Essential Oils from Apiaceae, Asteraceae, Cupressaceae and Lamiaceae Families Grown in Serbia: Comparative Chemical Profiling with In Vitro Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. EO Yield
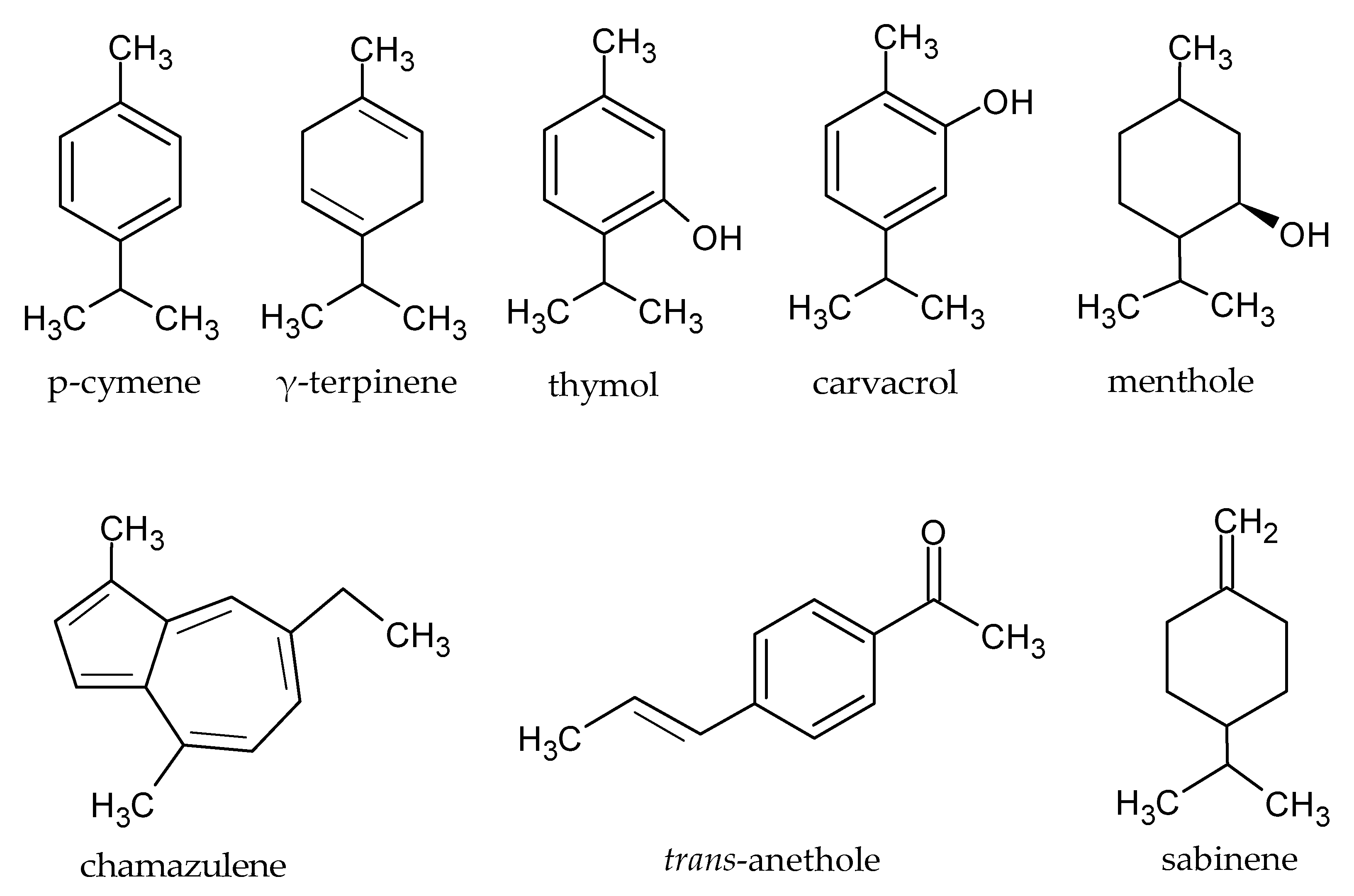
2.2. Chemical Profile of EOs
2.3. In Vitro Antioxidant Activity
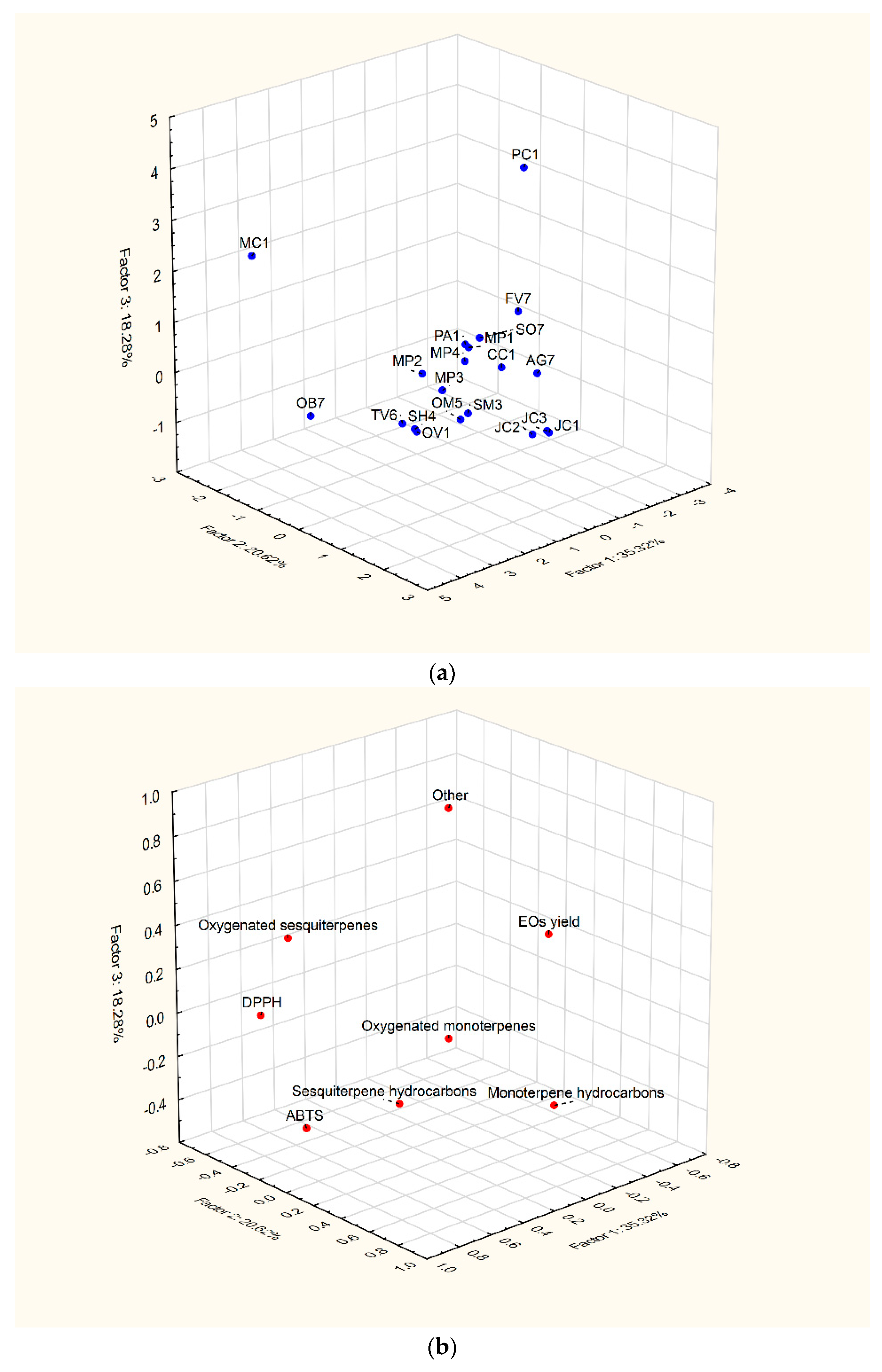
2.4. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Isolation of EO—Conventional Hydrodistillation (HD)
3.4. Gas Chromatography-Mass Spectrometry (GC–MS) Analysis
3.5. In Vitro Antioxidant Activity
3.6. Statistical Analysis and PCA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radovic, I.; Kozomara, M. Biodiversity Strategy of the Republic of Serbia for the Period 2011–2018; United Nations Development Programme: New York, NY, USA, 2011; p. 18. [Google Scholar]
- Vujanović, M.; Zengin, G.; Đurović, S.; Mašković, P.; Cvetanović, A.; Radojković, M. Biological activity of extracts of traditional wild medicinal plants from the Balkan Peninsula. S. Afr. J. Bot. 2019, 120, 213–218. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Harris, R. Biological Activities of Essential Oils: An Update. In Handbook of Essential Oils—Science, Technology, and Applications, 2nd ed.; Can Baser, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 298–339. [Google Scholar]
- Rajeswara Rao, B.R. Curry Leaf (Murraya koenigii) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 385–394. [Google Scholar]
- Šojić, B.; Putnik, P.; Danilović, B.; Teslić, N.; Kovačević, D.B.; Pavlić, B. Lipid Extracts Obtained by Supercritical Fluid Extraction and Their Application in Meat Products. Antioxidants 2022, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Mamadalieva, N.; Akramov, D.; Ovidi, E.; Tiezzi, A.; Nahar, L.; Azimova, S.; Sarker, S. Aromatic medicinal plants of the Lamiaceae family from Uzbekistan: Ethnopharmacology, essential oils composition, and biological activities. Medicines 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.; Cavaleiro, C.; Gonçalves, M.J.; Cruz, M.T.; Lopes, M.C.; Salgueiro, L. Otanthus maritimus (L.) Hoffmanns. & Link as a source of a bioactive and fragrant oil. Ind. Crops Prod. 2013, 43, 484–489. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Asteraceae species with most prominent bioactivity and their potential applications: A review. Ind. Crops Prod. 2015, 76, 604–615. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Faridchehr, A. Constituents and biological activities of selected genera of the Iranian Asteraceae family. J. Herb. Med. 2021, 25, 100405. [Google Scholar] [CrossRef]
- Acimovic, M.; Kostadinovic, L.; Popovic, S.; Dojcinovic, N. Apiaceae seeds as functional food. J. Agric. Sci. Belgrade 2015, 60, 237–246. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Judžentienė, A. A review of volatile organic compounds of wild and cultivated common juniper in Lithuania. Chemija 2019, 30, 184–193. [Google Scholar]
- Radivojac, A.; Bera, O.; Zeković, Z.; Teslić, N.; Mrkonjić, Ž.; Bursać Kovačević, D.; Putnik, P.; Pavlić, B. Extraction of Peppermint Essential Oils and Lipophilic Compounds: Assessment of Process Kinetics and Environmental Impacts with Multiple Techniques. Molecules 2021, 26, 2879. [Google Scholar] [CrossRef] [PubMed]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Mrkonjić, Ž.; Rakić, D.; Olgun, E.O.; Canli, O.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Optimization of antioxidants recovery from wild thyme (Thymus serpyllum L.) by ultrasound-assisted extraction: Multi-response approach. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100333. [Google Scholar] [CrossRef]
- Radivojac, A.; Bera, O.; Micić, D.; Đurović, S.; Zeković, Z.; Blagojević, S.; Pavlić, B. Conventional versus microwave-assisted hydrodistillation of sage herbal dust: Kinetics modeling and physico-chemical properties of essential oil. Food Bioprod. Process. 2020, 123, 90–101. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Chemat, F.; Giancarlo, C. Microwave-Assisted Extraction for Bioactive Compounds; Springer: Boston, MA, USA, 2013. [Google Scholar]
- Milojević, S.Ž.; Radosavljević, D.B.; Pavićević, V.P.; Pejanović, S.; Veljković, V.B. Modeling the kinetics of essential oil hydrodistillation from plant materials. Hem. Ind. 2013, 67, 843–859. [Google Scholar] [CrossRef]
