Different Effects of Reactive Species Generated from Chemical Donors on Seed Germination, Growth, and Chemical Contents of Oryza sativa L.
Abstract
:1. Introduction
2. Results
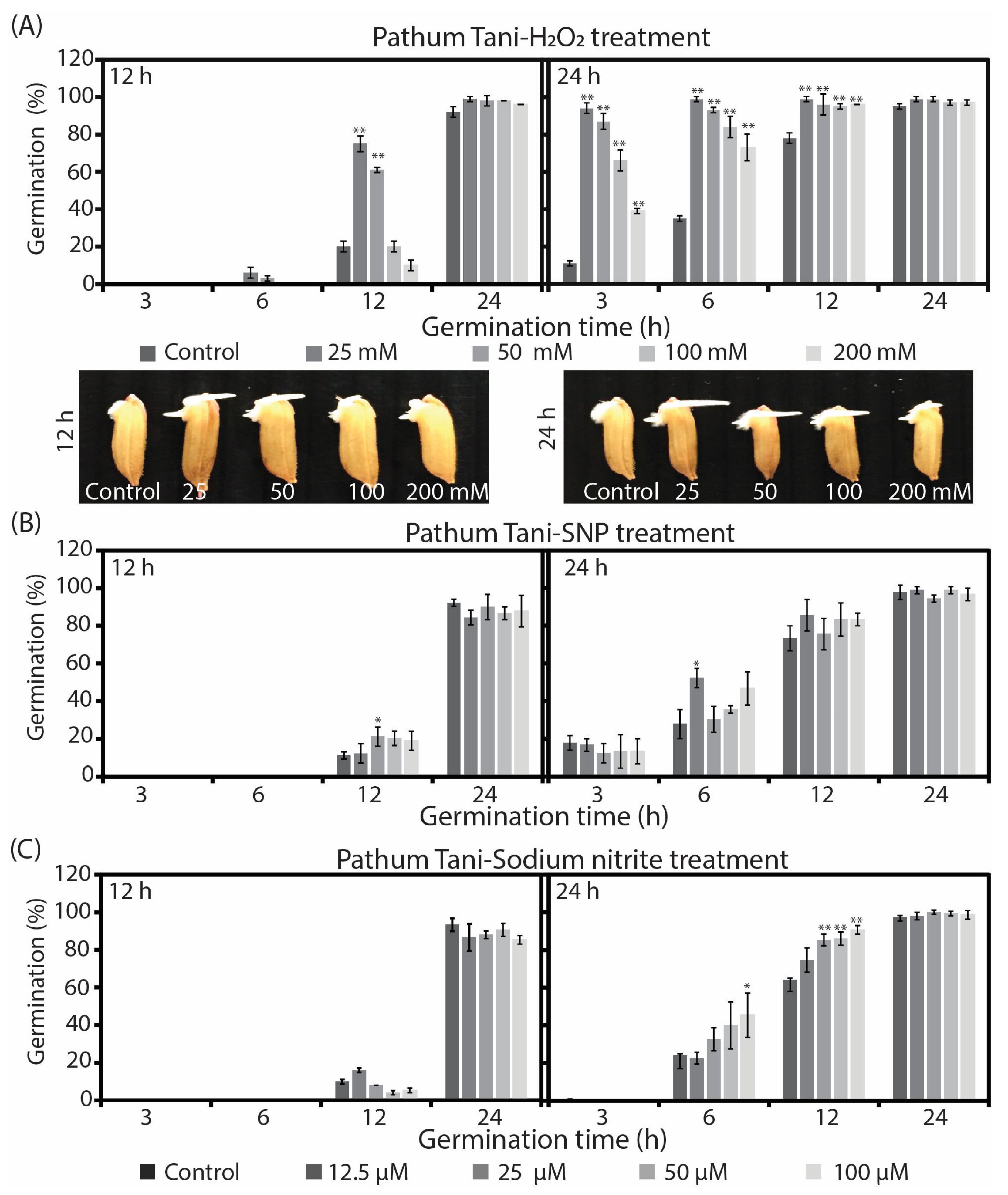
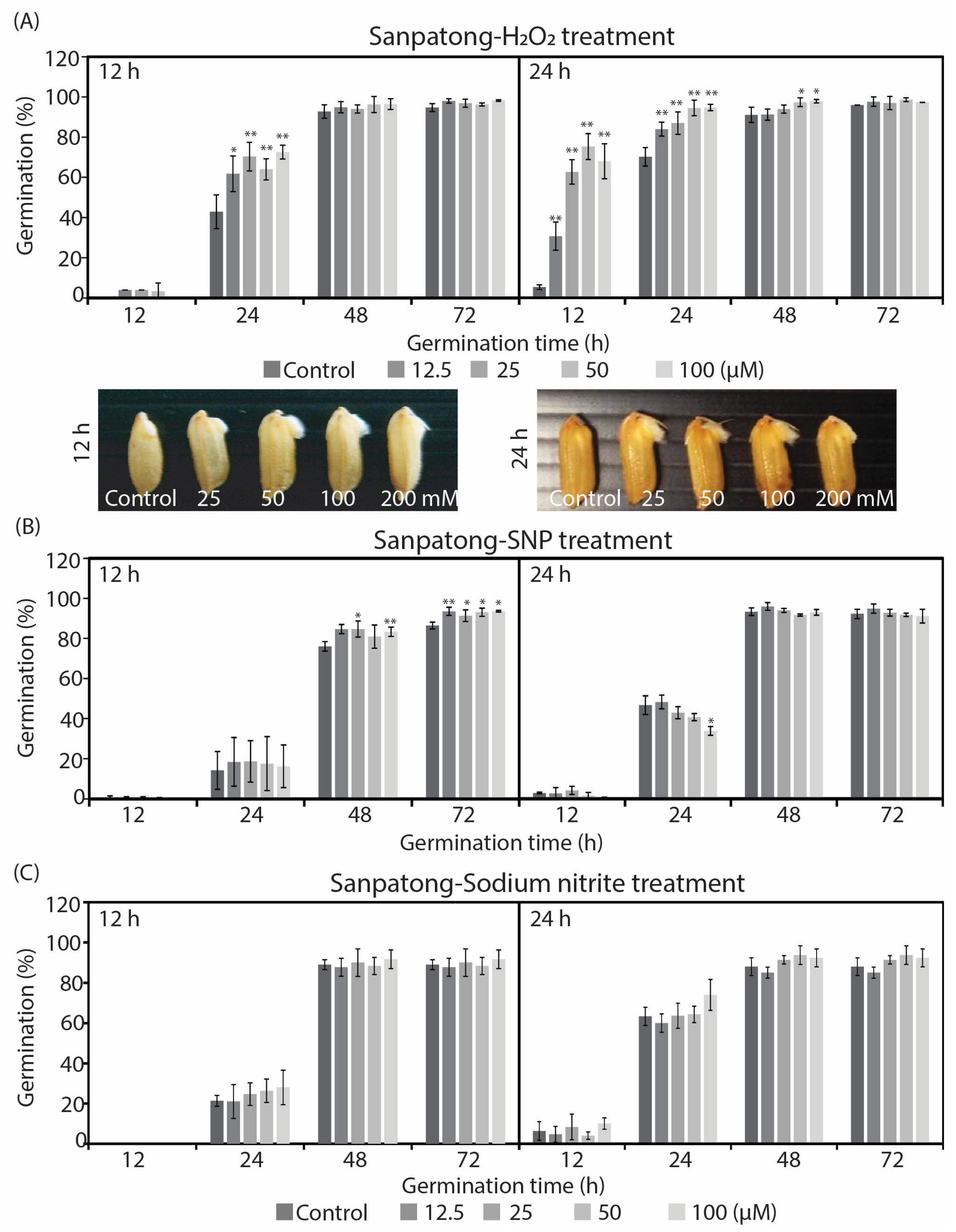
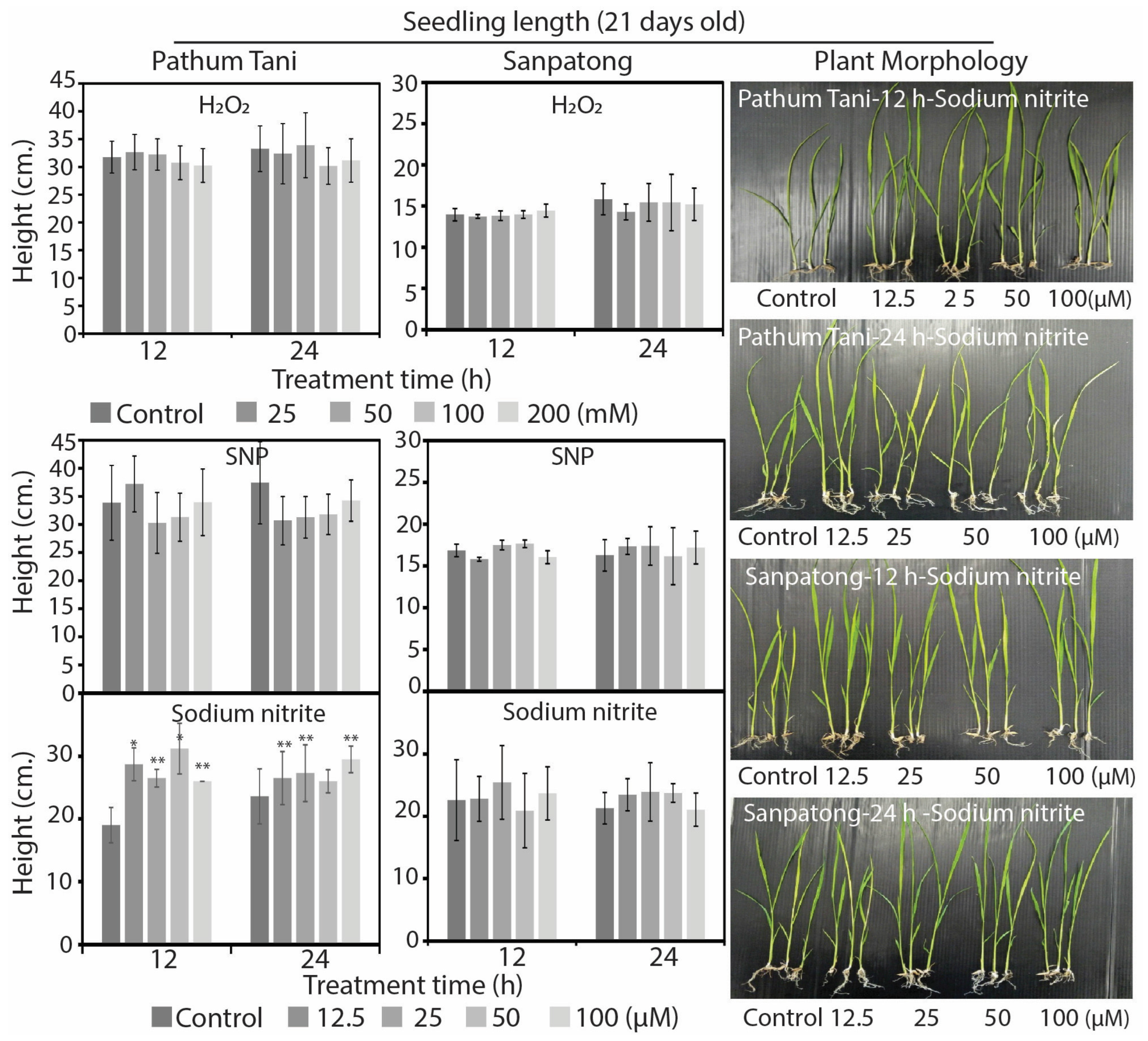
2.1. Reactive Oxygen and Nitrogen Species Differently Enhanced Seed Germination and Growth
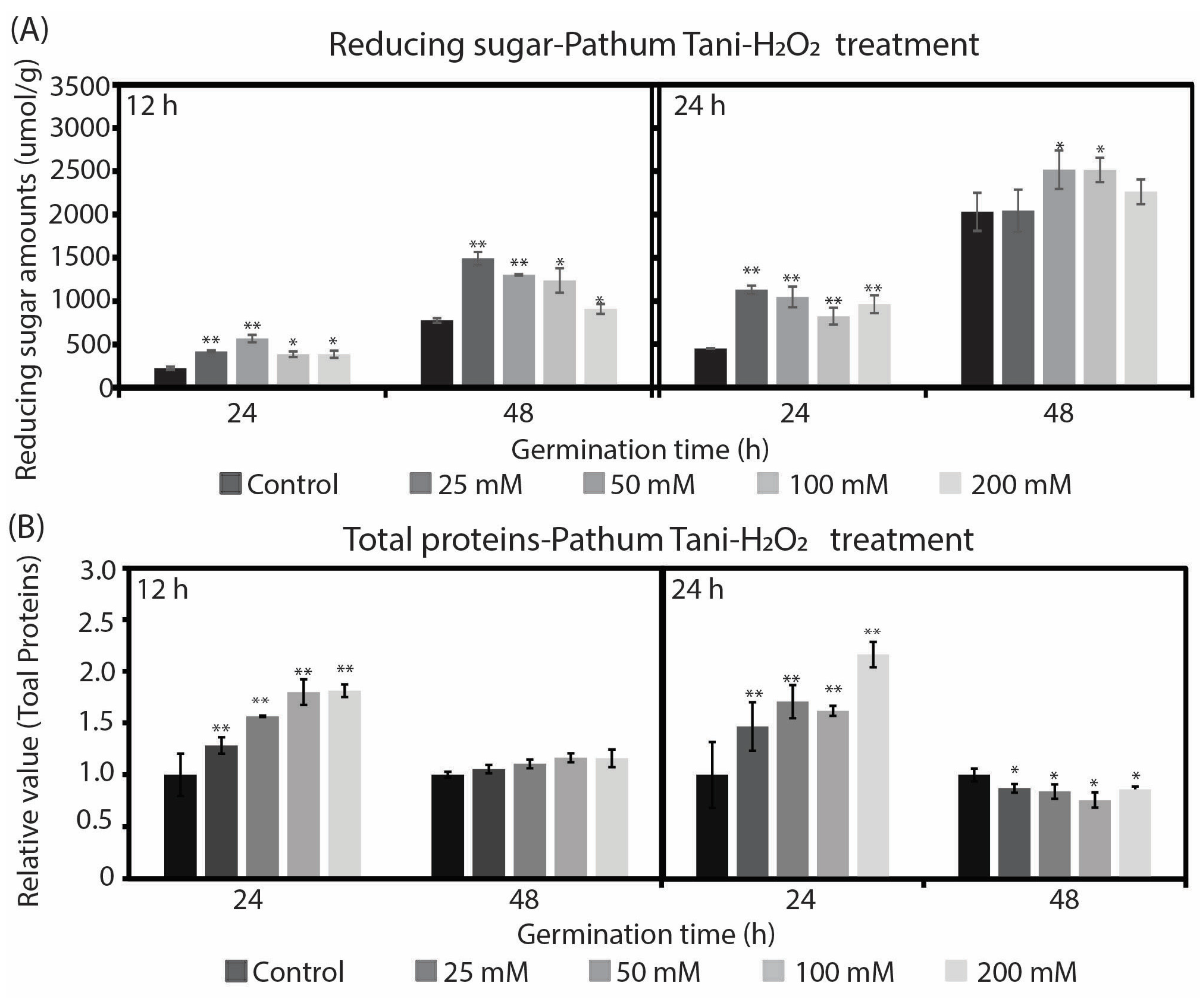
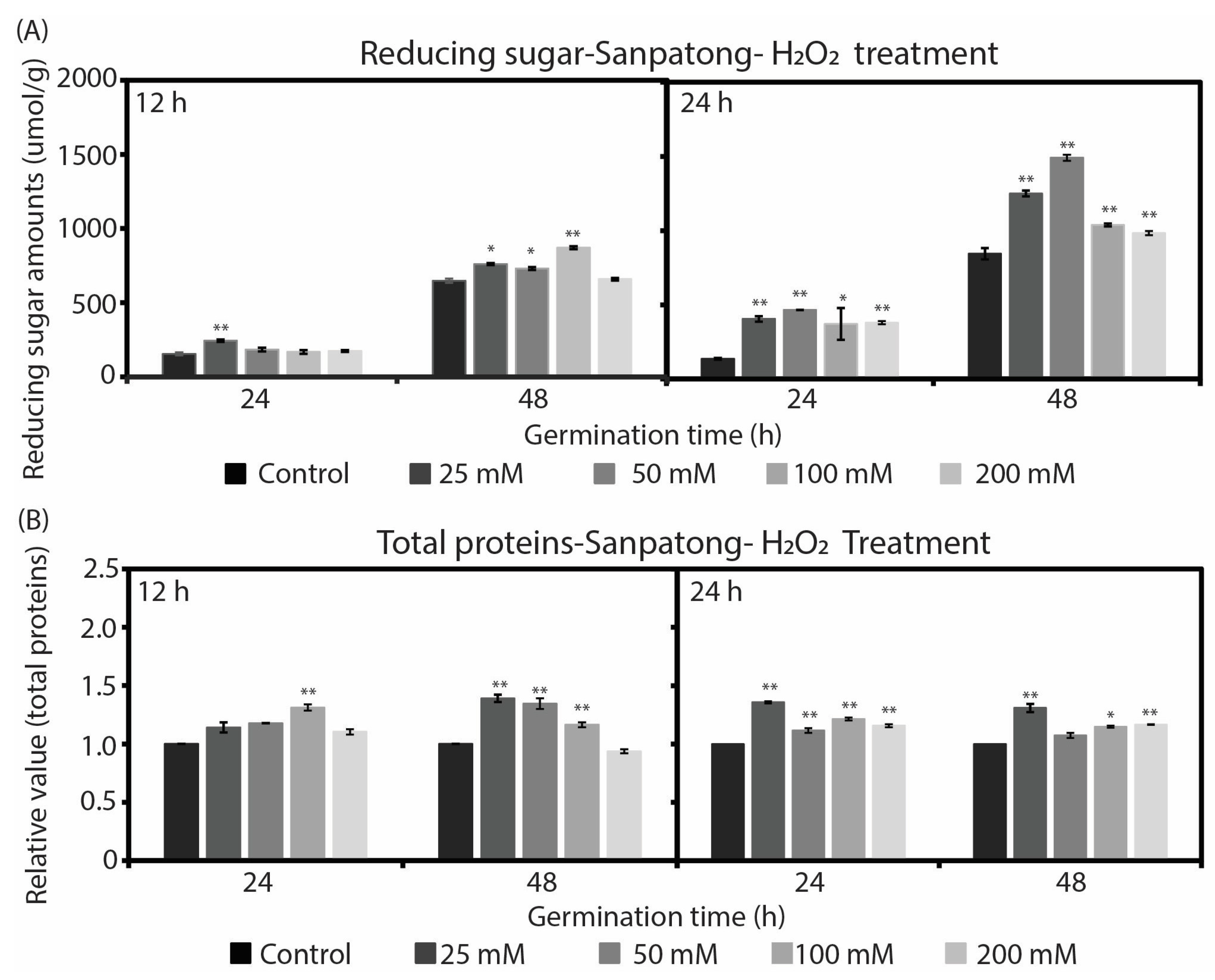
2.2. Changes in Reducing Sugar and Total Soluble Protein during Rice Seed Germination
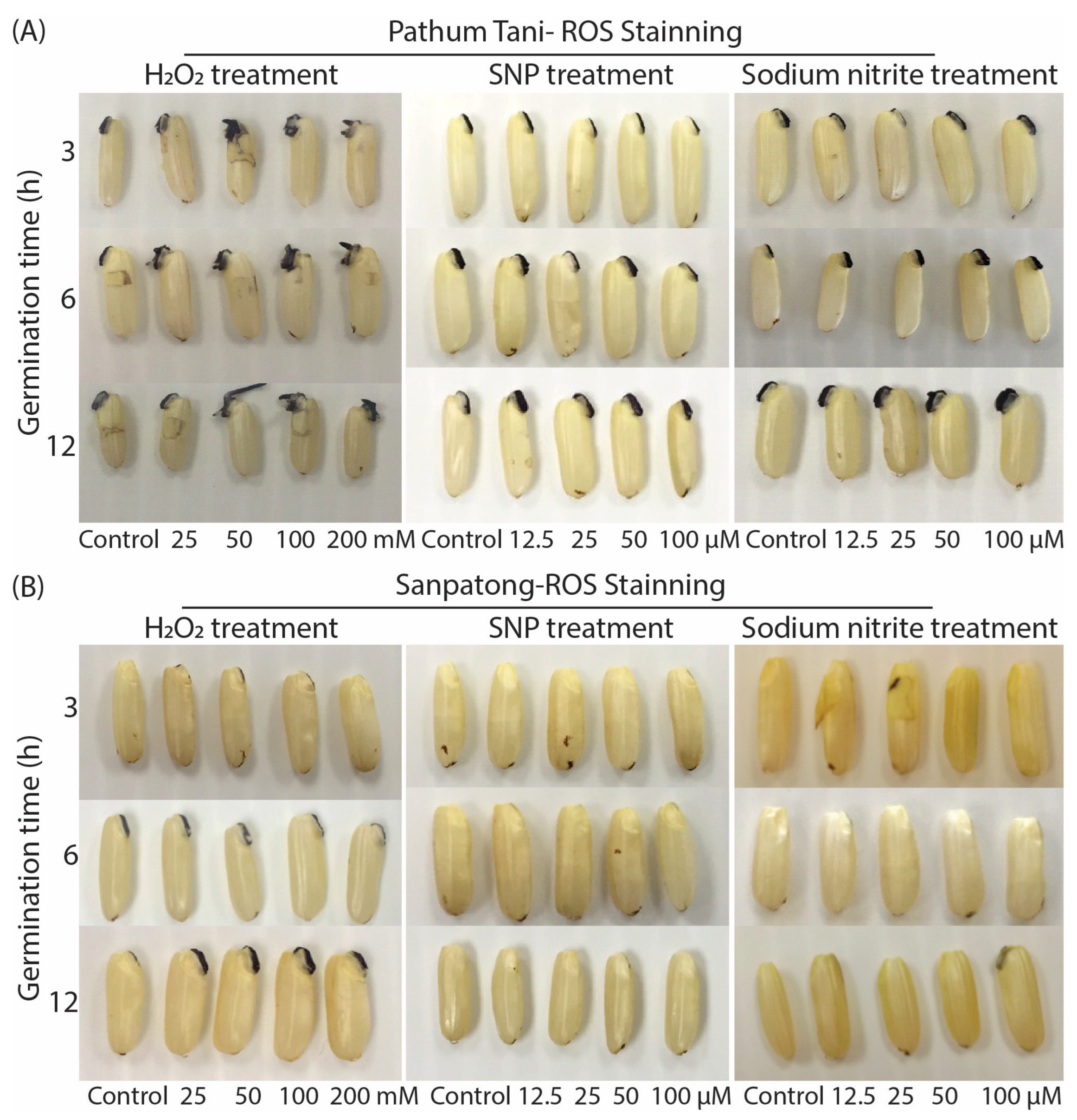
2.3. The Accumulation of ROS during Seed Germination on Two Rice Cultivars
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatment of Rice Seeds with Reactive Species Generated from Chemical Donors
4.2. Analysis of the Percentage of Seed Germination and Growth
4.3. Measurement of Reducing Sugar and Total Soluble Protein Contents
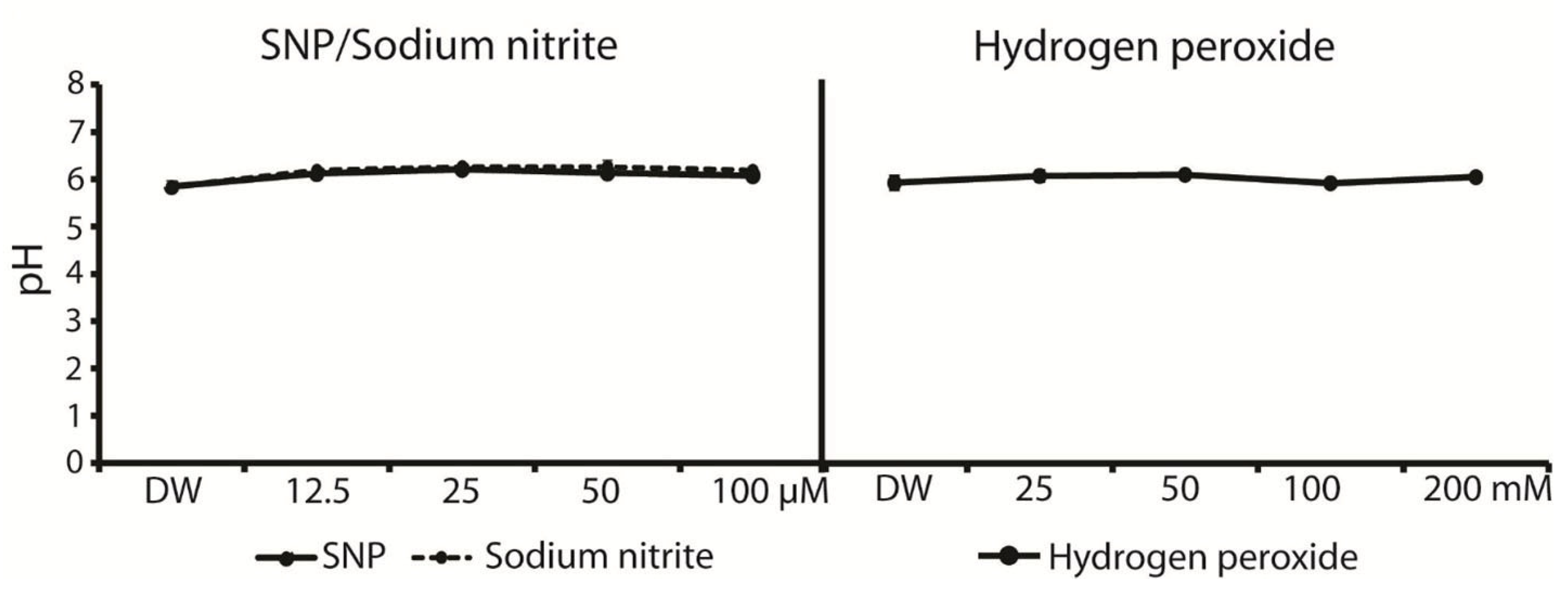
