Thresholds in the Species–Area–Habitat Model: Evidence from the Bryophytes on Continental Islands
Abstract
:1. Introduction
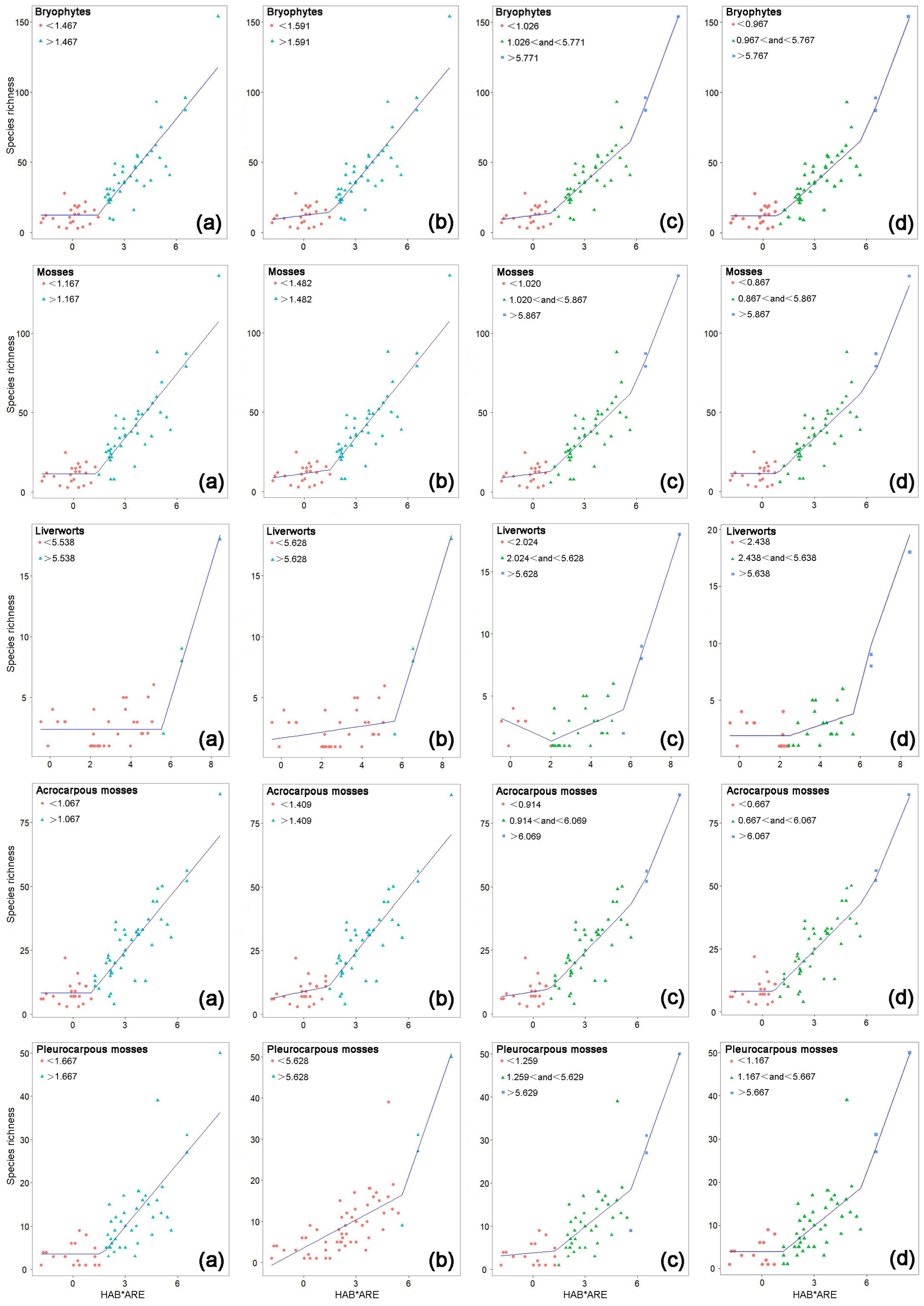
2. Results
3. Discussion
3.1. The Creditability of the SCEs and Thresholds
3.2. Thresholds in SKRs and Variations among Different Bryophyte Groups
3.3. Mechanisms of the Thresholds in SKRs
3.4. Implications of Choros and the Threshold SKRs
3.5. Some Notes of Caution
4. Materials and Methods
4.1. Study Area
4.2. Bryophyte Inventory
4.3. Surveys of Habitat Types
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dengler, J. Which function describes the species-area relationship best? A review and empirical evaluation. J. Biogeogr. 2009, 36, 728–744. [Google Scholar] [CrossRef]
- Williams, C.B. Patterns in the Balance of Nature and Related Problems in Quantitative Biology; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Preston, F.W. Time and Space and the Variation of Species. Ecology 1960, 41, 611–627. [Google Scholar] [CrossRef]
- Preston, F.W. The Canonical Distribution of Commonness and Rarity: Part I. Ecology 1962, 43, 185–215. [Google Scholar] [CrossRef]
- Preston, F.W. The Canonical Distribution of Commonness and Rarity: Part II. Ecology 1962, 43, 410–432. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Simberloff, D. Experimental Zoogeography of Islands: Effects of Island Size. Ecology 1976, 57, 629–648. [Google Scholar] [CrossRef]
- Rafe, R.W.; Usher, M.B.; Jefferson, R.G. Birds on Reserves: The Influence of Area and Habitat on Species Richness. J. Appl. Ecol. 1985, 22, 327. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Lovette, I.J. The roles of island area per se and habitat diversity in the species-area relationships of four Lesser Antillean faunal groups. J. Anim. Ecol. 1999, 68, 1142–1160. [Google Scholar] [CrossRef]
- Triantis, K.A.; Mylonas, M.; Lika, K.; Vardinoyannis, K. A model for the species-area-habitat relationship. J. Biogeogr. 2003, 30, 19–27. [Google Scholar] [CrossRef]
- Chen, Y.H. Combining the Species-Area-Habitat relationship and environmental cluster analysis to set conservation priori-ties: A study in the Zhoushan Archipelago, China. Conserv. Biol. 2008, 23, 537–545. [Google Scholar] [CrossRef]
- Duarte, M.C.; Rego, F.C.; Romeiras, M.M.; Moreira, I. Plant species richness in the Cape Verde Islands—Eco-geographical determinants. Biodivers. Conserv. 2008, 17, 453–466. [Google Scholar] [CrossRef]
- Hannus, J.J.; Von Numers, M. Vascular plant species richness in relation to habitat diversity and island area in the Finnish Archipelago. J. Biogeogr. 2008, 35, 1077–1086. [Google Scholar] [CrossRef]
- Kagiampaki, A.; Triantis, K.; Vardinoyannis, K.; Mylonas, M. Factors affecting plant species richness and endemism in the South Aegean (Greece). J. Biol. Res-Thessalon. 2011, 16, 282–295. [Google Scholar]
