Phytochemical Composition of the Fruit of Large Cranberry (Vaccinium macrocarpon Aiton) Cultivars Grown in the Collection of the National Botanic Garden of Latvia
Abstract
:1. Introduction
2. Results and Discussion
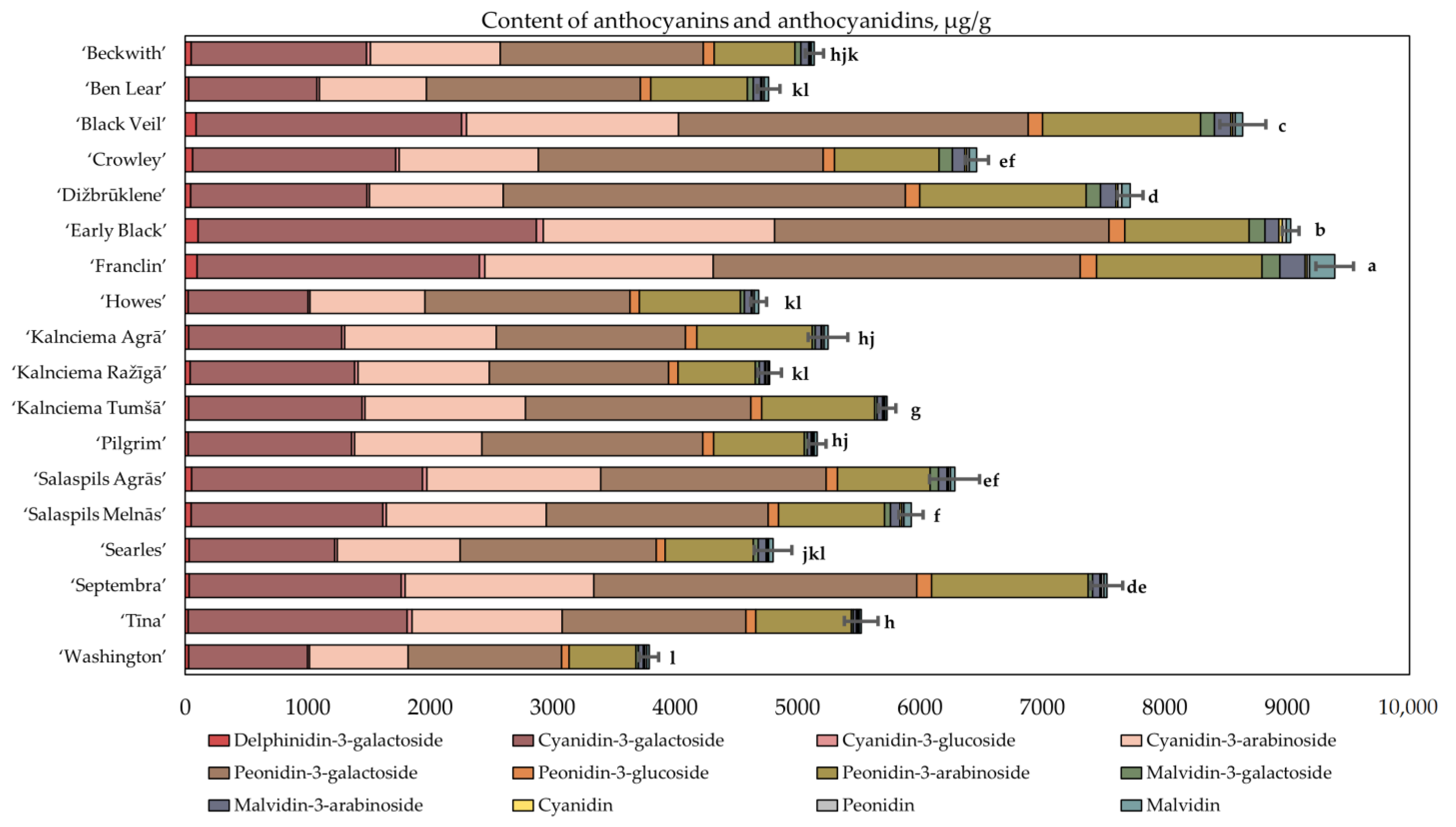
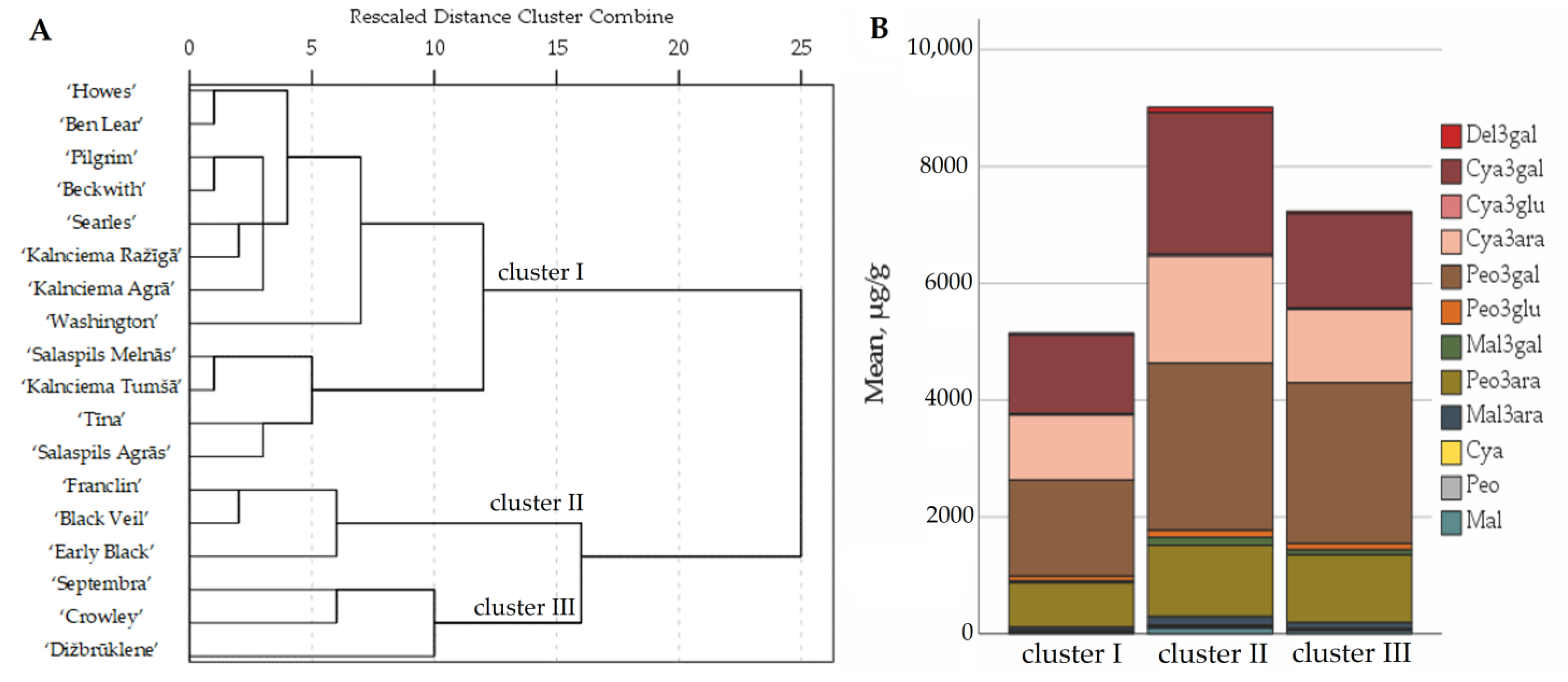
2.1. Determination of the Quantitative Composition of Anthocyanins and Anthocyanidins in Fruit Samples of Large Cranberry Cultivars
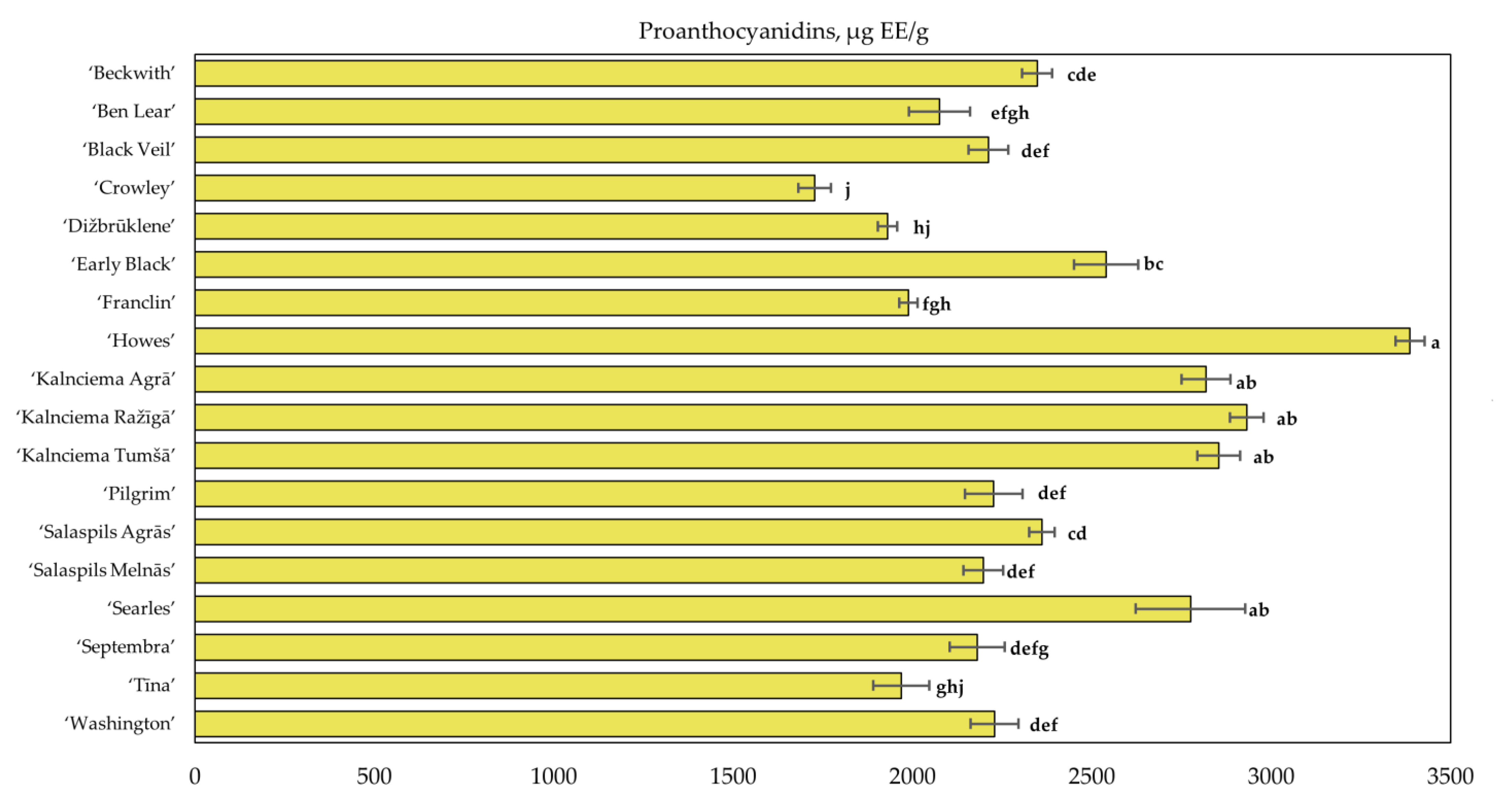
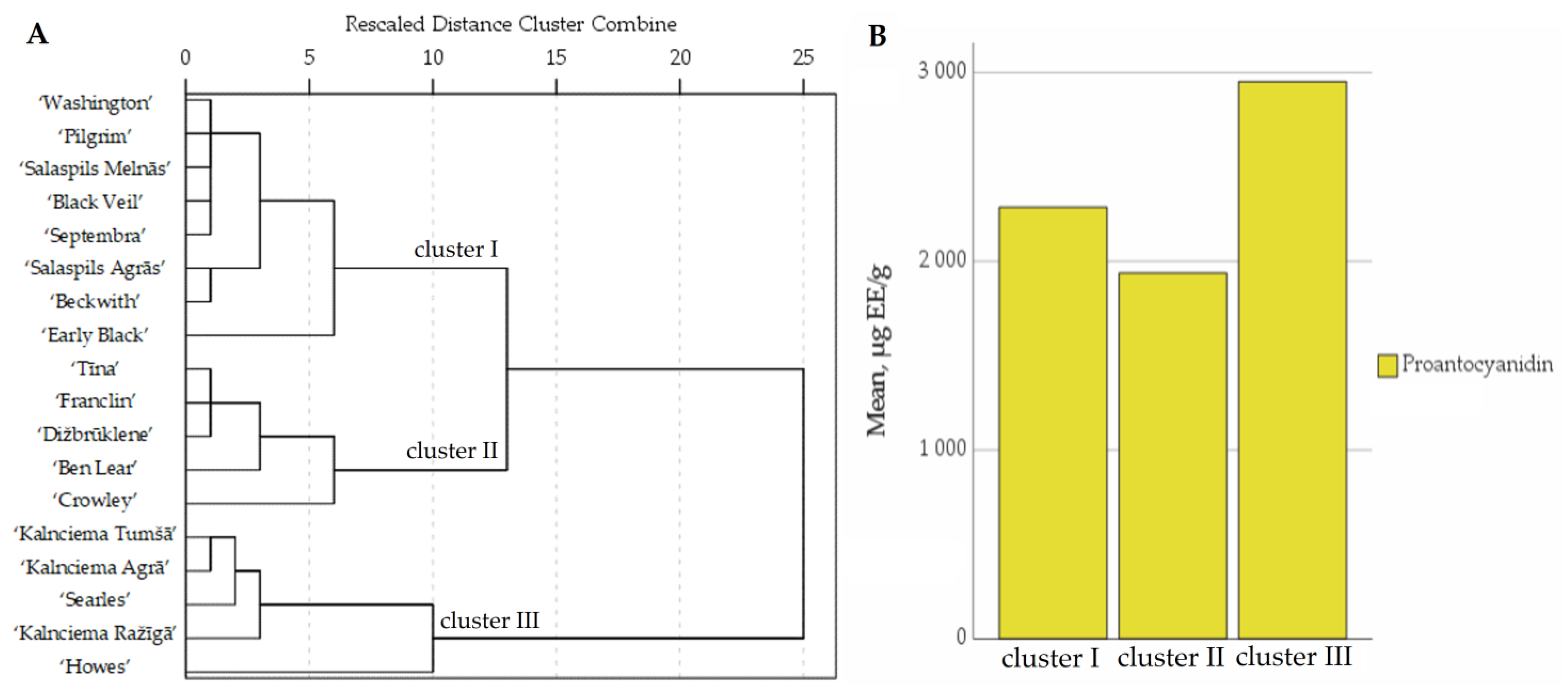
2.2. Determination of the Quantitative Composition of Proanthocyanidins in Fruit Samples of Large Cranberry Cultivars
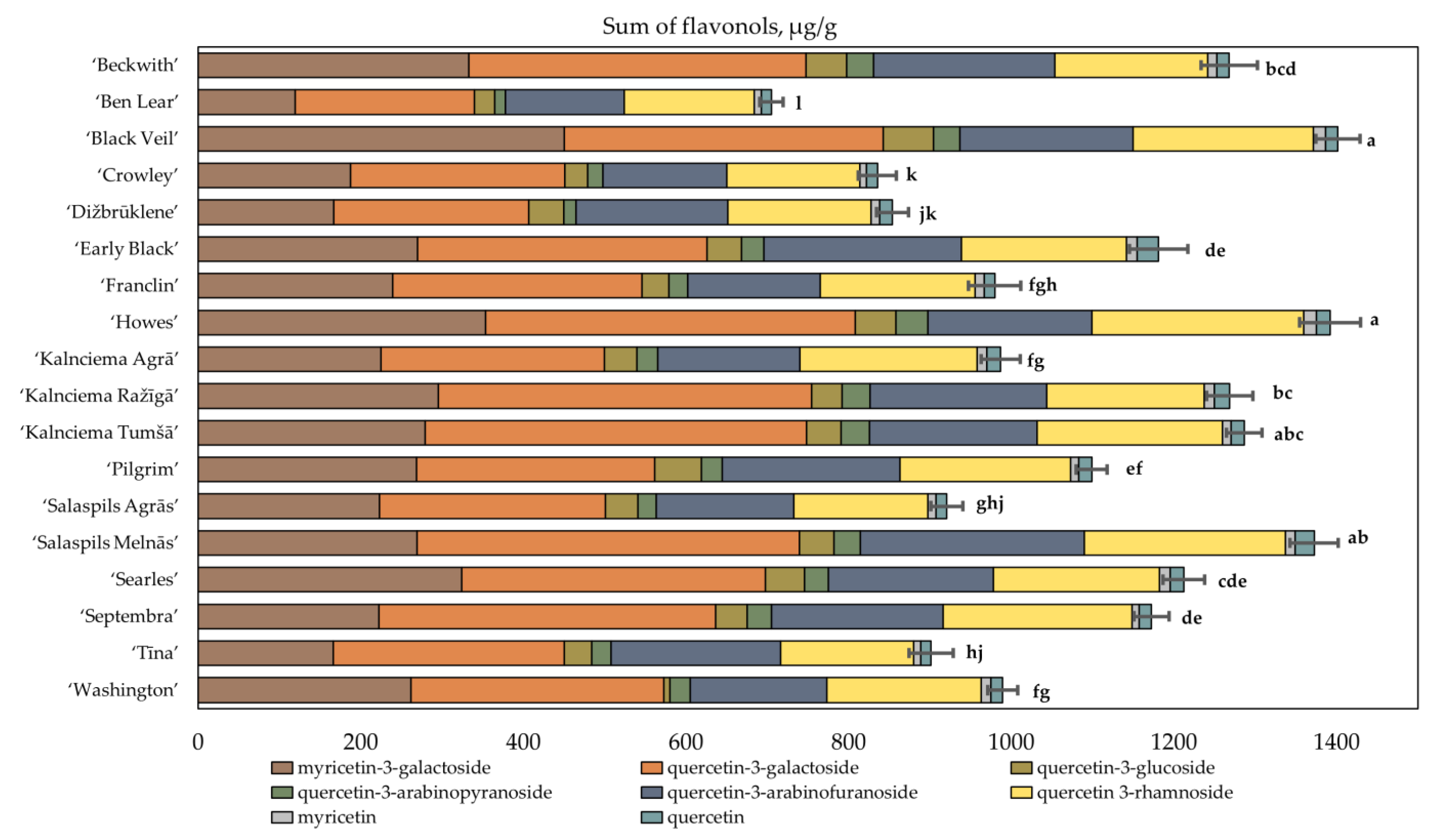
