Abscisic-Acid-Regulated Responses to Alleviate Cadmium Toxicity in Plants
Abstract
1. Introduction
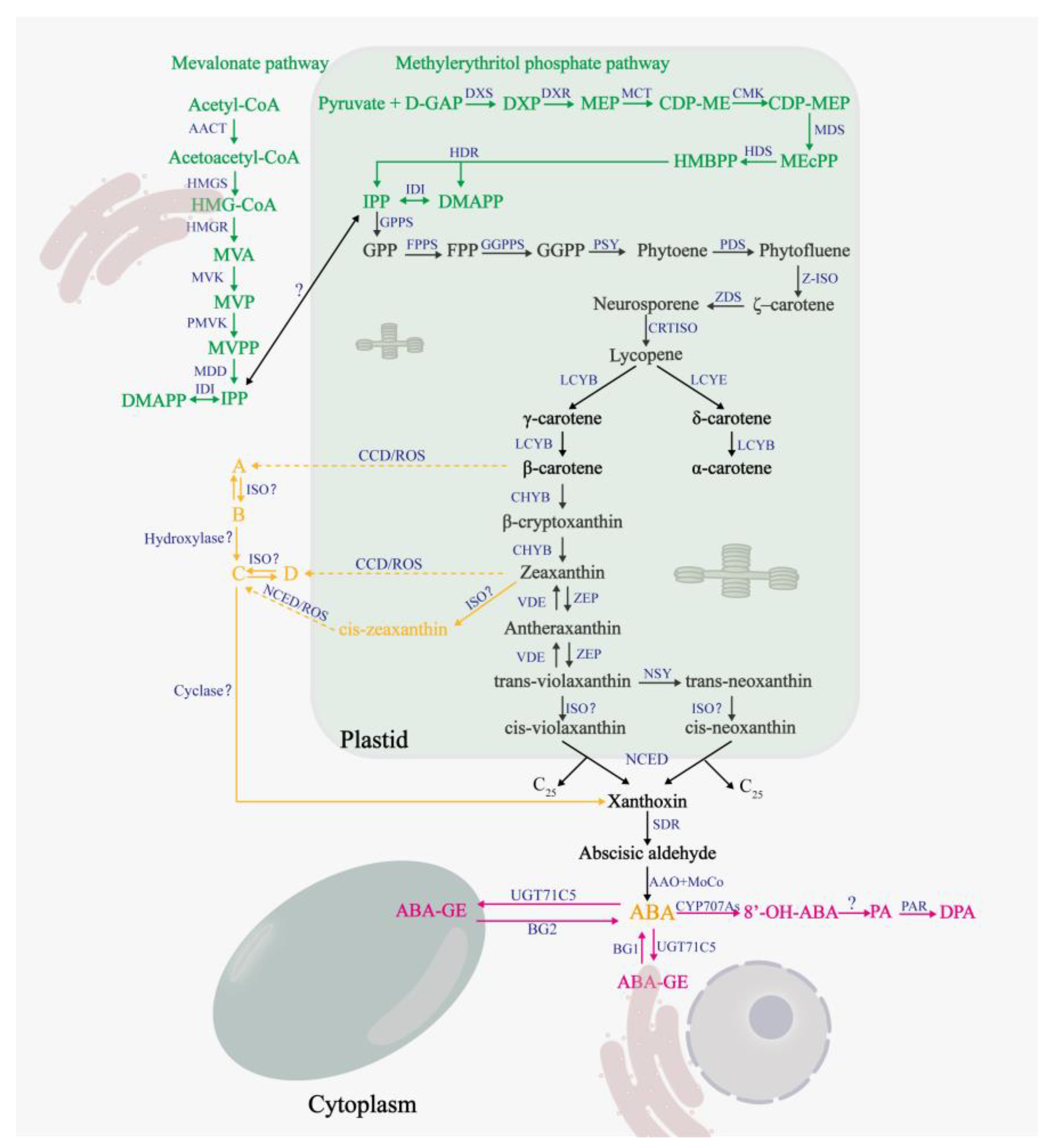
2. Synthesis and Catabolism of ABA in Plants
3. Physiological Mechanisms Underlying Enhancement in Cd Tolerance in Plants by ABA
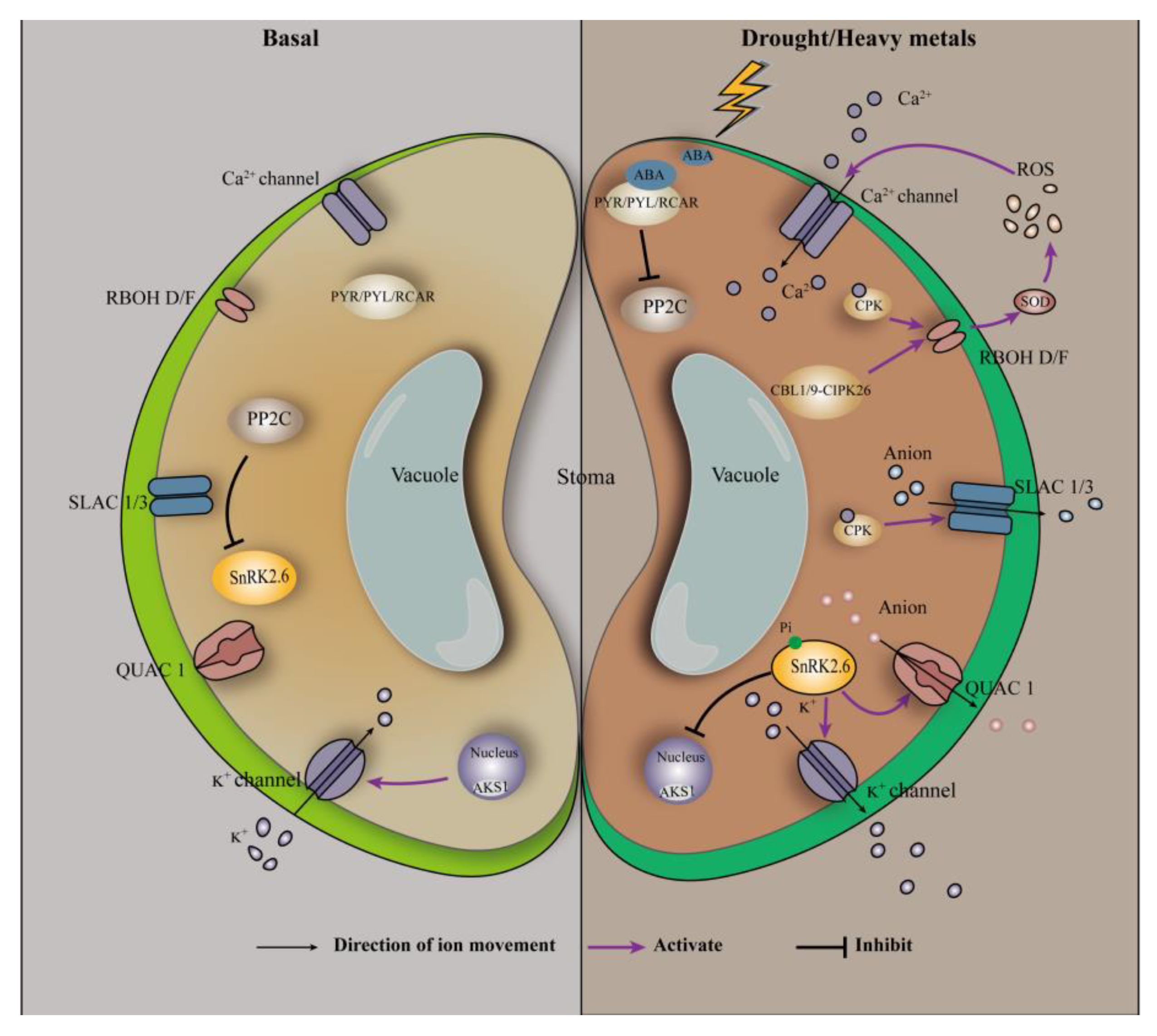
3.1. Regulation of Transpiration by ABA in Plants
3.2. Regulation of Metal Ion Transport by ABA in Plants
3.3. Regulation of Metal Ion Sequestration by ABA in Plants
3.4. Regulation of the Antioxidant System in Plants by ABA
3.5. Other Regulatory Effects of ABA
4. ABA-Mediated Signal Transduction
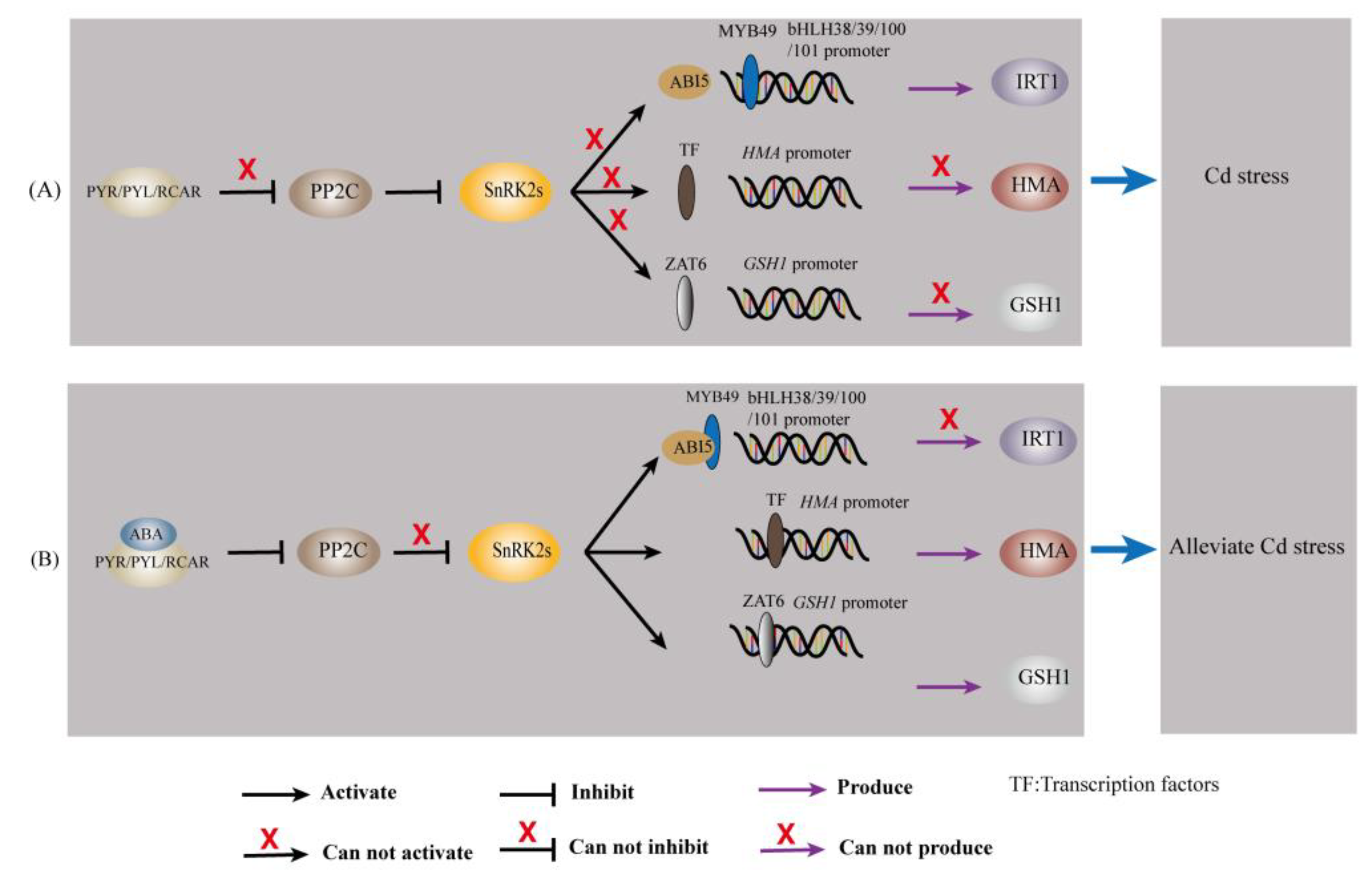
5. Regulation of Cd-Responsive Genes in Plants by ABA
| Species | Genes | Regulating Effects | References |
|---|---|---|---|
| Arabidopsis thaliana | ZAT6 | ABA could upregulate ZAT6, and the latter positively regulates Cd accumulation and tolerance in A. thaliana. | [107,108] |
| IRT1 | Compared with the control group, the expression of IRT1 in the roots of A. thaliana treated with CdCl2 and ABA decreased by approximately 90%. | [60] | |
| ABI5 | ABA-induced ABI5 interacts with Cd-induced MYB49 to reduce Cd uptake and accumulation. | [103] | |
| Brassica juncea L. Czern. et Coss. | BJCDR15 and TGA3 | ABA upregulated BJCDR15 and TGA3 (particularly the latter), and BJCDR15 overexpression in A. thaliana and tobacco increased Cd accumulation. The A. thaliana mutant TGA3-2 had a high Cd content in its roots, and Cd transport was blocked. | [109] |
| Boehmeria nivea | BnPCS1 | Cd and ABA significantly induced BnPCS1 expression. Plants overexpressing BnPCS1 accumulated more Cd. | [82] |
| BnbZIP3 | ABA treatment could induce BnbZIP3 expression. Overexpression of BnbZIP3 alleviated Cd stress. | [110] | |
| Hevea brasiliensis | HbMT2a | ABA could upregulate HbMT2a. Overexpression of MT2s could increase plants’ resistance to Cd. | [111] |
