Dynamics of Non-Structural Carbohydrates Release in Chinese Fir Topsoil and Canopy Litter at Different Altitudes
Abstract
:1. Introduction
2. Results
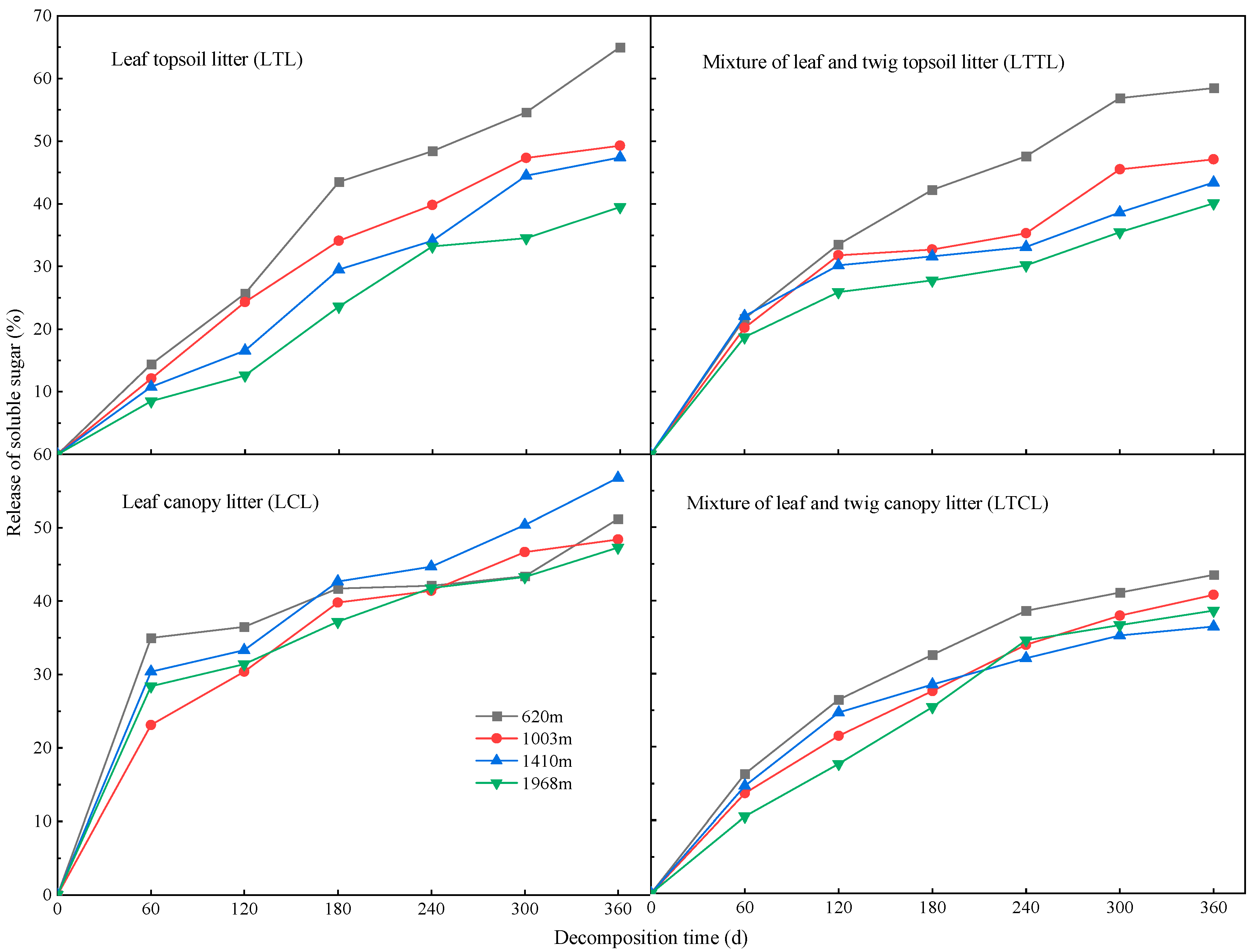
2.1. Dynamic Changes of Soluble Sugars at Different Altitudes
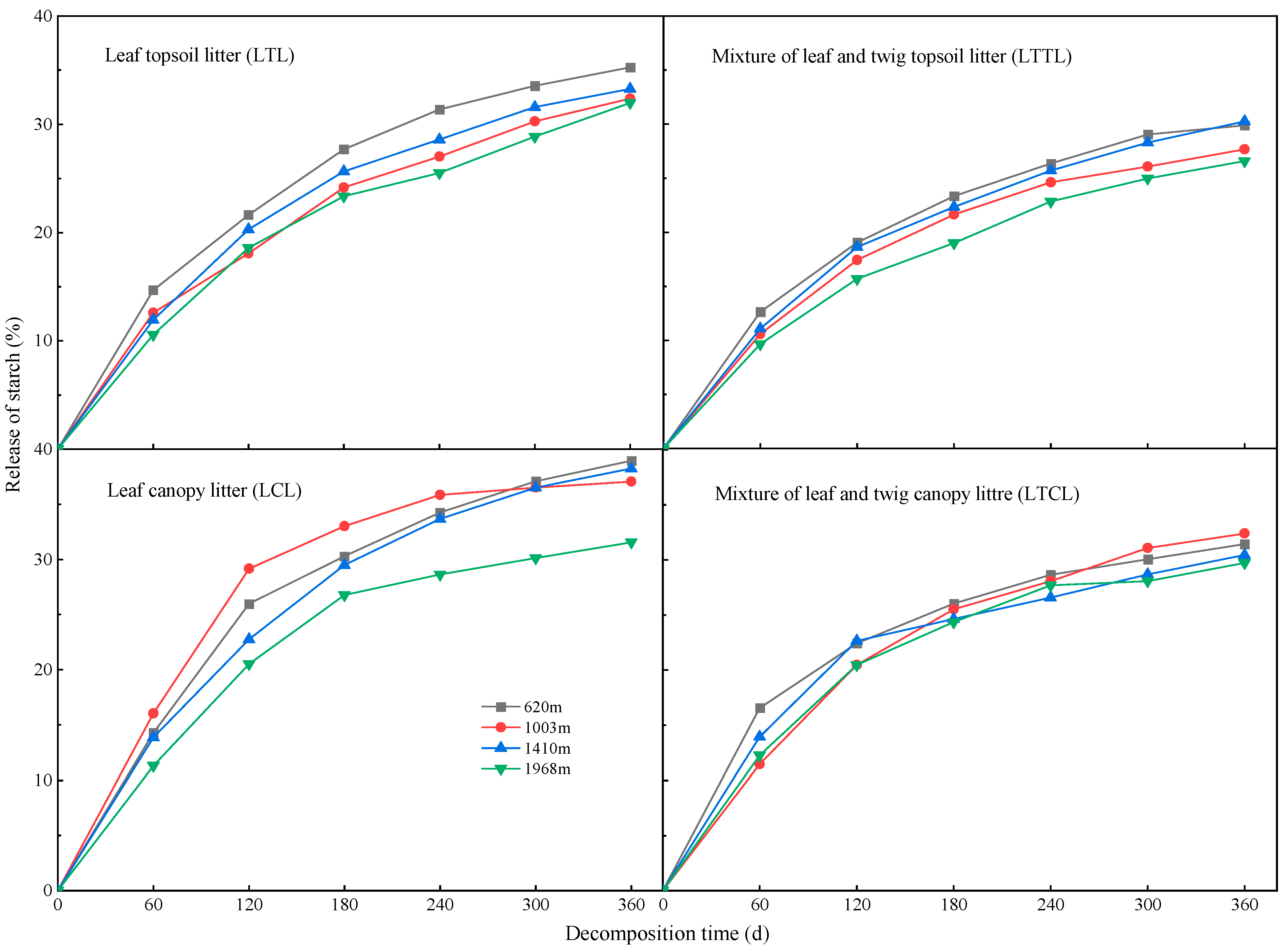
2.2. Dynamic Changes of Starch at Different Altitudes
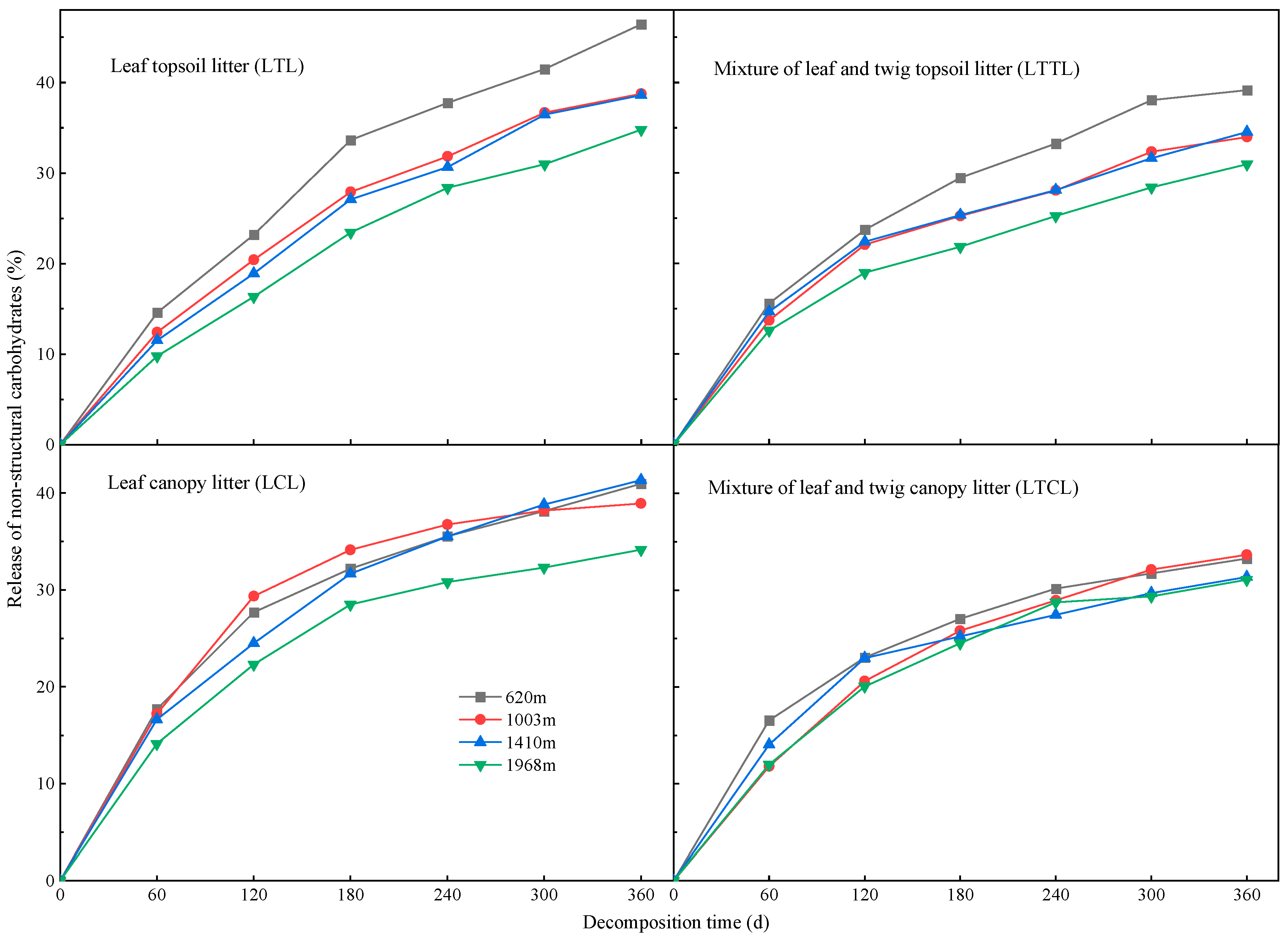
2.3. Dynamic Changes of NSC at Different Altitudes
2.4. Interaction between Altitude and Decomposition Time on NSC Content
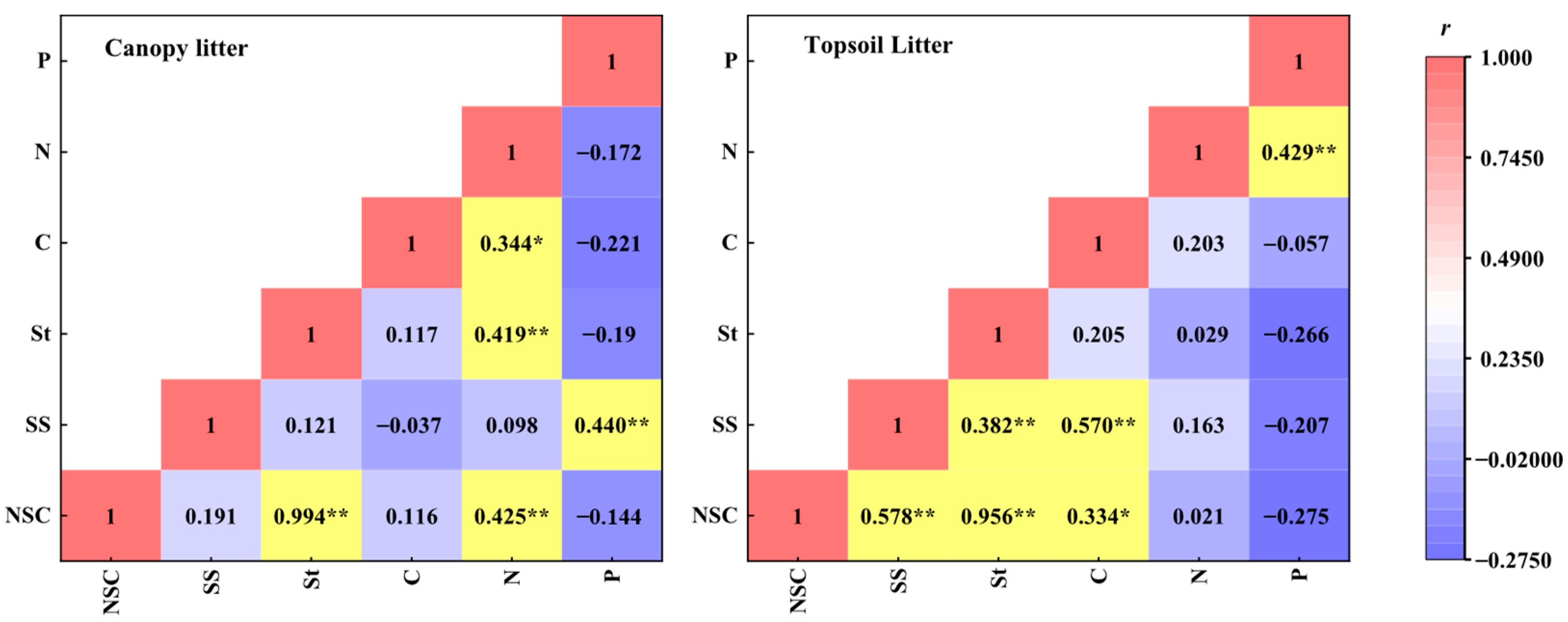
2.5. Correlation Analysis of the NSC and the C, N, and P Contents
3. Discussion
3.1. Changes of NSCs Content in Trees at Different Altitudes
3.2. Responses of the NSC Content in Different Types of Litter to Temperature Gradients
3.3. Correlation between the NSCs and the C, N, and P Contents
4. Materials and Methods
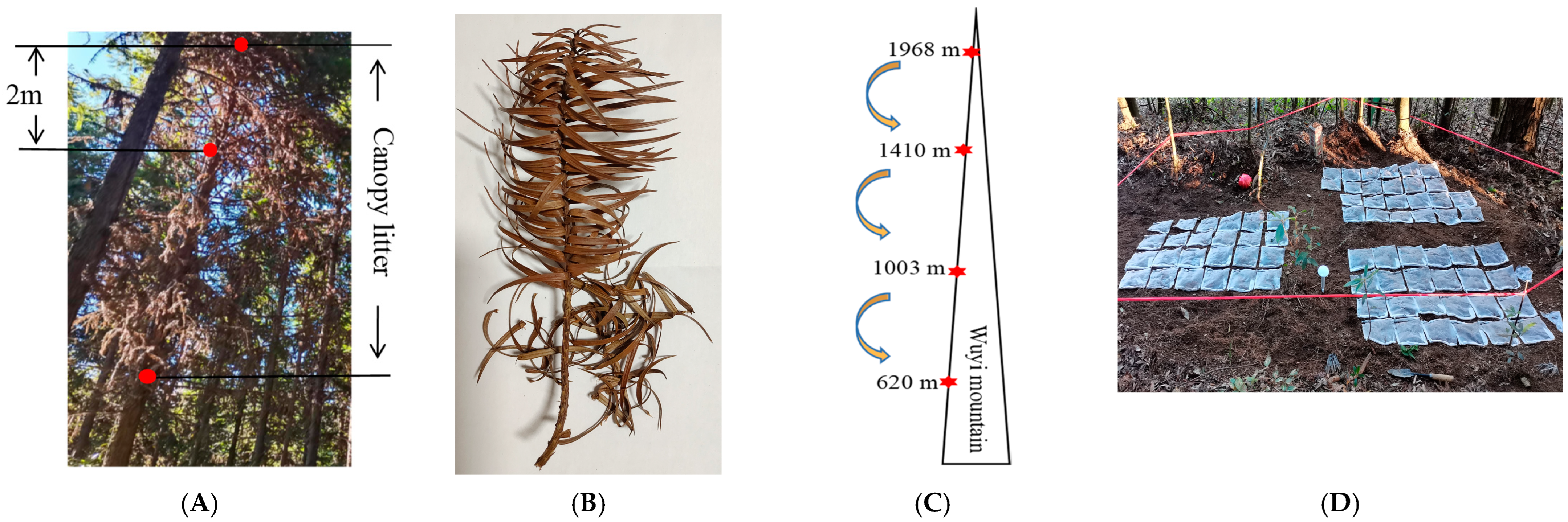
4.1. Study Site
4.2. Test Materials Preparation
4.3. Experimental Design
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tu, Z.H.; Chen, S.Y.; Ruan, D.S.; Chen, Z.X.; Huang, Y.P.; Chen, J.H. Differential Hydrological Properties of Forest Litter Layers in Artificial Afforestation of Eroded Areas of Latosol in China. Sustainability 2022, 14, 14869. [Google Scholar] [CrossRef]
- Wang, C.H.; Tao, C.; Yang, X.B.; Long, W.X.; Feng, D.D.; Zhou, W.S.; Yang, Q. Impact of forest community species composition on litter species composition. Acta Ecol. Sin. 2015, 35, 7435–7443. [Google Scholar]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Frida, I.; Piper, S.P. The Role of Nonstructural Carbohydrates Storage in Forest Resilience under Climate Change. Curr. For. Rep. 2020, 6, 1–13. [Google Scholar]
