Modification of Sugar Profile and Ripening in Atemoya (Annona × atemoya Mabb.) Fruits through Copper Hydroxide Application
Abstract
:1. Introduction
2. Results and Discussion
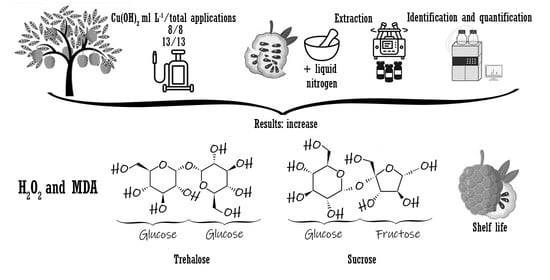
2.1. Hydrogen Peroxide and Lipid Peroxidation in Fruits
2.2. Carbohydrates
2.3. Fruit Quality
2.4. Heat Map
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahdeem, H. Custarde Apples (Annona Spp.). In Fruits of Warm Climates; Morton, J.F., Dowling, C., Eds.; Creative Resource Systems, Inc.: Miami, FL, USA, 1987; pp. 85–92. ISBN 0961018410. [Google Scholar]
- Watanabe, H.S.; Oliveira, S.L.D.; Camara, F.M.D.; Almeida, G.V.B.D.; Alves, A.A. Perfil de Comercialização Das Anonáceas Nas Ceasas Brasileiras. Rev. Bras. Frutic. 2014, 36, 65–70. [Google Scholar] [CrossRef]
- Judd, W.S.; Campbell, C.S.; Kellog, E.A.; Stevens, P.F.; Donoghue, M.J. Plant Systematics: A Phylogenetic Approach. Q Rev. Biol. 2003, 78, 208–262. [Google Scholar] [CrossRef]
- Junior, E.J.S.; Martins, A.B.G. Clonagem de Quatro Espécies de Annonaceae Potenciais Como Porta-Enxertos. Rev. Bras. Frutic. 2003, 25, 286–289. [Google Scholar] [CrossRef]
- Tokunaga, T.A. Manual Técnico, 233: Cultura Da Atemoia; CATI: Campinas, Brazil, 2000. [Google Scholar]
- Siqueira, C.A.T.; Oliani, J.; Sartoratto, A.; Queiroga, C.L.; Moreno, P.R.H.; Reimão, J.Q.; Tempone, A.G.; Fischer, D.C.H. Chemical Constituents of the Volatile Oil from Leaves of Annona Coriacea and in Vitro Antiprotozoal Activity. Rev. Bras. De Farmacogn. 2011, 21, 33–40. [Google Scholar] [CrossRef]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; de la Puerta, R. Potential Therapeutic Applications of the Genus Annona: Local and Traditional Uses and Pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef]
- Paull, R.E.; Duarte, O. Annonas: Cherimoya, Atemoya and Sweetsop. In Tropical Fruits; CABI: Wallingford, UK, 2011; Volume 1, pp. 123–152. [Google Scholar]
- Chatrou, L.W.; Erkens, R.H.J.; Richardson, J.E.; Saunders, R.M.K.; Fay, M.F. The Natural History of Annonaceae. Bot. J. Linn. Soc. 2012, 169, 1–4. [Google Scholar] [CrossRef]
- Pereira, M.C.T.; Nietsche, S.; São José, A.R.; Lemos, E.E.P.; Mizobutsi, E.H.; Corsato, C.D.A. Anonáceas: Pinha (Annona squamosa L.), Atemóia (Annonasquamosa L. × Annona cherimola mill) e Graviola (Annonamuricata L.). In 101 Culturas—Manual de Tecnologias Agrícolas; EPAMIG: Minas Gerais, Brazil, 2019; Volume 32, pp. 111–123. ISBN 978-85-99764-42-8. [Google Scholar]
- Pereira, M.C.T.; Nietsche, S.; Costa, M.R.; Crane, J.H.; Corsato, C.D.A.; Mizobutsi, E.H. Anonaceas: Pinha, Atemóia e Graviola. Inf. Agropecuário 2011, 32, 26–34. [Google Scholar]
- de Lemos, E.E.P. A Produção de Anonáceas No Brasil. Rev. Bras. Frutic 2014, 36, 77–85. [Google Scholar] [CrossRef]
- Alves, R.E.; Lima, M.A.C.; Mosca, J.L. Avances En Fisiología y Tecnología Postcosecha de Annonaceae. In Proceedings of the III Congreso Nacional de Anonáceas, Villahermosa, México, 11–15 September 2006; Embrapa Semiárido: Brasília, Brazil; pp. 13–18. [Google Scholar]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Oliveira, P.L., Ed.; Artmed Editora Ltda.: Porto Alegre, Brazil, 2017; ISBN 9788582713679. [Google Scholar]