- Abbas, A.; Anwar, F.; Ahmad, N. Variation in physico-chemical composition and biological attributes of common basil essential oils produced by hydro-distillation and super critical fluid extraction. J. Essent. Oil Bear. Plants 2017, 20, 95–109. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of Rosemary and Sage (Rosmarinus officinalis L. and Salvia officinalis L. Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Ben Salha, G.; Herrera Díaz, R.; Labidi, J.; Abderrabba, M. Deterpenation of Origanum majorana L. essential oil by reduced pressure steam distillation. Ind. Crops Prod. 2017, 109, 116–122. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Acimovic, M.; Stankovic, J.; Cvetkovic, M.; Kiprovski, B.; Todosijevic, M. Essential Oil Quality of Tetraploid Chamomile Cultivars Grown in Serbia. J. Essent. Oil Bear. Plants 2018, 21, 15–22. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Salehi, B.; Martorell, M.; del Mar Contreras, M.; Soltani-Nejad, A.; et al. Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phytother. Res. 2018, 32, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Trindade, H. Review on essential oil, extracts composition, molecular and phytochemical properties of Thymus species in Iran. Ind. Crops Prod. 2019, 134, 89–99. [Google Scholar] [CrossRef]
- Ramos da Silva, L.R.; Ferreira, O.O.; Cruz, J.N.; de Jesus Pereira Franco, C.; Oliveira dos Anjos, T.; Cascaes, M.M.; Almeida da Costa, W.; de Aguiar Andrade, H.E.; Santana de Oliveira, M.; Luís, Â.; et al. Lamiaceae Essential Oils, Phytochemical Profile, Antioxidant, and Biological Activities. Evid. Based Complement. Altern. Med. 2021, 2021, 6748052. [Google Scholar] [CrossRef]
- Nilo, M.C.S.; Riachi, L.G.; Simas, D.L.R.; Coelho, G.C.; da Silva, A.J.R.; Costa, D.C.M.; Alviano, D.S.; Alviano, C.S.; de Maria, C.A.B. Chemical composition and antioxidant and antifungal properties of Mentha x piperita L. (peppermint) and Mentha arvensis L. (cornmint) samples. Food Res. 2017, 1, 147–156. [Google Scholar] [CrossRef]
- Radusiene, J.; Judzentiene, A.; Janulis, V. Chemical composition of essential oil and antimicrobial activity of Origanum vulgare. Biologija 2005, 4, 53–58. [Google Scholar]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Guo, Y.; Pizzol, R.; Gabbanini, S.; Baschieri, A.; Amorati, R.; Valgimigli, L. Absolute Antioxidant Activity of Five Phenol-Rich Essential Oils. Molecules 2021, 26, 5237. [Google Scholar] [CrossRef]
- Karpiński, T.M. Essential oils of lamiaceae family plants as antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular mechanism of the anti-inflammatory effects of plant essential oils: A systematic review. J. Ethnopharmacol. 2023, 301, 115829. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Tirillini, B.; Pagiotti, R.; Menghini, L.; Pintore, G. Essential oil composition of ligulate and tubular flowers and receptacle from wild Chamomilla recutita (L.) rausch. Grown in Italy. J. Essent. Oil Res. 2006, 18, 42–45. [Google Scholar] [CrossRef]
- Wang, M.; Avula, B.; Wang, Y.-H.; Zhao, J.; Avonto, C.; Parcher, J.F.; Raman, V.; Zweigenbaum, J.A.; Wylie, P.L.; Khan, I.A.; et al. An integrated approach utilising chemometrics and GC/MS for classification of chamomile flowers, essential oils and commercial products. Food Chem. 2014, 152, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R. Essential Oil Composition of Wild Growing Apiaceae from Europe and the Mediterranean. Nat. Prod. Commun. 2010, 5, 1477–1492. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, E.; Maggi, F.; Bonacucina, G.; Pavela, R.; Boukouvala, M.C.; Kavallieratos, N.G.; Canale, A.; Romano, D.; Desneux, N.; Wilke, A.B.B.; et al. Apiaceae essential oils and their constituents as insecticides against mosquitoes—A review. Ind. Crops Prod. 2021, 171, 113892. [Google Scholar] [CrossRef]
- Rajčević, N.; Dodoš, T.; Novaković, J.; Boršić, I.; Janaćković, P.; Marin, P.D. Differentiation of North-Western Balkan Juniperus communis L. (Cupressaceae) populations–ecological and chemophenetic implications. J. Essent. Oil Res. 2020, 32, 562–570. [Google Scholar] [CrossRef]
