Light Intensity Affects the Assimilation Rate and Carbohydrates Partitioning in Spinach Grown in a Controlled Environment
Abstract
1. Introduction
2. Results
2.1. Plant Growth and Yield, and Product Characteristics
2.2. Gas Exchange
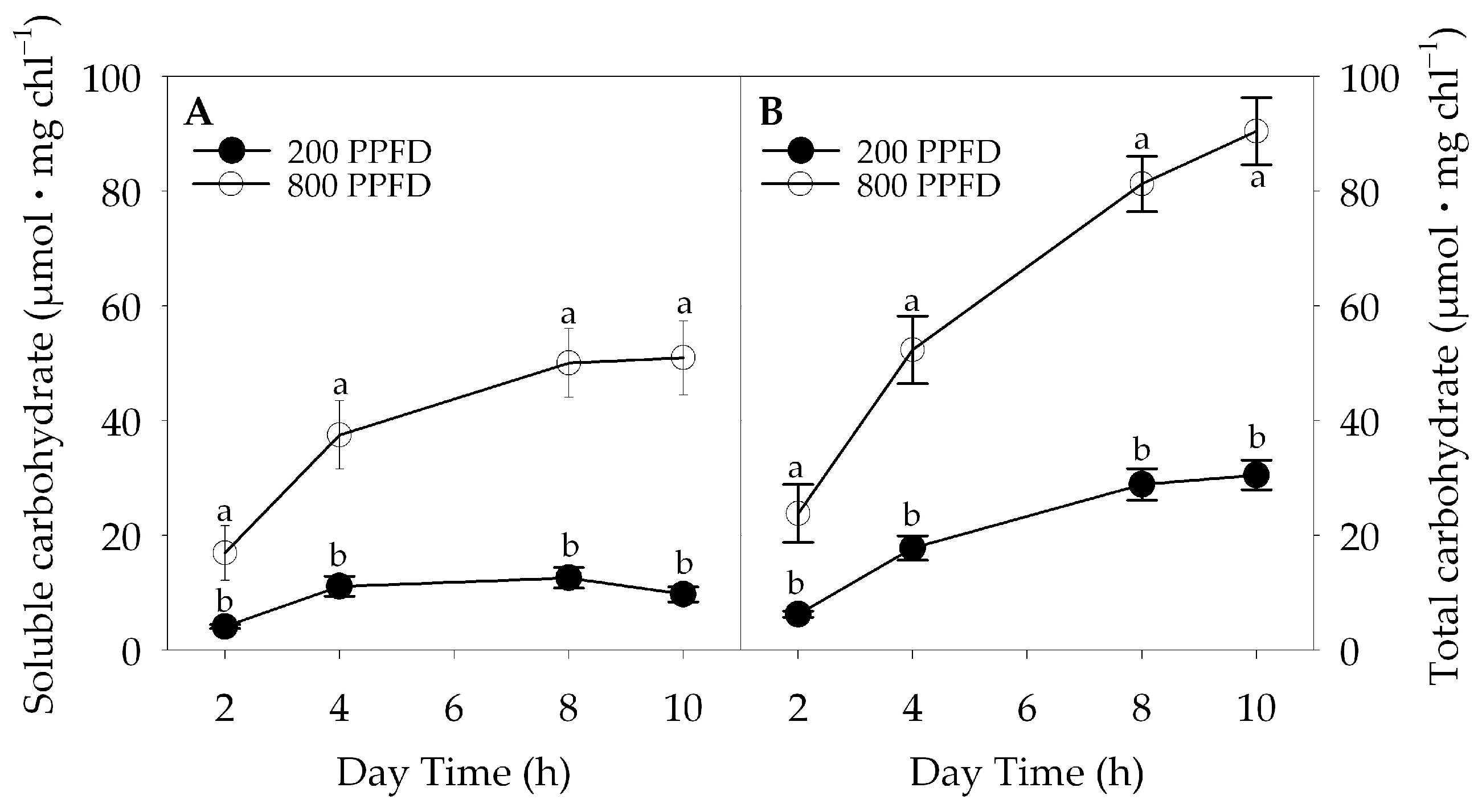
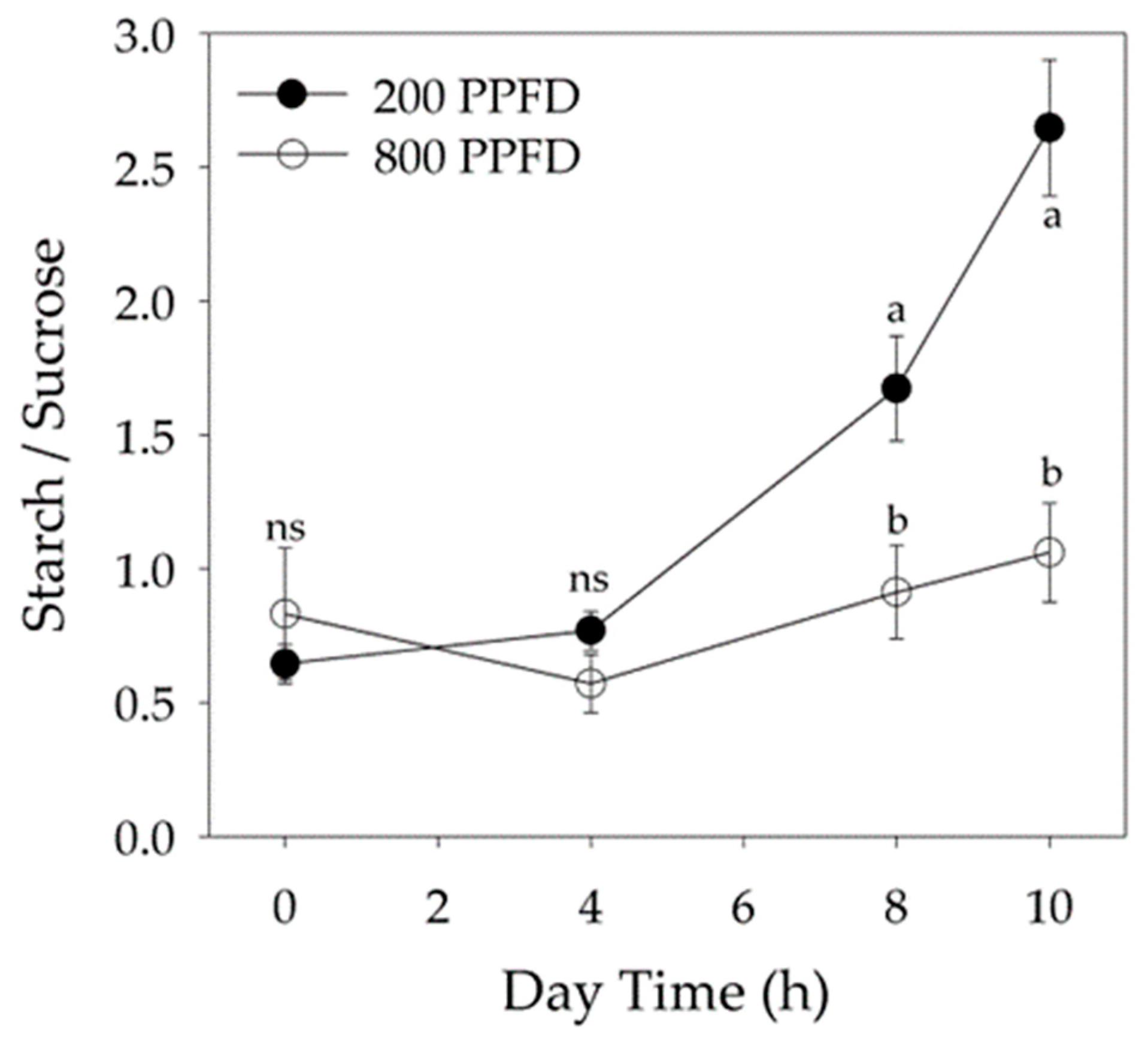
2.3. Non-Structural Carbohydrate Content
2.4. cFBPase, SPS, and AGPase Activity
3. Discussion
3.1. Plant Growth and Yield, and Product Characteristics and Gas Exchange
3.2. Accumulation of Assimilates in Leaves and Translocation Rate
3.3. Activity of cFBPase, SPS, and AGP
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Sampling and Analysis
4.3. Gas Exchange
4.4. Carbohydrate Content
4.5. Enzymatic Activity (cFBPase, SPS, AGP)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ojo, M.O.; Zahid, A. Deep Learning in Controlled Environment Agriculture: A Review of Recent Advancements, Challenges and Prospects. Sensors 2022, 22, 7965. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Proietti, S. Light quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. Review article. J. Plant Growth Regul. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Orsini, F. Innovation and sustainability in urban agriculture: The path forward. J. Consum. Prot. Food Saf. 2020, 15, 203–204. [Google Scholar] [CrossRef]
- Cocetta, G.; Casciani, D.; Bulgari, R.; Musante, F.; Kołton, A.; Rossi, M.; Ferrante, A. Light use efficiency for vegetables production in protected and indoor environments. Eur. Phys. J. Plus 2017, 132, 43. [Google Scholar] [CrossRef]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef]
- Hidaka, K.; Miyoshi, Y.; Ishii, S.; Suzui, N.; Yin, Y.G.; Kurita, K.; Nagao, K.; Araki, T.; Yasutake, D.; Kitano, M.; et al. Dynamic analysis of photosynthate translocation into strawberry fruits using non-invasive 11C-labeling supported with conventional destructive measurements using 13C-labeling. Front. Plant Sci. 2019, 9, 1946. [Google Scholar] [CrossRef]
- Shakya, R.; Manju, A.L. Photoassimilate translocation. In Plant Physiology, Development, and Metabolism; Springer: Singapore, 2018; pp. 227–251. [Google Scholar] [CrossRef]
- Foyer, C.H.; Galtier, N. Source-Sink interaction and communication in leaves. In Photoassimilate Distribution in Plants and Crops; Zamski, E., Schaffer, A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 311–340. [Google Scholar] [CrossRef]
