Promising Application of Automated Liquid Culture System and Arbuscular Mycorrhizal Fungi for Large-Scale Micropropagation of Red Dragon Fruit
Abstract
1. Introduction
2. Results and Discussion
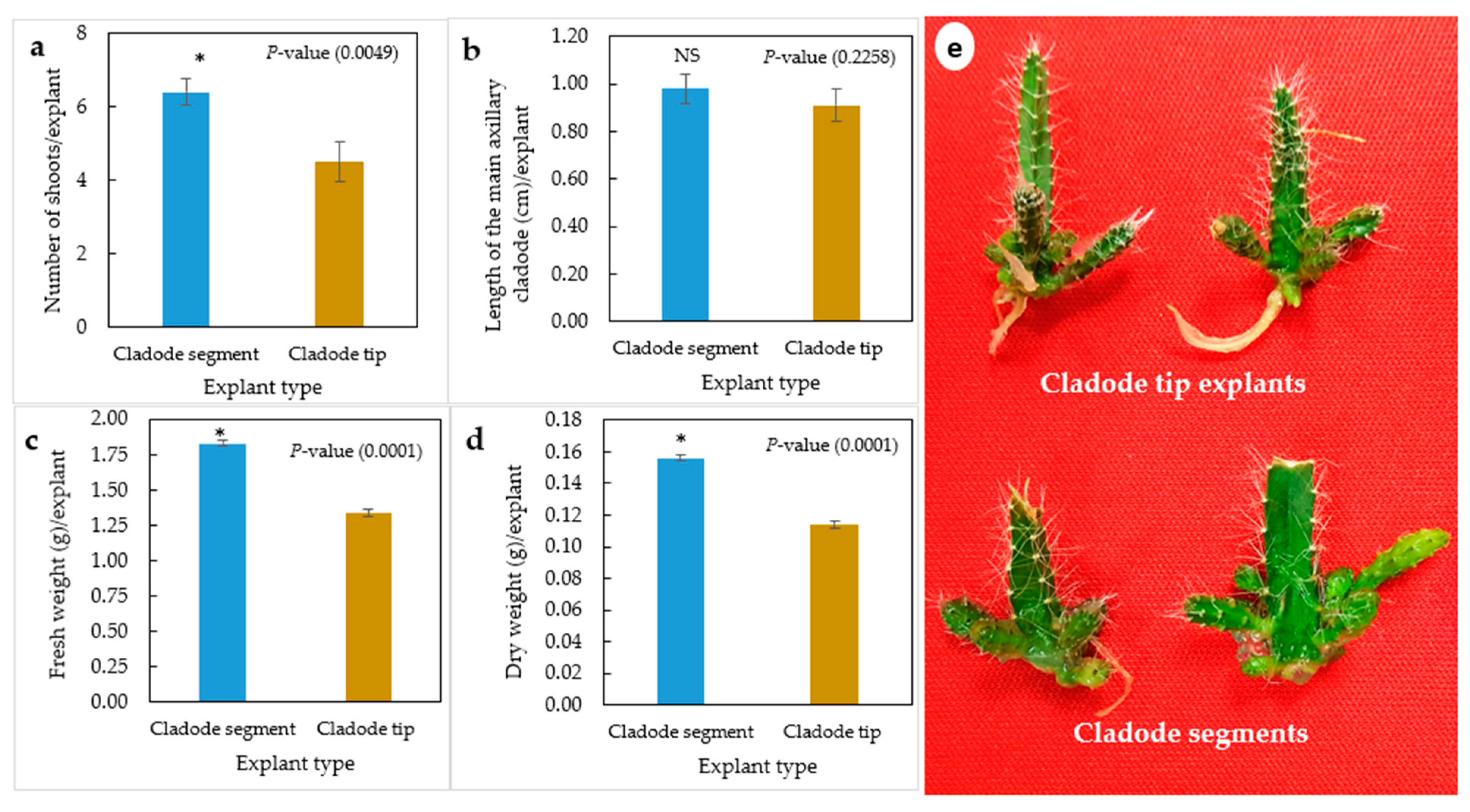
2.1. Effect of Explant Type and Axillary Cladode Multiplication in Gelled versus Liquid/Bioreactor Culture System
2.2. Effect of AMF on Acclimatization of Micropropagated H. polyrhizus Plantlets
3. Materials and Methods
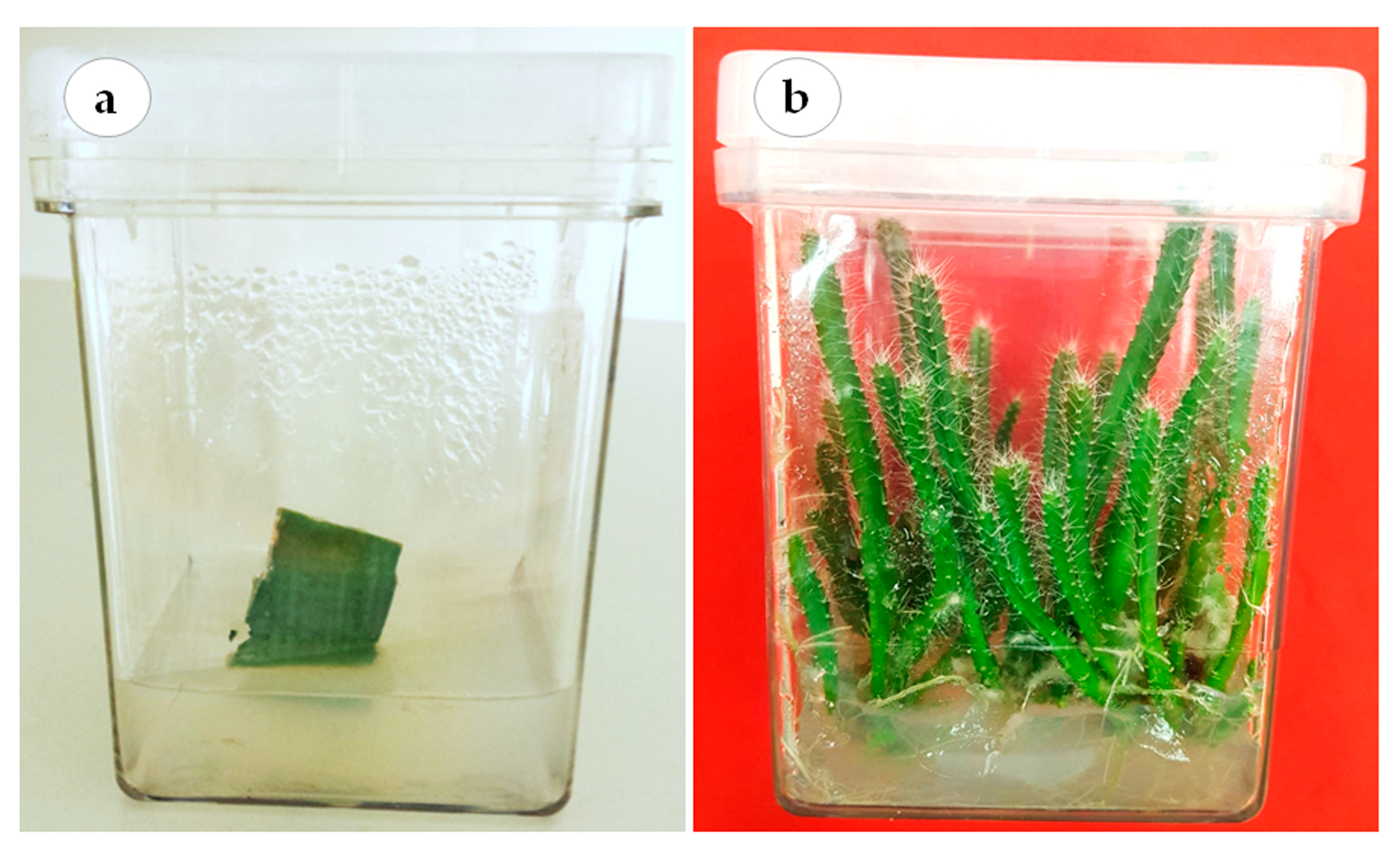
3.1. Plant Material and Establishment of In Vitro Culture
3.2. Effects of Explant Type on Axillary Cladode Multiplication of H. polyrhizus in Gelled Culture
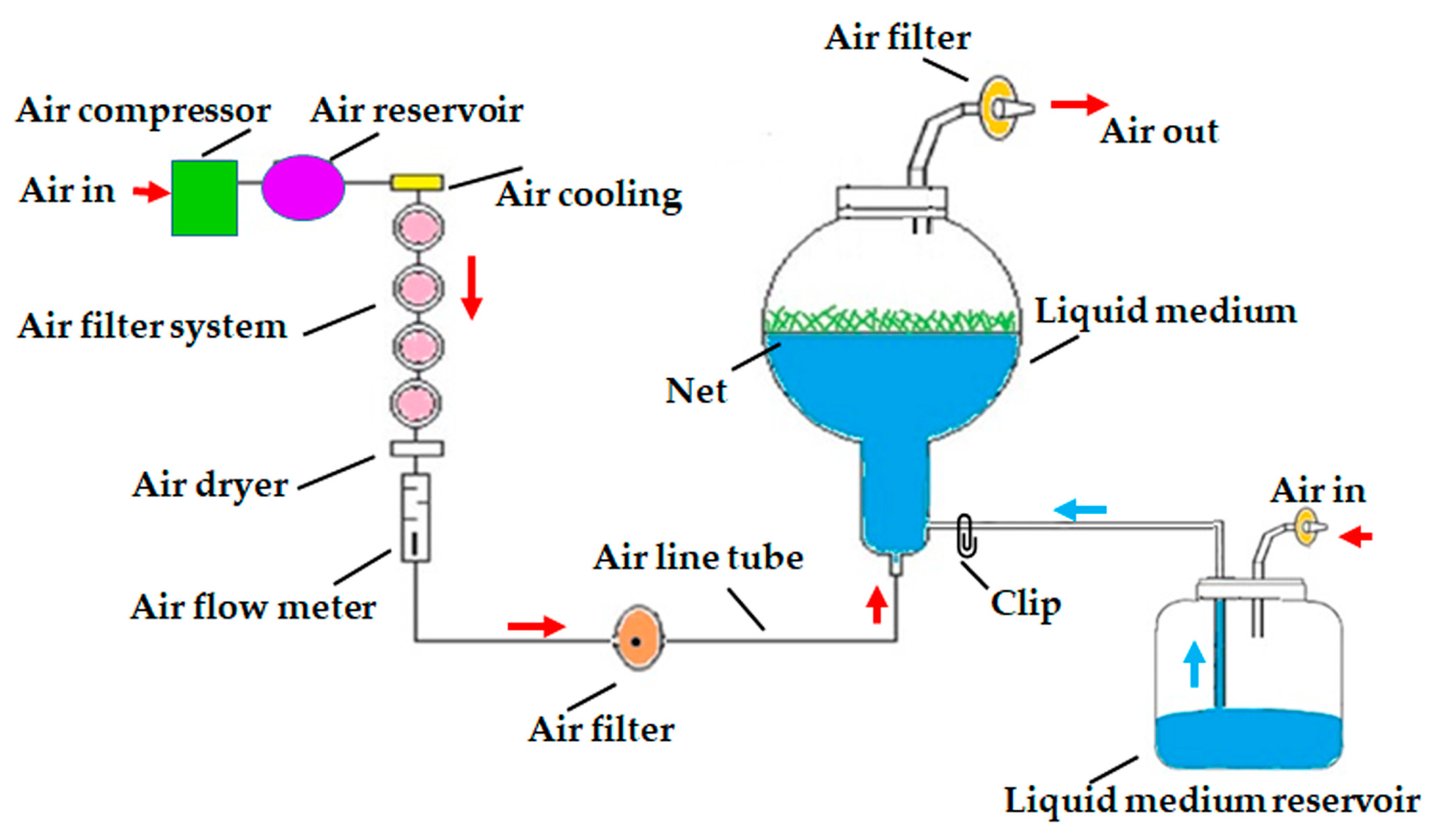
3.3. Effects of Gelled and Liquid Bioreactor Culture on Axillary Cladode Multiplication of H. polyrhizus
3.4. In Vitro Rooting, AMF Inoculation and Acclimatization of Micropropagated H. polyrhizus Plantlets
3.5. Symbiotic Development and AMF Spore Counts
3.6. Measurements of Leaf Pigments and Vegetative Parameters
3.7. Experimental Design and Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morton, J. Strawberry pear. In Fruits of Warm Climates; J.F. Morton: Miami, FL, USA, 1987; pp. 347–348. [Google Scholar]
- Esquivel, P.; Stintzing, F.C.; Carle, R. Comparison of morphological and chemical fruit traits from different pitaya genotypes (Hylocereus sp.) grown in Costa Rica. J. Appl. Bot. Food Qual. 2007, 81, 7–14. [Google Scholar]
- Le Bellec, F.; Vaillant, F. Pitahaya (pitaya) (Hylocereus spp.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 247–271, 272e–273e. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Betalains—Emerging prospects for food scientists. Trends Food Sci. Technol. 2007, 18, 514–525. [Google Scholar] [CrossRef]
- Mohd Adzim Khalili, R.; Norhayati, A.H.; Rokiah, M.Y.; Asmah, R.; Siti Muskinah, M.; Abdul Manaf, A. Hypocholesterolemic effect of red pitaya (Hylocereus sp.) on hypercholesterolemia induced rats. Int. Food Res. J. 2009, 16, 431–440. [Google Scholar]
- Le Bellec, F.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future. Fruits 2006, 61, 237–250. [Google Scholar] [CrossRef]