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Petronilho, S.; Maraschin, M.; Coimbra, M.A.; Rocha, S.M. In vitro and in vivo studies of natural products: A challenge for their valuation. The case study of chamomile (Matricaria recutita L.). Ind. Crops Prod. 2012, 40, 1–12. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, Antioxidant, Anti-Acetylcholinesterase, Antidiabetic, and Pharmacokinetic Properties of Carum carvi L. and Coriandrum sativum L. Essential Oils Alone and in Combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef]
- Riachi, L.G.; de Maria, C.A.B. Peppermint antioxidants revisited. Food Chem. 2015, 176, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.A.; Javadi, B.; Hassanzadeh, M.K. Antioxidant Activity of the Essential Oils of Different Parts of Juniperus excelsa M. Bieb. subsp. excelsa and J. excelsa M. Bieb. subsp. polycarpos (K. Koch) Takhtajan (Cupressaceae). Pharm. Biol. 2008, 45, 769–776. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef] [PubMed]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical composition and antioxidant properties of juniper berry (Juniperus communis L.) essential oil. action of the essential oil on the antioxidant protection of saccharomyces cerevisiae model organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef]
- Šojić, B.; Pavlić, B.; Zeković, Z.; Tomović, V.; Ikonić, P.; Kocić-Tanackov, S.; Džinić, N. The effect of essential oil and extract from sage (Salvia officinalis L.) herbal dust (food industry by-product) on the oxidative and microbiological stability of fresh pork sausages. LWT 2018, 89, 749–755. [Google Scholar] [CrossRef]
- Šojić, B.; Pavlić, B.; Tomović, V.; Ikonić, P.; Zeković, Z.; Kocić-Tanackov, S.; Đurović, S.; Škaljac, S.; Jokanović, M.; Ivić, M.; et al. Essential oil versus supercritical fluid extracts of winter savory (Satureja montana L.)—Assessment of the oxidative, microbiological and sensory quality of fresh pork sausages. Food Chem. 2019, 287, 280–286. [Google Scholar] [CrossRef]
- Šojić, B.; Tomović, V.; Kocić-Tanackov, S.; Kovačević, D.B.; Putnik, P.; Mrkonjić, Ž.; Đurović, S.; Jokanović, M.; Ivić, M.; Škaljac, S.; et al. Supercritical extracts of wild thyme (Thymus serpyllum L.) by-product as natural antioxidants in ground pork patties. LWT 2020, 130, 109661. [Google Scholar] [CrossRef]
- Pavlić, B.; Šojić, B.; Teslić, N.; Putnik, P.; Kovačević, D.B. Extraction of bioactive compounds and essential oils from herbs using green technologies. In Aromatic Herbs in Food Bioactive Compounds, Processing, and Applications; Academic Press: Cambridge, MA, USA, 2021; pp. 233–262. [Google Scholar] [CrossRef]
- Šojić, B.; Pavlić, B.; Ikonić, P.; Tomović, V.; Ikonić, B.; Zeković, Z.; Kocić-Tanackov, S.; Jokanović, M.; Škaljac, S.; Ivić, M.; et al. Coriander essential oil as natural food additive improves quality and safety of cooked pork sausages with different nitrite levels. Meat Sci. 2019, 157, 107879. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an Important Source of Antioxidants and Their Applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, Y.; Li, X.; Yuan, K.; Li, Y.; Tian, L.; Sun, J.; Bai, W. Modeling and application of sensory evaluation of blueberry wine based on principal component analysis. Curr. Res. Food Sci. 2023, 6, 100403. [Google Scholar] [CrossRef]
- Council of Europe. 2.9.18 Preparations for Inhalation: Aerodynamic Assessment of Fine Particles. In European Pharmacopoeia 7.0; Council of Europe: Strasbourg, France, 2010; pp. 274–285. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2017; pp. 1–809. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pavlić, B.; Bera, O.; Teslić, N.; Vidović, S.; Parpinello, G.; Zeković, Z. Chemical profile and antioxidant activity of sage herbal dust extracts obtained by supercritical fluid extraction. Ind. Crops Prod. 2018, 120, 305–312. [Google Scholar] [CrossRef]
- Dimić, I.; Teslić, N.; Putnik, P.; Kovačević, D.B.; Zeković, Z.; Šojić, B.; Mrkonjić, Ž.; Čolović, D.; Montesano, D.; Pavlić, B.; et al. Innovative and conventional valorizations of grape seeds from winery by-products as sustainable source of lipophilic antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef] [PubMed]
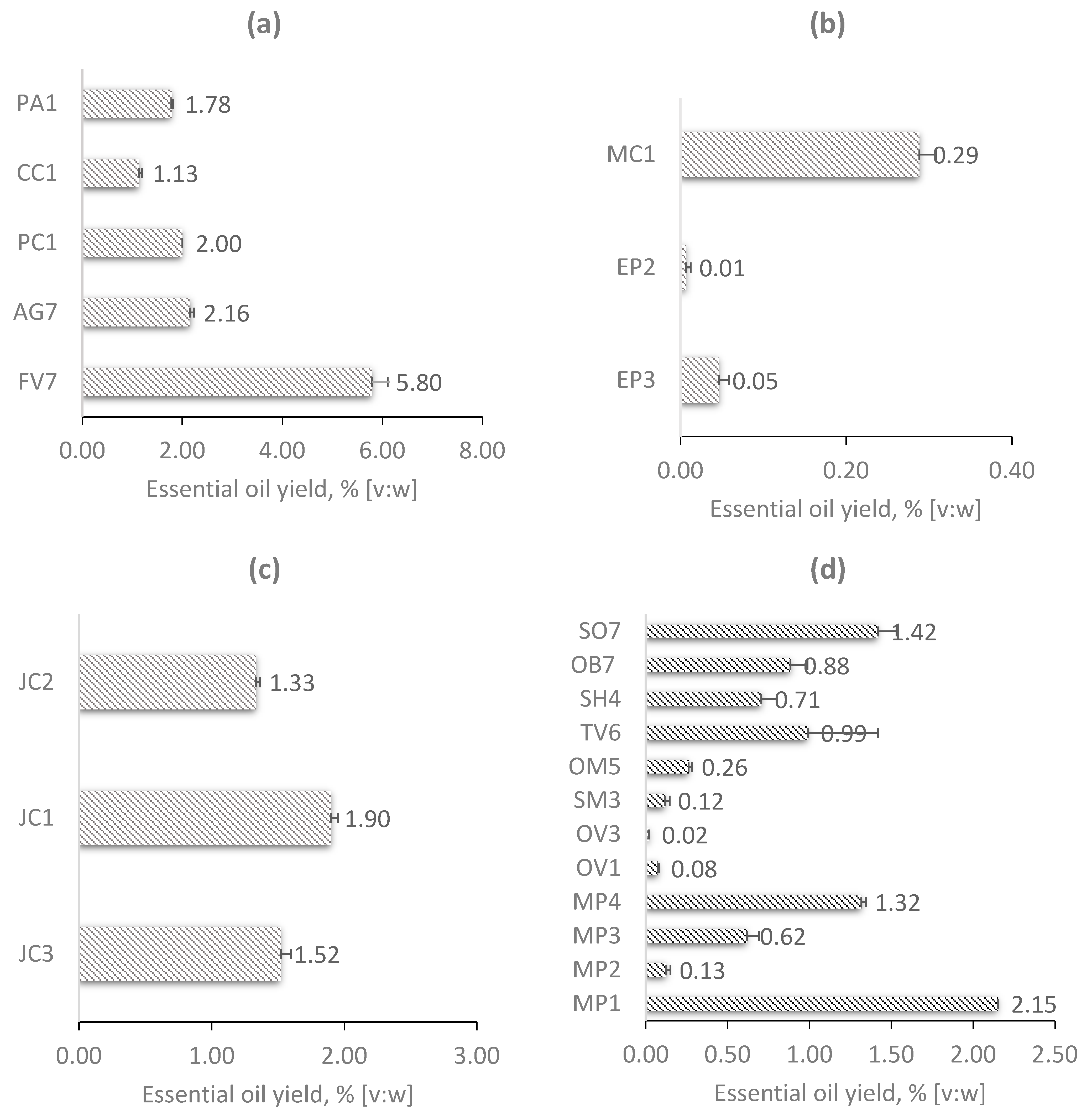
| Family | Plant Name English | Plant Name Latin | Analyzed Part of the Plant | Sample Name | Plant Properties | |
|---|---|---|---|---|---|---|
| MC [%, w:w] | d [mm] | |||||
| Apiaceae | Dill | Anethum graveolens | Chopped fruit/seed | AG7 | 8.49 | 0.3358 |
| Caraway | Carum carvi | Chopped fruit/seed | CC1 | 9.86 | 0.9163 | |