- Bradford, M.A.; Keiser, A.D.; Davies, C.A.; Mersmann, C.A.; Strickland, M.S. Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth. Biogeochemistry 2013, 113, 217–281. [Google Scholar] [CrossRef]
- Zhou, T.J. New physical science behind climate change: What does IPCC AR6 tell us? Innovation 2021, 2, 100173. [Google Scholar] [CrossRef] [PubMed]
- Kolomyts, E.G. Predictive modelling of boreal forest resources in regulation of the carbon cycle and mitigation of the global warming. Int. J. Glob. Warm. 2022, 27, 333–364. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Ruan, H.H.; Luo, Y.Q.; Wang, J.S. Temperature sensitivity increases with soil organic carbon recalcitrance along an elevational gradient in the Wuyi Mountains, China. Soil Biol. Biochem. 2010, 42, 1811–1815. [Google Scholar] [CrossRef]
- Salinas, N.; Malhi, Y.; Meir, P.; Silman, M.; Roman, C.R.; Huaman, J.; Salinas, D.; Huaman, V.; Gibaja, A.; Mamani, M.; et al. The sensitivity of tropical leaf litter decomposition to temperature: Results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol. 2011, 189, 967–977. [Google Scholar] [CrossRef]
- Su, Y.; Gong, Y.M.; Han, W.X.; Li, K.H.; Liu, X.J. Dependency of litter decomposition on litter quality, climate change, and grassland type in the alpine grassland of Tianshan Mountains, Northwest China. J. Arid Land 2022, 14, 691–703. [Google Scholar] [CrossRef]