4.4. Measurement of ROS Storage in Seed by Using Nitro Blue Tetrazolium Staining and pH Solution Measurement
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed germination, The biochemical and molecular mechanisms. Breed. Sci. 2006, 56, 93–105. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar]
- Gomes, M.P.; Gacia, Q.S. Reactive oxygen species and seed germination. Biologia 2013, 68, 351–357. [Google Scholar]
- Liu, H.Y.; Yu, X.; Cui, D.Y.; Sun, M.H.; Sun, W.N.; Tang, Z.C.; Kwak, S.S.; Su, W.A. The role of water channel proteins and nitric oxide signaling in rice seed germination. Cell Res. 2007, 17, 638–649. [Google Scholar] [CrossRef]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Mhamdi, A.; Breusegem, F.V. Reactive oxygen species in plant development. Development 2018, 145, 164376. [Google Scholar]
- Gondim, F.A.; Gomes-Filho, E.; Lacerda, C.F.; Prisco, J.T.; Azevedo, N.A.D.; Marques, E.C. Pretreatment with in maize seeds: Effects on germination and seedling acclimation to salt stress. Braz. J. Plant Physiol. 2010, 22, 103–112. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Hernández, J.A.; Diaz-Vivancos, P. Role of in pea seed germination. Plant Signal. Behav. 2012, 7, 193–195. [Google Scholar] [CrossRef]
- Panngom, K.; Chuesaard, T.; Tamchan, N.; Jiwchan, T.; Srikongsritong, K.; Park, G. Comparative assessment for the effects of reactive species on seed germination, growth and metabolisms of vegetables. Sci. Hortic. 2018, 227, 85–91. [Google Scholar]
- Kumar, S.P.J.; Chintagunta, A.D.; Reddy, Y.M.; Rajjou, L.; Garlapati, V.K.; Agarwal, D.K.; Prasad, S.R.; Simal-Gandara, J. Implications of reactive oxygen and nitrogen species in seed physiology for sustainable crop productivity under changing climate conditions. Curr. Plant Biol. 2021, 26, 100197. [Google Scholar]
- Din, A.R.J.M.; Hanapi, S.Z.; Supari, N.; Alam, S.A.Z.; Javed, M.A.; Tin, L.C.; Sarmidi, M.R. Germination, seedling growth, amylase, and protease activities in Malaysian upland rice seed under microbial inoculation condition. J. Pure Appl. Microbiol. 2014, 8, 2627–2635. [Google Scholar]
