Invasiveness, Monitoring and Control of Hakea sericea: A Systematic Review
Abstract
:1. Introduction
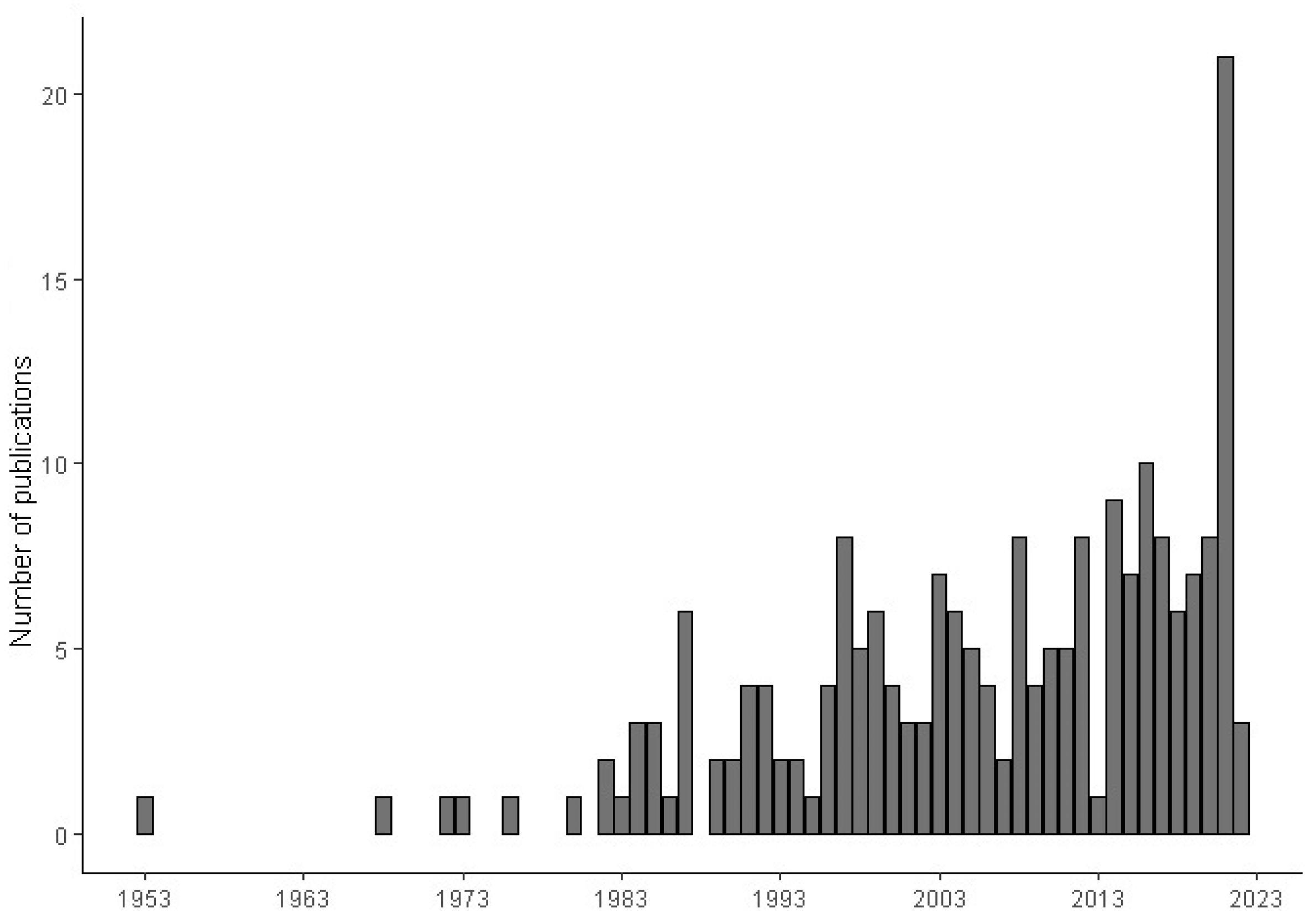
2. Results
3. Discussion
3.1. Ecological and Environmental Factors Contributing to H. sericea Invasiveness
3.1.1. Evolutionary History
3.1.2. Reproductive Strategies, Water Use Efficiency and Drought Resistance
3.1.3. Seeds, Follicles and Serotiny
3.1.4. Seedling Establishment
3.1.5. Proteoid Roots, Phosphorus and Nutrient Use Efficiency
3.1.6. Synthesis
3.2. Fire and H. sericea Invasions
3.2.1. Hakea Post-Fire Responses and Strategies
3.2.2. Seed Bank and Post-Fire Recruitment
3.2.3. Fire Modeling of Hakea Invasions
3.2.4. Synthesis
3.3. Control Methods, Monitoring and Management of H. sericea Invasions
3.3.1. Mechanical Control and Prescribed Fire
3.3.2. Chemical Control
3.3.3. Biological Control
3.3.4. Integrated Control Management
3.3.5. Modeling and Monitoring of H. sericea Invasions
3.3.6. Potential Attenuation of H. sericea Control Costs
3.3.7. Synthesis
4. Materials and Methods
4.1. Question Formulation
- What ecological and environmental factors (biotic and abiotic, morphological and ecophysiological aspects of the Hakea genus and species) contribute to the establishment of H. sericea populations?
- What is the response of H. sericea to fires in native and invaded ecosystems?
- What monitoring and control methods have already been used in H. sericea invasion management?
4.2. Search Strategy
- Hakea;
- Hakea sericea;
- Hakea sericea AND fire;
- Hakea sericea AND invasion;
- Hakea sericea AND fire AND invasion;
- Hakea sericea AND fire AND invasion AND management;
- Hakea sericea AND fire AND invasion AND Portugal;
- Hakea sericea AND ecophysiology;
- Hakea sericea AND control.
5. Final Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Plant List. A working List of All Species, Hakea sericea (Schrad. & J.C.Wendl.). 2022. Available online: http://www.theplantlist.org/tpl1.1/record/kew-2837858 (accessed on 15 January 2022).
- Moll, E.J.; Trinder-Smith, T. Invasion and control of alien woody plants on the Cape peninsula mountains South Africa—30 Years on. Biol. Conserv. 1992, 60, 135–143. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Forsyth, G.G.; Le Maitre, D.C.; Wannenburgh, A.; Kotzé, J.D.F.; Van Den Berg, E.; Henderson, L. An assessment of the effectiveness of a large, national-scale invasive alien plant control strategy in South Africa. Biol. Conserv. 2012, 148, 28–38. [Google Scholar] [CrossRef]
- Moodley, D.; Geerts, S.; Rebelo, T.; Richardson, D.M.; Wilson, J.R.U. Site-specific conditions influence plant naturalization: The case of alien Proteaceae in South Africa. Acta Oecol. 2014, 59, 62–71. [Google Scholar] [CrossRef]
- Morais, M.C.; Cabral, J.A.; Gonçalves, B. Seasonal variation in the leaf physiology of co-occurring invasive (Hakea sericea) and native (Pinus pinaster) woody species in a Mediterranean-Type Ecosystem. For. Ecol. Manage. 2021, 480, 118662. [Google Scholar] [CrossRef]
- EPPO (European and Mediterranean Plant Protection Organization). Pest Risk Analysis for Hakea sericea. 2018. Available online: http://www.iap-risk.eu/media/files/pra_exp_HKASE.pdf (accessed on 10 July 2022).