| Juglans regia L. | JrVHAG1 | CdCl2 and ABA significantly upregulated JrVHAG1. Overexpression of JrVHAG1 improved the growth of A. thaliana under ABA and/or CdCl2 treatment and increased the activity of antioxidant enzymes. | [112] |
| Solanum lycopersicum | TCMP-1 | TCMP-1 responded to Cd and ABA, and TCMP-1 interacted with heavy-metals-associated HIPP26 in tomatoes. Cd accumulation was lower in A. thaliana overexpressing TCMP-1. | [113] |
| Oryza sativa | OsSMP1 | ABA could upregulate OsSMP1, and OsSMP1 overexpression could improve the tolerance of rice to CdCl2 and CuSO4. | [114] |
| Poa Pratensis | Dof, MADS25, BCR-BPC, etc. | Dof, MADS25, BCR-BPC, B3, bZIP23, and MYB30 may be the central transcription factors under Cd stress. Hormonal signals, including ABA, interact with them to regulate the expression of multiple genes related to cell wall membrane stability and Cd tolerance. | [115] |
| Sedum alfredii | HsfA4c | Hsfs plays an important role in stress resistance. Treatment with ABA or Cd enhanced the expression of HsfA4c. | [69] |
| NAS | NAS encodes nicotianamine, which is involved in the long-distance transport of metals. The expression of NAS was positively correlated with endogenous ABA content. | ||
| HMA3 | HMA3 encodes a metal transporter whose expression in the shoots correlated with endogenous ABA content. | ||
| CAD | CAD encodes a protein associated with cell wall synthesis, and its expression positively correlated with endogenous ABA content. | ||
| HMA4 | HMA4 is a transporter that enhances Cd tolerance and promotes transfer of Cd to the shoots. Under the co-treatment of ABA and Cd, the expression of HMA4 was higher and positively correlated with endogenous ABA content and Cd accumulation. | ||