- Veluppillai, S.; Nithyanantharajah, K.; Vasantharuba, S.; Balakumar, S.; Arasaratnam, V. Biochemical changes associated with germinating rice grains and germination improvement. Rice Sci. 2009, 16, 240–242. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. H2O2 and nitric oxide as signaling molecules in plants. J. Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef]
- Chung, H.J.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Farooq, M.A.; Zhang, X.; Zafar, M.M.; Ma, W.; Zhao, J. Role of reactive oxygen species and mitochondria in seed germination. Front. Plant Sci. 2021, 12, 781734. [Google Scholar] [CrossRef]
- Pan, T.; Lin, L.; Liu, Q.; Wei, C. In situ Degradation and Characterization of Endosperm Starch in Waxy Rice with the Inhibition of Starch Branching Enzymes during Seedling Growth. Int. J. Mol. Sci. 2018, 19, 3397. [Google Scholar]
- Singh, K.L.; Chaudhuri, A.; Kar, R.K. Superoxide and its metabolism during germination and axis growth of Vigna radiata (L.) Wilczek seeds. Plant Signal. Behav. 2014, 9, e29278. [Google Scholar]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar]
- Bailly, C. The signaling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef]
- Vijaylaxmi. Biochemical changes in cotyledons of germinating mung bean seeds from summer and rainy seasons. Indian J. Plant Physiol. 2013, 18, 377–380. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Davoodi, M.G. Evaluation of changes in phytase, α-amylase and protease activities of some legume seeds during germination. In Proceedings of the International Conference on Bioscience, Biochemistry and Bioinformatics, Penang, Malaysia, 22–24 February 2011; Volume 5, pp. 353–356. [Google Scholar]
- Kaneko, M.; Itoh, H.; Miyako, U.T.; Ashikari, M.; Matsuka, M. The α-amylase induction in endosperm during rice germination is caused by gibberellin synthesized in epithelium. Plant Physiol. 2002, 128, 1264–1270. [Google Scholar] [CrossRef]