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species—A global review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Yelenik, S.G.; D’Antonio, C.M. Self-reinforcing impacts of plant invasions change over time. Nature 2013, 503, 517–520. [Google Scholar] [CrossRef] [PubMed]
- EPPO (European and Mediterranean Plant Protection Organization). Hakea sericea . EPPO Bull. 2022. Available online: https://gd.eppo.int/taxon/HKASE (accessed on 10 July 2022).
- Brunel, S.; Schrader, G.; Brundu, G.; Fried, G. Emerging invasive alien plants for the Mediterranean basin. EPPO Bull. 2010, 40, 219–238. [Google Scholar] [CrossRef]
- Tanner, R.; Branquart, E.; Brundu, G.; Buholzer, S.; Chapman, D.; Ehret, P.; Fried, G.; Starfinger, U.; van Valkenburg, J. The prioritisation of a short list of alien plants for risk analysis within the framework of the Regulation (EU) No. 1143/2014. NeoBiota 2017, 35, 87–118. [Google Scholar] [CrossRef]
- Ducatillion, C.; Badeau, V.; Bellanger, R.; Buchlin, S.; Diadema, K.; Gild, A.; Thevenet, J. Early detection of invasion risk by exotic plant species introduced in forest arboretum in south-eastern France. Emergence of species of the genus Hakea. Measures for management. Rev. Ecol. 2015, 70, 139–150. [Google Scholar]
- Decreto-Lei nº 92/2019 de 10 de Julho. Diário da República nº 130/2019—Série I; Ambiente e Transição Energética: Lisbon, Portugal, 2019. [Google Scholar]
- Skeels, A.; Dinnage, R.; Medina, I.; Cardillo, M. Ecological interactions shape the evolution of flower color in communities across a temperate biodiversity hotspot. Evol. Lett. 2021, 5, 277–289. [Google Scholar] [CrossRef]
- Lamont, B.B.; He, T.; Lim, S.L. Hakea, the world’s most Sclerophyllous genus, arose in Southwestern Australian Heathland and diversified throughout Australia over the past 12 million years. Aust. J. Bot. 2016, 64, 77–88. [Google Scholar] [CrossRef]
- Barker, W.R. Novelties and taxonomic notes relating to Hakea Sect. Hakea (Proteaceae), mainly of eastern Australia. J. Adelaide Bot. Gard. 1996, 17, 177–209. [Google Scholar]
- Causley, C.L.; Fowler, W.M.; Lamont, B.B.; He, T. Fitness benefits of serotiny in fire-and drought-prone environments. Plant Ecol. 2016, 217, 773–779. [Google Scholar] [CrossRef]
- Arnaud, A.; Chapman, D.; Le Roux, J.; Linnamagi, M.; Marchante, E.; Pasiecznik, N.; Pescott, O.; Singh, I.; Starfinger, U.; Vicente, J.; et al. Hakea sericea Schrad. & J.C.Wendl. EPPO Bull. 2019, 49, 273–279. [Google Scholar] [CrossRef]
- Bradstock, R.A.; Gill, A.M.; Hastings, S.M.; Moore, P.H.R. Survival of serotinous seedbanks during bushfires: Comparative studies of Hakea species from Southeastern Australia. Aust. J. Ecol. 1994, 19, 276–282. [Google Scholar] [CrossRef]
- Cramer, M.D.; Midgley, J.J. Maintenance costs of serotiny do not explain weak serotiny. Austral Ecol. 2009, 34, 653–662. [Google Scholar] [CrossRef]
- Schmidt, S.; Mason, M.; Sangtiean, T.; Stewart, G.R. Do cluster roots of Hakea actities (Proteaceae) acquire complex organic nitrogen? Plant Soil 2003, 248, 157–165. [Google Scholar] [CrossRef]
- Lamont, B.B. Structure, Ecology and physiology of root clusters—A review. Plant Soil 2003, 248, 1–19. [Google Scholar] [CrossRef]
- Hayes, P.E.; Nge, F.J.; Cramer, M.D.; Finnegan, P.M.; Fu, P.; Hopper, S.D.; Oliveira, R.S.; Turner, B.L.; Zemunik, G.; Zhong, H.; et al. Traits related to efficient acquisition and use of phosphorus promote diversification in Proteaceae in phosphorus-impoverished landscapes. Plant Soil 2021, 462, 67–88. [Google Scholar] [CrossRef]
- Tyler, C.; Pullin, A.S.; Stewart, G.B. Sistematic review effectiveness of management interventions to control invasion by Rhododendron ponticum. Environ. Manag. 2006, 37, 513–522. [Google Scholar] [CrossRef]
- Schindler, S.; Bayliss, H.R.; Essl, F.; Rabitsch, W.; Follak, S.; Pullin, A.S. Effectiveness of management interventions for control of invasive common ragweed Ambrosia artemisiifolia: A systematic review protocol. Environ. Evid. 2016, 5, 11. [Google Scholar] [CrossRef]
- Martin, P.A.; Shackelford, G.E.; Bullock, J.M.; Gallardo, B.; Aldridge, D.C.; Sutherland, W.J. Management of UK priority invasive alien plants: A systematic review protocol. Environ. Evid. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Pullin, A.S.; Knight, T.M. Support for decision-making in conservation practice: An evidence-based approach. J. Nat. Conserv. 2003, 11, 83–90. [Google Scholar] [CrossRef]
- Pullin, A.S.; Knight, T.M.; Stone, D.A.; Charman, K. Do conservation managers use scientific evidence to support their decision-making? Biol. Conserv. 2004, 119, 245–252. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Pullin, A.S.; Dolman, P.M.; Knight, T.M. The need for evidence-based conservation. Trends Ecol. Evol. 2004, 19, 305–308. [Google Scholar] [CrossRef]
- Gordon, A.J. A Review of Established and new insect agents for the biological control of Hakea sericea Schrader (Proteaceae) in South Africa. Afr. Entomol. Mem. 1999, 1, 35–43. [Google Scholar]
- Wood, A.R.; Den Breeÿen, A. Plant pathogens and biological control of invasive alien plants in South Africa: A Review of Projects and Progress (2011–2020). Afr. Entomol. Mem. 2021, 29, 983–1004. [Google Scholar] [CrossRef]
- Morris, M.J.; Wood, A.R.; Den Breeyen, A. Plant pathogens and biological control of weeds in South Africa: A review of projects and progress during the last decade. Afr. Entomol. Mem. 1999, 1, 129–137. [Google Scholar]
- Bell, D.T.; Vlahos, S.; Watson, L.E. Stimulation of seed-germination of understorey species of the Northern Jarrah Forest of Western-Australia. Aust. J. Bot. 1987, 35, 593–599. [Google Scholar] [CrossRef]
- Richardson, D.M.; Van Wilgen, B.W.; Mitchell, D.T. Aspects of the reproductive ecology of four australian Hakea Species (Proteaceae) in South Africa. Oecologia 1987, 71, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Milberg, P.; Lamont, B.B. Seed/cotyledon size and nutrient content play a major role in early performance of species on nutrient-poor soils. New Phytol. 1997, 137, 665–672. [Google Scholar] [CrossRef]
- McLay, T.G.B.; Bayly, M.J.; Ladiges, P.Y. Is South-Western Western Australia a centre of origin for Eastern Australian taxa or is the Centre an artefact of a method of analysis? A comment on Hakea and its supposed divergence over the past 12 million years. Aust. Syst. Bot. 2016, 29, 87–94. [Google Scholar] [CrossRef]
- Groom, P.K.; Lamont, B.B. Ecogeographical analysis of Hakea (Proteaceae) in South-Western Australia, with special reference to leaf morphology and life form. Aust. J. Bot. 1996, 44, 527–542. [Google Scholar] [CrossRef]