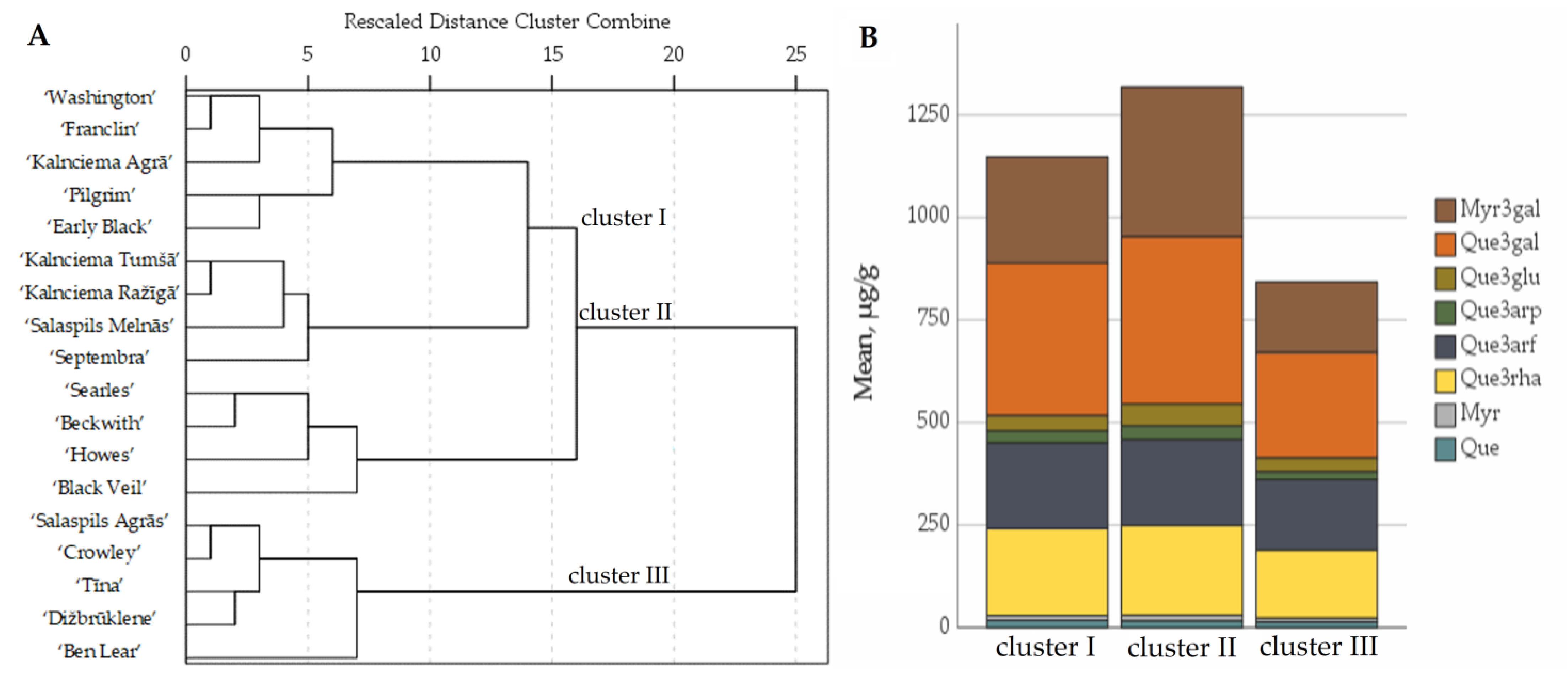
2.3. Determination of the Quantitative Composition of Flavanols in Fruit Samples of Large Cranberry Cultivars
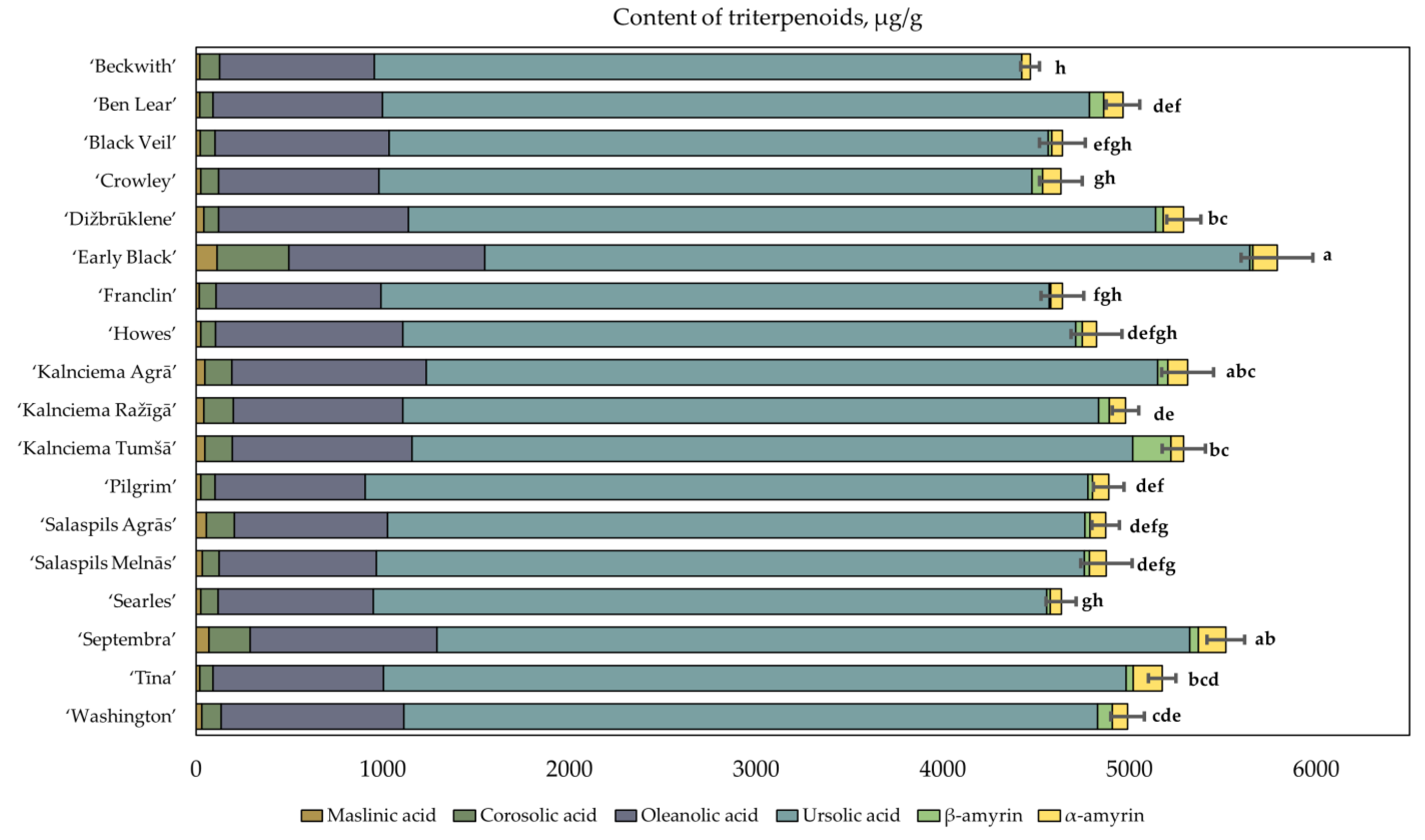
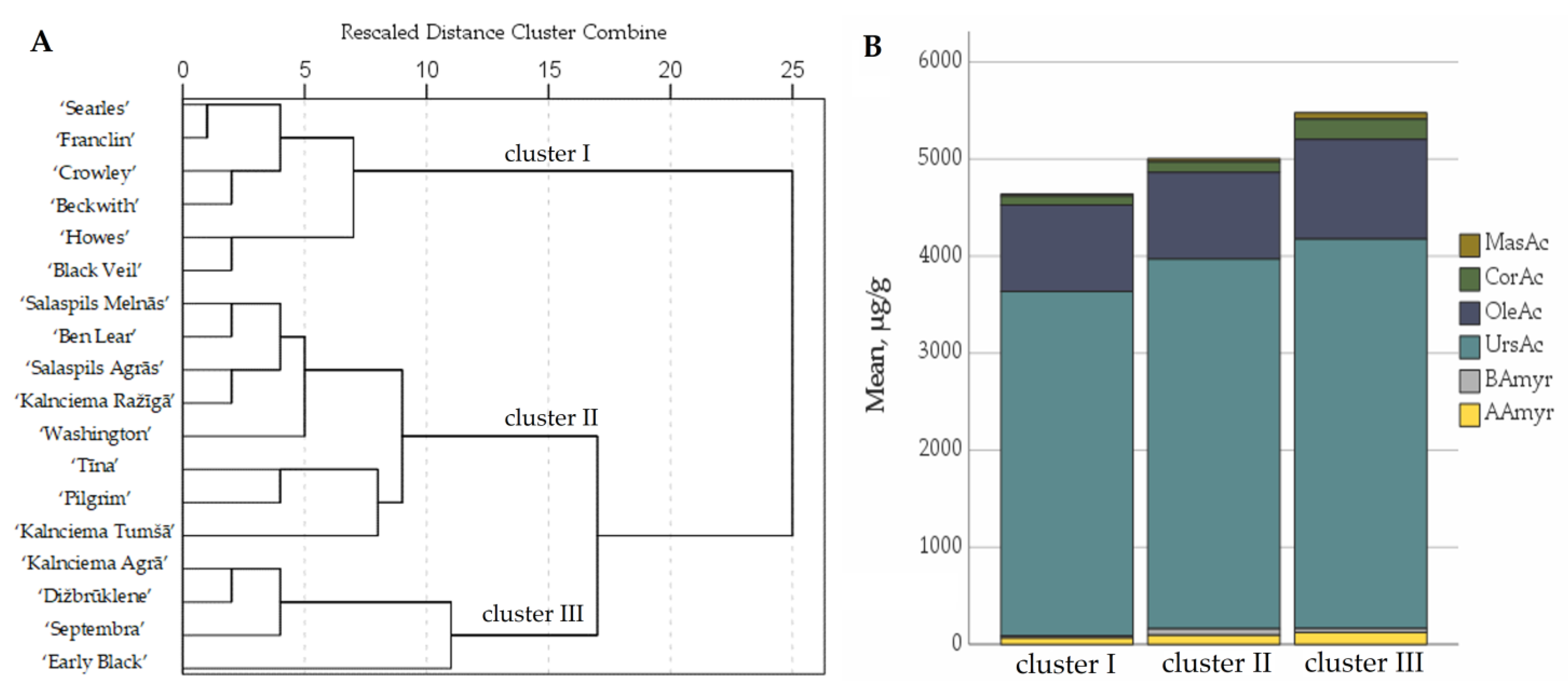
2.4. Determination of the Quantitative Composition of Triterpenoids in Fruit Samples of Large Cranberry Cultivars
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. Extraction Procedure
3.4. Chromatographic Methods
3.4.1. Determination of Anthocyanins and Anthocyanidins
3.4.2. Determination of Flavonols
3.4.3. Determination of Triterpenoids
3.5. Spectrophotometric Studies