- Aluko, O.O.; Li, C.; Wang, Q.; Liu, H. Sucrose utilization for improved crop yields: A review article. Int. J. Mol. Sci. 2021, 22, 4704. [Google Scholar] [CrossRef]
- Quick, W.P.; Schaffer, A.A. Sucrose metabolism in sources and sinks. In Photoassimilate Distribution in Plants and Crops; Zamski, E., Schaffer, A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 115–156. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9780203743539-6/sucrose-metabolism-sources-sinks-paul-quick-arthur-schaffer (accessed on 17 November 2022).
- Eichelmann, H.; Laisk, A.H. CO2 uptake and electron transport rates in wild-type and a starchless mutant of Nicotiana sylvestris. The role and regulation of starch synthesis at saturating CO2 concentration. Plant Physiol. 1994, 106, 679–687. [Google Scholar] [CrossRef]
- Stitt, M. Manipulation of carbohydrate partitioning. Curr. Opin. Biotechnol. 1994, 5, 137–143. [Google Scholar] [CrossRef]
- Schulze, W.; Stitt, M.; Schulze, E.D.; Neuhaus, H.E.; Fichtner, K. A quantification of the significance of assimilatory starch for Arabidopsis thaliana L. Heynh. Plant Physiol. 1991, 95, 890–895. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C.; Thorneycroft, D.; Smith, S.M. Starch mobilization in leaves. J. Exp. Bot. 2003, 54, 577–583. [Google Scholar] [CrossRef]
- Frommer, W.B.; Sonnewald, U. Molecular analysis of carbon partitioning in solanaceous species. J. Exp. Bot. 1995, 46, 587–607. [Google Scholar] [CrossRef]
- Vaughn, M.W.; Harrington, G.N.; Bush, D. Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc. Natl. Acad. Sci. USA 2002, 99, 10876–10880. [Google Scholar] [CrossRef]
- Milne, R.J.; Perroux, J.M.; Rae, A.L.; Reinders, A.; Ward, J.M.; Offler, C.E.; Patrick, J.W.; Grof, C.P. Sucrose transporter localization and function in phloem unloading in developing stems. Plant Physiol. 2017, 173, 1330–1341. [Google Scholar] [CrossRef]
- Evans, J.R.; Terashima, I. Photosynthetic characteristics of spinach leaves grown with different nitrogen treatments. Plant Cell Physiol. 1998, 29, 157–165. [Google Scholar] [CrossRef]
- Downton, W.J.S.; Grant, W.J.R.; Robinson, S.P. Photosynthetic and stomatal responses of spinach leaves to salt stress. Plant Physiol. 1985, 78, 85–88. [Google Scholar] [CrossRef]
- Yang, F.; Fan, Y.; Wu, X.; Cheng, Y.; Liu, Q.; Feng, L.; Chen, J.; Wang, Z.; Wang, X.; Yong, T.; et al. Auxin-to-gibberellin ratio as a signal for light intensity and quality in regulating soybean growth and matter partitioning. Front. Plant Sci. 2018, 9, 56. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Ann. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some relationship between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 424–426. [Google Scholar] [CrossRef]