- Cardillo, M.; Weston, P.H.; Reynolds, Z.K.M.; Olde, P.M.; Mast, A.R.; Lemmon, E.M.; Lemmon, A.R.; Bromham, L. The phylogeny and biogeography of Hakea (Proteaceae) reveals the role of biome shifts in a continental plant radiation. Evolution 2017, 71, 1928–1943. [Google Scholar] [CrossRef] [PubMed]
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.A.; Rentsch, D.; Robinson, N.; Christie, M.; Webb, R.I.; Gamage, H.K.; Carroll, B.J.; Schenk, P.M.; Schmidt, S. Plants can use protein as a nitrogen source without assistance from other organisms. Proc. Natl. Acad. Sci. USA 2008, 105, 4524–4529. [Google Scholar] [CrossRef] [PubMed]
- Groom, P.K.; Lamont, B.B.; Markey, A.S. Influence of leaf type and plant age on leaf structure and sclerophylly in Hakea (Proteaceae). Aust. J. Bot. 1997, 45, 827–838. [Google Scholar] [CrossRef]
- Richards, M.B.; Groom, P.K.; Lamont, B.B. A Trade-off between Fecundity and drought susceptibility in adults and seedlings of Hakea Species as influenced by leaf morphology. Aust. J. Bot. 1997, 45, 301–309. [Google Scholar] [CrossRef]
- Schmidt, S.; Stewart, G.R. Waterlogging and fire impacts on nitrogen availability and utilization in a subtropical wet heathland (wallum). Plant Cell Environ. 1997, 20, 1231–1241. [Google Scholar] [CrossRef]
- Collins, L.; Boer, M.M.; de Dios, V.R.; Power, S.A.; Bendall, E.R.; Hasegawa, S.; Hueso, R.O.; Nevado, J.P.; Bradstock, R.A. Effects of competition and herbivory over woody seedling growth in a temperate woodland trump the effects of elevated CO2. Oecologia 2018, 187, 811–823. [Google Scholar] [CrossRef]
- Schütte, K.H. Hakea eradication by means of new herbicides. J. South Afr. For. Assoc. 1953, 23, 30–36. [Google Scholar] [CrossRef]
- Fugler, S.R. Infestations of three Australian Hakea species in South Africa and their control. S. Afr. For. J. 1982, 120, 63–68. [Google Scholar] [CrossRef]
- Dennill, G.B.; Gordon, A.J.; Neser, S. Difficulties with the release and establishment of Carposina autologa Meyrick (Carposinidae) on the weed Hakea sericea (Proteaceae) in South Africa. J. Entomol. Soc. South. Afr. 1987, 50, 463–468. [Google Scholar]
- Kluge, R.L.; Marshall, C.R.; Siebert, M.W. Tebuthiuron as a selective herbicide for the control of Hakea gibbosa (Proteaceae) in Mountain Fynbos vegetation. S. Afr. For. J. 1987, 140, 35–38. [Google Scholar] [CrossRef]
- Gordon, A.J. The impact of the Hakea seed-moth Carposina autologa (Carposinidae) on the canopy-stored seeds of the weed Hakea sericea (Proteaceae). Agric. Ecosyst. Environ. 1993, 45, 105–113. [Google Scholar] [CrossRef]
- Holmes, P.M.; Marais, C. Impacts of alien plant clearance on vegetation in the mountain catchments of the Western Cape. S. Afr. For. J. 2000, 189, 113–117. [Google Scholar] [CrossRef]
- Esler, K.J.; van Wilgen, B.W.; te Roller, K.S.; Wood, A.R.; van der Merwe, J.H. A Landscape-scale assessment of the long-term integrated control of an invasive shrub in South Africa. Biol. Invasions 2010, 12, 211–218. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Thuiller, W.; Schonegevel, L. Developing an approach to defining the potential distributions of invasive plant species: A case study of Hakea Species in South Africa. Glob. Ecol. Biogeogr. 2008, 17, 569–584. [Google Scholar] [CrossRef]
- Forsyth, G.G.; Le Maitre, D.C.; O’Farrell, P.J.; van Wilgen, B.W. The prioritisation of invasive alien plant control projects using a multi-criteria decision model informed by stakeholder input and spatial data. J. Environ. Manag. 2012, 103, 51–57. [Google Scholar] [CrossRef]
- Moran, V.C.; Hoffmann, J.H. Conservation of the Fynbos Biome in the Cape Floral Region: The role of biological control in the management of invasive alien trees. BioControl 2012, 57, 139–149. [Google Scholar] [CrossRef]
- Williams, P.A. Hakea salicifolia: Biology and role in succession in Abel Tasman National Park, New Zealand. J. R. Soc. New Zeal. 1992, 22, 1–18. [Google Scholar] [CrossRef]
- Ayala, F.J.; Fitch, W.M. Genetics and the origin of species: An introduction. Proc. Natl. Acad. Sci. USA 1997, 94, 7691–7697. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Anderson, J.T.; Inouye, D.W.; McKinney, A.M.; Colautti, R.I.; Mitchell-Olds, T. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc. R. Soc. B Biol. Sci. 2012, 279, 3843–3852. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; Van Der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Lsuia, J.; et al. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Skeels, A.; Cardillo, M. Equilibrium and non-equilibrium phases in the radiation of Hakea and the drivers of diversity in Mediterranean-type ecosystems. Evolution 2019, 73, 1392–1410. [Google Scholar] [CrossRef] [PubMed]
- Dettmann, M.E.; Clifford, H.T. Fossil fruit of the Grevilleeae (Proteaceae) in the Tertiary of eastern Australia. Mem. Qld. Mus. 2005, 51, 359–374. [Google Scholar]
- Jordan, G.J.; Carpenter, R.J.; Brodribb, T.J. Using fossil leaves as evidence for open vegetation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 395, 168–175. [Google Scholar] [CrossRef]
- Van Rensburg, J.; Van Wilgen, B.W.; Richardson, D.M. Reconstructing the spread of invasive alien plants on privately-owned land in the Cape floristic region: Vergelegen Wine Estate as a Case Study. S. Afr. Geogr. J. 2018, 100, 180–195. [Google Scholar] [CrossRef]
- Ding, J.; Travers, S.K.; Eldridge, D.J. Occurrence of Australian woody species is driven by soil moisture and available phosphorus across a climatic gradient. J. Veg. Sci. 2021, 36, e13095. [Google Scholar] [CrossRef]
- Poot, P.; Lambers, H. Are Trade-Offs in allocation pattern and root morphology related to species abundance? A congeneric comparison between rare and common species in the south-western Australian flora. J. Ecol. 2003, 91, 58–67. [Google Scholar] [CrossRef]
- Sampson, J.F.; Byrne, M.; Gibson, N.; Yates, C. Limiting inbreeding in disjunct and isolated populations of a woody shrub. Ecol. Evol. 2016, 6, 5867–5880. [Google Scholar] [CrossRef] [PubMed]
- Moodley, D.; Geerts, S.; Richardson, D.M.; Wilson, J.R.U. The importance of pollinators and autonomous self-fertilisation in the early stages of plant invasions: Banksia and Hakea (Proteaceae) as case studies. Plant Biol. 2016, 18, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Marchante, E. Information on Measures and Related Costs in Relation to Species Considered for Inclusion on the Union List: Hakea sericea. Technical Note Prepared by IUCN for the European Commission. 2018. Available online: https://circabc.europa.eu/sd/a/7ebe09d6-a752-4b09-9b60-a394d27f7981/TSSR%20Task%202018%20Hakea%20sericea.pdf (accessed on 15 January 2022).