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sultana, N.; Menzel, G.; Seibt, K.M.; Garcia, S.; Serçe, S.; Heitkam, T. Genome-wide analysis of long terminal repeat retrotransposons from the cranberry Vaccinium macrocarpon. J. Berry Res. 2022, 12, 165–185. [Google Scholar] [CrossRef]
- Vorsa, N.; Zalapa, J. Domestication, genetics, and genomics of the American cranberry. Plant Breed. Rev. 2019, 43, 279–315. [Google Scholar] [CrossRef]
- Diaz-Garcia, L.; Covarrubias-Pazaran, G.; Johnson-Cicalese, J.; Vorsa, N.; Zalapa, J. Genotyping-by-Sequencing Identifies Historical Breeding Stages of the Recently Domesticated American Cranberry. Front. Plant Sci. 2020, 11, 607770. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive Compounds, Antioxidant Activity, and Biological Effects of European Cranberry (Vaccinium oxycoccos). Molecules 2018, 24, 24. [Google Scholar] [CrossRef]
- Viskelis, P.; Rubinskienė, M.; Jasutienė, I.; Šarkinas, A.; Daubaras, R.; Česonienė, L. Anthocyanins, Antioxidative, and Antimicrobial Properties of American Cranberry (Vaccinium macrocarpon Ait.) and their Press Cakes. J. Food Sci. 2009, 74, 157–161. [Google Scholar] [CrossRef]
- Karlsons, A.; Osvalde, A.; Čekstere, G.; Pormale, J. Research on the mineral composition of cultivated and wild blueberries and cranberries. Agron. Res. 2018, 16, 454–463. [Google Scholar] [CrossRef]
- Osvalde, A.; Karlsons, A.; Pormale, J.; Nollendorfs, V. Renovation of extracted high bogs in Latvia: Mineral nutrition and ecological aspects of American cranberry cultivation. Anadolu J. Agric. Sci. 2010, 25, 120–125. [Google Scholar] [CrossRef]
- Vilka, L.; Volkova, J. Morphological Diversity of Phomopsis vaccinii Isolates from Cranberry (Vaccinium macrocarpon Ait.) in Latvia. Proc. Latv. Univ. Agric. 2015, 33, 328. [Google Scholar] [CrossRef]
- UN Food and Agriculture Organization. Cranberry Production in 2020. Corporate Statistica Database. 2020. Available online: https://www.fao.org/faostat/en/#compare (accessed on 13 September 2022).