- El Obeidy, A.A. Mass propagation of pitaya (dragon fruit). Fruits 2006, 61, 313–319. [Google Scholar] [CrossRef]
- de Menezes, T.P.; Gomes, W.A.; Pio, L.A.S.; Pasqual, M.; Ramos, J.D. Micropropagation and endoreduplication in red pitaya, Hylocereus undatus haw. [Micropropagação e endorreduplicação em pitaya vermelha, Hylocereus undatus haw]. Biosci. J. 2012, 28, 868–876. [Google Scholar]
- Fan, Q.J.; Zheng, S.C.; Yan, F.X.; Zhang, B.X.; Qiao, G.; Wen, X.P. Efficient regeneration of dragon fruit (Hylocereus undatus) and an assessment of the genetic fidelity of in vitro-derived plants using ISSR markers. J. Hortic. Sci. Biotechnol. 2013, 88, 631–637. [Google Scholar] [CrossRef]
- Mohamed-Yasseen, Y. Micropropagation of pitaya (Hylocereus undatus Britton et Rose). In Vitro Cell. Dev. Biol.-Plant 2002, 38, 427–429. [Google Scholar] [CrossRef]
- Hua, Q.; Chen, P.; Liu, W.; Ma, Y.; Liang, R.; Wang, L.; Wang, Z.; Hu, G.; Qin, Y. A protocol for rapid in vitro propagation of genetically diverse pitaya. Plant Cell Tissue Organ Cult. 2015, 120, 741–745. [Google Scholar] [CrossRef]
- Viñas, M.; Fernández-Brenes, M.; Azofeifa, A.; Jiménez, V.M. In vitro propagation of purple pitahaya (Hylocereus costaricensis [F.A.C. weber] britton & rose) cv. cebra. In Vitro Cell. Dev. Biol. Plant 2012, 48, 469–477. [Google Scholar] [CrossRef]
- Pedda Kasim, D.; Sai Kishore, N.; Suneetha, P.; Bramareswara Rao, K.; Naresh Kumar, M.; Krishna, M.S.R. Multiple shoot regeneration in seed-derived immature leaflet explants of red dragon fruit (Hylocereus costaricensis). Res. J. Pharm. Technol. 2019, 12, 1491–1494. [Google Scholar] [CrossRef]
- Mállap-Detquizán, G.; Vilca-Valqui, N.C.; Meléndez-Mori, J.B.; Huaman-Huaman, E.; Oliva, M. In vitro multiplication of yellow dragon fruit (Hylocereus megalanthus) from seedlings obtained in vitro. [Multiplicación in vitro de pitahaya amarilla (Hylocereus megalanthus) a partir de plántulas obtenidas in vitro]. Agron. Mesoam. 2022, 33, 45472. [Google Scholar] [CrossRef]