- Groom, P.; Lamont, B. Leaf morphology and life form influence water relations of Hakea species on different soil substrates within southwestern Australia. Acta Oecol. 1995, 16, 609–620. [Google Scholar]
- Oyanoghafo, O.O.; O’ Brien, C.; Choat, B.; Tissue, D.; Rymer, P.D. Vulnerability to xylem cavitation of Hakea species (Proteaceae) from a range of biomes and life histories predicted by climatic niche. Ann. Bot. 2021, 127, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Whitworth-Hulse, J.I.; Magliano, P.N.; Zeballos, S.R.; Aguiar, S.; Baldi, G. Global patterns of rainfall partitioning by invasive woody plants. Glob. Ecol. Biogeogr. 2021, 30, 235–246. [Google Scholar] [CrossRef]
- Santini, N.S.; Cleverly, J.; Faux, R.; Lestrange, C.; Rumman, R.; Eamus, D. Xylem traits and water-use efficiency of woody species co-occurring in the Ti Tree Basin Arid Zone. Trees Struct. Funct. 2016, 30, 295–303. [Google Scholar] [CrossRef]
- Bell, D.T.; Van Der Moezel, P.G.; Delfs, J.C.; Loneragan, W.A. Northern sandplain Kwongan: Effect of fire on Hakea obliqua and Beaufortia elegans population structure. J. R. Soc. West. Aust. 1987, 69, 139–143. [Google Scholar]
- Bell, D.T.; Williams, D.S. Tolerance of thermal shock in seeds. Aust. J. Bot. 1998, 46, 221–233. [Google Scholar] [CrossRef]
- El-Amhir, S.H.M.; Lim, S.L.; Lamont, B.B.; He, T. Seed size, fecundity and postfire regeneration strategy are interdependent in Hakea. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef]
- Groom, P.K.; Lamont, B.B. Fruit-Seed relations in Hakea: Serotinous species invest more dry matter in predispersal seed protection. Austral Ecol. 1997, 22, 352–355. [Google Scholar] [CrossRef]
- Midgley, J.J.; Cowling, R.M.; Lamont, B.B. Relationship of follicle size and seed size in Hakea (Proteaceae); isometry, allometry and adaptation. S. Afr. J. Bot. 1991, 57, 107–110. [Google Scholar] [CrossRef]
- Lamont, B.B.; Milberg, P. Removal of the testa during germination or establishment increases germinant mortality, decay and water loss. Seed Sci. Res. 1997, 7, 245–252. [Google Scholar] [CrossRef]
- El-Amhir, S.H.; Lamont, B.B.; He, T.; Yan, G. Small-seeded Hakea species tolerate cotyledon loss better than large-seeded congeners. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Stock, W.D.; Pate, J.S.; Delfs, J. Influence of seed size and quality on seedling development under low nutrient conditions in five australian and south african members of the Proteaceae. J. Ecol. 1990, 78, 1005. [Google Scholar] [CrossRef]
- Kuo, J.; Hocking, P.J.; Pate, J.S. Nutrient reserves in seeds of selected proteaceous species from south-western Australia. Aust. J. Bot. 1982, 30, 231–249. [Google Scholar] [CrossRef]
- Lamont, B.B.; Groom, P.K. Seed and seedling biology of the woody-fruited Proteaceae. Aust. J. Bot. 1998, 46, 387–406. [Google Scholar] [CrossRef]
- Mitchell, D.T.; Allsopp, N. Changes in the phosphorus composition of seeds of Hakea sericea (Proteaceae) during germination under low phosphorus conditions. New Phytol. 1984, 96, 239–247. [Google Scholar] [CrossRef]
- Groom, P.K.; Lamont, B.B. Phosphorus accumulation in Proteaceae seeds: A synthesis. Plant Soil 2010, 334, 61–72. [Google Scholar] [CrossRef]
- Mason, R.A.B.; Cooke, J.; Moles, A.T.; Leishman, M.R. Reproductive output of invasive versus native plants. Glob. Ecol. Biogeogr. 2008, 17, 633–640. [Google Scholar] [CrossRef]
- Hammill, K.A.; Bradstock, R.A.; Allaway, W.G. Post-fire seed dispersal and species re-establishment in proteaceous heath. Aust. J. Bot. 1998, 46, 407–419. [Google Scholar] [CrossRef]
- Groom, P.K. Implications of terminal velocity and wing loading on Hakea (Proteaceae) seed dispersal. J. R. Soc. West. Aust 2010, 93, 175. [Google Scholar]
- Williams, P.A. Hakea sericea: Seed production and role in succession in Golden Bay, Nelson. J. R. Soc. New Zeal. 1992, 22, 307–320. [Google Scholar] [CrossRef]
- Morrison, D.A.; Renwick, J.A. Effects of variation in fire intensity on regeneration of co-occurring species of small trees in the Sydney Region. Aust. J. Bot. 2000, 48, 71–79. [Google Scholar] [CrossRef]
- Lamont, B.B.; Pausas, J.G.; He, T.; Witkowski, E.T.F.; Hanley, M.E. Fire as a selective agent for both serotiny and nonserotiny over space and time. CRC. Crit. Rev. Plant Sci. 2020, 39, 140–172. [Google Scholar] [CrossRef]
- Fischer, M.; Beismann, H. 3D Characterization of the complex vascular bundle system of Hakea fruits based on X-Ray Microtomography (ΜCT) for a better understanding of the opening mechanism. Flora Morphol. Distrib. Funct. Ecol. Plants 2022, 289, 1–9. [Google Scholar] [CrossRef]
- Lambers, H.; Cawthray, G.R.; Giavalisco, P.; Kuo, J.; Laliberté, E.; Pearse, S.J.; Scheible, W.R.; Stitt, M.; Teste, F.; Turner, B.L. Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytol. 2012, 196, 1098–1108. [Google Scholar] [CrossRef]
- Guilherme Pereira, C.; Hayes, P.E.; O’Sullivan, O.S.; Weerasinghe, L.K.; Clode, P.L.; Atkin, O.K.; Lambers, H. Trait convergence in photosynthetic nutrient-use efficiency along a 2-million year dune chronosequence in a global biodiversity hotspot. J. Ecol. 2019, 107, 2006–2023. [Google Scholar] [CrossRef]
- Lamont, B.B.; Groom, P.K. Green cotyledons of two Hakea Species control seedling mass and morphology by supplying mineral nutrients rather than organic compounds. New Phytol. 2002, 153, 101–110. [Google Scholar] [CrossRef]
- Standish, R.J.; Stokes, B.A.; Tibbett, M.; Hobbs, R.J. Seedling response to phosphate addition and inoculation with arbuscular mycorrhizas and the implications for old-field restoration in Western Australia. Environ. Exp. Bot. 2007, 61, 58–65. [Google Scholar] [CrossRef]
- Rafferty, C.; Lamont, B.B.; Hanley, M.E. Selective feeding by Kangaroos (Macropus fuliginosus) on seedlings of Hakea Species: Effects of chemical and physical defences. Plant Ecol. 2005, 177, 201–208. [Google Scholar] [CrossRef]
- Rafferty, C.M.; Lamont, B.B.; Hanley, M.E. Herbivore feeding preferences in captive and wild populations. Austral Ecol. 2010, 35, 257–263. [Google Scholar] [CrossRef]
- Poot, P.; Lambers, H. Growth responses to waterlogging and drainage of woody Hakea (Proteaceae) seedlings, originating from contrasting habitats in South-Western Australia. Plant Soil 2003, 253, 57–70. [Google Scholar] [CrossRef]
- Watt, M.; Evans, J. Proteoid roots. physiology and development. Plant Physiol. 1999, 121, 317–324. [Google Scholar] [CrossRef]
