ±4.42 a | 35.30 ±4.69 b | 50.50 ±4.25 a | 32.50 ±4.81 b | 39.70 ±4.65 b | 47.30 ±4.21 a | 33.50 ±4.16 b | % |
| Peonidin-3-acetylglucoside | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | 0.50 ±0.11 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | % |
| Peonidin-3-cumarylglucoside | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | 0.70 ±0.19 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | % |
| Peonidin-3-glucoside | 12.20 ±1.47 a | 12.20 ±1.37 a | 13.00 ±1.98 a | 12.80 ±2.96 a | 15.80 ±3.04 a | 11.40 ±2.38 a | 13.60 ±2.93 ab | 8.50 ±2.27 b | 15.90 ±2.45 a | 14.60 ±3.86 a | 11.70 ±2.04 a | 14.30 ±2.12 a | % |
| Petunidin-3-glucoside | 18.30 ±3.67 a | 17.20 ±3.23 a | 17.40 ±2.37 a | 18.00 ±4.23 a | 17.30 ±4.04 a | 19.10 ±3.34 a | 17.90 ±2.50 a | 9.50 ±2.13 b | 16.10 ±2.32 a | 17.50 ±2.45 a | 17.30 ±2.61 a | 18.60 ±1.44 a | % |
| Caffeic Acid | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | mg kg−1 |
| Coumaric Acid | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | mg kg−1 |
| Ferulic Acid | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | n.d. ±0.00 a | mg kg−1 |
| Gallic Acid | 3.28 ±1.04 a | 3.18 ±0.65 a | 1.02 ±0.85 a | 1.48 ±0.87 a | 2.39 ±0.93 a | 1.78 ±0.67 a | 1.60 ±1.23 a | 2.02 ±1.55 a | 1.24 ±1.23 a | 1.17 ±1.27 a | 1.78 ±1.89 a | 0.94 ±1.03 a | mg kg−1 |
| Quercetin-3-O-glucoside | 25.47 ±4.26 b | 33.70 ±5.27 b | 47.17 ±5.18 a | 49.77 ±4.21 a | 29.85 ±4.55 b | 41.93 ±6.36 a | 48.71 ±12.73 a | 34.53 ±14.45 b | 48.31 ±15.76 a | 64.05 ±16.34 b | 55.25 ±17.28 b | 76.96 ±14.61 a | mg kg−1 |
| Quercetin-3-O-galactoside | 5.75 ±3.56 a | 8.57 ±3.78 a | 12.73 ±4.56 a | 9.18 ±2.46 a | 5.80 ±3.68 a | 8.15 ±4.76 a | 8.06 ±3.45 a | 5.98 ±3.23 a | 9.46 ±2.87 a | 13.50 ±4.23 ab | 10.11 ±4.36 b | 22.04 ±4.11 a | mg kg−1 |
| Quercetin-3-O-glucuronide | 72.48 ±10.32 c | 89.04 ±15.95 b | 115.55 ±18.32 a | 66.60 ±11.87 b | 48.06 ±14.77 c | 90.05 ±18.39 a | 60.71 ±12.32 b | 74.67 ±17.14 a | 67.80 ±15.94 ab | 58.52 ±12.62 b | 44.51 ±13.46 c | 70.02 ±18.46 a | mg kg−1 |
| Quercetin-3-O-rutinoside | 2.69 ±1.23 a | 3.56 ±1.83 a | 6.96 ±2.27 a | 2.50 ±1.22 a | 1.44 ±1.63 a | 2.85 ±1.85 a | 1.74 ±1.24 a | 0.49 ±0.12 a | 3.33 ±1.38 a | 1.73 ±0.92 a | 0.96 ±0.21 a | 2.92 ±0.99 a | mg kg−1 |
| Kaempferol-3-O-glucoside | 4.87 ±1.04 b | 6.17 ±2.39 b | 12.79 ±2.82 a | 7.99 ±3.28 ab | 2.28 ±1.92 b | 10.15 ±3.58 a | 6.27 ±2.47 a | 6.61 ±2.86 a | 5.92 ±2.16 a | 7.53 ±4.34 ab | 6.08 ±3.52 b | 12.08 ±6.78 a | mg kg−1 |
| 18 July 2022 | 4 August 2022 | 17 August 2022 | 5 September 2022 | u.m. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zeowine | Zeolite | Compost | Zeowine | Zeolite | Compost | Zeowine | Zeolite | Compost | Zeowine | Zeolite | Compost | ||
| Cyanidin-3-glucoside | 16.80 ±5.21 a | 16.30 ±3.17 a | 7.40 ±2.85 b | 14.80 ±3.70 a | 16.70 ±4.72 a | 16.50 ±2.00 a | 12.10 ±3.64 a | 12.10 ±1.53 a | 13.90 ±4.20 a | 14.70 ±2.70 a | 16.30 ±3.91 a | 17.80 ±4.04 a | % |