| Saccharum | ScGluD2 | ScGluD2 is involved in responding to heavy metal stress in sugarcane, and ABA plays a role in ScGluD2 activation induced by CdCl2. | [116] |
| Triticum aestivum | WRAB15 and WRAB18 | WRAB15 and WRAB18 were regulated by ABA and induced by Cd, and their expression levels in wheat seedling leaves positively correlated with seedlings’ resistance to Cd. | [117] |
| Zea mays | GSH1 | GSH treatment restored plant growth, root cell viability, photosynthetic capacity, REDOX balance, and cell ultrastructure. Meanwhile, Cd-tolerance-related genes were strongly upregulated. Under Cd stress, ABA content significantly decreased after GSH application, except in leaves. | [90] |
| Tamarix hispida | ThUGT | ThUGT is a gene of the ABA signaling pathway, and overexpression of ThUGT could reduce Cd accumulation. | [34] |
6. Conclusions
- Search for genes that respond to both ABA and stress. Search for transcription factors upstream of genes, and improve the gene network involved in the regulation of ABA. This can be combined with transgenic technology (gene knockout and overexpression) to fundamentally breed stress-resistant crops, which is of great importance for molecular breeding.
- Identify unknown components of the ABA signaling pathway, improve the ABA signaling network, and understand the crosstalk between the ABA signaling pathway and other signaling pathways (e.g., MAPK). This will facilitate the understanding of how the adverse external environment causes the plant to develop a resistance response.
- Understand the mechanism of crosstalk between ABA and other hormones. This will be extremely helpful in understanding plant physiology and developing integrated resistance strategies. Like genes, hormones often do not work in isolation.
- Uncover new stress receptors. This is necessary to elucidate the mechanism of stress response.