| Fennel | Foeniculum vulgare | Chopped fruit/seed | FV7 | 9.96 | 0.6695 | |
| Parsley | Petroselinum crispum | Chopped fruit/seed | PC1 | 9.63 | 0.9525 | |
| Anise | Pimpinella anisum | Chopped fruit/seed | PA1 | 8.18 | 0.7563 | |
| Asteraceae | Echinacea | Ehinacea purpurea | Chopped herb | EP2 | 10.25 | 0.9784 |
| Chopped herb | EP3 | 10.74 | 0.898 | |||
| Chamomile | Matricaria chamomilla | Chopped flower | MC1 | 7.84 | 3.5930 | |
| Cupressaceae | Juniper | Juniperus comunis | Chopped fruit | JC1 | 10.98 | 0.9686 |
| Chopped fruit | JC2 | 10.29 | 0.8714 | |||
| Chopped fruit | JC3 | 10.65 | 0.9716 | |||
| Lamiaceae | Peppermint | Mentha x Piperita | Pharmacologically crushed leaf | MP1 | 13.02 | 1.5920 |
| Chopped herb | MP2 | 9.58 | 1.0260 | |||
| Chopped leaf | MP3 | 9.48 | 1.9045 | |||
| Chopped leaf | MP4 | 10.52 | / | |||
| Basil | Ocimum basilicum | Chopped herb | OB7 | 8.38 | 0.5493 | |
| Marjoram | Origanum majorana | Chopped leaf | OM5 | 8.02 | 0.8218 | |
| Oregano | Origanum vulgare | Chopped herb | OV1 | 0.00 | 0.9213 | |
| Chopped herb | OV3 | 8.39 | 0.7688 | |||
| Sage | Salvia officinalis | Chopped herb | SO7 | 9.95 | 1.5928 | |
| Winter savory | Satureja montana | Chopped herb | SM3 | 10.91 | 0.9342 | |
| Summer savory | Satureja hortensis | Chopped herb | SH4 | 12.22 | / | |
| Thyme | Thymus vulgaris | Chopped herb | TV6 | 11.19 | 0.4426 | |
| No | Component | RT [a] | Relative Percentage (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MP1 | MP2 | MP3 | MP4 | OV1 | SM3 | OM5 | TV6 | SH4 | OB7 | SO7 | |||
| 1 | α-Thujene | 3.8 | ND | ND | ND | ND | tr | ND | 0.69 | 0.64 | 0.64 | ND | ND |
| 2 | α-Pinene | 3.9 | 0.34 | 0.19 | 0.43 | 0.49 | 0.13 | 2.15 | 0.58 | 0.81 | 0.29 | ND | 2.93 |
| 3 | Camphene | 4.2 | ND | ND | ND | ND | 0.13 | 0.73 | ND | 0.23 | tr | ND | 5.33 |
| 4 | Sabinene | 4.8 | 0.13 | tr | tr | 0.17 | 1.66 | 0.28 | 3.44 | tr | ND | tr | tr |
| 5 | β-Pinene | 4.9 | 0.76 | 0.29 | 0.87 | 0.88 | ND | ND | ND | tr | 0.10 | ND | 1.77 |
| 6 | 1-Octen-3-ol | 5.0 | ND | ND | ND | ND | 0.49 | 0.27 | ND | ND | ND | ND | ND |
| 7 | Myrcene | 5.2 | tr | 0.12 | 0.34 | 1.61 | 0.28 | 0.36 | 0.73 | 0.84 | 0.91 | 0.14 | 0.42 |
| 8 | α-Phellandrene | 5.5 | ND | ND | ND | ND | ND | 0.11 | 0.60 | ND | ND | ND | ND |
| 9 | α-Terpinene | 5.8 | ND | 0.17 | ND | ND | 0.23 | 0.40 | 7.37 | 1.00 | 2.49 | ND | 0.15 |
| 10 | p-Cymene | 6.1 | ND | ND | ND | ND | 1.91 | 19.24 | 7.47 | 25.04 | 4.29 | ND | 0.09 |
| 11 | Limonene | 6.2 | 0.26 | 0.37 | 0.91 | 3.86 | ND | 7.83 | 3.23 | ND | ND | tr | 0.82 |
| 12 | β-Phellandrene | 6.2 | ND | ND | ND | ND | ND | ND | 4.64 | ND | ND | ND | ND |
| 13 | Eucalyptol (1,8-Cineole) | 6.2 | 6.56 | 3.62 | 8.27 | 11.19 | 1.64 | ND | ND | ND | ND | 1.83 | 7.27 |
| 14 | cis-β-Ocimene | 6.5 | ND | ND | ND | ND | 0.70 | 0.94 | ND | ND | ND | ND | ND |
| 15 | trans-β-Ocimene | 6.8 | tr | ND | ND | 0.17 | 0.23 | 0.93 | ND | ND | tr | 0.38 | ND |
| 16 | γ-Terpinene | 7.1 | 0.13 | 0.37 | tr | ND | 0.49 | 1.74 | 11.63 | 8.20 | 29.14 | ND | 0.28 |
| 17 | cis-Sabinene hydrate | 7.4 | 0.75 | 0.35 | 0.14 | 0.12 | ND | 0.72 | 1.49 | 0.69 | tr | ND | 0.10 |
| 18 | cis-Linalool oxide (furanoid) | 7.6 | ND | ND | ND | ND | ND | 0.36 | ND | ND | ND | tr | ND |
| 19 | Terpinolene | 8.0 | tr | 0.14 | ND | ND | ND | 0.19 | 2.52 | 0.13 | tr | ND | 0.42 |
| 20 | Fenchone | 8.0 | ND | ND | ND | ND | 0.67 | ND | ND | ND | ND | 0.18 | ND |
| 21 | trans-Linalool oxide (furanoid) | 8.1 | ND | ND | ND | ND | ND | 0.18 | ND | ND | ND | ND | ND |
| 22 | trans-Sabinene hydrate | 8.4 | ND | ND | ND | ND | ND | ND | 3.44 | ND | ND | ND | 0.07 |
| 23 | Linalool | 8.4 | 0.19 | 0.65 | 0.10 | 0.15 | 3.30 | 11.91 | 3.27 | 1.97 | tr | 24.62 | 0.12 |
| 24 | Isoamyl isovalerate | 8.5 | tr | ND | 0.27 | 0.41 | ND | ND | ND | ND | ND | ND | ND |
| 25 | α-Thujone | 8.6 | ND | ND | ND | ND | 0.88 | ND | ND | ND | ND | ND | 25.80 |
| 26 | β-Thujone | 9.0 | ND | ND | ND | ND | 0.08 | ND | ND | ND | ND | ND | 6.20 |
| 27 | cis-Menth-2-en-1-ol | 9.2 | ND | 0.16 | ND | ND | ND | 0.21 | 2.20 | ND | ND | ND | ND |
| 28 | allo-Ocimene | 9.5 | ND | ND | ND | ND | 0.11 | 0.11 | ND | ND | ND | ND | ND |
| 29 | trans-Menth-2-en-1-ol | 9.9 | ND | ND | ND | ND | ND | ND | 1.46 | ND | ND | ND | ND |
| 30 | Camphor | 9.9 | ND | 0.14 | ND | ND | 0.35 | 0.33 | ND | 0.35 | ND | 1.13 | 26.48 |