| Delphinidin-3-glucoside | 15.30 ±2.00 a | 13.30 ±1.80 a | 11.80 ±2.38 a | 16.60 ±2.00 a | 12.60 ±1.06 a | 14.50 ±1.88 a | 12.70 ±3.01 a | 12.50 ±1.73 a | 14.30 ±1.56 a | 12.60 ±2.18 a | 13.50±1.22 a | 12.30±1.09 a | % |
| Malvidin-3-acetylglucoside | <0.10 ±0.00 b | <0.10 ±0.00 b | 7.10 ±1.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | 2.50 ±0.60 a | <0.10 ±0.00 a | <0.10 ±0.00 a | 0.40±0.00 a | <0.10±0.00 a | % |
| Malvidin-3-cumarylglucoside | <0.10 ±0.00 b | <0.10 ±0.00 b | 9.4 ±1.50 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | 0.9 ±0.10 a | 3.4 ±0.15 a | 0.6 ±0.10 a | 0.70 ±0.04 a | 0.60±0.01 a | 0.50±0.01 a | % |
| Malvidin-3-glucoside | 38.50 ±6.10 a | 40.20 ±5.16 a | 42.70 ±4.11 a | 38.90 ±4.78 a | 39.40 ±5.42 a | 38.80 ±4.05 a | 45.50 ±5.78 a | 42.70 ±4.44 a | 40.40 ±6.89 a | 40.60 ±3.66 a | 37.70±5.23 a | 37.90±6.10 a | % |
| Peonidin-3-acetylglucoside | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10±0.00 a | <0.10±0.00 a | % |
| Peonidin-3-cumarylglucoside | <0.10 ±0.00 a | <0.10 ±0.00 a | 1.40 ±0.10 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | <0.10 ±0.00 a | 0.50 ±0.05 a | 0.40±0.05 a | <0.10±0.00 a | % |
| Peonidin-3-glucoside | 11.10 ±1.05 ab | 13.00 ±1.55 a | 7.10 ±0.80 b | 10.20 ±1.67 a | 15.60 ±3.34 a | 13.00 ±2.06 a | 12.00 ±2.00 a | 10.60 ±2.77 a | 12.90 ±1.08 a | 14.90 ±3.66 a | 14.50±2.98 a | 15.60±1.05 a | % |
| Petunidin-3-glucoside | 18.20 ±2.11 a | 17.30 ±3.28 a | 13.20 ±1.44 a | 19.40 ±4.55 a | 15.70 ±4.20 a | 17.20 ±3.05 a | 16.90 ±2.11 a | 16.30 ±2.06 a | 17.90 ±2.99 a | 16.00 ±3.36 a | 16.60±3.77 a | 15.90±1.50 a | % |
| Caffeic Acid | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | mg kg−1 |
| Coumaric Acid | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | mg kg−1 |
| Ferulic Acid | 0.08 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | 0.06 ±0.00 a | 0.08 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | mg kg−1 |
| Gallic Acid | 10.74 ±3.23 b | 21.68 ±8.15 a | 11.02 ±3.05 b | 8.11 ±1.20 b | 23.86 ±6.63 a | 8.94 ±4.60 b | 19.46 ±2.21 b | 23.08 ±4.32 a | 16.00 ±2.01 b | 32.73 ±1.67 a | 24.32 ±5.60 b | 11.48 ±2.36 c | mg kg−1 |
| Quercetin-3-O-glucoside | 36.79 ±5.25 b | 57.51 ±7.53 a | 62.13 ±7.29 a | 46.51 ±9.11 b | 82.38 ±10.72 a | 77.40 ±13.03 a | 82.06 ±19.81 b | 87.54 ±19.43 b | 101.23 ±22.61 a | 125.44 ±20.75 b | 133.77 ±21.30 b | 202.32 ±15.88 a | mg kg−1 |
| Quercetin-3-O-galattoside | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | <0.05 ±0.00 a | mg kg−1 |
| Quercetin-3-O-glucuronide | 79.34 ±13.25 c | 131.30 ±18.31 b | 166.59 ±15.71 a | 60.18 ±15.17 b | 115.61 ±19.70 a | 110.77 ±21.31 a | 77.67 ±16.13 b | 85.53 ±20.11 b | 129.22 ±25.42 a | 91.41 ±22.72 b | 102.17±23.16 b | 147.48 ±29.42 a | mg kg−1 |