- Sage, R.F.; Sharkey, T.D.; Seemann, J.R. Regulation of ribulose-1,5-bisphosphate carboxylase activity in response to light intensity and CO2 in C3 annuals Chenopodium album L. and Phaseolus vulgaris L. Plant Physiol. 1990, 94, 1735–1742. [Google Scholar] [CrossRef]
- Lauerer, M.; Saftic, D.; Quick, W.P.; Labate, C.; Fichtner, K.; Schulze, E.D.; Rodermel, S.R.; Bogorad, L.; Stitt, M. Decreased ribulose-1,5-bisphosphate carboxylase-oxigenase in transgenic tobacco transformed with “antisense” rbc S. VI. Effect on photosynthesis in plant grown at different irradiance. Planta 1993, 190, 332–345. [Google Scholar] [CrossRef]
- Evans, J.R. The relationship between electron transport components and photosynthetic capacity in pea leaves grown at different irradiances. Aust. J. Plant Physiol. 1987, 14, 157–170. [Google Scholar] [CrossRef]
- Rodríguez-López, N.F.; Martins, S.C.; Cavatte, P.C.; Silva, P.E.; Morais, L.E.; Pereira, L.F.; Reis, J.V.; Ávila, R.T.; Godoy, A.G.; Lavinski, A.O.; et al. Morphological and physiological acclimations of coffee seedlings to growth over a range of fixed or changing light supplies. Environ. Exp. Bot. 2014, 102, 1–10. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, W.; Yang, W. Shade inhibits leaf size by controlling cell proliferation and enlargement in soybean. Sci. Rep. 2017, 7, 9259. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Leccese, A.; Colla, G.; Battistelli, A. The effect of growing spinach (Spinacia oleracea L.) at two different light intensities on the amounts of oxalate, ascorbate and nitrate in their leaves. J. Hortic. Sci. Biotechnol. 2004, 79, 606–609. [Google Scholar] [CrossRef]
- Fabre, D.; Yin, X.; Dingkuhn, M.; Clément-Vidal, A.; Roques, S.; Rouan, L.; Soutiras, A.; Luquet, D. Is triose phosphate utilization involved in the feedback inhibition of photosynthesis in rice under conditions of sink limitation? J. Exp. Bot. 2019, 70, 5773–5785. [Google Scholar] [CrossRef]
- Fernandez, O.; Ishihara, H.; George, G.M.; Mengin, V.; Flis, A.; Sumner, D.; Arrivault, S.; Feil, R.; Lunn, J.E.; Zeeman, S.C.; et al. Leaf starch turnover occurs in long days and in falling light at the end of the day. Plant Physiol. 2017, 174, 2199–2212. [Google Scholar] [CrossRef]