- Explore whether ABA has a dosage effect and the differences in sensitivity among plant tissues. This will hopefully improve stress resistance without affecting yield.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yang, M.; Li, Y.; Tian, J.; Zhang, Y.; Liang, L.; Liu, Z.; Chen, K.; Li, Y.; Lv, K.; et al. Comprehensive analysis of variation of cadmium accumulation in rice and detection of a new weak allele of OsHMA3. J. Exp. Bot. 2019, 70, 6389–6400. [Google Scholar] [CrossRef]
- Deng, B.; Zhang, W.; Yang, H. Abscisic Acid Decreases Cell Death in Malus hupehensis Rehd. Under Cd Stress by Reducing Root Cd2+ Influx and Leaf Transpiration. J. Plant Growth Regul. 2021, 41, 639–646. [Google Scholar] [CrossRef]
- Das, U.; Rahman, M.A.; Ela, E.J.; Lee, K.-W.; Kabir, A.H. Sulfur triggers glutathione and phytochelatin accumulation causing excess Cd bound to the cell wall of roots in alleviating Cd-toxicity in alfalfa. Chemosphere 2021, 262, 128361. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Song, J.; Yue, S.; Yang, H. Cd2+ uptake inhibited by MhNCED3 from Malus hupehensis alleviates Cd-induced cell death. Environ. Exp. Bot. 2019, 166, 103802. [Google Scholar] [CrossRef]
- Lu, Q.; Weng, Y.; You, Y.; Xu, Q.; Li, H.; Li, Y.; Liu, H.; Du, S. Inoculation with abscisic acid (ABA)-catabolizing bacteria can improve phytoextraction of heavy metal in contaminated soil. Environ. Pollut. 2020, 257, 113497. [Google Scholar] [CrossRef]
- Aoshima, K. Itai-itai disease: Renal tubular osteomalacia induced by environmental exposure to cadmium—Historical review and perspectives. Soil Sci. Plant Nutr. 2016, 62, 319–326. [Google Scholar] [CrossRef]
- Baba, H.; Tsuneyama, K.; Yazaki, M.; Nagata, K.; Minamisaka, T.; Tsuda, T.; Nomoto, K.; Hayashi, S.; Miwa, S.; Nakajima, T.; et al. The liver in itai-itai disease (chronic cadmium poisoning): Pathological features and metallothionein expression. Mod. Pathol. 2013, 26, 1228–1234. [Google Scholar] [CrossRef]
- Addicott, F.T.; Lyon, J.L. Physiology of Abscisic Acid and Related Substances. Annu. Rev. Plant Physiol. 1969, 20, 139–164. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic Acid synthesis and response. Arab. Book 2013, 11, e0166. [Google Scholar] [CrossRef]
- Zeevaart, J.A.D.; Creelman, R.A. Metabolism and Physiology of Abscisic Acid. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 439–473. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Tiozon, R.N., Jr.; Fernie, A.R.; Sreenivasulu, N. Abscisic acid and its role in the modulation of plant growth, development, and yield stability. Trends Plant Sci. 2022, 27, 1283–1295. [Google Scholar] [CrossRef]
- Emenecker, R.J.; Strader, L.C. Auxin-Abscisic Acid Interactions in Plant Growth and Development. Biomolecules 2020, 10, 281. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A. Seed Dormancy: The Complex Process Regulated by Abscisic Acid, Gibberellins, and Other Phytohormones that Makes Seed Germination Work. In Phytohormones-Signaling Mechanisms and Crosstalk in Plant Development and Stress Responses; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Boursiac, Y.; Leran, S.; Corratge-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA transport and transporters. Trends Plant Sci. 2013, 18, 325–333. [Google Scholar] [CrossRef]
- Addicott, F.T. Plant Hormones in the Control of Abscission. Biol. Rev. 1970, 45, 485–524. [Google Scholar] [CrossRef]
- Cracker, L.E.; Abeles, F.B. Abscission: Role of abscisic Acid. Plant Physiol. 1969, 44, 1144–1149. [Google Scholar] [CrossRef]
- Ogawa, M.; Kay, P.; Wilson, S.; Swain, S.M. ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are Polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 2009, 21, 216–233. [Google Scholar] [CrossRef]
- Osborne, D.J.; Morgan, P.W. Abscission. Crit. Rev. Plant Sci. 1989, 8, 103–129. [Google Scholar] [CrossRef]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y.; et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Deng, F.; Chen, G.; Chen, X.; Gao, W.; Long, L.; Xia, J.; Chen, Z.-H. Evolution of Abscisic Acid Signaling for Stress Responses to Toxic Metals and Metalloids. Front. Plant Sci. 2020, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shah, S.H.; Vimala, Y.; Jatav, H.S.; Ahmad, P.; Chen, Y.; Siddique, K.H.M. Abscisic acid: Metabolism, transport, crosstalk with other plant growth regulators, and its role in heavy metal stress mitigation. Front. Plant Sci. 2022, 13, 972856. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Villagra, J.; Figueroa, C.; Luengo-Escobar, A.; Morales, M.; Inostroza-Blancheteau, C.; Reyes-Díaz, M. Abscisic Acid and Plant Response Under Adverse Environmental Conditions. In Plant Performance Under Environmental Stress; Springer Nature: Berlin/Heidelberg, Germany, 2021; pp. 17–47. [Google Scholar]
- Pan, W.; Lu, Q.; Xu, Q.-R.; Zhang, R.-R.; Li, H.-Y.; Yang, Y.-H.; Liu, H.-J.; Du, S.-T. Abscisic acid-generating bacteria can reduce Cd concentration in pakchoi grown in Cd-contaminated soil. Ecotoxicol. Environ. Saf. 2019, 177, 100–107. [Google Scholar] [CrossRef]