- Ng, Z.C.; Shiekh Mahmud, S.H.; Hian, T.S. Effects of cytokinin in enhancing the multiplication of vegetative Hylocereus polyrhizus. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2021. [Google Scholar] [CrossRef]
- Qin, J.; Wang, Y.; He, G.; Chen, L.; He, H.; Cheng, X.; Xu, K.; Zhang, D. High-efficiency micropropagation of dormant buds in spine base of red pitaya (Hylocereus polyrhizus) for industrial breeding. Int. J. Agric. Biol. 2017, 19, 193–198. [Google Scholar] [CrossRef]
- Román, R.S.S.; Caetano, C.M.; Ramírez, H. Multiplication of Selenicereus megalanthus (yellow pitahaya) and Hylocereus polyrhizus (red dragon fruit) by somatic organogenesis view. [Multiplicación de Selenicereus megalanthus (Pitahaya Amarilla) e Hylocereus polyrhizus (Pitahaya Roja) vía organogénesis somática]. Acta Agron. 2014, 63, 272–281. [Google Scholar]
- Bello-Bello, J.J.; Schettino-Salomón, S.; Ortega-Espinoza, J.; Spinoso-Castillo, J.L. A temporary immersion system for mass micropropagation of pitahaya (Hylocereus undatus). 3 Biotech 2021, 11, 437. [Google Scholar] [CrossRef]
- Declerck, S.; Risede, J.M.; Delvaux, B. Greenhouse response of micropropagated bananas inoculated with “in vitro” monoxenically produced arbuscular mycorrhizal fungi. Sci. Hortic. 2002, 93, 301–309. [Google Scholar] [CrossRef]
- Borkowska, B. Growth and photosynthetic activity of micropropagated strawberry plants inoculated with endomycorrhizal fungi (AMF) and growing under drought stress. Acta Physiol. Plant. 2002, 24, 365–370. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal symbiosis. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA; London, UK, 2008. [Google Scholar]
- Kapoor, R.; Sharma, D.; Bhatnagar, A.K. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci. Hortic. 2008, 116, 227–239. [Google Scholar] [CrossRef]
- Hazarika, B.N.; Bora, A. Use of bio-agents in acclimatizing micropropagated plants—A review. Agricultural Reviews-Agricultural Research Communications Centre India 2006, 27, 152–156. [Google Scholar]