- Česonienė, L.; Daubaras, R.; Jasutienė, I.; Venclovienė, J.; Miliauskienė, I. Evaluation of the Biochemical Components and Chromatic Properties of the Juice of Vaccinium macrocarpon Aiton and Vaccinium oxycoccos L. Plant Foods Hum. Nutr. 2011, 66, 238–244. [Google Scholar] [CrossRef]
- Ikase, L. Results of fruit breeding in baltic and nordic states. Nordic view to sustainable rural development. In Proceedings of the 25th NJF Congress, Ryga, Latvia, 16–18 June 2015. [Google Scholar]
- Karppinen, K.; Zoratti, L.; Nguyenquynh, N.; Häggman, H.; Jaakola, L. On the Developmental and Environmental Regulation of Secondary Metabolism in Vaccinium spp. Berries. Front. Plant Sci. 2016, 7, 655. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Phytochemical Compounds and Antioxidant Activity in Different Cultivars of Cranberry (Vaccinium Macrocarpon L). J. Food Sci. 2017, 82, 2569–2575. [Google Scholar] [CrossRef]
- Vlasova, I.; Gontova, T.; Grytsyk, L.; Zhumashova, G.; Sayakova, G.; Boshkayeva, A.; Shanaida, M.; Koshovyi, O. Determination of standardization parameters of Oxycoccus macrocarpus (Ait.) Pursh and Oxycoccus palustris Pers. Leaves. Sci. Pharm. Sci. 2022, 3, 48–57. [Google Scholar] [CrossRef]
- Zhang, J.; Kilmartin, P.A.; Peng, Y.; Chen, X.; Quek, S.Y. Identification of Key Aroma Compounds in Cranberry Juices as Influenced by Vinification. J. Agric. Food Chem. 2020, 68, 279–291. [Google Scholar] [CrossRef]
- Brendler, T.; Howell, A. American Cranberry (Vaccinium macrocarpon Ait.) and the Maintenance of Urinary Tract Health. In Medicinal and Aromatic Plants of North America; Máthé, Á., Ed.; Springer: Cham, Switzerland, 2020; Volume 6, pp. 81–117. [Google Scholar] [CrossRef]
- Navas, M.J.; Jiménez-Moreno, A.M.; Bueno, J.M.; Sáez-Plaza, P.; Asuero, A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part IV: Extraction of Anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 313–342. [Google Scholar] [CrossRef]
- Bueno, J.M.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jiménez, A.M.; Fett, R.; Asuero, A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part II: Chemical Structure, Color, and Intake of Anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151. [Google Scholar] [CrossRef]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhu, H.; Ma, N.; Ma, K.Y.; Chen, Z.-Y. Blueberry and cranberry anthocyanin extracts reduce bodyweight and modulate gut microbiota in C57BL/6 J mice fed with a high-fat diet. Eur. J. Nutr. 2021, 60, 2735–2746. [Google Scholar] [CrossRef]
- Brown, P.N.; Murch, S.J.; Shipley, P. Phytochemical Diversity of Cranberry (Vaccinium macrocarpon Aiton) Cultivars by Anthocyanin Determination and Metabolomic Profiling with Chemometric Analysis. J. Agric. Food Chem. 2012, 60, 261–271. [Google Scholar] [CrossRef]
- Gardana, C.; Scialpi, A.; Fachechi, C.; Simonetti, P. Identification of markers for the authentication of cranberry extract and cranberry-based food supplements. Heliyon 2020, 6, e03863. [Google Scholar] [CrossRef]
- Urbstaite, R.; Raudone, L.; Janulis, V. Phytogenotypic Anthocyanin Profiles and Antioxidant Activity Variation in Fruit Samples of the American Cranberry (Vaccinium macrocarpon Aiton). Antioxidants 2022, 11, 250. [Google Scholar] [CrossRef]
- Isaak, C.K.; Petkau, J.C.; Blewett, H.O.K.; Siow, Y.L. Lingonberry anthocyanins protect cardiac cells from oxidative-stress-induced apoptosis. Can. J. Physiol. Pharmacol. 2017, 95, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Sapers, G.M.; Phillips, J.G.; Rudolf, H.M.; DiVito, A.M. Cranberry Quality: Selection Procedures for Breeding Programs. J. Am. Soc. Hortic. Sci. 1983, 108, 241–246. [Google Scholar] [CrossRef]
- Tikuma, B.; Liepniece, M.; Sterne, D.; Abolins, M.; Seglina, D.; Krasnova, I. Preliminary results of biochemical composition of two cranberry species grown in Latvia. Acta Hortic. 2014, 1017, 209–214. [Google Scholar] [CrossRef]
- Shingisov, A.U.; Alibekov, R.S.; Tastemirova, U.U. Anthocyanins and anthocyanidins are multifunctional natural additives. In Proceedings of the IX International Science Conference—Problems and Tasks of Modern Science and Practice, Bordeaux, France, 15–17 November 2021; pp. 97–102. [Google Scholar]
- Lee, J. Anthocyanin analyses of Vaccinium fruit dietary supplements. Food Sci. Nutr. 2016, 4, 742–752. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Haya, J.; Chedraui, P. Vaccinium macrocarpon: An interesting option for women with recurrent urinary tract infections and other health benefits. J. Obstet. Gynaecol. Res. 2009, 35, 630–639. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Basu, A.; Krueger, C.G.; Lila, M.A.; Neto, C.C.; Novotny, J.A.; Reed, J.D.; Rodriguez-Mateos, A.; Toner, C.D. Impact of Cranberries on Gut Microbiota and Cardiometabolic Health: Proceedings of the Cranberry Health Research Conference 2015. Adv. Nutr. Int. Rev. J. 2016, 7, 759S–770S. [Google Scholar] [CrossRef]
