How Does Diurnal and Nocturnal Warming Affect the Freezing Resistance of Antarctic Vascular Plants?
Abstract
1. Introduction
2. Results
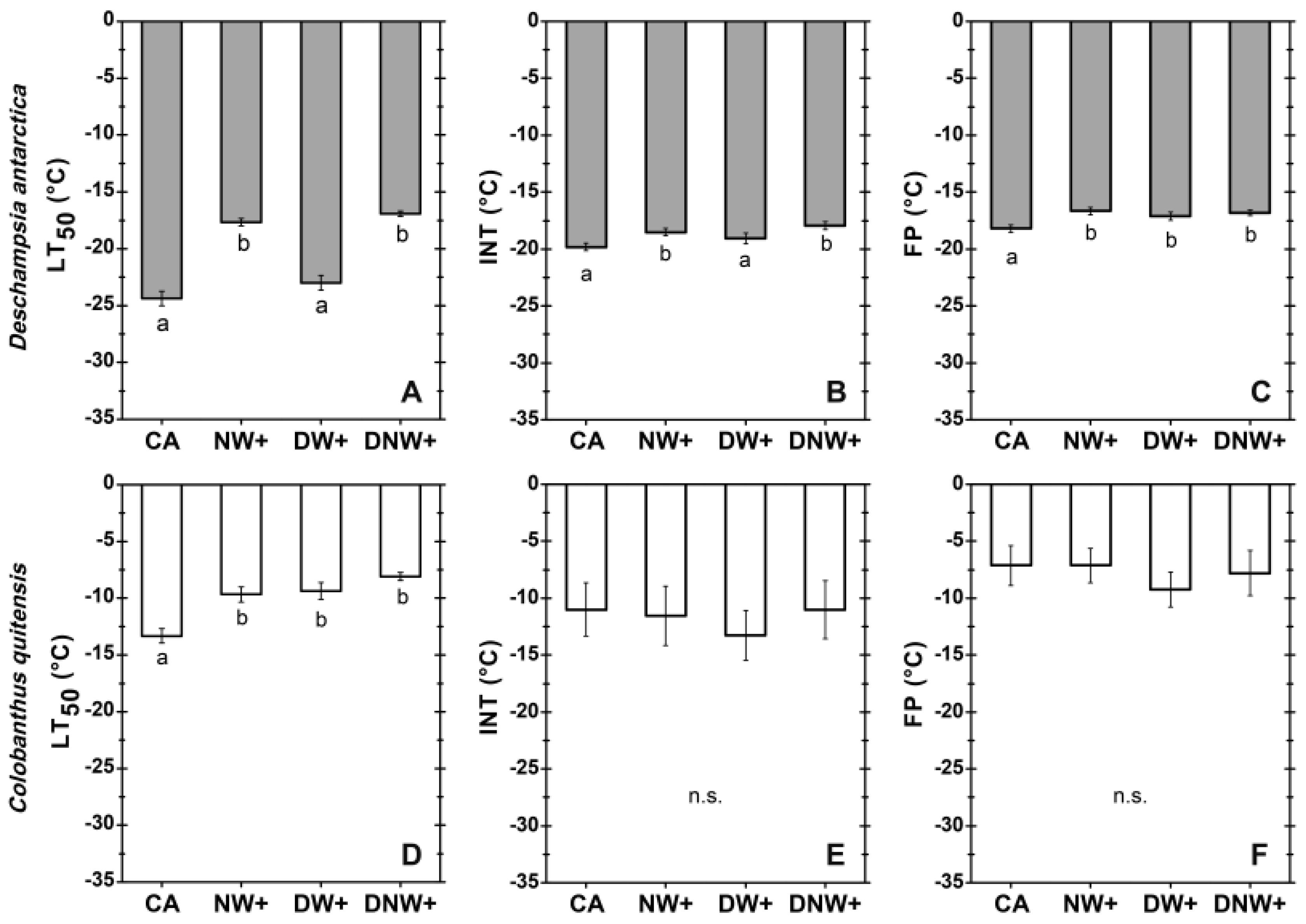
2.1. Freezing Resistance of D. antarctica and C. quitensis Leaves
2.2. Soluble Carbohydrates Content
2.3. Dehydrins Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material, Growth Conditions, and Warming Treatments
4.2. Freezing Tolerance (LT50)
4.3. Thermal Analysis
4.4. Carbohydrate Analyses
4.5. Immunoblot Analyses of Dehydrin-Like Peptides
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Mellado, G.A.; Jaña, R.; Casanova-Katny, M.A. Antarctic hairgrass expansion in the South Shetland archipelago and Antarctic Peninsula revisited. Polar Biol. 2011, 34, 1679–1688. [Google Scholar] [CrossRef]
- Cannone, N.; Guglielmin, M.; Convey, P.; Worland, M.R.; Favero Longo, S.E. Vascular plant changes in extreme environments: Effects of multiple drivers. Clim. Chang. 2016, 134, 651–665. [Google Scholar] [CrossRef]
- Day, T.A.; Ruhland, C.T.; Grobe, C.W.; Xiong, F. Growth and reproduction of Antarctic vascular plants in response to warming and UV radiation reductions in the field. Oecologia 1999, 119, 24–35. [Google Scholar] [CrossRef]
- Fowbert, J.A.; Smith, R.I.L. Rapid population increases in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arct. Alp. Res. 1994, 26, 290–296. [Google Scholar] [CrossRef]
- Smith, R.I.L. Vascular plants as bioindicators of regional warming in Antarctica. Oecologia 1994, 99, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.A.; Wasley, J.; Tobin, A.K. Living on the edge—Plants and global change in continental and maritime Antarctica. Glob. Chang. Biol. 2003, 9, 1681–1717. [Google Scholar] [CrossRef]
- Convey, P. The influence of environmental characteristics on life history attributes of Antarctic terrestrial biota. Biol. Rev. 1996, 71, 191–225. [Google Scholar] [CrossRef]
- Convey, P.; Chown, S.L.; Clarke, A.; Barnes, D.K.A.; Bokhorst, S.; Cummings, V.; Ducklow, H.W.; Frati, F.; Green, T.G.A.; Gordon, S.; et al. The spatial structure of antarctic biodiversity. Ecol. Monogr. 2014, 84, 203–244. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Sáez, P.; Sanhueza, C.; Sierra-Almeida, A.; Rabert, C.; Corcuera, L.J.; Alberdi, M.; Bravo, L.A. Ecophysiological traits of Antarctic vascular plants: Their importance in the responses to climate change. Plant Ecol. 2016, 217, 343–358. [Google Scholar] [CrossRef]
- Zúñiga-Feest, A.; Bascuñán-Godoy, L.; Reyes-Diaz, M.; Bravo, L.A.; Corcuera, L.J. Is survival after ice encasement related with sugar distribution in organs of the Antarctic plants Deschampsia antarctica Desv. (Poaceae) and Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae)? Polar Biol. 2009, 32, 583–591. [Google Scholar] [CrossRef]
- Piotrowicz-Cieślak, A.I.; Giełwanowska, I.; Bochenek, A.; Loro, P.; Górecki, R.J. Carbohydrates in Colobanthus quitensis and Deschampsia antarctica. Acta Soc. Bot. Pol. 2005, 74, 209–217. [Google Scholar] [CrossRef]
- Pastorczyk, M.; Giełwanowska, I.; Lahuta, L.B. Changes in soluble carbohydrates in polar Caryophyllaceae and Poaceae plants in response to chilling. Acta Physiol. Plant. 2014, 36, 1771–1780. [Google Scholar] [CrossRef]