- Rui, Y.; Eisenhauer, N.; Auge, H.; Purahong, W.; Schmidt, A.; Schädler, M. Additive effects of experimental climate change and land use on faunal contribution to litter decomposition. Soil Biol. Biochem. 2019, 131, 141–148. [Google Scholar]
- Wu, H.; Zheng, R.H.; Hao, Z.D.; Meng, Y.; Weng, Y.H.; Zhou, X.H.; Zhu, L.M.; Hu, X.Y.; Wang, G.B.; Shi, J.S.; et al. Cunninghamia lanceolata PSK Peptide Hormone Genes Promote Primary Root Growth and Adventitious Root Formation. Plants 2019, 8, 520. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.T.; Fan, S.H. Impact of Growth and Development Characters of Chinese Fir and Its Plantation on the Long-term Site Productivity. For. Res. 2002, 15, 629–636. [Google Scholar]
- Gao, S.L.; He, Z.M.; Huang, Z.Q.; Lin, S.Z.; Liu, Z.M. Decomposition, carbon and nitrogen stable isotopes and chemical composition of dead leaves clinging in a chinese fir (Cunninghamia lanceolata) plantation. Chin. J. Ecol. 2015, 34, 2457–2463. [Google Scholar]
- Zhang, J.C.; Sheng, W.T. The study on decay of dead branches and leaves on living trees taken from crown into litter environment in a Chinese fir plantation, compared with decay in canopy. Sci. Silvae Sin. 2001, 37, 2–10. [Google Scholar]
- Zhou, L.L.; Li, S.B.; Jia, Y.Y.; Heal, K.V.; He, Z.M.; Wu, P.F.; Ma, X.Q. Spatiotemporal distribution of canopy litter and nutrient resorption in a chronosequence of different development stages of Cunninghamia lanceolata in southeast China. Sci. Total Environ. 2020, 762, 143153. [Google Scholar] [CrossRef]