- Panitsa, M.; Tzanoudakis, D.; Triantis, K.A.; Sfenthourakis, S. Patterns of species richness on very small islands: The plants of the Aegean archipelago. J. Biogeogr. 2006, 33, 1223–1234. [Google Scholar] [CrossRef]
- Proença, V.; Pereira, H.M. Species–area models to assess biodiversity change in multi-habitat landscapes: The importance of species habitat affinity. Basic Appl. Ecol. 2013, 14, 102–114. [Google Scholar] [CrossRef]
- Shi, J.; Ma, K.; Wang, J.; Zhao, J.; He, K. Vascular plant species richness on wetland remnants is determined by both area and habitat heterogeneity. Biodivers. Conserv. 2010, 19, 1279–1295. [Google Scholar] [CrossRef]
- Triantis, K.A.; Mylonas, M.; Weiser, M.D.; Lika, K.; Vardinoyannis, K. Species richness, environmental heterogeneity and area: A case study based on land snails in Skyros archipelago (Aegean Sea, Greece). J. Biogeogr. 2005, 32, 1727–1735. [Google Scholar] [CrossRef]
- Triantis, K.; Vardinoyannis, K.; Mylonas, M. Area and habitat relationships in island land snail faunas: An Aegean case study exploring the choros model. Rec. West. Aust. Museum Suppl. 2005, 68, 133. [Google Scholar] [CrossRef]
- Panitsa, M.; Trigas, P.; Iatrou, G.; Sfenthourakis, S. Factors affecting plant species richness and endemism on land-bridge islands—An example from the East Aegean archipelago. Acta Oecol. 2010, 36, 431–437. [Google Scholar] [CrossRef]
- Van Tooren, B.F.; Van Dam, D.; During, H.J. The Relative Importance of Precipitation and Soil as Sources of Nutrients for Calliergonella cuspidata (Hedw.) Loeske in Chalk Grassland. Funct. Ecol. 1990, 4, 101. [Google Scholar] [CrossRef]
- Bates, J.W. Is ‘Life-Form’ a Useful Concept in Bryophyte Ecology? Oikos 1998, 82, 223. [Google Scholar] [CrossRef]
- He, X.; He, K.S.; Hyvönen, J. Will bryophytes survive in a warming world? Perspect. Plant Ecol. Evol. Syst. 2016, 19, 49–60. [Google Scholar] [CrossRef]
- During, H.; van Tooren, B. Recent developments in bryophyte population ecology. Trends Ecol. Evol. 1987, 2, 89–93. [Google Scholar] [CrossRef]
- Söderström, L. Bryology for the Twenty-First Century; CRC Press: New York, NY, USA, 1998. [Google Scholar]
- Snäll, T.; Ehrlén, J.; Rydin, H. Colonization–Extinction Dynamics of An Epiphyte Metapopulation in a Dynamic Landscape. Ecology 2005, 86, 106–115. [Google Scholar] [CrossRef]
- Proctor, M.C.F. Mosses and alternative adaptation to life on land. New Phytol. 2000, 148, 1–3. [Google Scholar] [CrossRef]
- Francesco Ficetola, G.; Denoël, M. Ecological thresholds: An assessment of methods to identify abrupt changes in species habitat relationships. Ecography 2009, 32, 1075–1084. [Google Scholar] [CrossRef]