- Sanhueza, C.; Fuentes, F.; Cortes, D.; Bascuñan, L.; Saez, P.; Bravo, L.A.; Cavieres, L. Contrasting thermal acclimation of leaf dark respiration and photosynthesis of Antarctic vascular plant species exposed to nocturnal warming. Physiol. Plant. 2019, 167, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga, G.E.; Alberdi, M.; Corcuera, L.J. Non-structural carbohydrates in Deschampsia antarctica Desv. from South Shetland Islands, Maritime Antarctic. Environ. Exp. Bot. 1996, 36, 393–399. [Google Scholar] [CrossRef]
- John, U.P.; Polotnianka, R.M.; Sivakumaran, K.A.; Chew, O.; MacKin, L.; Kuiper, M.J.; Talbot, J.P.; Nugent, G.D.; Mautord, J.; Schrauf, G.E.; et al. Ice recrystallization inhibition proteins (IRIPs) and freeze tolerance in the cryophilic Antarctic hair grass Deschampsia antarctica E. Desv. Plant Cell Environ. 2009, 32, 336–348. [Google Scholar] [CrossRef]
- Chew, O.; Lelean, S.; John, U.P.; Spangenberg, G.C. Cold acclimation induces rapid and dynamic changes in freeze tolerance mechanisms in the cryophile Deschampsia antarctica E. Desv. Plant Cell Environ. 2012, 35, 829–837. [Google Scholar] [CrossRef]
- Short, S.; Díaz, R.; Quiñones, J.; Beltrán, J.; Farías, J.G.; Graether, S.P.; Bravo, L.A. Effect of in vitro cold acclimation of Deschampsia antarctica on the accumulation of proteins with antifreeze activity. J. Exp. Bot. 2020, 71, 2933–2942. [Google Scholar] [CrossRef]
- Olave-Concha, N.; Ruiz-Lara, S.; Muñoz, X.; Bravo, L.A.; Corcuera, L.J. Accumulation of dehydrin transcripts and proteins in response to abiotic stresses in Deschampsia antarctica. Antarct. Sci. 2004, 16, 175–184. [Google Scholar] [CrossRef]
- Hoffman, L.; DaCosta, M.; Scott Ebdon, J. Examination of cold deacclimation sensitivity of annual bluegrass and creeping bentgrass. Crop Sci. 2014, 54, 413–420. [Google Scholar] [CrossRef]
- Jørgensen, M.; Østrem, L.; Höglind, M. De-hardening in contrasting cultivars of timothy and perennial ryegrass during winter and spring. Grass Forage Sci. 2010, 65, 38–48. [Google Scholar] [CrossRef]
- Pagter, M.; Arora, R. Winter survival and deacclimation of perennials under warming climate: Physiological perspectives. Physiol. Plant. 2012, 147, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Vyse, K.; Pagter, M.; Zuther, E.; Hincha, D.K. Deacclimation after cold acclimation- a crucial, but widely neglected part of plant winter survival. J. Exp. Bot. 2019, 70, 4595–4604. [Google Scholar] [CrossRef] [PubMed]
- Kalberer, S.R.; Wisniewski, M.; Arora, R. Deacclimation and reacclimation of cold-hardy plants: Current understanding and emerging concepts. Plant Sci. 2006, 171, 3–16. [Google Scholar] [CrossRef]
- Convey, P. Antarctic Ecosystems. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Elsevier: San Diego, CA, USA, 2013; Volume 1, pp. 179–188. ISBN 9780123847195. [Google Scholar] [CrossRef]
- Sierra-Almeida, A.; Cavieres, L.A.; Bravo, L.A. Warmer temperatures affect the in situ freezing resistance of the antarctic vascular plants. Front. Plant Sci. 2018, 9, 1456. [Google Scholar] [CrossRef] [PubMed]