- Palmiano, E.P.; Juliano, B.O. Biochemical changes in the rice grain during germination. Plant Physiol. 1972, 49, 751–756. [Google Scholar] [CrossRef]
- Zhang, N.; Jones, B.L. Characterization of germinated barley endoproteolytic enzymes by two-dimensional gel electrophoresis. J. Cereal Sci. 1995, 21, 145–153. [Google Scholar]
- Rahman, M.M.; Banu, L.A.; Rahman, M.M.; Shahjadee, U.F. Changes of the enzymes activity during germination of different mungbean varieties. Bangladesh J. Sci. Ind. Res. 2007, 42, 213–216. [Google Scholar]
- Wang, J.; Li, Y.; Lo, S.W.; Hillmer, S.; Sun, S.S.M.; Robinson, D.G.; Jiang, L. Protein mobilization in germinating mung bean seeds involves vacuolar sorting receptors and multivesicular bodies. Plant Physiol. 2007, 143, 1628–1639. [Google Scholar]
- Zhao, M.; Zhang, H.; Yan, H.; Qiu, L.; Baskin, C.C. Mobilization and role of starch, protein, and fat reserves during seed germination of six wild grassland species. Front. Plant Sci. 2018, 9, 234. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive oxygen species as potential drivers of the seed aging process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Wudick, M.M.; Li, X.; Valentini, V.; Geldner, N.; Chory, J.; Lin, J.; Maurel, C.; Luu, D.T. Subcellular redistribution of root aquaporins induced by hydrogen peroxide. Mol. Plant 2015, 8, 1103–1114. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [Green Version]
- Karkonen, A.; Kuchitsu, K. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 2015, 112, 22–32. [Google Scholar]
- Zeng, J.; Dong, Z.; Wu, H.; Tian, Z.; Zhao, Z. Redox regulation of plant stem cell fate. EMBO J. 2017, 36, 2844–2855. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuesaard, T.; Peankid, P.; Thaworn, S.; Jaradrattanapaiboon, A.; Veerana, M.; Panngom, K. Different Effects of Reactive Species Generated from Chemical Donors on Seed Germination, Growth, and Chemical Contents of Oryza sativa L. Plants 2023, 12, 765. https://doi.org/10.3390/plants12040765
Chuesaard T, Peankid P, Thaworn S, Jaradrattanapaiboon A, Veerana M, Panngom K. Different Effects of Reactive Species Generated from Chemical Donors on Seed Germination, Growth, and Chemical Contents of Oryza sativa L. Plants. 2023; 12(4):765. https://doi.org/10.3390/plants12040765
Chicago/Turabian StyleChuesaard, Thanyarat, Penpilai Peankid, Suwannee Thaworn, Anuwat Jaradrattanapaiboon, Mayura Veerana, and Kamonporn Panngom. 2023. "Different Effects of Reactive Species Generated from Chemical Donors on Seed Germination, Growth, and Chemical Contents of Oryza sativa L." Plants 12, no. 4: 765. https://doi.org/10.3390/plants12040765
APA StyleChuesaard, T., Peankid, P., Thaworn, S., Jaradrattanapaiboon, A., Veerana, M., & Panngom, K. (2023). Different Effects of Reactive Species Generated from Chemical Donors on Seed Germination, Growth, and Chemical Contents of Oryza sativa L. Plants, 12(4), 765. https://doi.org/10.3390/plants12040765