- Fahrig, L. How much habitat is enough? Biol. Conserv. 2001, 100, 65–74. [Google Scholar] [CrossRef]
- Burns, K.C.; McHardy, R.P.; Pledger, S. The small-island effect: Fact or artefact? Ecography 2009, 32, 269–276. [Google Scholar] [CrossRef]
- Chen, C.; Xu, A.; Ding, P.; Wang, Y. The small-island effect and nestedness in assemblages of medium- and large-bodied mammals on Chinese reservoir land-bridge islands. Basic Appl. Ecol. 2019, 38, 47–57. [Google Scholar] [CrossRef]
- Gao, D.; Perry, G. Detecting the small island effect and nestedness of herpetofauna of the West Indies. Ecol. Evol. 2016, 6, 5390–5403. [Google Scholar] [CrossRef]
- Matthews, T.J.; Rigal, F.; Kougioumoutzis, K.; Trigas, P.; Triantis, K.A. Unravelling the small-island effect through phylogenetic community ecology. J. Biogeogr. 2020, 47, 2341–2352. [Google Scholar] [CrossRef]
- Morrison, L.W. Why do some small islands lack vegetation? Evidence from long-term introduction experiments. Ecography 2010, 34, 384–391. [Google Scholar] [CrossRef]
- Triantis, K.A.; Vardinoyannis, K.; Tsolaki, E.P.; Botsaris, I.; Lika, K.; Mylonas, M. Re-approaching the small island effect. J. Biogeogr. 2006, 33, 914–923. [Google Scholar] [CrossRef]
- Wang, Y.; Millien, V.; Ding, P. On empty islands and the small-island effect. Glob. Ecol. Biogeogr. 2016, 25, 1333–1345. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wu, Q.; Chen, C.; Xu, A.; Ding, P. The small-island effect in amphibian assemblages on subtropical land-bridge islands of an inundated lake. Curr. Zool. 2018, 64, 303–309. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Tan, X.; Wang, Y. The role of habitat diversity in generating the small-island effect. Ecography 2020, 43, 1241–1249. [Google Scholar] [CrossRef]
- Chen, C.; Xu, A.; Wang, Y. Area threshold and trait–environment associations of butterfly assemblages in the Zhoushan Archipelago, China. J. Biogeogr. 2021, 48, 785–797. [Google Scholar] [CrossRef]
- Gao, D.; Wang, Y. A global synthesis of the small-island effect in amphibians and reptiles. Ecography 2022, 2022. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Wang, X.; Liu, C.; Wu, L.; Chen, C.; Ge, D.; Song, X.; Chen, C.; Xu, A.; et al. Small-island effect in snake communities on islands of an inundated lake: The need to include zeroes. Basic Appl. Ecol. 2015, 16, 19–27. [Google Scholar] [CrossRef]
- Dengler, J. Robust methods for detecting a small island effect. Divers. Distrib. 2010, 16, 256–266. [Google Scholar] [CrossRef]
- Lomolino, M.V. Ecology’s most general, yet protean 1 pattern: The species-area relationship. J. Biogeogr. 2000, 27, 17–26. [Google Scholar] [CrossRef]
- Kürschner, H. Life strategies and adaptations in bryophytes from the Near and Middle East. Turkey J. Bot. 2004, 28, 73–84. [Google Scholar]