- Krueger, C.G.; Reed, J.D.; Feliciano, R.P.; Howell, A.B. Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health. Anal. Bioanal. Chem. 2013, 405, 4385–4395. [Google Scholar] [CrossRef]
- Lu, Y.; Pekerti, B.N.; Toh, Z.S.; Broom, F.; Savage, G.; Liu, S.Q.; Huang, D. Physico-chemical parameters and proanthocyanidin profiles of cranberries cultivated in New Zealand. J. Food Compos. Anal. 2017, 63, 1–7. [Google Scholar] [CrossRef]
- Rupasinghe, V.; Neir, S.V.; Parmar, I. Polyphenol characterization, anti-oxidant, anti-proliferation and anti-tyrosinase activity of cranberry pomace. Funct. Foods Health Dis. 2016, 6, 754. [Google Scholar] [CrossRef]
- Šedbarė, R.; Siliņa, D.; Janulis, V. Evaluation of the Phytochemical Composition of Phenolic and Triterpene Compounds in Fruit of Large Cranberries (Vaccinium macrocarpon Aiton) Grown in Latvia. Plants 2022, 11, 2725. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prioret, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Vorsa, N.; Howell, A.B.; Foo, L.Y.; Lu, Y. Structure and genetic variation of cranberry proanthocyanidins that inhibit adherence of uropathogenic P-fimbriated E. coli. In Food Factors in Health Promotion and Disease Prevention; Shahidi, F., Ed.; ACS Books: Washington, DC, USA, 2003; pp. 298–311. [Google Scholar] [CrossRef]
- Wang, Y.; Johnson-Cicalese, J.; Singh, A.P.; Vorsa, N. Characterization and quantification of flavonoids and organic acids over fruit development in American cranberry (Vaccinium macrocarpon) cultivars using HPLC and APCI-MS/MS. Plant Sci. 2017, 262, 91–102. [Google Scholar] [CrossRef]
- Carpenter, J.L.; Caruso, F.L.; Tata, A.; Vorsa, N.; Neto, C.C. Variation in proanthocyanidin content and composition among commonly grown North American cranberry cultivars (Vaccinium macrocarpon): Proanthocyanidin content of cranberries. J. Sci. Food Agric. 2014, 94, 2738–2745. [Google Scholar] [CrossRef]
- Česonienė, L.; Daubaras, R. Phytochemical Composition of the Large Cranberry (Vaccinium macrocarpon) and the Small Cranberry (Vaccinium oxycoccos). In Nutritional Composition of Fruit Cultivars, 1st ed.; Simmonds, M.S.J., Preedy, V.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 173–194. [Google Scholar] [CrossRef]
- Narwojsz, A.; Tańska, M.; Mazur, B.; Borowska, E.J. Fruit Physical Features, Phenolic Compounds Profile and Inhibition Activities of Cranberry Cultivars (Vaccinium macrocarpon) Compared to Wild-Grown Cranberry (Vaccinium oxycoccus). Plant Foods Hum. Nutr. 2019, 74, 300–306. [Google Scholar] [CrossRef]
- Desrouillères, K.; Millette, M.; Bagheri, L.; Maherani, B.; Jamshidian, M.; Lacroix, M. The synergistic effect of cell wall extracted from probiotic biomass containing Lactobacillus acidophilus CL1285, L. casei LBC80R, and L. rhamnosus CLR2 on the anticancer activity of cranberry juice—HPLC fractions. J. Food Biochem. 2020, 44, e13195. [Google Scholar] [CrossRef]
- Ferguson, P.J.; Kurowska, E.; Freeman, D.J.; Chambers, A.F.; Koropatnick, D.J. A Flavonoid Fraction from Cranberry Extract Inhibits Proliferation of Human Tumor Cell Lines. J. Nutr. 2004, 134, 1529–1535. [Google Scholar] [CrossRef]
- Xue, L.; Liu, C.; Ma, H.; Seeram, N.P.; Neto, C.C. Anti-inflammatory Activities of Cranberry Fruit Extracts in Human THP-1 Monocytes are Influenced by Their Phytochemical Composition. ACS Food Sci. Technol. 2022, 2, 75–83. [Google Scholar] [CrossRef]
- Reed, J. Cranberry Flavonoids, Atherosclerosis and Cardiovascular Health. Crit. Rev. Food Sci. Nutr. 2002, 42, 301–316. [Google Scholar] [CrossRef]
- Urbstaite, R.; Raudone, L.; Liaudanskas, M.; Janulis, V. Development, Validation, and Application of the UPLC-DAD Methodology for the Evaluation of the Qualitative and Quantitative Composition of Phenolic Compounds in the Fruit of American Cranberry (Vaccinium macrocarpon Aiton). Molecules 2022, 27, 467. [Google Scholar] [CrossRef]
- Gudžinskaitė, I.; Stackevičienė, E.; Liaudanskas, M.; Zymonė, K.; Žvikas, V.; Viškelis, J.; Urbštaitė, R.; Janulis, V. Variability in the Qualitative and Quantitative Composition and Content of Phenolic Compounds in the Fruit of Introduced American Cranberry (Vaccinium macrocarpon Aiton). Plants 2020, 9, 1379. [Google Scholar] [CrossRef]
- Wang, Y.; Vorsa, N.; de, B. Harrington, P.; Chen, P. Nontargeted Metabolomic Study on Variation of Phenolics in Different Cranberry Cultivars Using UPLC-IM—HRMS. J. Agric. Food Chem. 2018, 66, 12206–12216. [Google Scholar] [CrossRef] [PubMed]