- Lamont, B. The effects of seasonality and waterlogging on the root systems of a number of Hakea Species. Aust. J. Bot. 1976, 24, 691–702. [Google Scholar] [CrossRef]
- Sousa, M.F.; Façanha, A.R.; Tavares, R.M.; Lino-Neto, T.; Gerós, H. Phosphate transport by proteoid roots of Hakea sericea. Plant Sci. 2007, 173, 550–558. [Google Scholar] [CrossRef]
- Shane, M.W.; Lambers, H. Manganese accumulation in leaves of Hakea prostrata (Proteaceae) and the significance of cluster roots for micronutrient uptake as dependent on phosphorus supply. Physiol. Plant. 2005, 124, 441–450. [Google Scholar] [CrossRef]
- Lambers, H.; Wright, I.J.; Guilherme Pereira, C.; Bellingham, P.J.; Bentley, L.P.; Boonman, A.; Cernusak, L.A.; Foulds, W.; Gleason, S.M.; Gray, E.F.; et al. Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant Soil 2021, 461, 43–61. [Google Scholar] [CrossRef]
- Delgado, M.; Suriyagoda, L.; Zúñiga-Feest, A.; Borie, F.; Lambers, H. Divergent functioning of Proteaceae species: The South American Embothrium coccineum displays a combination of adaptive traits to survive in high-phosphorus soils. Funct. Ecol. 2014, 28, 1356–1366. [Google Scholar] [CrossRef]
- Dell, B.; Kuo, J.; Thomson, G.J. Development of proteoid roots in Hakea obliqua R. Br.(Proteaceae) grown in water culture. Aust. J. Bot. 1980, 28, 27–37. [Google Scholar] [CrossRef]
- Jeffrey, D.W. Phosphate nutrition of Australian heath plants. II. The formation of polyphosphate by five heath species. Aust. J. Bot. 1968, 16, 603–613. [Google Scholar] [CrossRef]
- Lamont, B. Factors affecting the distribution of proteoid roots within the root systems of two Hakea species. Aust. J. Bot. 1973, 21, 165–187. [Google Scholar] [CrossRef]
- Lamont, B. The Effect of soil nutrients on the production of proteoid roots by Hakea Species. Aust. J. Bot. 1972, 20, 27–40. [Google Scholar] [CrossRef]
- Standish, R.J.; Albornoz, F.E.; Morald, T.K.; Hobbs, R.J.; Tibbett, M. Mycorrhizal symbiosis and phosphorus supply determine interactions among plants with contrasting nutrient-acquisition strategies. J. Ecol. 2021, 109, 3892–3902. [Google Scholar] [CrossRef]
- Hoang, S.A.; Lamb, D.; Sarkar, B.; Seshadri, B.; Kit Yu, R.M.; Anh Tran, T.K.; O’Connor, J.; Rinklebe, J.; Kirkham, M.B.; Vo, H.T.; et al. Phosphorus application enhances alkane hydroxylase gene abundance in the rhizosphere of wild plants grown in petroleum-hydrocarbon-contaminated Soil. Environ. Res. 2022, 204, 1–10. [Google Scholar] [CrossRef]
- Shane, M.W.; McCully, M.E.; Lambers, H. Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae). J. Exp. Bot. 2004, 55, 1033–1044. [Google Scholar] [CrossRef]
- Shane, M.W.; De Vos, M.; De Roock, S.; Cawthray, G.R.; Lambers, H. Effects of external phosphorus supply on internal phosphorus concentration and the initiation, growth and exudation of cluster roots in Hakea prostrata R.Br. Plant Soil 2003, 248, 209–219. [Google Scholar] [CrossRef]
- Guilherme Pereira, C.; Hayes, P.E.; Clode, P.L.; Lambers, H. Phosphorus Toxicity, not deficiency, explains the calcifuge habit of phosphorus-efficient Proteaceae. Physiol. Plant. 2021, 172, 1724–1738. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Stock, W.D.; Verboom, G.A. Phylogenetic ecology of foliar N and P concentrations and N:P Ratios across Mediterranean-Type ecosystems. Glob. Ecol. Biogeogr. 2012, 21, 1147–1156. [Google Scholar] [CrossRef]
- Prodhan, M.A.; Jost, R.; Watanabe, M.; Hoefgen, R.; Lambers, H.; Finnegan, P.M. Tight Control of nitrate acquisition in a plant species that evolved in an extremely phosphorus-impoverished environment. Plant Cell Environ. 2016, 39, 2754–2761. [Google Scholar] [CrossRef] [PubMed]
- Prodhan, M.A.; Jost, R.; Watanabe, M.; Hoefgen, R.; Lambers, H.; Finnegan, P.M. Tight control of sulfur assimilation: An adaptive mechanism for a plant from a severely phosphorus-impoverished habitat. New Phytol. 2017, 215, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Sulpice, R.; Ishihara, H.; Schlereth, A.; Cawthray, G.R.; Encke, B.; Giavalisco, P.; Ivakov, A.; Arrivault, S.; Jost, R.; Krohn, N.; et al. Low levels of ribosomal RNA partly account for the very high photosynthetic phosphorus-use efficiency of Proteaceae species. Plant Cell Environ. 2014, 37, 1276–1298. [Google Scholar] [CrossRef] [PubMed]
- Kotula, L.; Clode, P.L.; Ranathunge, K.; Lambers, H. Role of roots in adaptation of soil-indifferent Proteaceae to calcareous soils in south-western Australia. J. Exp. Bot. 2021, 72, 1490–1505. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, X.; Han, Z.; Pang, J.; Lambers, H.; Finnegan, P.M. Responses of foliar phosphorus fractions to soil age are diverse along a 2 Myr dune chronosequence. New Phytol. 2019, 223, 1621–1633. [Google Scholar] [CrossRef]
- Lamont, B.B.; He, T.; Yan, Z. Evolutionary history of fire-stimulated resprouting, flowering, seed release and germination. Biol. Rev. 2019, 94, 903–928. [Google Scholar] [CrossRef]
- Miller, R.G.; Tangney, R.; Enright, N.J.; Fontaine, J.B.; Merritt, D.J.; Ooi, M.K.J.; Ruthrof, K.X.; Miller, B.P. Mechanisms of fire seasonality effects on plant populations. Trends Ecol. Evol. 2019, 34, 1104–1117. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’Antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. Bioscience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Krawchuk, M.A.; Moritz, M.A.; Parisien, M.A.; Van Dorn, J.; Hayhoe, K. Global pyrogeography: The current and future distribution of wildfire. PLoS ONE 2009, 4, 1–12. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Balch, J.K.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; Defries, R.S.; Doyle, J.C.; Harrison, S.P.; Johnston, F.H.; Keeley, J.E.; et al. Fire in the earth system. Science 2009, 324, 481–484. [Google Scholar] [CrossRef]
- Emery, S.M.; Uwimbabazi, J.; Flory, S.L. Fire intensity effects on seed germination of native and invasive eastern deciduous forest understory plants. For. Ecol. Manag. 2011, 261, 1401–1408. [Google Scholar] [CrossRef]
- Silva, J.S.; Deus, E.; Nereu, M.; Davim, D.; Rossa, C. Aliens & Flames: A new research initiative joining fire behaviour and invasion ecology. In Advances in Forest Fire Research; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2018; Volume 1, pp. 1219–1222. [Google Scholar] [CrossRef]
- Silva, J.S.; Nereu, M.; Pinho, S.; Queirós, L.; Jesús, C.; Deus, E. Post-fire demography, growth, and control of Eucalyptus globulus wildlings. Forests 2021, 12, 156. [Google Scholar] [CrossRef]