- Durand, M.; Mainson, D.; Porcheron, B.; Maorousset, L.; Lemoine, R.; Pourtau, N. Carbon source–sink relationship in Arabidopsis thaliana: The role of sucrose transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef]
- Heineke, D.; Kruse, A.; Flügge, U.I.; Frommer, W.B.; Riesmeier, J.W.; Willmitzer, L.; Heldt, H.W. Effect of antisense repression of the chloroplast triose-phosphate translocator on photosynthetic metabolism in transgenic potato plants. Planta 1994, 193, 174–180. [Google Scholar] [CrossRef]
- Koch, K.E. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef]
- Hendrix, D.L.; Huber, S.C. Diurnal fluctuations in cotton leaf carbon export, carbohydrate content, and sucrose synthesising enzymes. Plant Physiol. 1986, 81, 584–586. [Google Scholar] [CrossRef]
- Rojas-González, J.A.; Soto-Súarez, M.; García-Díaz, Á.; Romero-Puertas, M.C.; Sandalio, L.M.; Mérida, Á.; Thormählen, I.; Geigenberger, P.; Serrato, A.J.; Sahrawy, M. Disruption of both chloroplastic and cytosolic FBPase genes results in a dwarf phenotype and important starch and metabolite changes in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 2673–2689. [Google Scholar] [CrossRef]
- Battistelli, A.; Adcock, M.D.; Leegood, R.C. The relationship between the activation state of sucrose-phosphate synthase and the rate of CO2 assimilation in spinach leaves. Planta 1991, 183, 620–622. [Google Scholar] [CrossRef]
- Ballicora, M.A.; Iglesias, A.A.; Preiss, J. ADP-glucose pyrophosphorylase: A regulatory enzyme for plant starch synthesis. Photosynth. Res. 2004, 79, 1–24. [Google Scholar] [CrossRef]
- Strand, A.; Zrenner, R.; Trevanion, S.; Stitt, M.; Gustafsson, P.; Gardestrom, P. Decreased expression of two key enzymes in the sucrose biosynthesis pathway, cytosolic fructose-1,6-bisphosphatase and sucrose phosphate synthase, has remarkably different consequences for photosynthetic carbon metabolism in transgenic Arabidopsis thaliana. Plant J. 2000, 23, 759–770. [Google Scholar] [CrossRef]
- Battistelli, A.; Moscatello, S.; Proietti, S.; Salvini, D.; Scartazza, A.; Augusti, A. Growth Light Intensity Affects Photosynthetic Carbon Metabolism in Spinach. In Photosynthesis: Mechanisms and Effects; Springer: Dordrecht, The Netherlands, 1998; pp. 3467–3470. [Google Scholar] [CrossRef]