- Stocker, T. (Ed.) Intergovernmental Panel on Climate Change. In Climate Change 2013 – The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781107415324. [Google Scholar] [CrossRef]
- Davy, R.; Esau, I.; Chernokulsky, A.; Outten, S.; Zilitinkevich, S. Diurnal asymmetry to the observed global warming. Int. J. Climatol. 2017, 37, 79–93. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, X.; Du, G. Nonuniform time-lag effects of asymmetric warming on net primary productivity across global terrestrial biomes. Earth Interact. 2018, 22, 1–26. [Google Scholar] [CrossRef]
- Wan, S.; Xia, J.; Liu, W.; Niu, S. Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 2009, 90, 2700–2710. [Google Scholar] [CrossRef]
- Turnbull, M.H.; Murthy, R.; Griffin, K.L. The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant Cell Environ. 2002, 25, 1729–1737. [Google Scholar] [CrossRef]
- Hu, C.; Tian, Z.; Gu, S.; Guo, H.; Fan, Y.; Abid, M.; Chen, K.; Jiang, D.; Cao, W.; Dai, T. Winter and spring night-warming improve root extension and soil nitrogen supply to increase nitrogen uptake and utilization of winter wheat (Triticum aestivum L.). Eur. J. Agron. 2018, 96, 96–107. [Google Scholar] [CrossRef]
- Bokhorst, S.; Huiskes, A.D.; Aerts, R.; Convey, P. Variable temperature effects of Open Top Chambers at polar and alpine sites explained by irradiance and snow depth. Glob. Chang. Biol. 2013, 19, 64–74. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, P.; Tawaraya, K.; Tokida, T.; Fukuoka, M.; Yoshimoto, M.; Sakai, H.; Hasegawa, T.; Xu, X.; Cheng, W. Winter nocturnal warming affects the freeze-thaw frequency, soil aggregate distribution, and the contents and decomposability of C and N in paddy fields. Sci. Total Environ. 2022, 802, 149870. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Almeida, A.; Cavieres, L.A. Summer freezing resistance decreased in high-elevation plants exposed to experimental warming in the central Chilean Andes. Oecologia 2010, 163, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Rapacz, M.; Jurczyk, B.; Sasal, M. Deacclimation may be crucial for winter survival of cereals under warming climate. Plant Sci. 2017, 256, 5–15. [Google Scholar] [CrossRef]
- Bravo, L.A.; Ulloa, N.; Zuñiga, G.E.; Casanova, A.; Corcuera, L.J.; Alberdi, M. Cold resistance in antarctic angiosperms. Physiol. Plant. 2001, 111, 55–65. [Google Scholar] [CrossRef]
- Gianoli, E.; Inostroza, P.; Zúñiga-Feest, A.; Reyes-Díaz, M.; Cavieres, L.A.; Bravo, L.A.; Corcuera, L.J. Ecotypic Differentiation in Morphology and Cold Resistance in Populations of Colobanthus quitensis (Caryophyllaceae) from the Andes of Central Chile and the Maritime Antarctic. Arctic Antarct. Alp. Res. 2004, 36, 484–489. [Google Scholar] [CrossRef]
- Sakai, A.; Larcher, W. Frost Survival of Plants Responses and Adaptation to Freezing Stress; Springer: Berlin, Germany, 1987; ISBN 9783642717475. [Google Scholar] [CrossRef]
- Borovik, O.A.; Pomortsev, A.V.; Korsukova, A.V.; Polyakova, E.A.; Fomina, E.A.; Zabanova, N.S.; Grabelnych, O.I. Effect of Cold Acclimation and Deacclimation on the Content of Soluble Carbohydrates and Dehydrins in the Leaves of Winter Wheat. J. Stress Physiol. Biochem. 2019, 15, 62–67. [Google Scholar]