- Prasain, J.K.; Grubbs, C.; Barnes, S. Cranberry anti-cancer compounds and their uptake and metabolism: An updated review. J. Berry Res. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Prasain, J.K.; Rajbhandari, R.; Keeton, A.B.; Piazza, G.A.; Barnes, S. Metabolism and growth inhibitory activity of cranberry derived flavonoids in bladder cancer cells. Food Funct. 2016, 7, 4012–4019. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C.; Amoroso, J.W.; Liberty, A.M. Anticancer activities of cranberry phytochemicals: An update. Mol. Nutr. Food Res. 2008, 52, S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, A.; Chen, E.; Singh, R.K.; Chichester, C.O.; Moore, R.G.; Singh, A.P.; Vorsa, N. The cranberry flavonoids PAC DP-9 and quercetin aglycone induce cytotoxicity and cell cycle arrest and increase cisplatin sensitivity in ovarian cancer cells. Int. J. Oncol. 2015, 46, 1924–1934. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health: Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- Kondo, M.; MacKinnon, S.L.; Craft, C.C.; Matchett, M.D.; Hurta, R.A.R.; Neto, C.C. Ursolic acid and its esters: Occurrence in cranberries and other Vaccinium fruit and effects on matrix metalloproteinase activity in DU145 prostate tumor cells: Anti-tumor activity and content of ursolic acid from Vaccinium fruit. J. Sci. Food Agric. 2011, 91, 789–796. [Google Scholar] [CrossRef]
- Neto, C.C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef]
- Klavins, L.; Klavins, M. Cuticular Wax Composition of Wild and Cultivated Northern Berries. Foods 2020, 9, 587. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. The effect of different maturity stages on phytochemical composition and antioxidant capacity of cranberry cultivars. Eur. Food Res. Technol. 2018, 244, 705–719. [Google Scholar] [CrossRef]
- Tsai, S.J.; Yin, M.C. Antioxidative and Anti-Inflammatory Protection of Oleanolic Acid and Ursolic Acid in PC12 Cells. J. Food Sci. 2008, 73, H174–H178. [Google Scholar] [CrossRef]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Gautam, S.; Aggarwal, B.B.; Sainis, K.B. Potent Anti-Inflammatory Activity of Ursolic Acid, a Triterpenoid Antioxidant, Is Mediated through Suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef]
- Murphy, B.T.; MacKinnon, S.L.; Yan, X.; Hammond, G.B.; Vaisberg, A.J.; Neto, C.C. Identification of Triterpene Hydroxycinnamates with in Vitro Antitumor Activity from Whole Cranberry Fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 2003, 51, 3541–3545. [Google Scholar] [CrossRef]
- Sakipova, S.; Jakovičs, A.; Gendelis, S. Studies Energy Efficiency of the Renewable Sources use Considering Climate in Latvia. Energy Procedia 2015, 78, 1980–1984. [Google Scholar] [CrossRef]
- Stivrins, N.; Briede, A.; Steinberga, D.; Jasiunas, N.; Jeskins, J.; Kalnina, L.; Maksims, A.; Rendenieks, Z.; Trasune, L. Natural and Human-Transformed Vegetation and Landscape Reflected by Modern Pollen Data in the Boreonemoral Zone of Northeastern Europe. Forests 2021, 12, 1166. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; p. 51. [Google Scholar]
- Sedbare, R.; Raudone, L.; Zvikas, V.; Viskelis, J.; Liaudanskas, M.; Janulis, V. Development and Validation of the UPLC-DAD Methodology for the Detection of Triterpenoids and Phytosterols in Fruit Samples of Vaccinium macrocarpon Aiton and Vaccinium oxycoccos L. Molecules 2022, 27, 4403. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Motiekaityte, V.; Vainoriene, R.; Liaudanskas, M.; Raudone, L. Development, validation, and application of UPLC-PDA method for anthocyanins profiling in Vaccinium L. berries. J. Berry Res. 2021, 11, 583–599. [Google Scholar] [CrossRef]
- Heil, M.; Baumann, B.; Andary, C.; Linsenmair, E.K.; McKey, D. Extraction and quantification of ‘condensed tannins’ as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften 2002, 89, 519–524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šedbarė, R.; Jakštāne, G.; Janulis, V. Phytochemical Composition of the Fruit of Large Cranberry (Vaccinium macrocarpon Aiton) Cultivars Grown in the Collection of the National Botanic Garden of Latvia. Plants 2023, 12, 771. https://doi.org/10.3390/plants12040771
Šedbarė R, Jakštāne G, Janulis V. Phytochemical Composition of the Fruit of Large Cranberry (Vaccinium macrocarpon Aiton) Cultivars Grown in the Collection of the National Botanic Garden of Latvia. Plants. 2023; 12(4):771. https://doi.org/10.3390/plants12040771
Chicago/Turabian StyleŠedbarė, Rima, Ginta Jakštāne, and Valdimaras Janulis. 2023. "Phytochemical Composition of the Fruit of Large Cranberry (Vaccinium macrocarpon Aiton) Cultivars Grown in the Collection of the National Botanic Garden of Latvia" Plants 12, no. 4: 771. https://doi.org/10.3390/plants12040771
APA StyleŠedbarė, R., Jakštāne, G., & Janulis, V. (2023). Phytochemical Composition of the Fruit of Large Cranberry (Vaccinium macrocarpon Aiton) Cultivars Grown in the Collection of the National Botanic Garden of Latvia. Plants, 12(4), 771. https://doi.org/10.3390/plants12040771