- Vanderpoorten, A.; Goffinet, B. Introduction to Bryophytes; Cambridge University Press: London, UK, 2009; 312p. [Google Scholar]
- Chen, P.C. Genera Muscorum Sinicorum (1); Science Press: Beijing, China, 1963; pp. 1–304. [Google Scholar]
- Chen, P.C. Genera Muscorum Sinicorum (2); Science Press: Beijing, China, 1978; pp. 1–331. [Google Scholar]
- Hylander, K.; Dynesius, M.; Jonsson, B.G.; Nilsson, C. Substrate Form Determines the Fate of Bryophytes in Riparian Buffer Strips. Ecol. Appl. 2005, 15, 674–688. [Google Scholar] [CrossRef]
- Kürschner, H. Life strategies of epiphytic bryophytes in Mediterranean Pinus woodlands and Platanus orientalis alluvial forests of Turkey. Cryptogam. Bryol. 1999, 20, 17–33. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Engels, P. The effects of environmental variation on bryophytes at a regional scale. Ecography 2002, 25, 513–522. [Google Scholar] [CrossRef]
- Zhejiang Islands Investigation Staff. Comprehensive Investigation of Island Resources in Zhejiang Province; Zhejiang Science and Technology Press: Hangzhou, China, 1995; 482p. [Google Scholar]
- Gavish, Y.; Ziv, Y.; Rosenzweig, M.L. Decoupling Fragmentation from Habitat Loss for Spiders in Patchy Agricultural Landscapes. Conserv. Biol. 2011, 26, 150–159. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Weiser, M.D. Towards a more general species-area relationship: Diversity on all islands, great and small. J. Biogeogr. 2001, 28, 431–445. [Google Scholar] [CrossRef]
- Yu, J.; Li, D.; Zhang, Z.; Guo, S. Species–area relationship and small-island effect of bryophytes on the Zhoushan Archipelago, China. J. Biogeogr. 2020, 47, 978–992. [Google Scholar] [CrossRef]
- Staniaszek-Kik, M.; Chmura, D.; Żarnowiec, J. What factors influence colonization of lichens, liverworts, mosses and vascular plants on snags? Biologia 2019, 74, 375–384. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967; pp. 1–205. [Google Scholar]
- Frahm, J.P. Diversity, dispersal and biogeography of bryophytes (mosses). Biodivers. Conserv. 2008, 17, 277–284. [Google Scholar] [CrossRef]
- Medina, N.G.; Draper, I.; Lara, F. Biogeography of Mosses and Allies: Does Size Matter? Biogeography of Microorganisms: Is Everything Small Everywhere? Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Fenton, N.J.; Frego, K.A. Bryophyte (moss and liverwort) conservation under remnant canopy in managed forests. Biol. Conserv. 2005, 122, 417–430. [Google Scholar] [CrossRef]
- McCune, J.L.; Frendo, C.J.; Ramadan, M.; Baldwin, L.K. Comparing the effect of landscape context on vascular plant and bryophyte communities in a human-dominated landscape. J. Veg. Sci. 2021, 32, 12932. [Google Scholar] [CrossRef]