- Morais, M.C.; Gonçalves, B.; Cabral, J.A. A Dynamic modeling framework to evaluate the efficacy of control actions for a woody invasive plant, Hakea sericea. Front. Ecol. Evol. 2021, 9, 1–9. [Google Scholar] [CrossRef]
- Silva, J.S.; Nereu, M.; Queirós, L.; Deus, E.; Fernandes, P. Fire hazard and plant invasions—The cases of Hakea sericea and Acacia dealbata in Portugal. In Proceedings of the 15th Conference on Ecology and Management of Alien Plant Invasions, Prague, Czech Republic, 9–13 September 2019. [Google Scholar]
- Bell, D.T. Ecological Response Syndromes in the flora of southwestern Western Australia: Fire resprouters versus reseeders. Bot. Rev. 2001, 67, 417–440. [Google Scholar] [CrossRef]
- Pickard, J.O.H.N.; Jacobs, S.W.L. Vegetation Patterns on the Sassafras Plateau; Australian and New Zealand Geomorphology Group: Wollongong, Australia, 1983; pp. 54–65. [Google Scholar]
- Pepo, C.; Monteiro, A.; Forte, P.; Teixeira, G. Biologia da germinação das invasoras Hakea salicifolia e H. sericeae. In Proceedings of the XII Congreso da SEMh/XIX Congreso da ALAM/II Congreso da IBCM, Lisbon, Portugal, 10 November 2009. [Google Scholar]
- Brown, C.L.; Whelan, R.J. Seasonal occurrence of fire and availability of germinable seeds in Hakea sericea and Petrophile sessilis. J. Ecol. 1999, 87, 932–941. [Google Scholar] [CrossRef]
- Tonnabel, J.; Van Dooren, T.J.M.; Midgley, J.; Haccou, P.; Mignot, A.; Ronce, O.; Olivieri, I. Optimal resource allocation in a serotinous non-resprouting plant species under different fire regimes. J. Ecol. 2012, 100, 1464–1474. [Google Scholar] [CrossRef]
- Kirkpatrick, J.; Gilfedder, L.; Duncan, F.; Wapstra, M. Frequent planned fire can prevent succession to woody plant dominance in montane temperate grasslands. Austral Ecol. 2020, 45, 872–879. [Google Scholar] [CrossRef]
- Perry, G.L.W.; Wilmshurst, J.M.; McGlone, M.S. Ecology and Long-term history of fire in New Zealand. N. Z. J. Ecol. 2014, 38, 157–176. [Google Scholar]
- Clarkson, B.R.; Smale, M.C.; Williams, P.A.; Wiser, S.K.; Buxton, R.P. Drainage, soil fertility and fire frequency determine composition and structure of gumland heaths in northern New Zealand. N. Z. J. Ecol. 2011, 35, 96–113. [Google Scholar]
- Knox, K.J.E.; Clarke, P.J. Response of resprouting shrubs to repeated fires in the dry sclerophyll forest of Gibraltar Range National Park. Proc. Linn. Soc. New South Wales 2006, 127, 49–56. [Google Scholar]
- Whelan, R.J.; York, J. Post-fire germination of Hakea sericea and Petrophile sessilis after spring burning. Aust. J. Bot. 1998, 46, 367–376. [Google Scholar] [CrossRef]
- Wyse, S.V.; Perry, G.L.W.; Curran, T.J. Shoot-level flammability of species mixtures is driven by the most flammable species: Implications for vegetation-fire feedbacks favouring invasive species. Ecosystems 2018, 21, 886–900. [Google Scholar] [CrossRef]
- Penman, T.D.; Penman, S.H. Influence of prescribed burning on fruit production in Proteaceae. Pac. Conserv. Biol. 2010, 16, 46–53. [Google Scholar] [CrossRef]
- Knox, K.J.E.; Clarke, P.J. Fire season and intensity affect shrub recruitment in temperate sclerophyllous woodlands. Oecologia 2006, 149, 730–739. [Google Scholar] [CrossRef]
- Van Wilgen, B.; Richardson, D.M. The effects of alien shrub invasions on vegetation structure and fire behaviour in South African Fynbos Shrublands: A simulation study. J. Appl. Ecol. 1985, 22, 955–966. [Google Scholar] [CrossRef]
- Turco, M.; Jerez, S.; Augusto, S.; Tarín-Carrasco, P.; Ratola, N.; Jiménez-Guerrero, P.; Trigo, R.M. Climate drivers of the 2017 devastating fires in Portugal. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Van Wilgen, B.W.; Gelderblom, C.M.; Bailey, C.; Chapman, R.A.; Nel, J.A. Invasive alien trees and water resources in South Africa: Case studies of the costs and benefits of management. For. Ecol. Manag. 2002, 160, 143–159. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Fill, J.M.; Baard, J.; Cheney, C.; Forsyth, A.T.; Kraaij, T. Historical costs and projected future scenarios for the management of invasive alien plants in protected areas in the Cape floristic region. Biol. Conserv. 2016, 200, 168–177. [Google Scholar] [CrossRef]
- Wells, M.J. Introduced plants of the fynbos biome. In Biogeography of Mediterranean Invasions; Cambridge University Press: Cambridge, United Kingdom, 1991; pp. 115–129. [Google Scholar]
- Gordon, A.J.; Fourie, A. Biological control of Hakea sericea Schrad. & JC Wendl. and Hakea gibbosa (Sm.) Cav.(Proteaceae) in South Africa. Afr. Entomol. 2011, 19, 303–314. [Google Scholar] [CrossRef]
- Teixeira, G.; Monteiro, A.; Pepo, C. Leaf morphoanatomy in Hakea sericeae and H. salicifolia. Microsc. Microanal. 2008, 14, 109–110. [Google Scholar] [CrossRef]
- Martins, J.; Richardson, D.M.; Henriques, R.; Marchante, E.; Marchante, H.; Alves, P.; Gaertner, M.; Honrado, J.P.; Vicente, J.R. A multi-scale modelling framework to guide management of plant invasions in a transboundary context. For. Ecosyst. 2016, 3, 1–14. [Google Scholar] [CrossRef]
- Alvarez-Taboada, F.; Paredes, C.; Julián-Pelaz, J. Mapping of the invasive species Hakea sericea using Unmanned Aerial Vehicle (UAV) and Worldview-2 Imagery and an object-oriented approach. Remote Sens. 2017, 9, 913. [Google Scholar] [CrossRef]
- Van Der Weide, R.Y.; Bleeker, P.O.; Achten, V.T.J.M.; Lotz, L.A.P.; Fogelberg, F.; Melander, B. Innovation in mechanical weed control in crop rows. Weed Res. 2008, 48, 215–224. [Google Scholar] [CrossRef]
- Richardson, D.M.; Van Wilgen, B.W. Factors affecting the regeneration success of Hakea sericea. S. Afr. For. J. 1984, 131, 63–68. [Google Scholar] [CrossRef]
- Breytenbach, G.J. Alien control: Can we afford to slash and burn Hakea in Fynbos Ecosystems? S. Afr. For. J. 1989, 151, 6–16. [Google Scholar] [CrossRef]
- Richardson, D.M.; Van Wilgen, B.W. The Effects of fire in felled Hakea sericea and Natural Fynbos implications for weed control in Mountain Catchments. S. Afr. For. J. 1986, 139, 4–14. [Google Scholar] [CrossRef]
- Serralheiro, D.A. Impacte da Hakea sericea no Ecossistema da ZIF do Marão: Situação Atual e Cenários Evolutivos. Master’s Thesis, University of Porto, Porto, Portugal, 2018. [Google Scholar]
- Bosch, A. The Influence of Prescribed Fire Treatments on the Abundance of Hakea sericea in Fire-Prone Areas in Northern Portugal. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2020. [Google Scholar]