| 31 | Menthone | 10.3 | 31.75 | 21.02 | 2.88 | 7.05 | 0.84 | 0.29 | ND | ND | ND | ND | ND |
| 32 | Isomenthone | 10.7 | 6.55 | 4.05 | 0.64 | 1.82 | 0.12 | ND | ND | ND | ND | ND | ND |
| 33 | Neomenthol | 10.8 | 3.58 | 2.71 | tr | 0.85 | ND | ND | ND | ND | ND | ND | ND |
| 34 | Borneol | 10.8 | ND | ND | ND | ND | 0.77 | 5.29 | tr | 1.11 | tr | tr | 2.26 |
| 35 | δ-Terpineol | 11.0 | ND | ND | ND | ND | ND | ND | ND | ND | ND | tr | ND |
| 36 | Menthol | 11.1 | 37.63 | 33.54 | 6.30 | 7.94 | 0.79 | ND | ND | ND | ND | ND | ND |
| 37 | Terpinen-4-ol | 11.3 | ND | ND | ND | ND | 0.92 | 2.44 | 30.20 | 0.61 | 0.18 | tr | 0.19 |
| 38 | Isomenthol | 11.5 | 0.40 | 0.75 | 0.10 | 0.16 | ND | ND | ND | ND | ND | ND | ND |
| 39 | Neoisomenthol | 11.7 | ND | tr | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 40 | α-Terpineol | 11.8 | tr | 0.13 | 0.16 | ND | 4.55 | 0.50 | 3.75 | tr | ND | 0.25 | ND |
| 41 | cis-Dihydrocarvone | 12.0 | ND | 1.11 | 3.01 | 4.38 | ND | 0.31 | ND | ND | ND | ND | ND |
| 42 | cis-Piperitol | 12.0 | ND | ND | ND | ND | ND | ND | 0.97 | ND | ND | ND | ND |
| 43 | Estragole | 12.1 | ND | ND | ND | ND | 0.68 | ND | 0.77 | ND | ND | 16.99 | ND |
| 44 | trans-Dihydrocarvone | 12.3 | ND | ND | 0.25 | 0.24 | ND | 0.47 | ND | ND | ND | ND | ND |
| 45 | trans-Piperitol | 12.5 | ND | ND | ND | ND | ND | ND | 0.50 | ND | ND | ND | ND |
| 46 | trans-Carveol | 13.0 | ND | 0.25 | tr | tr | ND | 0.35 | ND | ND | ND | ND | ND |
| 47 | Nerol | 13.3 | ND | ND | ND | ND | ND | 0.36 | ND | ND | ND | ND | ND |
| 48 | Thymol methyl ether | 13.5 | ND | ND | ND | ND | 0.14 | 0.23 | ND | 0.10 | ND | ND | ND |
| 49 | cis-3-Hexenyl isovalerate | 13.5 | ND | ND | 0.16 | 0.19 | ND | ND | ND | ND | ND | ND | ND |
| 50 | Pulegone | 13.7 | 1.74 | 1.26 | 0.17 | 0.41 | 0.11 | ND | ND | ND | ND | ND | ND |
| 51 | Neral (Citral) | 13.8 | ND | ND | ND | ND | ND | 0.21 | ND | ND | ND | ND | ND |
| 52 | Carvone | 13.9 | ND | 10.96 | 45.61 | 47.04 | 0.62 | 1.11 | 1.06 | ND | ND | 0.12 | ND |
| 53 | Piperitone | 14.3 | 0.67 | 1.97 | 1.04 | 0.22 | ND | ND | ND | ND | ND | ND | ND |
| 54 | Linalool acetate | 14.3 | ND | ND | ND | ND | 0.64 | ND | 0.70 | ND | ND | ND | ND |
| 55 | Geraniol | 14.4 | ND | ND | ND | ND | ND | 10.15 | ND | ND | ND | 0.51 | ND |
| 56 | Geranial | 15.0 | ND | ND | ND | ND | ND | 0.41 | ND | ND | ND | ND | ND |
| 57 | trans-Carane | 15.0 | 0.19 | 0.21 | tr | tr | ND | ND | ND | ND | ND | ND | ND |
| 58 | Bornyl acetate | 15.4 | ND | ND | ND | ND | 0.72 | 0.39 | 0.09 | ND | ND | 0.48 | 1.11 |
| 59 | Dihydroedulane I | 15.5 | tr | 1.07 | 0.45 | 0.13 | ND | ND | ND | ND | ND | ND | ND |
| 60 | Dihydroedulane I | 15.7 | ND | 0.76 | 0.58 | 0.20 | ND | ND | ND | ND | ND | ND | ND |
| 61 | trans-Anethole | 15.6 | ND | ND | ND | ND | 16.04 | ND | ND | ND | ND | 3.09 | ND |
| 62 | cis-Carane | 15.8 | 4.25 | 3.51 | 1.18 | 0.82 | ND | ND | ND | ND | ND | ND | ND |
| 63 | Thymol derivative | 16.2 | ND | ND | ND | ND | ND | ND | ND | 0.24 | ND | ND | ND |
| 64 | Menthyl acetate | 16.4 | 0.20 | 0.17 | ND | tr | ND | ND | ND | ND | ND | ND | ND |
| 65 | Thymol | 16.6 | ND | ND | ND | ND | 0.44 | 0.43 | ND | 55.65 | tr | ND | ND |
| 66 | Carvacrol | 16.9 | ND | ND | ND | ND | 1.19 | ND | 0.43 | ND | 61.32 | ND | ND |
| 67 | 1,5,5-Trimethyl-6-methylene-cyclohexene | 17.4 | ND | tr | 0.30 | 0.12 | ND | 0.22 | ND | ND | ND | 0.35 | ND |
| 68 | α-Cubebene | 18.0 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.13 | ND |
| 69 | α-Terpinyl acetate | 18.1 | ND | ND | ND | ND | ND | 0.86 | ND | ND | ND | ND | ND |
| 70 | Eugenol | 18.7 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 71 | α-Copaene | 19.0 | ND | ND | ND | ND | 0.49 | 0.22 | 0.16 | ND | ND | 0.46 | ND |
| 72 | β-Bourbonene | 19.3 | 0.16 | 0.43 | 1.85 | 1.16 | 1.48 | 1.53 | ND | ND | ND | 0.48 | ND |
| 73 | Geranyl acetate | 19.5 | ND | ND | ND | ND | ND | 12.64 | ND | ND | ND | ND | ND |
| 74 | β-Cubebene | 19.6 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.13 | ND |
| 75 | β-Elemene | 19.7 | 0.04 | tr | 0.27 | tr | 0.46 | ND | ND | ND | ND | 7.46 | ND |
| 76 | cis-Caryophyllene | 20.2 | ND | ND | ND | tr | ND | ND | ND | ND | ND | ND | ND |
| 77 | trans-Caryophyllene | 20.6 | 1.63 | tr | 12.54 | 5.69 | 14.60 | 2.50 | 2.74 | 0.97 | ND | 0.98 | 1.26 |