- McMurchie, E.J.; McGlasson, W.B.; Eaks, I.L. Treatment of Fruit with Propylene Gives Information about the Biogenesis of Ethylene. Nature 1972, 237, 235–236. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory Metabolism: Glycolysis, the TCA Cycle and Mitochondrial Electron Transport. Curr Opin Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Manoochehri, H.; Hosseini, N.F.; Saidijam, M.; Taheri, M.; Rezaee, H.; Nouri, F. A Review on Invertase: Its Potentials and Applications. Biocatal. Agric. Biotechnol. 2020, 25, 101599. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Tyagi, P.; Sindhi, V.; Yadavilli, K.S. Invertase and Its Applications—A Brief Review. J. Pharm Res. 2013, 7, 792–797. [Google Scholar] [CrossRef]
- Laporte, D.; González, A.; Moenne, A. Copper-Induced Activation of MAPKs, CDPKs and CaMKs Triggers Activation of Hexokinase and Inhibition of Pyruvate Kinase Leading to Increased Synthesis of ASC, GSH and NADPH in Ulva Compressa. Front Plant Sci. 2020, 11, 990. [Google Scholar] [CrossRef]
- Binder, B.M.; Rodríguez, F.I.; Bleecker, A.B. The Copper Transporter RAN1 Is Essential for Biogenesis of Ethylene Receptors in Arabidopsis. J. Biol. Chem. 2010, 285, 37263–37270. [Google Scholar] [CrossRef]
- Rodríguez, F.I.; Esch, J.J.; Hall, A.E.; Binder, B.M.; Schaller, G.E.; Bleecker, A.B. A Copper Cofactor for the Ethylene Receptor ETR1 from Arabidopsis. Science 1999, 283, 996–998. [Google Scholar] [CrossRef]
- Cao, X.; Wen, Z.; Shang, C.; Cai, X.; Hou, Q.; Qiao, G. Copper Amine Oxidase (CuAO)-Mediated Polyamine Catabolism Plays Potential Roles in Sweet Cherry (Prunus avium L.) Fruit Development and Ripening. Int. J. Mol. Sci. 2022, 23, 12112. [Google Scholar] [CrossRef]
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit Development and Ripening. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef]
- Taylor, J.E. Exotics. In Biochemistry of Fruit Ripening; Seymour, G.B., Taylor, J.E., Tucker, G.A., Eds.; Springer: Dordrecht, The Netherlands, 1993; Volume 1, pp. 151–187. ISBN 978-94-010-4689-3. [Google Scholar]
- O’Leary, B.; Plaxton, W.C. Multifaceted Functions of Post-Translational Enzyme Modifications in the Control of Plant Glycolysis. Curr Opin Plant Biol. 2020, 55, 28–37. [Google Scholar] [CrossRef]
- da Cunha, K.T.; Mendes, T.D.S.R.; da Costa, C.L.S. Detecção de Carboidratos de Baixo Peso Molecular Em Diferentes Variedades de Manga, Em Dois Estágios de Maturação. Res. Soc. Dev. 2022, 11, e27411225664. [Google Scholar] [CrossRef]
- Barry, C.S. Factors Influencing the Ripening and Quality of Fleshy Fruits. In Annual Plant Reviews Online; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 296–325. [Google Scholar]
- Hameed, A.; Iqbal, N.; Malik, S.A. Mannose-Induced Modulations in Antioxidants, Protease Activity, Lipid Peroxidation, and Total Phenolics in Etiolated Wheat Leaves. J. Plant Growth Regul. 2009, 28, 58–65. [Google Scholar] [CrossRef]