- Pan, W.; You, Y.; Shentu, J.-L.; Weng, Y.-N.; Wang, S.-T.; Xu, Q.-R.; Liu, H.-J.; Du, S.-T. Abscisic acid (ABA)-importing transporter 1 (AIT1) contributes to the inhibition of Cd accumulation via exogenous ABA application in Arabidopsis. J. Hazard. Mater. 2020, 391, 122189. [Google Scholar] [CrossRef]
- Leng, Y.; Li, Y.; Ma, Y.-H.; He, L.-F.; Li, S.-W. Abscisic acid modulates differential physiological and biochemical responses of roots, stems, and leaves in mung bean seedlings to cadmium stress. Environ. Sci. Pollut. Res. 2020, 28, 6030–6043. [Google Scholar] [CrossRef]
- Han, Y.; Wang, S.; Zhao, N.; Deng, S.; Zhao, C.; Li, N.; Sun, J.; Zhao, R.; Yi, H.; Shen, X.; et al. Exogenous Abscisic Acid Alleviates Cadmium Toxicity by Restricting Cd2+ Influx in Populus euphratica Cells. J. Plant Growth Regul. 2016, 35, 827–837. [Google Scholar] [CrossRef]
- Mizutani, M.; Todoroki, Y. ABA 8’-hydroxylase and its chemical inhibitors. Phytochem. Rev. 2006, 5, 385–404. [Google Scholar] [CrossRef]
- Humplik, J.F.; Bergougnoux, V.; Van Volkenburgh, E. To Stimulate or Inhibit? That Is the Question for the Function of Abscisic Acid. Trends Plant Sci. 2017, 22, 830–841. [Google Scholar] [CrossRef]
- Dong, T.; Park, Y.; Hwang, I. Abscisic acid: Biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 2015, 58, 29–48. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Liu, Y.; Luo, J.; Li, J.; Kovac, J.; Li, B.; Li, Q.; Wu, K.; Liang, Y.; et al. Abscisic acid-mediated modifications of radial apoplastic transport pathway play a key role in cadmium uptake in hyperaccumulator Sedum alfredii. Plant Cell Environ. 2019, 42, 1425–1440. [Google Scholar] [CrossRef]
- Wang, P.L.; Lei, X.J.; Wang, Y.Y.; Liu, B.C.; Wang, D.N.; Liu, Z.Y.; Gao, C.Q. Transcriptomic Analysis of Cadmium Stressed Tamarix hispida Revealed Novel Transcripts and the Importance of Abscisic Acid Network. Front. Plant Sci. 2022, 13, 843725. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef]
- Pu, X.; Dong, X.; Li, Q.; Chen, Z.; Liu, L. An update on the function and regulation of methylerythritol phosphate and mevalonate pathways and their evolutionary dynamics. J. Integr. Plant Biol. 2021, 63, 1211–1226. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, W.-c.; Xiao, Y.; Liu, H.-w.; Liu, P. Methylerythritol Phosphate Pathway of Isoprenoid Biosynthesis. Annu. Rev. Biochem. 2013, 82, 497–530. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Frank, A.; Groll, M. The Methylerythritol Phosphate Pathway to Isoprenoids. Chem. Rev. 2017, 117, 5675–5703. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Hirschberg, J. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A.D. Elucidation of the Indirect Pathway of Abscisic Acid Biosynthesis by Mutants, Genes, and Enzymes. Plant Physiol. 2003, 131, 1591–1601. [Google Scholar] [CrossRef]
- Jia, K.-P.; Mi, J.; Ali, S.; Ohyanagi, H.; Moreno, J.C.; Ablazov, A.; Balakrishna, A.; Berqdar, L.; Fiore, A.; Diretto, G.; et al. An alternative, zeaxanthin epoxidase-independent abscisic acid biosynthetic pathway in plants. Mol. Plant 2022, 15, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the Basal Role of ABA–Roles Outside of Stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Ma, Y.; Wang, H.; Shi, Y. Role of transpiration and metabolism in translocation and accumulation of cadmium in tobacco plants (Nicotiana tabacum L.). Chemosphere 2016, 144, 1960–1965. [Google Scholar] [CrossRef]
- Naeem, A.; Saifullah; Zia-ur-Rehman, M.; Akhtar, T.; Zia, M.H.; Aslam, M. Silicon nutrition lowers cadmium content of wheat cultivars by regulating transpiration rate and activity of antioxidant enzymes. Environ. Pollut. 2018, 242, 126–135. [Google Scholar] [CrossRef]
- Chen, S.; Sharmin, S.; Lipka, U.; Polle, A.; Eckert, C. The influence of transpiration on foliar accumulation of salt and nutrients under salinity in poplar (Populus × canescens). PLoS ONE 2021, 16, e0253228. [Google Scholar] [CrossRef]
- Ge, L.; Cang, L.; Yang, J.; Zhou, D. Effects of root morphology and leaf transpiration on Cd uptake and translocation in rice under different growth temperature. Environ. Sci. Pollut. Res. 2016, 23, 24205–24214. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Dong, Q.; Yang, X.; Liu, Y.; Li, B.; Yuan, S.; Yin, J.; Xu, Q.; Li, T.; et al. Abscisic acid-mediated modifications in water transport continuum are involved in cadmium hyperaccumulation in Sedum alfredii. Chemosphere 2021, 268, 129339. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Brodribb, T.J. Separating Active and Passive Influences on Stomatal Control of Transpiration. Plant Physiol. 2014, 164, 1578–1586. [Google Scholar] [CrossRef]
- Vaidya, A.S.; Peterson, F.C.; Yarmolinsky, D.; Merilo, E.; Verstraeten, I.; Park, S.-Y.; Elzinga, D.; Kaundal, A.; Helander, J.; Lozano-Juste, J.; et al. A Rationally Designed Agonist Defines Subfamily IIIA Abscisic Acid Receptors as Critical Targets for Manipulating Transpiration. ACS Chem. Biol. 2017, 12, 2842–2848. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef]