- Zúñiga-Feest, A.; Inostroza, P.; Vega, M.; Bravo, L.A.; Corcuera, L.J. Sugars and enzyme activity in the grass Deschampsia antarctica. Antarct. Sci. 2003, 15, 483–491. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; Uribe, E.; Zúñiga-Feest, A.; Corcuera, L.J.; Bravo, L.A. Low temperature regulates sucrose-phosphate synthase activity in Colobanthus quitensis (Kunth) Bartl. by decreasing its sensitivity to Pi and increased activation by glucose-6-phosphate. Polar Biol. 2006, 29, 1011–1017. [Google Scholar] [CrossRef]
- Hagiwara, T.; Hartel, R.W.; Matsukawa, S. Relationship between recrystallization rate of ice crystals in sugar solutions and water mobility in freeze-concentrated matrix. Food Biophys. 2006, 1, 74–82. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Kundu, A. Sugars and Sugar Polyols in Overcoming Environmental Stresses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, A., Kumar Tripathi, D., Eds.; Wiley Blackwell: New York, NY, USA, 2020; pp. 71–101. ISBN 9781119552154. [Google Scholar] [CrossRef]
- Strauss, G.; Hauser, H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Biophysics 1986, 83, 2422–2426. [Google Scholar] [CrossRef] [PubMed]
- Nägele, T.; Heyer, A.G. Approximating subcellular organisation of carbohydrate metabolism during cold acclimation in different natural accessions of Arabidopsis thaliana. New Phytol. 2013, 198, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Findling, S.; Zanger, K.; Krueger, S.; Lohaus, G. Subcellular distribution of raffinose oligosaccharides and other metabolites in summer and winter leaves of Ajuga reptans (Lamiaceae). Planta 2015, 241, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Knaupp, M.; Mishra, K.B.; Nedbal, L.; Heyer, A.G. Evidence for a role of raffinose in stabilizing photosystem II during freeze-thaw cycles. Planta 2011, 234, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, A.; Garcia, L.; Charrier, G.; Buttò, V.; Pichette, A.; Paré, M. Cold acclimation and deacclimation in wild blueberry: Direct and indirect influence of environmental factors and non-structural carbohydrates. Agric. For. Meteorol. 2021, 301–302, 108349. [Google Scholar] [CrossRef]
- Keller, I.; Müdsam, C.; Rodrigues, C.M.; Kischka, D.; Zierer, W.; Sonnewald, U.; Harms, K.; Czarnecki, O.; Fiedler-Wiechers, K.; Koch, W.; et al. Cold-Triggered Induction of ROS- and Raffinose Metabolism in Freezing-Sensitive Taproot Tissue of Sugar Beet. Front. Plant Sci. 2021, 12, 715767. [Google Scholar] [CrossRef] [PubMed]
- Strimbeck, G.R.; Kjellsen, T.D.; Schaberg, P.G.; Murakami, P.F. Dynamics of low-temperature acclimation in temperate and boreal conifer foliage in a mild winter climate. Tree Physiol. 2008, 28, 1365–1374. [Google Scholar] [CrossRef]
- Zuther, E.; Juszczak, I.; Ping Lee, Y.; Baier, M.; Hincha, D.K. Time-dependent deacclimation after cold acclimation in Arabidopsis thaliana accessions. Sci. Rep. 2015, 5, 12199. [Google Scholar] [CrossRef]