- Shaner, D.L.; Beckie, H.J. The Future for Weed Control and Technology. Pest Manag. Sci. 2014, 70, 1329–1339. [Google Scholar] [CrossRef]
- Davis, A.S.; Frisvold, G.B. Are herbicides a once in a century method of weed control? Pest Manag. Sci. 2017, 73, 2209–2220. [Google Scholar] [CrossRef]
- Kluge, R.L.; Neser, S. Biological control of Hakea sericea (Proteaceae) in South Africa. Agric. Ecosyst. Environ. 1991, 37, 91–113. [Google Scholar] [CrossRef]
- Adair, R.J. Biological control of Australian native plants, in Australia, with an emphasis on Acacias. Muelleria 2008, 26, 67–78. [Google Scholar] [CrossRef]
- Gordon, A.J.; Lyons, C.L. Current status of Carposina autologa (Lepidoptera: Carposinidae), a biological control agent of silky Hakea, Hakea sericea (Proteaceae) and rock Hakea, Hakea gibbosa (Proteaceae) in the Western Cape, South Africa. Afr. Entomol. 2017, 25, 250–253. [Google Scholar] [CrossRef]
- Kluge, R.L.; Siebert, M.W. Erytenna Consputa pascoe (Coleoptera: Curculionidae) as the main mortality factor of developing fruits of the weed, Hakea sericea Schrader, in South Africa. J. Entomol. Soc. South. Afr. 1985, 48, 241–245. [Google Scholar]
- Kluge, R.L.; Gordon, A.J. The Fixed plot survey method for determining the host range of the flowerbud-feeding weevil dicomada rufa, a candidate for the biological control of Hakea sericea in South Africa. BioControl 2004, 49, 341–355. [Google Scholar] [CrossRef]
- Gordon, A.J. Biology and host range of the stem-boring beetle Aphanasium australe, a promising agent for the biological control of Hakea sericea in South Africa. BioControl 2003, 48, 113–122. [Google Scholar] [CrossRef]
- Lyons, C.L.; Tshibalanganda, M.; Plessis, A. Du using CT-Scanning technology to quantify damage of the stem-boring beetle, Aphanasium australe, a biocontrol agent of Hakea sericea in South Africa. Biocontrol Sci. Technol. 2020, 30, 33–41. [Google Scholar] [CrossRef]
- Lyons, C.L.; English, K.F.; Hoffmann, J.H. Research on the biological control of Hakea sericea over the past ten years: Lessons informing future management of the species in the Western Cape province, South Africa. Afr. Entomol. 2021, 29, 768–774. [Google Scholar] [CrossRef]
- Smith, L.; Gordon, A.J. A need for an additional biological control agent on Hakea sericea Schrad. & J.C. Wendl. (Proteaceae) in South Africa. Afr. Entomol. 2009, 17, 200–206. [Google Scholar] [CrossRef]
- Wood, A.R.; Breeyen, A. Incidence of gummosis disease in silky Hakea under natural conditions in South Africa. S. Afr. J. Plant Soil 2021, 38, 126–133. [Google Scholar] [CrossRef]
- Lubbe, C.M.; Denman, S.; Cannon, P.F.; Groenewald, J.Z.; Lamprecht, S.C.; Crous, P.W. Characterization of Colletotrichum species associated with diseases of Proteaceae. Mycologia 2004, 96, 1268–1279. [Google Scholar] [CrossRef]
- Lubbe, C.M.; Denman, S.; Lamprecht, S.C.; Crous, P.W. Pathogenicity of Colletotrichum species to Protea cultivars. Australas. Plant Pathol. 2006, 35, 37–41. [Google Scholar] [CrossRef]
- Richardson, D.M.; Manders, P.T. Predicting pathogen-induced mortality in Hakea sericea (Proteaceae), an aggressive alien plant invader in South Africa. Ann. Appl. Biol. 1985, 106, 243–254. [Google Scholar] [CrossRef]
- Morris, M.J. A method for controlling Hakea sericea Schrad. seedlings using the fungus Colletotrichum gloeosporioides (Penz.) Sacc. Weed Res. 1989, 29, 449–454. [Google Scholar] [CrossRef]
- Pearce, C.A.; Reddell, P.; Hyde, K.D. Revision of the Phyllachoraceae (Ascomycota) on hosts in the angiosperm family, Proteaceae. Aust. Syst. Bot. 2001, 14, 283–328. [Google Scholar] [CrossRef]
- Morris, M.J. The use of plant pathogens for biological weed control in South Africa. Agric. Ecosyst. Environ. 1991, 37, 239–255. [Google Scholar] [CrossRef]
- Sousa, M.F.; Tavares, R.M.; Gerós, H.; Lino-Neto, T. First report of Hakea sericea leaf infection caused by Pestalotiopsis funerea in Portugal. Plant Pathol. 2004, 53, 535. [Google Scholar] [CrossRef]
- Johnston, P.R. Potential of fungi for the biological control of some New Zealand Weeds. New Zeal. J. Agric. Res. 1990, 33, 1–14. [Google Scholar] [CrossRef]
- Rathé, A.A.; Pilkington, L.J.; Gurr, G.M.; Daugherty, M.P. Potential for persistence and within-plant movement of Xylella fastidiosa in Australian native plants. Australas. Plant Pathol. 2012, 41, 405–412. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Krug, R.M.; Hoffmann, J.H.; Gordon, A.J.; Mgidi, T.N. Hakea sericea: Development of a model of the impacts of biological control on population dynamics and rates of spread of an invasive species. Ecol. Modell. 2008, 212, 342–358. [Google Scholar] [CrossRef]
- Richardson, D.M. A Cartographic analysis of physiographic factors influencing the distribution of Hakea sp. in the South Western Cape. S. Afr. For. J. 1984, 128, 36–40. [Google Scholar] [CrossRef]
- Canavan, K.; Canavan, S.; Clark, V.R.; Gwate, O.; Richardson, D.M.; Sutton, G.F.; Martin, G.D. The alien plants that threaten South Africa’s mountain ecosystems. Land 2021, 10, 1393. [Google Scholar] [CrossRef]
- Hoang, S.A.; Lamb, D.; Seshadri, B.; Sarkar, B.; Cheng, Y.; Wang, L.; Bolan, N.S. Petroleum hydrocarbon rhizoremediation and soil microbial activity improvement via cluster root formation by wild proteaceae plant species. Chemosphere 2021, 275, 1–13. [Google Scholar] [CrossRef]
- Máximo, P.; Ferreira, L.M.; Branco, P.S.; Lourenço, A. Invasive plants: Turning enemies into value. Molecules 2020, 25, 3529. [Google Scholar] [CrossRef]
- Queirós, C.S.G.P.; Cardoso, S.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of Hakea sericea fruits regarding chemical composition and extract properties. Waste Biomass Valorization 2020, 11, 4859–4870. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Rodrigues, A.M.; Loureiro, L.M.E.F.; Sá, L.C.R.; Matias, J.C.O. Energy recovery from invasive species: Creation of value chains to promote control and eradication. Recycling 2021, 6, 21. [Google Scholar] [CrossRef]
- Warren, C.R.; Adams, M.A. Capillary electrophoresis for the determination of major amino acids and sugars in foliage: Application to the nitrogen nutrition of Sclerophyllous species. J. Exp. Bot. 2000, 51, 1147–1157. [Google Scholar] [CrossRef]
- Yusiharni, E.; Gilkes, R. Minerals in the ash of Australian native plants. Geoderma 2012, 189–190, 369–380. [Google Scholar] [CrossRef]