- Berg, B. Decomposition patterns for foliar litter—A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Anna, M.; Lucia, N.; Andrea, C.; Flavia, M.; Giovanni, C.; Riccardo, G.; Jaroslav, H.; Rodolfo, B. Moderate Salinity Stress Affects Expression of Main Sugar Metabolism and Transport Genes and Soluble Carbohydrate Content in Ripe Fig Fruits (Ficus carica L. cv. Dottato). Plants 2021, 10, 1861. [Google Scholar]
- Chen, Y.X.; Chen, B.; Sun, H.; Ma, L.H.; Shen, D.J.; Liu, Y.Y. Impacts of warming and nitrogen addition on organic fraction release in fresh litter of Larix potaninii in alpine zone of the western Sichuan Province, China. Chin. J. Appl. Ecol. 2017, 28, 1753–1760. [Google Scholar]
- Bufacchi, P.; Bizzo, W.A.; Buckeridge, M.S.; Franco-Jacome, D.L.; Grandis, A.; Cambler, A.B.; Krieger Filho, G.C. Thermal degradation of leaves from the Amazon rainforest litter considering non-structural, structural carbohydrates and lignin composition. Bioresour. Technol. Rep. 2020, 11, 100490. [Google Scholar] [CrossRef]
- Xie, H.T.; Yu, M.K.; Cheng, X.R. Leaf non-structural carbohydrate allocation and C:N:P stoichiometry in response to light acclimation in seedlings of two subtropical shade-tolerant tree species. Plant Physiol. Biochem. 2018, 124, 146–154. [Google Scholar] [CrossRef]
- Du, Y.; Lu, R.L.; Xia, J.Y. Impacts of global environmental change drivers on non-structural carbohydrates in terrestrial plants. Funct. Ecol. 2020, 34, 1525–1536. [Google Scholar] [CrossRef]