| 78 | β-Caryophyllene | 20.7 | ND | ND | ND | ND | ND | ND | ND | ND | 0.52 | ND | ND |
| 79 | β-Gurjunene | 21.1 | ND | tr | 0.24 | tr | 0.75 | 0.33 | ND | ND | ND | 0.31 | ND |
| 80 | α-trans-Bergamotene | 21.4 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.81 | ND |
| 81 | α-Guaiene | 21.5 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 2.69 | ND |
| 82 | Aromadendrene | 21.8 | ND | ND | ND | ND | 0.26 | ND | ND | ND | ND | ND | ND |
| 83 | α-Humulene (α-Caryophyllene) | 22.0 | tr | 0.13 | 0.50 | 0.21 | 2.42 | 0.20 | 0.16 | ND | ND | 1.87 | 3.72 |
| 84 | allo-Aromadendrene | 22.3 | ND | ND | ND | ND | 0.69 | ND | ND | ND | ND | ND | ND |
| 85 | cis-Muurola-4(14),5-diene | 22.5 | ND | 0.15 | 0.92 | 0.34 | ND | ND | ND | ND | ND | 0.63 | ND |
| 86 | Germacrene D | 23.2 | 0.91 | 1.08 | 2.32 | 0.77 | 14.56 | 3.64 | ND | ND | ND | 4.98 | ND |
| 87 | Bicyclogermacrene | 23.7 | 0.20 | 0.22 | 0.71 | 0.20 | 0.79 | 0.60 | 0.63 | ND | ND | 0.97 | ND |
| 88 | α-Muurolene | 24.0 | ND | ND | ND | ND | 0.30 | ND | ND | ND | ND | ND | ND |
| 89 | α-Bulnesene (δ-Guaiene) | 24.1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 4.90 | ND |
| 90 | γ-Cadinene | 24.6 | ND | 0.10 | tr | ND | ND | ND | 0.12 | ND | ND | 4.40 | ND |
| 91 | β-Bisabolene | 24.4 | ND | ND | ND | ND | 1.67 | ND | ND | ND | 0.11 | ND | ND |
| 92 | γ-Cadinene | 24.5 | ND | ND | ND | ND | 1.07 | ND | ND | ND | ND | ND | ND |
| 93 | β-Bisabolene+γ-Cadinene | 24.6 | ND | ND | ND | ND | ND | 0.34 | ND | ND | ND | ND | ND |
| 94 | δ-Cadinene | 24.9 | ND | 0.19 | 0.60 | 0.19 | 2.53 | 0.26 | ND | 0.11 | ND | 0.49 | ND |
| 95 | Elemol | 26.0 | ND | ND | ND | ND | 1.01 | ND | ND | ND | ND | ND | ND |
| 96 | Germacrene D-4-ol | 26.9 | ND | ND | ND | ND | 0.23 | ND | ND | ND | ND | ND | ND |
| 97 | Spathulenol | 27.0 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 2.02 | ND |
| 98 | Caryophyllene oxide | 27.1 | 0.29 | 1.93 | 3.80 | 0.63 | 8.82 | 4.16 | 1.41 | 0.51 | tr | ND | 0.17 |
| 99 | Viridiflorol | 27.5 | 0.50 | 1.37 | 0.39 | ND | ND | ND | ND | ND | ND | ND | 3.46 |
| 100 | Humulene epoxide II | 28.1 | ND | ND | ND | ND | 0.76 | ND | ND | ND | ND | ND | 0.64 |
| 101 | τ-Cadinol | 29.4 | ND | ND | ND | ND | 0.60 | ND | ND | ND | ND | 10.93 | ND |
| 102 | α-Cadinol | 30.0 | ND | 0.36 | 0.51 | 0.22 | 1.50 | ND | ND | ND | ND | 0.62 | ND |
| 103 | Bifomene | 33.6 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.04 |
| 104 | Manool | 34.6 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 8.07 |
| Monoterpene hydrocarbons | 6.06 | 5.37 | 4.04 | 8.11 | 5.88 | 35.23 | 42.89 | 36.89 | 37.86 | 0.87 | 12.21 | ||
| Sesquiterpene hydrocarbons | 2.93 | 2.30 | 19.97 | 8.56 | 42.06 | 9.63 | 3.81 | 1.08 | 0.64 | 31.72 | 4.97 | ||
| Oxygenated monoterpenes | 89.82 | 82.68 | 68.95 | 81.96 | 35.34 | 49.91 | 50.32 | 60.37 | 61.51 | 49.22 | 69.61 | ||
| Oxygenated sesquterpenes | 0.80 | 3.66 | 4.70 | 0.85 | 12.93 | 4.16 | 1.41 | 0.51 | 0.00 | 13.57 | 4.27 | ||
| Other | 0.20 | 2.01 | 1.19 | 0.52 | 0.63 | 0.50 | 0.00 | 0.34 | 0.00 | 0.00 | 8.11 | ||
| Total | 99.80 | 96.02 | 98.84 | 100.00 | 96.84 | 99.43 | 98.43 | 99.19 | 100.00 | 95.38 | 99.17 | ||
| No. | Component | RT [a] | Relative Percentage (%) |
|---|---|---|---|
| MC1 | |||
| 1 | Camphor | 9.9 | 0.14 |
| 2 | Menthone | 10.3 | 0.07 |
| 3 | Isomenthone | 10.7 | 0.05 |
| 4 | Menthol | 11.1 | 0.07 |
| 5 | trans-Caryophyllene | 20.7 | 0.11 |
| 6 | α-Humulene (α-Caryophyllene) | 22.0 | tr |
| 7 | trans-β-Farnesene | 22.4 | 4.84 |
| 8 | Dihydro sesquicineole | 22.8 | 0.39 |
| 9 | Germacrene D | 23.2 | tr |
| 10 | β-Selinene | 23.4 | tr |
| 11 | δ-Cadinene | 24.9 | tr |
| 12 | Spathulenol | 26.9 | 1.95 |
| 13 | α-Bisabolol oxide B | 29.8 | 19.93 |
| 14 | α-Bisabolone oxide A | 30.7 | 4.57 |
| 15 | α-Bisabolol | 30.8 | 6.17 |
| 16 | Chamazulene | 31.7 | 5.54 |
| 17 | α-Bisabolol oxide A | 31.9 | 30.64 |
| 18 | cis-Spiroether | 33.4 | 22.10 |
| 19 | trans-Spiroether | 33.6 | 0.44 |
| Monoterpene hydrocarbons | 0.00 | ||
| Sesquiterpene hydrocarbons | 10.49 | ||
| Oxygenated monoterpenes | 0.34 | ||
| Oxygenated sesquiterpenes | 63.26 | ||
| Other | 22.92 | ||
| Total | 97.01 |
| No. | Component | RT [a] | Relative Percentage (%) | ||||
|---|---|---|---|---|---|---|---|
| PA1 | FV7 | CC1 | AG7 | PC1 | |||
| 1 | α-Pinene | 3.9 | ND | 2.33 | ND | 0.15 | 8.17 |
| 2 | Camphene | 4.2 | ND | Tr | ND | ND | ND |