- Pullin, A.S.; Stewart, G.B. Guidelines for systematic review in conservation and environmental management. Conserv. Biol. 2006, 20, 1647–1656. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. he PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 1–11. [Google Scholar] [CrossRef]

| Search String | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | TOTAL | |
| SCOPUS | 271 | 62 | 72 | 23 | 11 | 4 | 1 | 3 | 56 | 503 |
| Web of Science | 239 | 52 | 53 | 18 | 7 | 3 | 1 | 4 | 52 | 429 |
| CAPES periodicals | 19 | 5 | 11 | 9 | 1 | 1 | 0 | 2 | 5 | 53 |
| Overall | 985 | |||||||||
| Question(s) | Publications | |
|---|---|---|
| No. | % | |
| 1 | 98 | 47.35 |
| 2 | 24 | 11.59 |
| 3 | 54 | 26.09 |
| 1, 2 | 12 | 5.80 |
| 1, 3 | 13 | 6.27 |
| 1, 2, 3 | 6 | 2.90 |
| Total | 207 | 100 |
| No. | % | |
|---|---|---|
| Publications including Hakea sericea | 114 | 55.1 |
| Publications including unspecified Hakea sp. or Hakea spp. | 23 | 11.1 |
| Publications including other Hakea species | 70 | 33.8 |
| Total | 207 | 100 |
| Question(s) | Country | |||||
|---|---|---|---|---|---|---|
| South Africa | Australia | Portugal | Global | New Zealand | Other | |
| 1 | 7 (13.2) | 67 (66.3) | 3 (25) | 6 (66.7) | 0 | 15 (57.6) |
| 2 | 2 (3.7) | 16 (15.8) | 1 (8.3) | 0 | 4 (66.6) | 1 (3.9) |
| 3 | 39 (73.5) | 2 (1.9) | 6 (50.0) | 0 | 1 (16.6) | 6 (23.1) |
| 1, 2 | 0 | 10 (9.9) | 0 | 1 (11.1) | 0 | 1 (3.9) |
| 1, 3 | 4 (7.5) | 4 (3.9) | 1 (8.3) | 1 (11.1) | 1 (16.6) | 2 (7.6) |
| 1, 2, 3 | 1 (1.9) | 2 (1.9) | 1 (8.3) | 1 (11.1) | 0 | 1 (3.9) |
| Total | 53 (25.6) | 101 (48.7) | 12 (5.8) | 9 (4.4) | 6 (2.9) | 26 (12.6) |
| Group | Trait | Implications |
|---|---|---|
| Evolutionary history (Hakea genus) | Hakea long evolutionary history (35 MY) | High genetic diversity, high phenotypic plasticity, high niche amplitude, widespread biogeographic range |
| Reproductive strategies | Dense and persistent canopy seed bank | High fecundity ability, high reproductive fitness |
| Water use efficiency | Leaf sclerophylly | Low transpiration rates |
| Drought resistance | High stomatal conductance, low hydraulic conductivity potential | Prevention of hydric stress, resistance to aridity, high photosynthetic capacity |
| Seeds and follicles | Seeds with water retention mechanism, germination in low water potential and in high temperature range | High environmental adaptability and plasticity, resistance to aridity |
| High nutrient reserves in seeds cotyledon | Adaptability to dystrophic soils | |
| Winged seeds, anemochoric dispersion | Long distance dispersion | |
| High follicle production, thick and lignified follicles | Dense canopy seed bank, resistance to dry conditions, high resilience in response to fire | |
| Serotiny | Strong serotiny, seed retention in follicles up to 10 years | Avoidance of seed predators, adaptation to fire-prone ecosystems, maintenance of dense canopy seed bank |
| Seedling establishment | High concentration of phenolic compounds | Seedling resistance to herbivory and pathogens |
| High nutrient cotyledon content | Fast seedling foliar expansion | |
| Proteoid roots | High contact surface | High P and Mn uptake |
| Organic acids/enzymes exudation | Increasing nutrient uptake (inaccessible to other plants) | |
| Nutrient use efficiency | High P use efficiency | Low tissue construction cost, high photosynthetic rates |
| High P resorption to new leaves | Adaptation to dystrophic environments |
| Group | Trait | Implications |
|---|---|---|
| H. sericea post-fire responses and strategies | Large heat-resistant follicles | Seed protection to high temperatures, high seeds survival rates, even after plant death |
| High post-fire seed production | High probability of survival, high post-fire recruitment in fire-prone ecosystems | |
| Seed bank/post-fire recruitment | Accumulation of seed storage with increasing fire intervals | High germination rates from seeds in dead plants follicles |
| Post-fire seed liberation | Enhanced post-fire population establishment |
| Group | Type | Implications |
|---|---|---|
| Control methods | Mechanical cleaning usually followed by prescribed fires (at different fire intervals) | Widespread and effective depending on access to invaded areas, negative impacts of fire on soil and biodiversity |
| Chemical (herbicides) | Expensive and difficult to apply, impact in non-target species; high tolerance due to H. sericea thick cuticle, immersed stomata and lower stomatal densities | |
| Biological (insects) | Coleoptera and lepidoptera species contributed to H. sericea population decrease in South Africa | |
| Biological (Fungi and bacteria) | Development and use of bioherbicides and used as part of sustainable weed management, risk of migrating to other non-target (susceptible) plants | |
| Modeling and monitoring of H. sericea invasions | Modeling of invasion factors (biotic and abiotic) | Identification and selection of biotic and abiotic limiting variables, prediction of spread behavior and trends |
| Modeling of control agent (biological) impacts on plant fecundity | Prevision of seed load and population growth rates, dispersal distances and new invasive foci | |
| Modeling costs and benefits | Optimal cost–benefit addressing biodiversity conservation and provision of ecosystem services and invasion reduction in priority areas | |
| Modeling of H. sericea population dynamics in fire risk and control scenarios | System dynamics of control efficacy evaluation in non-planned fire scenarios to be used in decision making process | |
| Modeling of fire scenarios | Different fuel scenarios and fire behavior and its impacts on ecosystem functioning and services |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobson, T.K.B.; Gerber, D.; Azevedo, J.C. Invasiveness, Monitoring and Control of Hakea sericea: A Systematic Review. Plants 2023, 12, 751. https://doi.org/10.3390/plants12040751
Jacobson TKB, Gerber D, Azevedo JC. Invasiveness, Monitoring and Control of Hakea sericea: A Systematic Review. Plants. 2023; 12(4):751. https://doi.org/10.3390/plants12040751
Chicago/Turabian StyleJacobson, Tamiel Khan Baiocchi, Dionatan Gerber, and João Carlos Azevedo. 2023. "Invasiveness, Monitoring and Control of Hakea sericea: A Systematic Review" Plants 12, no. 4: 751. https://doi.org/10.3390/plants12040751
APA StyleJacobson, T. K. B., Gerber, D., & Azevedo, J. C. (2023). Invasiveness, Monitoring and Control of Hakea sericea: A Systematic Review. Plants, 12(4), 751. https://doi.org/10.3390/plants12040751