- Stitt, M. Fructose-2,6-bisphosphate as a regulatory molecule in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 153185. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Riccio, F.; Downey, P.; Battistelli, A. Continuous lighting promotes plant growth, light conversion efficiency, and nutritional quality of Eruca vesicaria (L.) Cav. in controlled environment with minor effects due to light quality. Front. Plant Sci. 2021, 12, 730119. [Google Scholar] [CrossRef]
- Ripullone, F.; Grassi, G.; Lauteri, M.; Borghetti, M. Photosynthesis-nitrogen relationships: Interpretation of different patterns between Pseudotsuga menziesii and Populus x euroamericana in a mini-stand experiment. Tree Physiol. 2003, 23, 137–144. [Google Scholar] [CrossRef]
- Moscatello, S.; Famiani, F.; Proietti, S.; Farinelli, D.; Battistelli, A. Sucrose synthase dominates carbohydrate metabolism and relative growth rate in growing kiwifruit (Actinidia deliciosa, cv Hayward). Sci. Hortic. 2011, 128, 197–205. [Google Scholar] [CrossRef]
- Holaday, A.S.; Martindale, W.; Alred, R.; Brooks, R.L.; Leegood, R. Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant Physiol. 1992, 98, 1105–1114. [Google Scholar] [CrossRef]
- Kleczkowshi, L.A.; Villand, P.; Preiss, J.; Olsen, O. Kinetic mechanism and regulation of ADP- glucose pyrophosphorylase from barley (Hordeum vulgare) leaves. J. Biol. Chem. 1993, 268, 6228–6233. [Google Scholar] [CrossRef]



| F.W. (g Plant −1) | D.W. (g Plant −1) | D.M. (%) | |
|---|---|---|---|
| Leaf | |||
| 200 PPFD | 2.78 ± 0.89 b | 0.29 ± 0.03 b | 10.70 ± 0.18 b |
| 800 PPFD | 7.26 ± 0.89 a | 0.88 ± 0.11 a | 12.00 ± 0.24 a |
| Root | |||
| 200 PPFD | 0.88 ± 0.09 b | 0.05 ± 0.01 b | 6.35 ± 0.31 b |
| 800 PPFD | 3.68 ± 0.49 a | 0.26 ± 0.03 a | 7.35 ± 0.16 a |
| Time of Exposure | PPFD | ||
|---|---|---|---|
| 200 | 800 | ||
| Assimilation rate (µmol CO2 m2s−1) | 2 h | 5.0 ± 0.1 b | 15.4 ± 0.4 a |
| 4 h | 5.0 ± 0.2 b | 13.3 ± 0.5 a | |
| 8 h | 4.1 ± 0.2 b | 13.4 ± 0.1 a | |
| Sub stomatal CO2 partial pressure (µbar bar−1) | 2 h | 360.3 ± 0.8 a | 310.6 ± 2.6 b |
| 4 h | 357.7 ± 1.9 a | 319.4 ± 1.4 b | |
| 8 h | 352.6 ± 2.4 a | 297.3 ± 1.8 b | |
| Stomatal conductance (mmol CO2 m2s−1) | 2 h | 470.0 ± 15.5 | 517.4 ± 35.3 |
| 4 h | 460.9 ± 27.7 | 500.4 ± 26.5 | |
| 8 h | 270.8 ± 16.8 | 337.7 ± 7.7 | |
| Starch | Soluble Carbohydrates | Total Carbohydrates | Starch (% of Total Carbohydrate) | Soluble (% of Total Carbohydrate) | ||
|---|---|---|---|---|---|---|
| Beginning of the day | 12.9 ± 1.7 b | 24.5 ± 1.9 b | 37.4 ± 3.3 b | 34.5 ± 2.3 n.s. | 65.5 ± 2.2 n.s. | |