| 3 | Sabinene | 4.8 | ND | Tr | ND | ND | 0.14 |
| 4 | β-Pinene | 4.8 | ND | 0.15 | ND | ND | 6.02 |
| 5 | Myrcene | 5.2 | ND | 0.55 | tr | 0.16 | tr |
| 6 | α-Phellandrene | 5.5 | ND | 0.73 | ND | 3.54 | tr |
| 7 | p-Cymene | 6.1 | ND | 0.08 | ND | 0.58 | 0.08 |
| 8 | Limonene | 6.2 | ND | 1.81 | 27.63 | 45.24 | ND |
| 9 | β-Phellandrene | 6.2 | ND | ND | ND | ND | 1.87 |
| 10 | γ-Terpinene | 7.1 | ND | 0.79 | ND | ND | 0.11 |
| 11 | cis-Sabinene hydrate | 7.4 | ND | tr | ND | ND | ND |
| 12 | Fenchone | 8.0 | ND | 15.48 | ND | 0.09 | ND |
| 13 | Linalool | 8.5 | 0.75 | ND | ND | ND | ND |
| 14 | Camphor | 9.9 | ND | 0.35 | ND | ND | ND |
| 15 | Terpinen-4-ol | 11.3 | ND | tr | ND | ND | ND |
| 16 | Dill ether | 11.5 | ND | ND | ND | 1.07 | ND |
| 17 | α-Terpineol | 11.9 | ND | ND | 0.11 | ND | ND |
| 18 | Myrtenal | 11.9 | ND | ND | ND | ND | 0.19 |
| 19 | cis-Dihydrocarvone | 12.0 | ND | ND | 0.48 | 0.73 | ND |
| 20 | Estragole | 12.1 | 1.20 | 3.81 | ND | ND | ND |
| 21 | trans-Dihydrocarvone | 12.3 | ND | ND | 0.14 | 1.77 | ND |
| 22 | Isodihydrocarveol | 12.8 | ND | ND | 0.12 | 0.11 | ND |
| 23 | trans-Carveol | 13.0 | ND | ND | tr | 0.08 | ND |
| 24 | Neoisodihydrocarveol | 13.3 | ND | ND | 0.29 | 0.26 | ND |
| 25 | cis-Carveol | 13.5 | ND | ND | ND | 0.12 | ND |
| 26 | Carvone | 13.8 | 0.14 | ND | 70.25 | 45.90 | ND |
| 27 | cis-Anethole | 14.3 | 0.10 | 0.06 | ND | ND | ND |
| 28 | p-Anisaldehyde | 14.5 | tr | ND | ND | ND | ND |
| 29 | trans-Anethole | 15.6 | 96.40 | 73.85 | 0.90 | 0.19 | ND |
| 30 | trans-Caryophyllene | 20.7 | ND | ND | 0.08 | ND | ND |
| 31 | α-Himachalene | 21.8 | 0.13 | ND | ND | ND | ND |
| 32 | trans-β-Farnesene | 22.4 | ND | ND | ND | ND | 0.02 |
| 33 | γ-Himachalene | 23.0 | tr | ND | ND | ND | ND |
| 34 | Germacrene D | 23.2 | ND | tr | ND | tr | ND |
| 35 | Unknown | 23.2 | 1.29 | ND | ND | ND | ND |
| 36 | Dill apiole | 29.3 | ND | ND | ND | tr | ND |
| 37 | Myristicin | 24.9 | ND | ND | ND | ND | 35.81 |
| 38 | Elemicin | 26.4 | ND | ND | ND | ND | 5.53 |
| 39 | Carotol | 27.6 | ND | ND | ND | ND | 0.26 |
| 40 | 6-Methoxyelemicin | 27.9 | ND | ND | ND | ND | 17.44 |
| 41 | Apiol | 30.8 | ND | ND | ND | ND | 24.35 |
| Monoterpene hydrocarbons | 0.00 | 6.45 | 27.63 | 49.68 | 16.39 | ||
| Sesquiterpene hydrocarbons | 0.13 | 0.00 | 0.08 | 0.00 | 0.29 | ||
| Oxygenated monoterpenes | 98.58 | 93.55 | 72.29 | 50.32 | 0.19 | ||
| Oxygenated sesquiterpenes | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Other | 0.00 | 0.00 | 0.00 | 0.00 | 83.14 | ||
| Total | 98.71 | 100.00 | 100.00 | 100.00 | 100.00 | ||
| No. | Component | RT [a] | Relative Percentage (%) | ||
|---|---|---|---|---|---|
| JC2 | JC3 | JC1 | |||
| 1 | α-Thujene | 3.8 | 0.30 | 0.45 | 0.67 |
| 2 | α-Pinene | 3.9 | 33.06 | 44.73 | 29.57 |
| 3 | Sabinene | 4.7 | 13.12 | 11.83 | 19.64 |
| 4 | β-Pinene | 5.2 | ND | ND | 11.57 |
| 5 | Myrcene | 5.4 | 12.28 | 7.50 | 0.39 |
| 6 | α-Terpinene | 5.8 | tr | tr | ND |
| 7 | p-Cymene | 6.1 | 0.30 | 0.30 | 0.21 |
| 8 | Limonene | 6.2 | 4.80 | 4.45 | 4.92 |
| 9 | γ-Terpinene | 7.1 | 0.32 | 0.26 | 0.81 |
| 10 | cis-Sabinene hydrate | 7.4 | tr | tr | tr |
| 11 | Terpinolene | 8.0 | 0.62 | 0.62 | 1.02 |
| 12 | Linalool | 8.4 | tr | tr | tr |
| 13 | Isoamyl isovalerate | 8.5 | tr | ND | ND |
| 14 | Borneol | 10.8 | tr | tr | tr |
| 15 | Terpinen-4-ol | 11.2 | 1.30 | 1.23 | 1.42 |
| 16 | α-Terpineol | 11.9 | 0.28 | tr | ND |
| 17 | Bornyl acetate | 14.2 | 0.33 | 0.24 | tr |
| 18 | 1,5,5-Trimethyl-6-methylene-cyclohexene | 17.4 | 0.17 | 0.13 | 0.43 |
| 19 | α-Cubebene | 17.9 | 0.99 | 0.57 | 0.63 |
| 20 | α-Terpinyl acetate | 18.1 | 0.17 | tr | ND |
| 21 | α-Copaene | 19.0 | 0.74 | 0.36 | 0.22 |
| 22 | β-Elemene | 19.7 | 2.22 | 1.68 | 1.75 |
| 23 | Longifolene | 20.0 | 0.30 | 0.10 | 0.13 |
| 24 | trans-Caryophyllene | 20.7 | 2.69 | 3.01 | 2.58 |
| 25 | β-Gurjunene | 21.1 | 0.40 | 0.21 | 0.27 |
| 26 | γ-Elemene | 21.3 | 3.36 | 5.03 | 3.78 |
| 27 | α-Humulene (α-Caryophyllene) | 22.0 | 2.19 | 2.25 | 2.17 |
| 28 | trans-β-Farnesene | 22.4 | 0.66 | 0.37 | 0.34 |
| 29 | γ-Muurolene | 23.1 | 0.19 | 0.23 | 0.30 |
| 30 | Germacrene D | 23.2 | 8.02 | 4.36 | 7.59 |
| 31 | β-Selinene | 23.4 | 0.33 | 0.46 | 0.25 |
| 32 | Bicyclogermacrene | 23.7 | 0.72 | 0.62 | 1.01 |
| 33 | α-Muurolene | 24.0 | 0.32 | 0.37 | 0.34 |