- Liñero, O.; Cornu, J.-Y.; Candaudap, F.; Pokrovsky, O.S.; Bussière, S.; Coriou, C.; Humann-Guilleminot, T.; Robert, T.; Thunot, S.; de Diego, A.; et al. Short-term partitioning of Cd recently taken up between sunflowers organs (Helianthus annuus) at flowering and grain filling stages: Effect of plant transpiration and allometry. Plant Soil 2016, 408, 163–181. [Google Scholar] [CrossRef]
- Li, D.; He, T.; Saleem, M.; He, G. Metalloprotein-Specific or Critical Amino Acid Residues: Perspectives on Plant-Precise Detoxification and Recognition Mechanisms under Cadmium Stress. Int. J. Mol. Sci. 2022, 23, 1734. [Google Scholar] [CrossRef]
- He, X.L.; Fan, S.K.; Zhu, J.; Guan, M.Y.; Liu, X.X.; Zhang, Y.S.; Jin, C.W. Iron supply prevents Cd uptake in Arabidopsis by inhibiting IRT1 expression and favoring competition between Fe and Cd uptake. Plant Soil 2017, 416, 453–462. [Google Scholar] [CrossRef]
- Shi, W.-G.; Liu, W.; Yu, W.; Zhang, Y.; Ding, S.; Li, H.; Mrak, T.; Kraigher, H.; Luo, Z.-B. Abscisic acid enhances lead translocation from the roots to the leaves and alleviates its toxicity in Populus × canescens. J. Hazard. Mater. 2019, 362, 275–285. [Google Scholar] [CrossRef]
- Fan, S.K.; Fang, X.Z.; Guan, M.Y.; Ye, Y.Q.; Lin, X.Y.; Du, S.T.; Jin, C.W. Exogenous abscisic acid application decreases cadmium accumulation in Arabidopsis plants, which is associated with the inhibition of IRT1-mediated cadmium uptake. Front. Plant Sci. 2014, 5, 721. [Google Scholar] [CrossRef]
- Xu, Q.; Pan, W.; Zhang, R.; Lu, Q.; Xue, W.; Wu, C.; Song, B.; Du, S. Inoculation with Bacillus subtilis and Azospirillum brasilense Produces Abscisic Acid That Reduces Irt1-Mediated Cadmium Uptake of Roots. J. Agric. Food Chem. 2018, 66, 5229–5236. [Google Scholar] [CrossRef]
- You, Y.; Wang, Y.; Zhang, S.; Sun, X.; Liu, H.; Guo, E.Y.; Du, S. Different pathways for exogenous ABA-mediated down-regulation of cadmium accumulation in plants under different iron supplies. J Hazard. Mater. 2022, 440, 129769. [Google Scholar] [CrossRef]
- Chaudhary, K.; Jan, S.; Khan, S. Heavy Metal ATPase (HMA2, HMA3, and HMA4) Genes in Hyperaccumulation Mechanism of Heavy Metals. In Plant Metal Interaction; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 545–556. [Google Scholar]
- Yu, E.; Wang, W.; Yamaji, N.; Fukuoka, S.; Che, J.; Ueno, D.; Ando, T.; Deng, F.; Hori, K.; Yano, M.; et al. Duplication of a manganese/cadmium transporter gene reduces cadmium accumulation in rice grain. Nat. Food 2022, 3, 597–607. [Google Scholar] [CrossRef]
- Wong, C.K.E.; Cobbett, C.S. HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol. 2008, 181, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Nagasawa, N.; Mori, M.; Nakazawa, N.; Kawamoto, T.; Nagato, Y.; Sakurai, K.; Takahashi, H.; Watanabe, A.; Akagi, H. Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol. 2012, 53, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L.; Deng, Y.; Bai, Y.; Sun, C.; Huang, S.; Zhou, J.; Shi, L.; Yang, X.; Li, L.; et al. BrpNAC895 and BrpABI449 coregulate the transcription of the afflux-type Cd transporter BrpHMA2 in Brassica parachinensis. Hortic. Res. 2022, 9, uhac044. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, S.; Li, Y.; Zheng, F.; He, B.; Gu, M. Exogenous abscisic acid (ABA) promotes cadmium (Cd) accumulation in Sedum alfredii Hance by regulating the expression of Cd stress response genes. Environ. Sci. Pollut. Res. 2020, 27, 8719–8731. [Google Scholar] [CrossRef]
- Tian, W.; He, G.; Qin, L.; Li, D.; Meng, L.; Huang, Y.; He, T. Genome-wide analysis of the NRAMP gene family in potato (Solanum tuberosum): Identification, expression analysis and response to five heavy metals stress. Ecotoxicol. Environ. Saf. 2021, 208, 111661. [Google Scholar] [CrossRef]
- Qin, L.; Han, P.; Chen, L.; Walk, T.C.; Li, Y.; Hu, X.; Xie, L.; Liao, H.; Liao, X. Genome-Wide Identification and Expression Analysis of NRAMP Family Genes in Soybean (Glycine Max L.). Front. Plant Sci. 2017, 8, 1436. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, Y. Differential expression of rice Nramp genes in response to pathogen infection, defense signal molecules and metal ions. Physiol. Mol. Plant Pathol. 2004, 65, 235–243. [Google Scholar] [CrossRef]
- Pál, M.; Csávás, G.; Szalai, G.; Oláh, T.; Khalil, R.; Yordanova, R.; Gell, G.; Birinyi, Z.; Németh, E.; Janda, T. Polyamines may influence phytochelatin synthesis during Cd stress in rice. J. Hazard. Mater. 2017, 340, 272–280. [Google Scholar] [CrossRef]
- Degola, F.; De Benedictis, M.; Petraglia, A.; Massimi, A.; Fattorini, L.; Sorbo, S.; Basile, A.; Sanità di Toppi, L. A Cd/Fe/Zn-Responsive Phytochelatin Synthase is Constitutively Present in the Ancient Liverwort Lunularia cruciata (L.) Dumort. Plant Cell Physiol. 2014, 55, 1884–1891. [Google Scholar] [CrossRef]
- Senoura, T.; Sakashita, E.; Kobayashi, T.; Takahashi, M.; Aung, M.S.; Masuda, H.; Nakanishi, H.; Nishizawa, N.K. The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol. Biol. 2017, 95, 375–387. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Clemens, S.; Schroeder, J.I.; Degenkolb, T. Caenorhabditis elegans expresses a functional phytochelatin synthase. Eur. J. Biochem. 2001, 268, 3640–3643. [Google Scholar] [CrossRef]