- Müller, J.; Boch, S.; Prati, D.; Socher, S.A.; Pommer, U.; Hessenmöller, D.; Schall, P.; Schulze, E.D.; Fischer, M. Effects of forest management on bryophyte species richness in Central European forests. For. Ecol. Manag. 2019, 432, 850–859. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, Z.; Wang, C.; Hao, Z.; Sun, B.; Zuo, Q.; Zhang, C.; Sun, R.; Jin, J.; Wang, H. Urban orchards provide a suitable habitat for epiphytic bryophytes. For. Ecol. Manag. 2021, 483. [Google Scholar] [CrossRef]
- Zielińska, K. Vascular plants and bryophytes in managed forests—Analysis of the impact of the old ditches on the species diversity (central european plain). Appl. Ecol. Environ. Res. 2017, 15, 1375–1392. [Google Scholar] [CrossRef]
- Yu, J.; Shen, L.; Zang, C.; Cai, J.; Guo, S. Geographical, anthropogenic and climatic determinants of bryophyte species composition and richness in the Shengsi archipelago, East China Sea. J. Bryol. 2019, 41, 107–120. [Google Scholar] [CrossRef]
- Triantis, K.A.; Nogués-Bravo, D.; Borges, P.A.V. Measurements of area and the (island) species-area relationship: New direc-tions fro an old pattern. Oikos 2008, 117, 1553–1559. [Google Scholar] [CrossRef]
- Kreft, H.; Jetz, W.; Mutke, J.; Kier, G.; Barthlott, W. Global diversity of island floras from a macroecological perspective. Ecol. Lett. 2007, 11, 116–127. [Google Scholar] [CrossRef]
- Pan, Y.; Deng, J.S.; Cai, J.; Wang, K.; Wu, C.; Li, J.; Li, J. Spatiotemporal dynamics of island land expansion response to intensive island development and reclamation. J. Coast. Res. 2015, 31, 1439–1448. [Google Scholar] [CrossRef]
- Chi, Y.; Zhang, Z.; Xie, Z.; Wang, J. How human activities influence the island ecosystem through damaging the natural ecosystem and supporting the social ecosystem? J. Clean. Prod. 2020, 248, 119203. [Google Scholar] [CrossRef]
- Jia, Y.; He, S. Species Catalogue of China, Volume 1: Plants: Bryophytes; Science Press: Beijing, Beijing, 2013. [Google Scholar]
- Fenton, N.J.; Bergeron, Y. Facilitative succession in a boreal bryophyte community driven by changes in available moisture and light. J. Veg. Sci. 2006, 17, 65–76. [Google Scholar] [CrossRef]
- Ross-Davis, A.L.; Frego, K.A. Comparison of plantations and naturally regenerated clearcuts in the Acadian Forest: Forest floor bryophyte community and habitat features. Can. J. Bot. 2002, 80, 21–33. [Google Scholar] [CrossRef]
- Newmaster, S.G.; Belland, R.J.; Arsenault, A.; Vitt, D.H.; Stephens, T.R. The ones we left behind: Comparing plot sampling and floristic habitat sampling for estimating bryophyte diversity. Divers. Distrib. 2005, 11, 57–72. [Google Scholar] [CrossRef]
- Paciorek, T.; Stebel, A.; Jankowska-Błaszczuk, M.; Wojciechowska, A.; Wojciechowska, T. Bryophyte Species Diversity in Human-Influenced Habitats Within Protected Areas—A Case Study from the Świętokrzyski National Park in Poland. Herzogia 2016, 29, 668–687. [Google Scholar] [CrossRef]