- Pankaj, T.; Pamela, B.; Singh, R.G.; Datt, R.I.; Gautam, T. Experimental warming increases ecosystem respiration by increasing above-ground respiration in alpine meadows of Western Himalaya. Sci. Rep. 2021, 11, 2640. [Google Scholar]
- Way, D.A.; Sage, R.F. Elevated growth temperatures reduce the carbon gain of black spruce [Picea mariana (Mill.) BSP]. Glob. Change Biol. 2008, 14, 624–636. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Wu, Q.X.; Wu, F.Z.; Hu, Y.; Kang, Z.J.; Zhang, Y.Y.; Yang, J.; Yue, K.; Ni, X.Y.; Yang, Y.S. Difference in non-structural carbohydrates between fresh and senescent leaves of 11 tree species in a subtropical common-garden. Chin. J. Plant Ecol. 2021, 45, 771–779. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, F.Z.; Wu, Q.X.; Kang, Z.J.; Yue, K.; Yang, Y.S.; Ni, X.Y. Seasonal variations of non-structural carbohydrates in fresh litters of three dominant tree species in subtropical forests. Acta Ecol. Sin. 2022, 42, 1901–1910. [Google Scholar]
- Lin, T.; Zheng, H.Z.; Huang, Z.H.; Wang, J.; Zhu, J.M. Non-Structural Carbohydrate Dynamics in Leaves and Branches of Pinus massoniana (Lamb.) Following 3-Year Rainfall Exclusion. Forests 2018, 9, 315. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, F.P.; Zeng, Z.X.; Du, H.; Su, L.; Zhang, L.J.; Lu, M.Z.; Zhang, H. Impact of Selected Environmental Factors on Variation in Leaf and Branch Traits on Endangered Karst Woody Plants of Southwest China. Forests 2022, 13, 1080. [Google Scholar] [CrossRef]