- Kavoo-Mwangi, A.M.; Kahangi, E.M.; Ateka, E.; Onguso, J.; Mukhongo, R.W.; Mwangi, E.K.; Jefwa, J.M. Growth effects of microorganisms based commercial products inoculated to tissue cultured banana cultivated in three different soils in Kenya. Appl. Soil Ecol. 2013, 64, 152–162. [Google Scholar] [CrossRef]
- Koffi, M.C.; Declerck, S. In vitro mycorrhization of banana (Musa acuminata) plantlets improves their growth during acclimatization. In Vitro Cell. Dev. Biol. Plant 2015, 51, 265–273. [Google Scholar] [CrossRef]
- Villarreal, T.C.; Medina, M.E.; Ulloa, S.M.; Darwin, R.O.; Bangeppagari, M.; Selvaraj, T.; Sikandar, I.M. Effect of arbuscular mycorrhizal fungi (AMF) and Azospirillum on growth and nutrition of banana plantlets during acclimatization phase. J. Appl. Pharm. Sci. 2016, 6, 131–138. [Google Scholar] [CrossRef]
- Campanelli, A.; Ruta, C.; Tagarelli, A.; Morone-Fortunato, I.; De Mastro, G. Effectiveness of mycorrhizal fungi on globe artichoke (Cynara cardunculus L. var. scolymus) micropropagation. J. Plant Interact. 2014, 9, 100–106. [Google Scholar] [CrossRef]
- Yadav, K.; Aggarwal, A.; Singh, N. Arbuscular mycorrhizal fungi (AMF) induced acclimatization, growth enhancement and colchicine content of micropropagated Gloriosa superba L. plantlets. Ind. Crops Prod. 2013, 45, 88–93. [Google Scholar] [CrossRef]
- Estrada-Luna, A.A.; Davies, F.T., Jr. Mycorrhizal fungi enhance growth and nutrient uptake of prickly-pear cactus (Opuntia albicarpa Scheinvar ‘Reyna’) plantlets after ex vitro transplantation. J. Hortic. Sci. Biotechnol. 2001, 76, 739–745. [Google Scholar] [CrossRef]
- Dabekaussen, M.A.A.; Pierik, R.L.M.; Van der Laken, J.D.; Spaans, J.H. Factors affecting areole activation in vitro in the cactus Sulcorebutia alba Rausch. Sci. Hortic. 1991, 46, 283–294. [Google Scholar] [CrossRef]
- Brukhin, V.; Morozova, N. Plant growth and development-basic knowledge and current views. Math. Model. Nat. Phenom. 2011, 6, 1–53. [Google Scholar] [CrossRef]
- Paek, K.Y.; Hahn, E.J.; Son, S.H. Application of bioreactors of large scale micropropagation systems of plants. In Vitro Cell. Dev. Biol.-Plant 2001, 37, 149–157. [Google Scholar] [CrossRef]