- Pi, C.Y.; Liu, Y. Bryophyte composition and diversity within anthropogenic habitats in a residential area of Chongqing municipality city. Biodiv. Sci. 2014, 22, 583–588. [Google Scholar]
- Vitt, D.H.; Halsey, L.A.; Bray, J.; Kinser, A. Patterns of Bryophyte Richness in a Complex Boreal Landscape: Identifying Key Habitats at McClelland Lake Wetland. Bryologist 2003, 106, 372–382. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Dengler, J.; Matthews, T.J.; Steinbauer, M.J.; Wolfrum, S.; Boch, S.; Chiarucci, A.; Conradi, T.; Dembicz, I.; Marcenò, C.; García-Mijangos, I.; et al. Species–area relationships in continuous vegetation: Evidence from Palaearctic grasslands. J. Biogeogr. 2020, 47, 72–86. [Google Scholar] [CrossRef]
- Morin, D.J.; Yackulic, C.B.; Diffendorfer, J.E.; Lesmeister, D.B.; Nielsen, C.K.; Reid, J.; Schauber, E.M. Is your ad hoc model selection strategy affecting your multimodel inference? Ecosphere 2020, 11, e02997. [Google Scholar] [CrossRef]
- Matthews, J.; Triantis, K.A.; Whittaker, R.J.; Guihaumon, F. Sars: An R package for fitting, evaluating and comparing species-area relationship models. Ecography 2019, 42, 1446–1455. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]

| Models | Parameters | Total Bryophytes | Total Mosses | Liverworts | Acrocarpous Mosses | Pleurocarpous Mosses |
|---|---|---|---|---|---|---|
| Power model | R2adj | 0.84 | 0.82 | 0.74 | 0.80 | 0.70 |
| AICc | 503.93 | 496.06 | 147.70 | 450.75 | 355.90 | |
| ∆AICc | 0 | 0.92 | 9.35 | 0 | 0 | |
| Simple logarithmic model | R2adj | 0.71 | 0.73 | 0.36 | 0.73 | 0.54 |
| AICc | 540.18 | 524.46 | 182.38 | 468.78 | 380.07 | |
| ∆AICc | 36.25 | 29.32 | 44.03 | 18.03 | 24.17 | |
| Left-horizontal one-threshold model | C | 12.45 | 10.82 | 2.38 | 8.52 | 3.57 |
| T1 | 1.47/4.34 | 1.17/3.21 | 5.54/254.17 | 1.07/2.91 | 1.67/5.30 | |
| Z2 | 15.14 | 13.08 | 5.55 | 8.37 | 4.82 | |
| R2adj | 0.80 | 0.81 | 0.79 | 0.80 | 0.65 | |
| AICc | 518.68 | 504.60 | 142.47 | 454.08 | 367.82 | |
| ∆AICc | 14.75 | 9.46 | 4.12 | 3.33 | 11.92 | |
| One-threshold model | C | 12.18 | 11.58 | 1.71 | 8.95 | 3.54 |
| T1 | 1.59/4.91 | 1.48/4.40 | 5.63/278.11 | 1.41/4.09 | 5.63/278.11 | |
| Z1 | 1.54 | 1.51 | 0.24 | 1.42 | 2.28 | |
| Z2 | 15.03 | 13.45 | 5.43 | 8.50 | 12.31 | |
| R2adj | 0.80 | 0.81 | 0.80 | 0.79 | 0.69 | |
| AICc | 512.05 | 503.73 | 142.47 | 456.24 | 361.57 | |
| ∆AICc | 8.12 | 8.59 | 4.12 | 5.49 | 5.67 | |
| Left-horizontal two-threshold model | C | 12.29 | 11.31 | 1.880 | 8.28 | 3.82 |
| T1 | 0.97/2.63 | 0.87/2.38 | 2.44/11.45 | 0.67/1.95 | 1.17/3.21 | |
| T2 | 5.77/319.58 | 5.87/353.19 | 5.64/280.90 | 6.07/431.39 | 5.67/289.17 | |