- Wątły, J.; Łuczkowski, M.; Padjasek, M.; Krężel, A. Phytochelatins as a Dynamic System for Cd(II) Buffering from the Micro- to Femtomolar Range. Inorg. Chem. 2021, 60, 4657–4675. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Grill, E.; Winnacker, E.L.; Zenk, M.H. Phytochelatins: The principal heavy-metal complexing peptides of higher plants. Science 1985, 230, 674–676. [Google Scholar] [CrossRef]
- Rauser, W.E. Phytochelatins. Annu. Rev. Biochem. 1990, 59, 61–86. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, W.; Jie, Y. Overexpression of BnPCS1, a Novel Phytochelatin Synthase Gene from Ramie (Boehmeria nivea), Enhanced Cd Tolerance, Accumulation, and Translocation in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 639189. [Google Scholar] [CrossRef]
- Stroiński, A.; Giżewska, K.; Zielezińska, M. Abscisic acid is required in transduction of cadmium signal to potato roots. Biol. Plant. 2013, 57, 121–127. [Google Scholar] [CrossRef]
- Hernández, L.E.; Ortega-Villasante, C.; Montero-Palmero, M.B.; Escobar, C.; Carpena, R.O. Heavy Metal Perception in a Microscale Environment: A Model System Using High Doses of Pollutants. In Metal Toxicity in Plants: Perception, Signaling and Remediation; Gupta, D.K., Sandalio, L.M., Eds.; Springe: Berlin/Heidelberg, Germany, 2012; pp. 23–39. [Google Scholar]
- Koramutla, M.K.; Negi, M.; Ayele, B.T. Roles of Glutathione in Mediating Abscisic Acid Signaling and Its Regulation of Seed Dormancy and Drought Tolerance. Genes 2021, 12, 1620. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Y.; Ma, X.; Guo, L.; He, Y.; Ren, Z.; Kuang, Z.; Zhang, X.; Zhang, Z. Analysis of potential strategies for cadmium stress tolerance revealed by transcriptome analysis of upland cotton. Sci. Rep. 2019, 9, 86. [Google Scholar] [CrossRef]
- Li, S.-W.; Leng, Y.; Feng, L.; Zeng, X.-Y. Involvement of abscisic acid in regulating antioxidative defense systems and IAA-oxidase activity and improving adventitious rooting in mung bean [Vigna radiata (L.) Wilczek] seedlings under cadmium stress. Environ. Sci. Pollut. Res. 2013, 21, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan, C.; Turkan, I.; Sekmen, A.H.; Seckin, B. Abscisic acid-regulated responses of aba2-1 under osmotic stress: The abscisic acid-inducible antioxidant defence system and reactive oxygen species production. Plant Biol. 2012, 14, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Niu, J.; Deng, Z. Abscisic acid treatment alleviates cadmium toxicity in purple flowering stalk (Brassica campestris L. ssp. chinensis var. purpurea Hort.) seedlings. Plant Physiol. Biochem. 2017, 118, 471–478. [Google Scholar] [CrossRef]
- Li, M.; Hao, P.; Cao, F. Glutathione-induced alleviation of cadmium toxicity in Zea mays. Plant Physiol. Biochem. 2017, 119, 240–249. [Google Scholar] [CrossRef]
- Wang, T.; Hua, Y.; Chen, M.; Zhang, J.; Guan, C.; Zhang, Z. Mechanism Enhancing Arabidopsis Resistance to Cadmium: The Role of NRT1.5 and Proton Pump. Front. Plant Sci. 2018, 9, 1892. [Google Scholar] [CrossRef]
- Zhang, G.-B.; Yi, H.-Y.; Gong, J.-M. The Arabidopsis Ethylene/Jasmonic Acid-NRT Signaling Module Coordinates Nitrate Reallocation and the Trade-Off between Growth and Environmental Adaptation. Plant Cell 2014, 26, 3984–3998. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Lozano-Juste, J.; Albert, A. PYR/PYL/RCAR ABA receptors. In Abscisic Acid in Plants; Advances in Botanical Research; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 51–82. [Google Scholar]
- Yang, Q.; Liu, K.; Niu, X.; Wang, Q.; Wan, Y.; Yang, F.; Li, G.; Wang, Y.; Wang, R. Genome-wide Identification of PP2C Genes and Their Expression Profiling in Response to Drought and Cold Stresses in Medicago truncatula. Sci. Rep. 2018, 8, 12841. [Google Scholar] [CrossRef]
- Ng, L.M.; Melcher, K.; Teh, B.T.; Xu, H.E. Abscisic acid perception and signaling: Structural mechanisms and applications. Acta Pharm. Sin. 2014, 35, 567–584. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef]
- Tavares, L.S.C.; Reis, S.P.; Marques, D.N.; Tavares, E.J.M.; Cunha Ferreira, S.; Coelho, F.M.; Souza, C.R.B. Abscisic Acid in Abiotic Stress-responsive Gene Expression. In Molecular Plant Abiotic Stress; Aryadeep Roychoudhury, D.D.T., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 145–184. [Google Scholar]
- Kim, T.-H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.J.; Kim, M.H.; Kwak, J.M. MAPK Cascades in Guard Cell Signal Transduction. Front. Plant Sci. 2016, 7, 80. [Google Scholar] [CrossRef]
- Brandt, B.; Munemasa, S.; Wang, C.; Nguyen, D.; Yong, T.; Yang, P.G.; Poretsky, E.; Belknap, T.F.; Waadt, R.; Aleman, F.; et al. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. Elife 2015, 4, e03599. [Google Scholar] [CrossRef]
- Pei, D.; Hua, D.; Deng, J.; Wang, Z.; Song, C.; Wang, Y.; Wang, Y.; Qi, J.; Kollist, H.; Yang, S.; et al. Phosphorylation of the plasma membrane H+-ATPase AHA2 by BAK1 is required for ABA-induced stomatal closure in Arabidopsis. Plant Cell 2022, 34, 2708–2729. [Google Scholar] [CrossRef]