- Keel, S.G.; Schadel, C. Expanding leaves of mature deciduous forest trees rapidly become autotrophic. Tree Physiol. 2010, 30, 1253–1259. [Google Scholar] [CrossRef] [Green Version]
- Millard, P.; Sommerkorn, M.; Grelet, G.A. Environmental change and carbon limitation in trees: A biochemical, ecophysiological and ecosystem appraisal. New Phytol. 2007, 175, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.X. Research on the Effect of Litter on the Nutrient Characteristics in Chinese Fir Plantation. For. Res. 1997, 10, 48–52. [Google Scholar]
- Xu, Y.X.; Wang, Z.C.; Zhang, L.L.; Zhu, W.K.; Du, A.P. The Stoichiometric Characteristics of C, N and P in Leaf-litter-soil of Different Aged Eucalyptus urophylla × E. grandis Plantations. For. Res. 2018, 31, 168–174. [Google Scholar]
- Chen, F.S.; Wang, G.G.; Fang, X.M.; Wan, S.Z.; Zhang, Y.; Liang, C. Nitrogen deposition effect on forest litter decomposition is interactively regulated by endogenous litter quality and exogenous resource supply. Plant Soil 2019, 437, 413–426. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, Y.F. Change trend analysis of the time-series shadow-eliminated vegetation index (SEVI) for the Wuyishan Nature Reserve with the Sen+Mann-Kendall method. IOP Conf. Ser. Earth Environ. Sci. 2021, 658, 012016. [Google Scholar] [CrossRef]