- Hwang, H.-D.; Kwon, S.-H.; Murthy, H.N.; Yun, S.-W.; Pyo, S.-S.; Park, S.-Y. Temporary immersion bioreactor system as an efficient method for mass production of in vitro plants in horticulture and medicinal plants. Agronomy 2022, 12, 346. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Chakrabarty, D.; Hahn, E.J.; Paek, K.Y. Reversion of inflorescence development in Euphorbia millii and its application to large-scale micropropagation in an air-lift bioreactor. J. Hortic. Sci. Biotechnol. 2005, 80, 581–587. [Google Scholar] [CrossRef]
- Dewir, Y.H. Mass Propagation of the Christ Plant Using Bioreactor and Hydroponics; Scholars’ Press: Saarbrücken, Germany, 2016; p. 108. ISBN 978-3-659-84521-5. [Google Scholar]
- Dewir, Y.H.; Indoliya, Y.; Chakrabarty, D.; Paek, K.Y. Biochemical and physiological aspects of hyperhydricity in liquid culture system. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Springer: Dordrecht, The Netherlands, 2014; pp. 693–709. [Google Scholar]
- Dewir, Y.H.; Chakrabarty, D.; Ali, M.B.; Hahn, E.J.; Paek, K.Y. Lipid peroxidation and antioxidant enzyme activities of Euphorbia millii hyperhydric shoots. Environ. Exp. Bot. 2006, 58, 93–99. [Google Scholar] [CrossRef]
- Malda, G.; Backhaus, R.A.; Martin, C. Alterations in growth and crassulacean acid metabolism (CAM) activity of in vitro cultured cactus. Plant Cell, Tissue Organ Cult. 1999, 58, 1–9. [Google Scholar] [CrossRef]
- Silveira, A.P.D. Micorrizas. In Microbiologia Do Solo; Cardoso, E.J.B.N., Tsai, S.M., Neves, M.C., Eds.; SBCS: Campinas, Brazil, 1992; pp. 257–282. [Google Scholar]
- Sato, A.Y.; Nannetti, D.d.C.; Pinto, J.E.B.P.; Siqueira, O.; Blank, M.d.F.A. Fungos micorrízicos-arbusculares no desenvolvimento de mudas de helicônia e gerbera micropropagadas. Hortic. Bras. 1999, 17, 25–28. [Google Scholar] [CrossRef]
- Da Silva, A.R.; De Melo, N.F.; Yano-Melo, A.M. Acclimatization of micropropagated plants of Etlingera elatior (Jack) RM Sm. inoculated with arbuscular mycorrhizal fungi. S. Afr. J. Bot. 2017, 113, 164–169. [Google Scholar] [CrossRef]