| 200 PPFD | End of the day | 124.6 ± 10.2 b | 58.3 ± 7.9 b | 182.9 ± 15.6 b | 68.5 ± 2.4 a | 31.5 ± 2.3 b |
| Variation | 111.6 ± 10.9 b | 33.8 ± 7.7 b | 145.4 ± 16.3 b | |||
| Beginning of the day | 41.6 ± 7.9 a | 101.4 ± 28.7 a | 143.0 ± 30.3 a | 29.1 ± 5.8 n.s. | 70.9 ± 5.8 n.s. | |
| 800 PPFD | End of the day | 237.1 ± 26.1 a | 305.7 ± 38.8 a | 542.8 ± 35.1 a | 43.7 ± 4.8 b | 56.3 ± 4.8 a |
| Variation | 195.6 ± 28.6 a | 204.3 ± 23.8 a | 399.9 ± 39.0 a |
| 200 PPFD | 800 PPFD | |
|---|---|---|
| Assimilation of CO2 (µmol C mg−1 chl h−1) | 39.0 ± 3.9 b | 105 ± 7.3 a |
| Amount of Photoassimilates (µmol C mg−1 chl) | ||
| Produced during the day | 390.0 ± 39.0 b | 1050.0 ± 73.2 a |
| Accumulated during the day | 146.0 ± 16.3 b | 400.0 ± 39.0 a |
| As % on the total | 37.4 ± 4.2 n.s. | 38.0 ± 3.7 n.s. |
| Exported during the day | 244.9 ± 16.3 b | 650.0 ± 39.0 a |
| Rate of assimilates use (µmol C mg−1 chl h−1) | ||
| During the day | 24.5 ± 1.6 b | 65.0 ± 3.9 a |
| During the night | 10.4 ± 1.2 b | 28.6 ± 2.8 a |
| PPFD | Time of Exposure | cFBPase | SPSmax | AGPase |
|---|---|---|---|---|
| 200 | 48.2 ± 4.94 a | 21.63 ± 0.83 b | 10.94 ± 0.77 b | |
| 800 | 63.33 ± 5.65 a | 33.80 ± 2.15 a | 14.20 ± 1.46 a | |
| 4 h | 56.10 ± 7.40 a | 27.30 ± 2.56 a | 14.27 ± 1.48 a | |
| 8 h | 52.00 ± 8.56 a | 28.13 ± 3.58 a | 10.85 ± 0.63 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proietti, S.; Paradiso, R.; Moscatello, S.; Saccardo, F.; Battistelli, A. Light Intensity Affects the Assimilation Rate and Carbohydrates Partitioning in Spinach Grown in a Controlled Environment. Plants 2023, 12, 804. https://doi.org/10.3390/plants12040804
Proietti S, Paradiso R, Moscatello S, Saccardo F, Battistelli A. Light Intensity Affects the Assimilation Rate and Carbohydrates Partitioning in Spinach Grown in a Controlled Environment. Plants. 2023; 12(4):804. https://doi.org/10.3390/plants12040804
Chicago/Turabian StyleProietti, Simona, Roberta Paradiso, Stefano Moscatello, Francesco Saccardo, and Alberto Battistelli. 2023. "Light Intensity Affects the Assimilation Rate and Carbohydrates Partitioning in Spinach Grown in a Controlled Environment" Plants 12, no. 4: 804. https://doi.org/10.3390/plants12040804
APA StyleProietti, S., Paradiso, R., Moscatello, S., Saccardo, F., & Battistelli, A. (2023). Light Intensity Affects the Assimilation Rate and Carbohydrates Partitioning in Spinach Grown in a Controlled Environment. Plants, 12(4), 804. https://doi.org/10.3390/plants12040804