- Guo, F.; Han, A.; Gao, H.; Liang, J.; Zhao, K.; Cao, S.; Wang, H.; Wei, Y.; Shao, X.; Xu, F. Mannose Alleviates Yellowing Process of Broccoli Florets by Regulating Chlorophyll Catabolism and Delaying Programmed Cell Death. Sci. Hortic 2022, 295, 110888. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of Trehalose on Protein Structure. Protein Sci. 2008, 18, 24–36. [Google Scholar] [CrossRef]
- Villanueva, E.; Fujibayashi-Yoshii, N.; Matsuzaki, S.; Yamazaki, K.; Burana, C.; Yamane, K. Effects of Trehalose and Sucrose on the Vase Life and Physiology of Cut Astilbe (Astilbe × Arendsii Arends) Flowers. Hort J. 2019, 88, 276–283. [Google Scholar] [CrossRef]
- Vichaiya, T.; Faiyue, B.; Rotarayanont, S.; Uthaibutra, J.; Saengnil, K. Exogenous Trehalose Alleviates Chilling Injury of ‘Kim Ju’ Guava by Modulating Soluble Sugar and Energy Metabolisms. Sci. Hortic. 2022, 301, 111138. [Google Scholar] [CrossRef]
- Allwood, J.W.; Gibon, Y.; Osorio, S.; Araújo, W.L.; Vallarino, J.G.; Pétriacq, P.; Moing, A. Developmental Metabolomics to Decipher and Improve Fleshy Fruit Quality. In Advances in Botanical Research; Academic Press Inc.: Cambridge, MA, USA, 2021; Volume 98, pp. 3–34. [Google Scholar]
- Apaliya, M.T.; Zhang, H.; Yang, Q.; Zheng, X.; Zhao, L.; Kwaw, E.; Mahunu, G.K. Hanseniaspora Uvarum Enhanced with Trehalose Induced Defense-Related Enzyme Activities and Relative Genes Expression Levels against Aspergillus Tubingensis in Table Grapes. Postharvest Biol. Technol. 2017, 132, 162–170. [Google Scholar] [CrossRef]
- Topcu, H.; Degirmenci, I.; Sonmez, D.A.; Paizila, A.; Karci, H.; Kafkas, S.; Kafkas, E.; Ercisli, S.; Alatawi, A. Sugar, Invertase Enzyme Activities and Invertase Gene Expression in Different Developmental Stages of Strawberry Fruits. Plants 2022, 11, 509. [Google Scholar] [CrossRef]
- Phimolsiripol, Y.; Suppakul, P. Techniques in Shelf Life Evaluation of Food Products. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- ASEAN. Standard for Sugar Apple; ASEAN: Jakarta, Indonesia, 2014. [Google Scholar]
- UNECE. Concerning the Marketing and Commercial Quality Control of Annonas; UNECE: Geneva, Switzerland, 2017. [Google Scholar]
- CQH. Normas de Classificação—Anonáceas; CQH: São Paulo, Brazil, 2013. [Google Scholar]
- Cooke, T.; Persley, D.M.; House, S. Custard Apple (Atemoya). In Disease of Fruit Crops in Australia; Cooke, T., Persley, D.M., House, S., Eds.; CSIRO Pub: Collingwood, Australia, 2009; Volume 1, pp. 123–129. ISBN 9780643069718. [Google Scholar]
- Junqueira, N.T.V.; Junqueira, K.P. Principais Doenças de Anonáceas No Brasil: Descrição e Controle. Rev. Bras. Frutic 2014, 36, 55–64. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants Andstress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and Biotic Stresses and Changes in the Lignin Content and Composition in Plants. J. Integr Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- MAPA. Agrolinkfito: Todos Os Agrotóxicos Do Brasil Para Controlar Doenças e Pragas Em Sua Lavoura. Available online: https://www.agrolink.com.br/agrolinkfito/produto/tutor_7888.html (accessed on 1 February 2023).