- Sáez, P.L.; Cavieres, L.A.; Galmés, J.; Gil-Pelegrín, E.; Peguero-Pina, J.J.; Sancho-Knapik, D.; Vivas, M.; Sanhueza, C.; Ramírez, C.F.; Rivera, B.K.; et al. In situ warming in the Antarctic: Effects on growth and photosynthesis in Antarctic vascular plants. New Phytol. 2018, 218, 1406–1418. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Group II late embryogenesis abundant (LEA) proteins: Structural and functional aspects in plant abiotic stress. Plant Growth Regul. 2016, 79, 1–17. [Google Scholar] [CrossRef]
- Vítámvás, P.; Prášil, I.T. WCS120 protein family and frost tolerance during cold acclimation, deacclimation and reacclimation of winter wheat. Plant Physiol. Biochem. 2008, 46, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, K.; Ervin, E.H.; Waltz, C.; Murphy, T. Metabolic changes during cold acclimation and deacclimation in five bermudagrass varieties. I. Proline, total amino acid, protein, and dehydrin expression. Crop Sci. 2011, 51, 838–846. [Google Scholar] [CrossRef]
- Rathore, N.; Kumar, P.; Mehta, N.; Swarnkar, M.K.; Shankar, R.; Chawla, A. Time-series RNA-Seq transcriptome profiling reveals novel insights about cold acclimation and de-acclimation processes in an evergreen shrub of high altitude. Sci. Rep. 2022, 12, 15553. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Jagła, M.; Daszkowska-Golec, A.; Fiust, A.; Kopeć, P.; Rapacz, M. Identification of the genetic basis of response to de-acclimation in winter barley. Int. J. Mol. Sci. 2021, 22, 1057. [Google Scholar] [CrossRef]
- Sandve, S.R.; Fjellheim, S. Did gene family expansions during the Eocene-Oligocene boundary climate cooling play a role in Pooideae adaptation to cool climates? Mol. Ecol. 2010, 19, 2075–2088. [Google Scholar] [CrossRef]
- Pagter, M.; Alpers, J.; Erban, A.; Kopka, J.; Zuther, E.; Hincha, D.K. Rapid transcriptional and metabolic regulation of the deacclimation process in cold acclimated Arabidopsis thaliana. BMC Genom. 2017, 18, 731. [Google Scholar] [CrossRef]
- Fowler, S.; Thomashow, M.F. Arabidopsis Transcriptome Profiling Indicates that Multiple Regulatory Pathways are Activated during Cold Acclimation in Addition to the CBF Cold Response Pathway. Plant Cell 2002, 14, 1675–1690. [Google Scholar] [CrossRef]
- Kume, S.; Kobayashi, F.; Ishibashi, M.; Ohno, R.; Nakamura, C.; Takumi, S. Differential and coordinated expression of Cbf and Cor/Lea genes during long-term cold acclimation in two wheat cultivars showing distinct levels of freezing tolerance. Genes Genet. Syst. 2005, 80, 185–197. [Google Scholar] [CrossRef]
- Jia, Y.; Ding, Y.; Shi, Y.; Zhang, X.; Gong, Z.; Yang, S. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016, 212, 345–353. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Al-Issawi, M.; Fuller, M.P. Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Interact. 2017, 12, 143–157. [Google Scholar] [CrossRef]
- Byun, M.Y.; Cui, L.H.; Lee, J.; Park, H.; Lee, A.; Kim, W.T.; Lee, H. Identification of Rice Genes Associated with Enhanced Cold Tolerance by Comparative Transcriptome Analysis with Two Transgenic Rice Plants Isolated from Antarctic Flowering Plant Deschampsia antarctica. Front. Plant Sci. 2018, 9, 601. [Google Scholar] [CrossRef] [PubMed]