- Hauser, F.; Li, Z.; Waadt, R.; Schroeder, J.I. SnapShot: Abscisic Acid Signaling. Cell 2017, 171, 1708. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, R.; Ju, Q.; Li, W.; Tran, L.-S.P.; Xu, J. The R2R3-MYB Transcription Factor MYB49 Regulates Cadmium Accumulation. Plant Physiol. 2019, 180, 529–542. [Google Scholar] [CrossRef]
- Huang, Y.T.; Cai, S.Y.; Ruan, X.L.; Chen, S.Y.; Mei, G.F.; Ruan, G.H.; Cao, D.D. Salicylic acid enhances sunflower seed germination under Zn2+ stress via involvement in Zn2+ metabolic balance and phytohormone interactions. Sci. Hortic. 2021, 275, 109702. [Google Scholar] [CrossRef]
- Li, D.; Xu, X.; Hu, X.; Liu, Q.; Wang, Z.; Zhang, H.; Wang, H.; Wei, M.; Wang, H.; Liu, H.; et al. Genome-Wide Analysis and Heavy Metal-Induced Expression Profiling of the HMA Gene Family in Populus trichocarpa. Front. Plant Sci. 2015, 6, 1149. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Yang, C.; Yan, W.; Liu, C.; Tang, X.; Gao, C. Genome-wide Identification and Characterization of FCS-Like Zinc Finger (FLZ) Family Genes in Maize (Zea mays) and Functional Analysis of ZmFLZ25 in Plant Abscisic Acid Response. Int. J. Mol. Sci. 2021, 22, 3529. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y.; et al. Zinc-Finger Transcription Factor ZAT6 Positively Regulates Cadmium Tolerance through the Glutathione-Dependent Pathway in Arabidopsis. Plant Physiol. 2016, 171, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Huang, S.-s.C.; Wise, A.; Castanon, R.; Nery, J.R.; Chen, H.; Watanabe, M.; Thomas, J.; Bar-Joseph, Z.; Ecker, J.R. A transcription factor hierarchy defines an environmental stress response network. Science 2016, 354, aag1550. [Google Scholar] [CrossRef] [PubMed]
- Farinati, S.; DalCorso, G.; Varotto, S.; Furini, A. The Brassica juncea BjCdR15, an ortholog of Arabidopsis TGA3, is a regulator of cadmium uptake, transport and accumulation in shoots and confers cadmium tolerance in transgenic plants. New Phytol. 2009, 185, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, J.; Jie, Y.; Xing, H.; Zhong, Y.; She, W.; Wei, G.; Yu, W.; Ma, Y. A ramie (Boehmeria nivea) bZIP transcription factor BnbZIP3 positively regulates drought, salinity and heavy metal tolerance. Mol. Breed. 2016, 36, 120. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.Y.; Yang, S.G.; Tian, W.M. Cloning and characterization of HbMT2a, a metallothionein gene from Hevea brasiliensis Muell. Arg differently responds to abiotic stress and heavy metals. Biochem. Biophys. Res. Commun. 2015, 461, 95–101. [Google Scholar] [CrossRef]
- Xu, Z.; Ge, Y.; Zhang, W.; Zhao, Y.; Yang, G. The walnut JrVHAG1 gene is involved in cadmium stress response through ABA-signal pathway and MYB transcription regulation. BMC Plant Biol. 2018, 18, 19. [Google Scholar] [CrossRef]
- Manara, A.; Fasani, E.; Molesini, B.; DalCorso, G.; Pennisi, F.; Pandolfini, T.; Furini, A. The Tomato Metallocarboxypeptidase Inhibitor I, which Interacts with a Heavy Metal-Associated Isoprenylated Protein, Is Implicated in Plant Response to Cadmium. Molecules 2020, 25, 700. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, S.; Feng, J.; Wang, W.; Wang, Y.; Yu, Q.; Liao, Y.; Mo, Y.; Xu, Z.; Li, L.; et al. Overexpression of a stress response membrane protein gene OsSMP1 enhances rice tolerance to salt, cold and heavy metal stress. Environ. Exp. Bot. 2021, 182, 104327. [Google Scholar] [CrossRef]
- Xian, J.; Wang, Y.; Niu, K.; Ma, H.; Ma, X. Transcriptional regulation and expression network responding to cadmium stress in a Cd-tolerant perennial grass Poa Pratensis. Chemosphere 2020, 250, 126158. [Google Scholar] [CrossRef]
- Su, Y.; Wang, Z.; Liu, F.; Li, Z.; Peng, Q.; Guo, J.; Xu, L.; Que, Y. Isolation and Characterization of ScGluD2, a New Sugarcane beta-1,3-Glucanase D Family Gene Induced by Sporisorium scitamineum, ABA, H2O2, NaCl, and CdCl2 Stresses. Front. Plant Sci. 2016, 7, 1348. [Google Scholar] [CrossRef]
- Talanova, V.V.; Titov, A.F.; Repkina, N.S.; Topchieva, L.V. Cold-responsive COR/LEA genes participate in the response of wheat plants to heavy metals stress. Dokl. Biol. Sci. 2013, 448, 28–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, J.; Huang, W.; Zhang, D.; Wu, J.; Li, B.; Li, M.; Liu, L.; Yan, M. Abscisic-Acid-Regulated Responses to Alleviate Cadmium Toxicity in Plants. Plants 2023, 12, 1023. https://doi.org/10.3390/plants12051023
Zhao Y, Wang J, Huang W, Zhang D, Wu J, Li B, Li M, Liu L, Yan M. Abscisic-Acid-Regulated Responses to Alleviate Cadmium Toxicity in Plants. Plants. 2023; 12(5):1023. https://doi.org/10.3390/plants12051023
Chicago/Turabian StyleZhao, Yuquan, Jiaqi Wang, Wei Huang, Dawei Zhang, Jinfeng Wu, Bao Li, Mei Li, Lili Liu, and Mingli Yan. 2023. "Abscisic-Acid-Regulated Responses to Alleviate Cadmium Toxicity in Plants" Plants 12, no. 5: 1023. https://doi.org/10.3390/plants12051023
APA StyleZhao, Y., Wang, J., Huang, W., Zhang, D., Wu, J., Li, B., Li, M., Liu, L., & Yan, M. (2023). Abscisic-Acid-Regulated Responses to Alleviate Cadmium Toxicity in Plants. Plants, 12(5), 1023. https://doi.org/10.3390/plants12051023