- Hoch, G.; Richter, A.; Körner, C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2010, 26, 1067–1081. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An Improved Colorimetric Method to Quantify Sugar Content of Plant Tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- He, Y.; Liao, H.; Yan, X.L. Localized supply of phosphorus induces root morphological and architectural changes of rice in split and stratified soil cultures. Plant Soil 2003, 248, 247–256. [Google Scholar] [CrossRef]
| Treatment | Source of Variation | SS | df | F | p |
|---|---|---|---|---|---|
| Leaf topsoil litter (LTL) | Time | 11.22 | 5 | 15.77 | <0.001 |
| Altitude | 4.63 | 3 | 10.84 | <0.001 | |
| Time × Altitude | 21.36 | 15 | 10.01 | <0.001 | |
| Mixture of leaf and twig topsoil litter (LTTL) | Time | 66.16 | 5 | 75.50 | <0.001 |
| Altitude | 4.06 | 3 | 7.71 | <0.001 | |
| Time × Altitude | 18.52 | 15 | 7.04 | <0.001 | |
| Leaf canopy litter (LCL) | Time | 46.88 | 5 | 20.80 | <0.001 |
| Altitude | 12.70 | 3 | 9.39 | <0.001 | |
| Time × Altitude | 39.43 | 15 | 5.83 | <0.001 | |
| Mixture of leaf and twig canopy litter (LTCL) | Time | 26.75 | 5 | 13.91 | <0.001 |
| Altitude | 2.65 | 3 | 2.30 | 0.089 | |
| Time × Altitude | 43.68 | 15 | 7.57 | <0.001 |
| Altitude/m | Average Annual Air Temperature/°C | Average Annual Soil Temperature/°C | Average Annual Soil Humidity/% | Soil Total Nitrogen (g·kg−1) | Soil Total Phosphorus/ (g·kg−1) | Soil pH |
|---|---|---|---|---|---|---|
| 692 | 16.9 | 17.9 | 14.2 | 0.43 ± 0.07 | 0.32 ± 0.01 | 4.76 |
| 1003 | 15.0 | 14.8 | 16.8 | 0.77 ± 0.12 | 0.30 ± 0.01 | 4.41 |
| 1410 | 12.9 | 12.7 | 18.7 | 0.71 ± 0.13 | 0.46 ± 0.06 | 4.38 |