| Z2 | 11.37 | 10.63 | 0.610 | 6.97 | 3.29 | |
| Z3 | 32.57 | 27.84 | 5.090 | 16.95 | 11.41 | |
| R2adj | 0.85 | 0.84 | 0.830 | 0.820 | 0.72 | |
| AICc | 504.04 | 495.14 | 139.57 | 451.03 | 359.40 | |
| ∆AICc | 0.11 | 0 | 1.22 | 0.28 | 3.5 | |
| Two-threshold model | C | 12.17 | 11.59 | 2.872 | 8.742 | 3.83 |
| T1 | 1.03/2.79 | 1.02/2.77 | 2.024/7.57 | 0.91/2.49 | 1.26/3.52 | |
| T2 | 5.77/320.86 | 5.87/353.19 | 5.63/278.11 | 6.07/432.25 | 5.63/278.38 | |
| Z1 | 1.54 | 1.44 | −0.73 | 1.04 | 0.35 | |
| Z2 | 11.09 | 10.59 | 0.70 | 7.07 | 3.24 | |
| Z3 | 32.87 | 27.88 | 5.03 | 16.83 | 11.31 | |
| R2adj | 0.85 | 0.84 | 0.84 | 0.81 | 0.70 | |
| AICc | 506.09 | 497.38 | 138.35 | 453.32 | 361.85 | |
| ΔAICc | 2.16 | 2.24 | 0 | 2.57 | 5.95 |
| Model Types | Groups | Average | ||||
|---|---|---|---|---|---|---|
| Total Bryophytes | Total Mosses | LiverWorts | Acrocarpous Mosses | Pleurocarpous Mosses | ||
| Power model | 0.03 | 0.03 | −0.01 | 0.04 | 0.02 | 0.022 |
| Logarithmic model | 0.01 | 0.02 | −0.02 | 0.02 | −0.01 | 0.004 |
| Left-horizontal one-threshold model | 0.02 | 0.02 | 0 | 0.03 | 0.01 | 0.016 |
| One-threshold model | −0.011 | 0.025 | 0.003 | 0.028 | 0.01 | 0.011 |
| Left-horizontal two-threshold model | 0.02 | 0.02 | 0.01 | 0.03 | 0.02 | 0.020 |
| Two-threshold model | 0.026 | 0.03 | 0.008 | 0.032 | 0.016 | 0.0224 |
| Model Types | Groups | Average | ||||
|---|---|---|---|---|---|---|
| Total Bryophytes | Total Mosses | Liverworts | Acrocarpous Mosses | Pleurocarpous Mosses | ||
| Power model | −9.6 | −10.43 | 1.00 | −9.94 | −2.46 | −6.286 |
| Logarithmic model | −2.33 | −3.52 | 1.17 | −4.65 | 0.76 | −1.714 |
| Left-horizontal one-threshold model | −6.4 | −7.81 | −0.38 | −8.03 | −1.27 | −4.778 |
| One-threshold model | −7.57 | −8.07 | −0.65 | −8.15 | −1.87 | −5.262 |
| Left-horizontal two-threshold model | −10.2 | −11.1 | −1.3 | −9.92 | −3.01 | −7.106 |
| Two-threshold model | −10.35 | −11.11 | −1.82 | −9.96 | −3.04 | −7.256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Huang, R.; Guo, S.; Li, D.; Yang, J.; Zhang, F.; Yu, J. Thresholds in the Species–Area–Habitat Model: Evidence from the Bryophytes on Continental Islands. Plants 2023, 12, 837. https://doi.org/10.3390/plants12040837
Luo G, Huang R, Guo S, Li D, Yang J, Zhang F, Yu J. Thresholds in the Species–Area–Habitat Model: Evidence from the Bryophytes on Continental Islands. Plants. 2023; 12(4):837. https://doi.org/10.3390/plants12040837
Chicago/Turabian StyleLuo, Guangyu, Ruoling Huang, Shuiliang Guo, Dandan Li, Jun Yang, Feng Zhang, and Jing Yu. 2023. "Thresholds in the Species–Area–Habitat Model: Evidence from the Bryophytes on Continental Islands" Plants 12, no. 4: 837. https://doi.org/10.3390/plants12040837