- Close, T.J.; Fenton, R.D.; Moonan, F. A view of plant dehydrins using antibodies specific to the carboxy terminal peptide. Plant Mol. Biol. 1993, 23, 279–286. [Google Scholar] [CrossRef]
- Kejna, M. Air temperature on King George Island, South Shetland Islands, Antarctica. Polish Polar Res. 1999, 20, 183–201. [Google Scholar]
- Plenzler, J.; Budzik, T.; Puczko, D.; Bialik, R.J. Climatic conditions at Arctowski Station (King George Island, West Antarctica) in 2013–2017 against the background of regional changes. Polish Polar Res. 2019, 40, 1–27. [Google Scholar] [CrossRef]
- Larcher, W. Temperature stress and survival ability of Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosyst. 2000, 134, 279–295. [Google Scholar] [CrossRef]
- Arnold, P.A.; Bricenõ, V.F.; Gowland, K.M.; Catling, A.A.; Bravo, L.A.; Nicotra, A.B. A high-throughput method for measuring critical thermal limits of leaves by chlorophyll imaging fluorescence. Funct. Plant Biol. 2021, 48, 634–646. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: New York, NY, USA, 2003. [Google Scholar]
- Dickson, R.E. Analytical Procedures for the Sequential Extraction of 14C-Labeled Constituents from Leaves, Bark and Wood of Cottonwood Plants. Physiol. Plant. 1979, 45, 480–488. [Google Scholar] [CrossRef]
- Oberlerchner, J.T.; Böhmdorfer, S.; Rosenau, T.; Potthast, A. A matrix-resistant HPTLC method to quantify monosaccharides in wood-based lignocellulose biorefinery streams. De Gruyter 2018, 72, 645–652. [Google Scholar] [CrossRef]
- Oliveira, E.; Amara, I.; Bellido, D.; Odena, M.A.; Domínguez, E.; Pagès, M.; Goday, A. LC-MSMS identification of Arabidopsis thaliana heat-stable seed proteins: Enriching for LEA-type proteins by acid treatment. J. Mass Spectrom. 2007, 42, 1485–1495. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Neuhoff, V.; Arold, N.; Taube, D.; Ehrhardt, W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 1988, 9, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Close, T.J.; Lammers, P.J. An osmotic stress protein of cyanobacteria is immunologically related to plant dehydrins. Plant Physiol. 1993, 101, 773–779. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, D.; Sanhueza, C.; Salvo-Garrido, H.; Bascunan-Godoy, L.; Bravo, L.A. How Does Diurnal and Nocturnal Warming Affect the Freezing Resistance of Antarctic Vascular Plants? Plants 2023, 12, 806. https://doi.org/10.3390/plants12040806
López D, Sanhueza C, Salvo-Garrido H, Bascunan-Godoy L, Bravo LA. How Does Diurnal and Nocturnal Warming Affect the Freezing Resistance of Antarctic Vascular Plants? Plants. 2023; 12(4):806. https://doi.org/10.3390/plants12040806
Chicago/Turabian StyleLópez, Dariel, Carolina Sanhueza, Haroldo Salvo-Garrido, Luisa Bascunan-Godoy, and León A. Bravo. 2023. "How Does Diurnal and Nocturnal Warming Affect the Freezing Resistance of Antarctic Vascular Plants?" Plants 12, no. 4: 806. https://doi.org/10.3390/plants12040806
APA StyleLópez, D., Sanhueza, C., Salvo-Garrido, H., Bascunan-Godoy, L., & Bravo, L. A. (2023). How Does Diurnal and Nocturnal Warming Affect the Freezing Resistance of Antarctic Vascular Plants? Plants, 12(4), 806. https://doi.org/10.3390/plants12040806