| 34 | γ-Cadinene | 24.5 | 0.39 | 0.45 | 0.36 |
| 35 | δ-Cadinene | 24.9 | 2.53 | 1.66 | 1.99 |
| 36 | Germacrene D-4-ol | 27.0 | 0.81 | 0.67 | 0.75 |
| 37 | Caryophyllene oxide | 27.1 | 1.30 | 0.73 | 0.70 |
| 38 | τ-Cadinol | 29.5 | 1.42 | 0.97 | 0.69 |
| 39 | α-Cardinol | 30.0 | 1.98 | 1.50 | 1.30 |
| Monoterpene hydrocarbons | 64.96 | 70.27 | 69.24 | ||
| Sesquiterpene hydrocarbons | 26.04 | 21.74 | 23.70 | ||
| Oxygenated monoterpenes | 2.09 | 1.47 | 1.42 | ||
| Oxygenated sesquiterpenes | 5.51 | 3.86 | 3.45 | ||
| Other | 0.00 | 0.00 | 0.00 | ||
| Total | 98.60 | 97.34 | 97.81 | ||
| Family | Plant Name English | Plant Name Latin | Sample Name | DPPH Assay | ABTS Assay | ||||
|---|---|---|---|---|---|---|---|---|---|
| c [mg/mL] | DPPH [µM TE/g] | Std | c [mg/mL] | ABTS [µM TE/g] | Std | ||||
| Apiaceae | Dill | Anethum graveolens | AG7 | 10 | 2.86 | 0.3898 | 10 | 50.89 | 1.6416 |
| Caraway | Carum carvi | CC1 | 15 | 0.46 | 0.4836 | 10 | 27.61 | 0.3570 | |
| Fennel | Foeniculum vulgare | FV7 | 10 | 1.37 | 0.3898 | 10 | 28.40 | 0.6554 | |
| Parsley | Petroselinum crispum | PC1 | 20 | 1.52 | 0.9029 | 10 | 24.95 | 0.3825 | |
| Anise | Pimpinella anisum | PA1 | 20 | 2.26 | 0.3683 | 10 | 40.34 | 0.7803 | |
| Asteraceae | Echinacea | Ehinacea purpurea | EP2 | 10 | 3.97 | 0.8931 | 1 | 343.89 | 1.1899 |
| Chamomile | Matricaria chamomilla | MC1 | 10 | 44.20 | 0.8202 | 1 | 469.23 | 3.6353 | |
| Cupressaceae | Juniper | Juniperus comunis | JC3 | 15 | 3.38 | 0.2455 | 1 | 284.79 | 0.6870 |
| JC1 | 15 | 2.96 | 0.8173 | 1 | 324.06 | 1.8176 | |||
| JC2 | 15 | 4.15 | 0.8729 | 1 | 315.33 | 3.5698 | |||
| Lamiaceae | Peppermint | Mentha x Piperita | MP1 | 15 | 5.71 | 0.5893 | 10 | 57.79 | 2.0135 |
| MP2 | 15 | 8.46 | 0.1299 | 1 | 301.45 | 4.1789 | |||
| MP3 | 20 | 1.96 | 0.1328 | 1 | 296.69 | 2.9946 | |||
| MP4 | 15 | 5.82 | 1.4542 | 10 | 50.77 | 2.9252 | |||
| Basil | Ocimum basilicum | OB7 | 15 | 44.97 | 1.1948 | 1 | 757.98 | 1.1899 | |
| Marjoram | Origanum majorana | OM5 | 15 | 11.35 | 1.5806 | 1 | 477.56 | 4.1789 | |
| Oregano | Origanum vulgare | OV1 | 10 | 2.22 | 1.0700 | 1 | 556.88 | 5.1868 | |
| Sage | Salvia officinalis | SO7 | 10 | 1.03 | 2.2135 | 10 | 40.02 | 0.1818 | |
| Winter savory | Satureja montana | SM3 | 15 | 3.55 | 0.2140 | 1 | 398.23 | 3.6353 | |
| Summer savory | Satureja hortensis | SH4 | 10 | 23.32 | 1.0312 | 1 | 757.58 | 0.6870 | |
| Thyme | Thymus vulgaris | TV6 | 10 | 29.78 | 1.3522 | 1 | 757.19 | 0.6870 | |
| Variable | F1 (35.33%) | F2 (20.62%) | F3 (18.28%) |
|---|---|---|---|
| EO yield | 14.67 | 0.11 | 2.73 |
| DPPH [µM TE/g] | 19.69 | 13.82 | 0.02 |
| ABTS [µM TE/g] | 23.14 | 0.32 | 9.65 |
| Monoterpene hydrocarbons | 0.30 | 46.66 | 2.28 |
| Sesquiterpene hydrocarbons | 12.38 | 7.32 | 2.72 |
| Oxygenated monoterpenes | 12.98 | 24.85 | 14.80 |
| Oxygenated sesquiterpenes | 16.83 | 6.83 | 10.45 |
| Other | 0.00 | 0.09 | 57.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladikostić, N.; Ikonić, B.; Teslić, N.; Zeković, Z.; Božović, D.; Putnik, P.; Bursać Kovačević, D.; Pavlić, B. Essential Oils from Apiaceae, Asteraceae, Cupressaceae and Lamiaceae Families Grown in Serbia: Comparative Chemical Profiling with In Vitro Antioxidant Activity. Plants 2023, 12, 745. https://doi.org/10.3390/plants12040745
Gladikostić N, Ikonić B, Teslić N, Zeković Z, Božović D, Putnik P, Bursać Kovačević D, Pavlić B. Essential Oils from Apiaceae, Asteraceae, Cupressaceae and Lamiaceae Families Grown in Serbia: Comparative Chemical Profiling with In Vitro Antioxidant Activity. Plants. 2023; 12(4):745. https://doi.org/10.3390/plants12040745
Chicago/Turabian StyleGladikostić, Nevena, Bojana Ikonić, Nemanja Teslić, Zoran Zeković, Danica Božović, Predrag Putnik, Danijela Bursać Kovačević, and Branimir Pavlić. 2023. "Essential Oils from Apiaceae, Asteraceae, Cupressaceae and Lamiaceae Families Grown in Serbia: Comparative Chemical Profiling with In Vitro Antioxidant Activity" Plants 12, no. 4: 745. https://doi.org/10.3390/plants12040745
APA StyleGladikostić, N., Ikonić, B., Teslić, N., Zeković, Z., Božović, D., Putnik, P., Bursać Kovačević, D., & Pavlić, B. (2023). Essential Oils from Apiaceae, Asteraceae, Cupressaceae and Lamiaceae Families Grown in Serbia: Comparative Chemical Profiling with In Vitro Antioxidant Activity. Plants, 12(4), 745. https://doi.org/10.3390/plants12040745