- la Torre, A.; Iovino, V.; Caradonia, F. Copper in Plant Protection: Current Situation and Prospects. Phytopathol Mediterr 2018, 57, 201–236. [Google Scholar] [CrossRef]
- FRAC. Fungal Control Agents Sorted by Cross-Resistance Pattern and Mode of Action; FRAC: Switzerland, 2022. [Google Scholar]
- McCallan, S.E.A. The Nature of the Fungicidal Action of Copper and Sulfur. Bot. Rev. 1949, 15, 629–643. [Google Scholar] [CrossRef]
- Graham, R.D.; Webb, M.J. Micronutrients and Disease Resistance and Tolerance in Plants. In Micronutrients in Agriculture, 2nd ed.; Mortvedt, J.J., Ed.; SSSA Book Series: Peoria, AZ, USA, 2018; pp. 329–370. [Google Scholar]
- Yruela, I. Copper in Plants: Acquisition, Transport and Interactions. Funct. Plant Biol. 2009, 36, 409. [Google Scholar] [CrossRef]
- Reis, A.C.; Forcelini, C.A.; Reis, E.M. Manual de Fungicidas—Guia Para o Controle de Doenças de Plantas, 5th ed.; UPF Editora: Passo Fundo, Brazil, 2019; Volume 1. [Google Scholar]
- Lopez, A.M. Doenças de Anonáceas e Do Urucuzeiro. In Manual de Fitopatologia; Agronômica Ceres: São Paulo, Brazil, 1997; ISBN 8531800080. [Google Scholar]
- Boudet, A.-M. Lignins and Lignification: Selected Issues. Plant Physiol. Biochem. 2000, 38, 81–96. [Google Scholar] [CrossRef]
- Dizhbite, T. Characterization of the Radical Scavenging Activity of Lignins—Natural Antioxidants. Bioresour Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Applicability of Lignins from Different Sources as Antioxidants Based on the Protective Effects on Lipid Peroxidation Induced by Oxygen Radicals. Ind. Crops Prod. 2009, 30, 184–187. [Google Scholar] [CrossRef]
- Jung, Y.; Ha, M.; Lee, J.; Ahn, Y.G.; Kwak, J.H.; Ryu, D.H.; Hwang, G.S. Metabolite Profiling of the Response of Burdock Roots to Copper Stress. J. Agric. Food Chem. 2015, 63, 1309–1317. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Silva, J.G.; Silva, L.P.; Souza, J.R.; Pires De Castro, C.S. Interação Entre a Enzima Catalase e Os Íons Cu(II): Sítios de Ligação, Estequiometria, Kd e E° Por CV, DPV e Ferramentas Teóricas; EMBRAPA: São Paulo, Brazil, 2008. [Google Scholar]
- Cao, Y.; Ma, C.; Chen, G.; Zhang, J.; Xing, B. Physiological and Biochemical Responses of Salix Integra Thunb. under Copper Stress as Affected by Soil Flooding. Environ. Pollut. 2017, 225, 644–653. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive Oxygen Species, Essential Molecules, during Plant-Pathogen Interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as Key Players in Plant Stress Signalling. J. Exp. Bot 2014, 65, 1229–1240. [Google Scholar]
- Eloy, Y.R.G.; Vasconcelos, I.M.; Barreto, A.L.H.; Freire-Filho, F.R.; Oliveira, J.T.A. H2O2 Plays an Important Role in the Lifestyle of Colletotrichum Gloeosporioides during Interaction with Cowpea [Vigna unguiculata (L.) Walp.]. Fungal Biol. 2015, 119, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of Lead and Copper on Photosynthetic Apparatus in Citrus (Citrus aurantium L.) Plants. the Role of Antioxidants in Oxidative Damage as a Response to Heavy Metal Stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Dowling, D.K.; Simmons, L.W. Reactive Oxygen Species as Universal Constraints in Life-History Evolution. Proc. R. Soc. B Biol. Sci. 2009, 276, 1737–1745. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Tounekti, T.; Vadel, A.M.; Oñate, M.; Khemira, H.; Munné-Bosch, S. Salt-Induced Oxidative Stress in Rosemary Plants: Damage or Protection? Environ. Exp. Bot. 2011, 71, 298–305. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, L.; Dai, T.; Zhou, J.; Kang, Q.; Chen, H.; Li, K.; Li, Z. Effects of Copper on the Growth, Antioxidant Enzymes and Photosynthesis of Spinach Seedlings. Ecotoxicol Environ. Saf. 2019, 171, 771–780. [Google Scholar] [CrossRef]