| Quercetin-3-O-rutinoside | 4.73 ±0.85 b | 9.28 ±1.17 ab | 11.62 ±2.73 a | 2.58 ±0.35 b | 13.32 ±1.26 a | 12.65 ±2.00 a | 2.32 ±0.38 b | 2.97 ±0.38 b | 9.56 ±2.51 a | 1.48 ±0.33 c | 7.56 ±1.37 b | 13.53 ±2.90 a | mg kg−1 |
| Kaempferol-3-O-glucoside | 22.63 ±1.81 a | 13.86 ±2.30 b | 11.64 ±1.42 b | 12.67 ±1.36 b | 18.56 ±1.72 a | 20.21 ±2.20 a | 18.62 ±2.95 b | 27.57 ±3.23 a | 15.60 ±1.04 b | 28.39 ±3.02 b | 42.93 ±3.44 a | 21.85 ±1.92 b | mg kg−1 |
| Zeowine 1:2.5 | Zeowine 1:10 | Control | D. Lgs. N° 75/2010 Green Compost | ||
|---|---|---|---|---|---|
| pH | 8.26 | 7.95 | 7.37 | 6–8.8 | |
| EC | dS m−1 | 0.22 | 0.35 | 1.51 | |
| CSC | C mol c kg−1 | 45.9 | 43.8 | 36.4 | |
| TOC | C % | 25.68 | 29.41 | 27.01 | ≥ 20 |
| TN | TN % | 1,48 | 1.55 | 1.28 | |
| N-NO3 | mg kg−1 | 73 | 118 | 196 | |
| N-NH4 | mg kg−1 | 611 | 540 | 469 | |
| C/N | 17.35 | 18.98 | 21.1 | ≤ 50 | |
| Humic carbon | C % | 3.5 | 3.6 | 3.2 | ≥ 2.5 |
| TK | % | 1.19 | 0.738 | 0.559 | |
| TP | % | 0.144 | 0.172 | 0.116 | |
| Available K | mg K kg−1 | 317 | 531 | 453 | |
| Available P | mg P kg−1 | 328 | 370 | 568 | |
| Cu | mg Cu kg−1 | 44 | 70 | 78 | < 230 |
| Zn | mg Zn kg−1 | 35 | 45 | 49 | < 500 |
| Cd | mg Cd kg−1 | < 0.1 | < 0.1 | < 0.1 | < 1.5 |
| Ni | mg Ni kg−1 | 13 | 23 | 27 | < 100 |
| Pb | mg Pb kg−1 | 7.9 | 8.84 | 8.26 | < 140 |
| Cr | mg Cr kg−1 | 21 | 33 | 46 | < 100 |
| Germination Index | % | 142 | 126 | 72 | > 60% |
| Salmonella | CFU g−1 | absent | absent | absent | absent |
| Escherichia Coli | CFU g−1 | 100 | 100 | 100 | ≤ 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cataldo, E.; Fucile, M.; Manzi, D.; Masini, C.M.; Doni, S.; Mattii, G.B. Sustainable Soil Management: Effects of Clinoptilolite and Organic Compost Soil Application on Eco-Physiology, Quercitin, and Hydroxylated, Methoxylated Anthocyanins on Vitis vinifera. Plants 2023, 12, 708. https://doi.org/10.3390/plants12040708
Cataldo E, Fucile M, Manzi D, Masini CM, Doni S, Mattii GB. Sustainable Soil Management: Effects of Clinoptilolite and Organic Compost Soil Application on Eco-Physiology, Quercitin, and Hydroxylated, Methoxylated Anthocyanins on Vitis vinifera. Plants. 2023; 12(4):708. https://doi.org/10.3390/plants12040708
Chicago/Turabian StyleCataldo, Eleonora, Maddalena Fucile, Davide Manzi, Cosimo Maria Masini, Serena Doni, and Giovan Battista Mattii. 2023. "Sustainable Soil Management: Effects of Clinoptilolite and Organic Compost Soil Application on Eco-Physiology, Quercitin, and Hydroxylated, Methoxylated Anthocyanins on Vitis vinifera" Plants 12, no. 4: 708. https://doi.org/10.3390/plants12040708
APA StyleCataldo, E., Fucile, M., Manzi, D., Masini, C. M., Doni, S., & Mattii, G. B. (2023). Sustainable Soil Management: Effects of Clinoptilolite and Organic Compost Soil Application on Eco-Physiology, Quercitin, and Hydroxylated, Methoxylated Anthocyanins on Vitis vinifera. Plants, 12(4), 708. https://doi.org/10.3390/plants12040708