| 1968 | 10.2 | 11.5 | 21.4 | 0.54 ± 0.04 | 0.30 ± 0.03 | 4.59 |
| Treatment | Soluble Sugar Content/% | Starch Content/% | NSC Content/% | Total Carbon/(g·kg−1) | Total Nitrogen/(g·kg−1) | Total Phosphorus/(g·kg−1) |
|---|---|---|---|---|---|---|
| LTL | 1.56 ± 0.03 A | 2.59 ± 0.08 C | 4.15 ± 0.11 C | 506.03 ± 20.54 A | 15.22 ± 0.20 C | 0.35 ± 0.11 A |
| LTTL | 1.54 ± 0.04 A | 3.22 ± 0.32 D | 4.76 ± 0.29 BC | 503.69 ± 13.62 A | 13.98 ± 0.22 D | 0.29 ± 0.07 A |
| LCL | 1.34 ± 0.19 B | 6.73 ± 0.28 A | 8.07 ± 0.68 A | 496.42 ± 16.53 A | 17.60 ± 0.31 A | 0.34 ± 0.02 A |
| LTCL | 0.80 ± 0.04 C | 4.46 ± 0.25 B | 5.26 ± 0.25 B | 494.50 ± 14.96 A | 16.16 ± 0.22 B | 0.25 ± 0.02 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Cao, Y.; Jiang, Y.; Chen, M.; Zhang, H.; Wu, P.; Ma, X. Dynamics of Non-Structural Carbohydrates Release in Chinese Fir Topsoil and Canopy Litter at Different Altitudes. Plants 2023, 12, 729. https://doi.org/10.3390/plants12040729
Wu X, Cao Y, Jiang Y, Chen M, Zhang H, Wu P, Ma X. Dynamics of Non-Structural Carbohydrates Release in Chinese Fir Topsoil and Canopy Litter at Different Altitudes. Plants. 2023; 12(4):729. https://doi.org/10.3390/plants12040729
Chicago/Turabian StyleWu, Xiaojian, Yue Cao, Yu Jiang, Mingxu Chen, Huiguang Zhang, Pengfei Wu, and Xiangqing Ma. 2023. "Dynamics of Non-Structural Carbohydrates Release in Chinese Fir Topsoil and Canopy Litter at Different Altitudes" Plants 12, no. 4: 729. https://doi.org/10.3390/plants12040729