- An, Q.; He, X.; Zheng, N.; Hou, S.; Sun, S.; Wang, S.; Li, P.; Li, X.; Song, X. Physiological and Genetic Effects of Cadmium and Copper Mixtures on Carrot under Greenhouse Cultivation. Ecotoxicol Environ. Saf. 2020, 206, 111363. [Google Scholar] [CrossRef]
- Yruela, I. Copper in Plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Li, M.; Xiong, Z.; Liu, H.; Kuo, Y.; Tong, L. Copper-Induced Alteration in Sucrose Partitioning and Its Relationship to the Root Growth of Two Elsholtzia Haichowensis Sun Populations. Int. J. Phytoremediation 2016, 18, 966–976. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Giardina, T. Sucrose and Invertases, a Part of the Plant Defense Response to the Biotic Stresses. Front Plant Sci. 2014, 5, 293. [Google Scholar]
- Yusuf, M.; Saeed Almehrzi, A.S.; Nasir Alnajjar, A.J.; Alam, P.; Elsayed, N.; Khalil, R.; Hayat, S. Glucose Modulates Copper Induced Changes in Photosynthesis, Ion Uptake, Antioxidants and Proline in Cucumis Sativus Plants. Carbohydr. Res. 2021, 501, 108271. [Google Scholar] [CrossRef]
- Bray, G.A. How Bad Is Fructose? Am. J. Clin. Nutr. 2007, 86, 895–896. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and Plant Stress Responses: Friend or Foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Bruton, B.D.; Russo, V.M.; Garcia-Jimenez, J.; Miller, M.E. Carbohydrate Partitioning, Cultural Practices, and Vine Decline Diseases of Cucurbits.; McCreight, J.D., Ed.; ASHS Press: Alexandria, VA, USA, 1998; Volume 1. [Google Scholar]
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Hassan, M.U.; Nawaz, M.; Shah, A.N.; Raza, A.; Barbanti, L.; Skalicky, M.; Hashem, M.; Brestic, M.; Pandey, S.; Alamri, S.; et al. Trehalose: A Key Player in Plant Growth Regulation and Tolerance to Abiotic Stresses. J. Plant Growth Regul. 2022, 41, 1–23. [Google Scholar] [CrossRef]
- Xu, F.; Tang, Y.; Dong, S.; Shao, X.; Wang, H.; Zheng, Y.; Yang, Z. Reducing Yellowing and Enhancing Antioxidant Capacity of Broccoli in Storage by Sucrose Treatment. Postharvest Biol. Technol. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Horn, D.; Barrientos, A. Mitochondrial Copper Metabolism and Delivery to Cytochrome c Oxidase. IUBMB Life 2008, 60, 421–429. [Google Scholar] [CrossRef]
- Cruz, L.S.D.; Lima, R.Z.; Abreu, C.M.P.D.; Corrêa, A.D.; Pinto, L.D.M.A. Caracterização Física e Química Das Frações Do Fruto Atemoia Gefner. Ciência Rural. 2013, 43, 2280–2284. [Google Scholar] [CrossRef]
- Batista, P.F.; Lima, M.A.C.D.; Trindade, D.C.G.D.; Alves, R.E. Quality of Different Tropical Fruit Cultivars Produced in the Lower Basin of the São Francisco Valley. Rev. Cienc. Agron. 2015, 46, 176–184. [Google Scholar] [CrossRef]
- do Prado Verotti, T.; de Oliveira, C.G.; de Souza Parreiras, N.; Gonçalves, F.C.M.; Corrêa, C.V.; Ferreira, G.; Campos, F.G.; Boaro, C.S.F. Vegetal Regulators Increase the Quality of Atemoya Fruits and Recover the Photosynthetic Metabolism of Stressed Plants. Acta Physiol. Plant 2019, 41, 165. [Google Scholar] [CrossRef]
- Chitarra, M.I.F. Pós-Colheita de Frutas e Hortaliças: Fisiologia e Manuseio, 2nd ed.; Chitarra, A.B., Ed.; Universidade Federal de Lavras: Lavras, Brazil, 2005; Volume 1. [Google Scholar]
- Jia, K.; Zhang, Q.; Xing, Y.; Yan, J.; Liu, L.; Nie, K. A Development-Associated Decrease in Osmotic Potential Contributes to Fruit Ripening Initiation in Strawberry (Fragaria ananassa). Front Plant Sci. 2020, 11, 1035. [Google Scholar] [CrossRef]
- Cecchi, H.M. Fundamentos Teóricos e Práticos Em Análise de Alimentos, 2nd ed.; Editora da Unicamp: Campinas, Brazil, 2003; ISBN 9788526814721. [Google Scholar]
- Silva, A.V.C.; Andrade, D.G.D.; Yaguiu, P.; Carnelossi, M.A.G.; Muniz, E.N.; Narain, N. Uso de Embalagens e Refrigeração Na Conservação de Atemóia. Ciência E Tecnol. De Aliment. 2009, 29, 300–304. [Google Scholar] [CrossRef] [Green Version]
- Orsi, D.C.; Carvalho, V.S.; Nishi, A.C.F.; Damiani, C.; Asquieri, E.R. Use of Sugar Apple, Atemoya and Soursop for Technological Development of Jams: Chemical and Sensorial Composition. Ciência E Agrotecnologia 2012, 36, 560–566. [Google Scholar] [CrossRef]
- de Souza, V.R.; Pereira, P.A.P.; Queiroz, F.; Borges, S.V.; de Deus Souza Carneiro, J. Determination of Bioactive Compounds, Antioxidant Activity and Chemical Composition of Cerrado Brazilian Fruits. Food Chem. 2012, 134, 381–386. [Google Scholar] [CrossRef]
- López-Vargas, E.R.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar Application of Copper Nanoparticles Increases the Fruit Quality and the Content of Bioactive Compounds in Tomatoes. Appl. Sci. (Switz.) 2018, 8, 20. [Google Scholar] [CrossRef]
- Silveira, L.K.; Pavão, G.C.; dos Santos Dias, C.T.; Quaggio, J.A.; de Matos Pires, R.C. Deficit Irrigation Effect on Fruit Yield, Quality and Water Use Efficiency: A Long-Term Study on Pêra-IAC Sweet Orange. Agric. Water Manag. 2020, 231, 106019. [Google Scholar] [CrossRef]
- Batten, D.J. Effect of Temperature on Ripening and Post-Harvest Life of Fruit of Atemoya (Annona cherimola mill. × A. squamosa L.) Cv. ‘African Pride’. Sci. Hortic 1990, 45, 129–136. [Google Scholar] [CrossRef]
- Shewfelt, R.L.; Prussia, S.E. Challenges in Handling Fresh Fruits and Vegetables. In Postharvest Handling; Elsevier: Amsterdam, The Netherlands, 2022; pp. 167–186. [Google Scholar]
- Frank Bollen, A.; Prussia, S.E. Sorting for Defects. In Postharvest Handling; Elsevier: Amsterdam, The Netherlands, 2014; pp. 341–362. [Google Scholar]
- Stepansky, A.; Kovalski, I.; Schaffer, A.A.; Perl-Treves, R. Variation in Sugar Levels and Invertase Activity in Mature Fruit Representing a Broad Spectrum of Cucumis Melo Genotypes. Genet. Resour Crop. Evol 1999, 46, 53–62. [Google Scholar] [CrossRef]
- Ozaki, K.; Uchida, A.; Takabe, T.; Shinagawa, F.; Tanaka, Y.; Takabe, T.; Hayashi, T.; Hattori, T.; Rai, A.K.; Takabe, T. Enrichment of Sugar Content in Melon Fruits by Hydrogen Peroxide Treatment. J. Plant Physiol. 2009, 166, 569–578. [Google Scholar] [CrossRef]
- da Cunha, A.R.; Martins, D. Classificação Climática Para Os Municípios de Botucatu e São Manuel, SP. IRRIGA 2009, 14, 1–11. [Google Scholar] [CrossRef]
- Campo-Dall’Orto, F.A.; Barbosa, W.; Ojima, M.; Raij, B. Frutas de Clima Temperado—II: Figo, Maçã, Marmelo, Pêra e Pessego Em Pomar Compacto. In Boletim Técnico 100—Recomendações de Adubação e Calagem para o Estado de São Paulo; Raij, B., Cantarella, H., Quaggio, J.A., Furlani, Â.M., Eds.; EMBRAPA: Planaltina, Brazil, 1997; Volume 1, pp. 139–141. [Google Scholar]
- Neto, J.E.B.; Kavati, R.; Filho, M.F.d.S.; Sanches, J.; Fischer, I.H.; Rozane, D.E.; Natale, W. Anona. In Boletim 200—Instruções Agrícolas Para as Principais Culturas Econômicas; Aguiar, A.T.d.E., Gonçalves, C., Paterniani, M.E.A.G.Z., Tucci, M.L.S., Castro, C.E.F., Eds.; Instituto Agronômico (IAC): Campinas, Brazil, 2014; Volume 1, pp. 28–33. [Google Scholar]
- Tavares, S.D.H.; Costa, V.D.O.; dos Santos, V.F.C. Manejo Da Antracnose Na Produção Integrada de Manga; EMBRAPA: Petrolina, Brazil, 2005. [Google Scholar]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The Effect of Drought and Ultraviolet Radiation on Growth and Stress Markers in Pea and Wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Teisseire, H.; Guy, V. Copper-Induced Changes in Antioxidant Enzymes Activities in Fronds of Duckweed (Lemna minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Garcia, I.S.; Souza, A.; Barbedo, C.J.; Dietrich, S.M.C.; Figueiredo-Ribeiro, R.C.L. Changes in Soluble Carbohydrates during Storage of Caesalpinia Echinata LAM. (Brazilwood) Seeds, an Endangered Leguminous Tree from the Brazilian Atlantic Forest. Braz. J. Biol. 2006, 66, 739–745. [Google Scholar] [CrossRef]
- AOAC International. Methods of Analysis for Nutrition Labeling; Sullivan, D.M., Carpenter, D.E., Eds.; AOAC International: Arlington, VA, USA, 1995; Volume 78. [Google Scholar]
- AOAC International. Official Methods of Analysis, 15th ed.; AOAC International: Arlington, VA, USA, 1990; Volume 1, ISBN 0-935584-42-0. [Google Scholar]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-Enabled Heat Mapping for All. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]




| Treatment * | Cu(OH)2 Concentration per Application (mL L−1) | Cu(OH)2 Total Concentration (mL L−1) | Number of Cu(OH)2 Application |
|---|---|---|---|
| T1 | 0.0 | 0.0 | 0 |
| T2 | 3.9 | 7.8 | 2 |
| T3 | 3.9 | 15.6 | 4 |
| T4 | 1.0 | 8.0 | 8 |
| T5 | 1.0 | 13.0 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, C.P.; Campos, F.G.; Napoleão, G.M.; Barzotto, G.R.; Campos, L.P.; Ferreira, G.; Boaro, C.S.F. Modification of Sugar Profile and Ripening in Atemoya (Annona × atemoya Mabb.) Fruits through Copper Hydroxide Application. Plants 2023, 12, 768. https://doi.org/10.3390/plants12040768
Cardoso CP, Campos FG, Napoleão GM, Barzotto GR, Campos LP, Ferreira G, Boaro CSF. Modification of Sugar Profile and Ripening in Atemoya (Annona × atemoya Mabb.) Fruits through Copper Hydroxide Application. Plants. 2023; 12(4):768. https://doi.org/10.3390/plants12040768
Chicago/Turabian StyleCardoso, Caroline P., Felipe G. Campos, Gabriel M. Napoleão, Gustavo R. Barzotto, Lauro P. Campos, Gisela Ferreira, and Carmen S. F. Boaro. 2023. "Modification of Sugar Profile and Ripening in Atemoya (Annona × atemoya Mabb.) Fruits through Copper Hydroxide Application" Plants 12, no. 4: 768. https://doi.org/10.3390/plants12040768