- Lima, F.C.D.; Ulisses, C.; Camara, T.R.; Cavalcante, U.M.T.; Albuquerque, C.C.; Willadino, L. Anthurium andraeanum Lindl. cv. Eidibel in vitro rooting and acclimatization with arbuscular mycorrhizal fungi. Rev. Bras. Ciênc Agrár. 2006, 1, 13–16. Available online: https://www.redalyc.org/articulo.oa?id=119018241003 (accessed on 15 December 2022).
- Krishna, H.; Singh, S.K.; Sharma, R.R.; Khawale, R.N.; Grover, M.; Patel, V.B. Biochemical changes in micropropagated grape (Vitis vinifera L.) plantlets due to arbuscular-mycorrhizal fungi (AMF) inoculation during ex vitro acclimatization. Sci. Hortic. 2005, 106, 554–567. [Google Scholar] [CrossRef]
- Bavaresco, L.; Fogher, C. Lime-induced chlorosis of grape-vine as affected by rootstock and root infection with arbuscular mycorrhiza and Pseudomonas flurescens. Vitis 1996, 35, 119–123. [Google Scholar]
- Gaur, A.; Adholeya, A. Mycorrhizal effects on the acclimatization, survival, growth and chlorophyll of micropropagated Syngonium and Draceana inoculated at weaning and hardening stages. Mycorrhiza 1999, 9, 215–219. [Google Scholar] [CrossRef]
- Young, A.J. The photoprotective role of carotenoids in higher plants. Physiol. Plant. 1991, 83, 702–708. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Al-Qarawi, A.; Mridha, M.; Alghamdi, O. Diversity of structural colonization and spore population of arbuscular mycorrhizal fungi in some plants from Riyadh, Saudi Arabia. J. Pure Appl. Microbiol. 2012, 6, 1119–1125. [Google Scholar]
- Gerdemann, J.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Schüssler, A.; Walker, C. The Glomeromycota: A Species List with New Families and New Gener. In The Glomeromycota; Create Space Independent Publishing Platform: Scotts Valley, CA, USA, 2010. [Google Scholar]
- Lichtenethaler, H.K. Biosynthesis, accumulation and emission of carotenoids, α-tocopherol, plastoquinone and isoprene in leaves under high photosynthetic irradiance. Phytosynth. Res. 2007, 92, 163–179. [Google Scholar] [CrossRef]
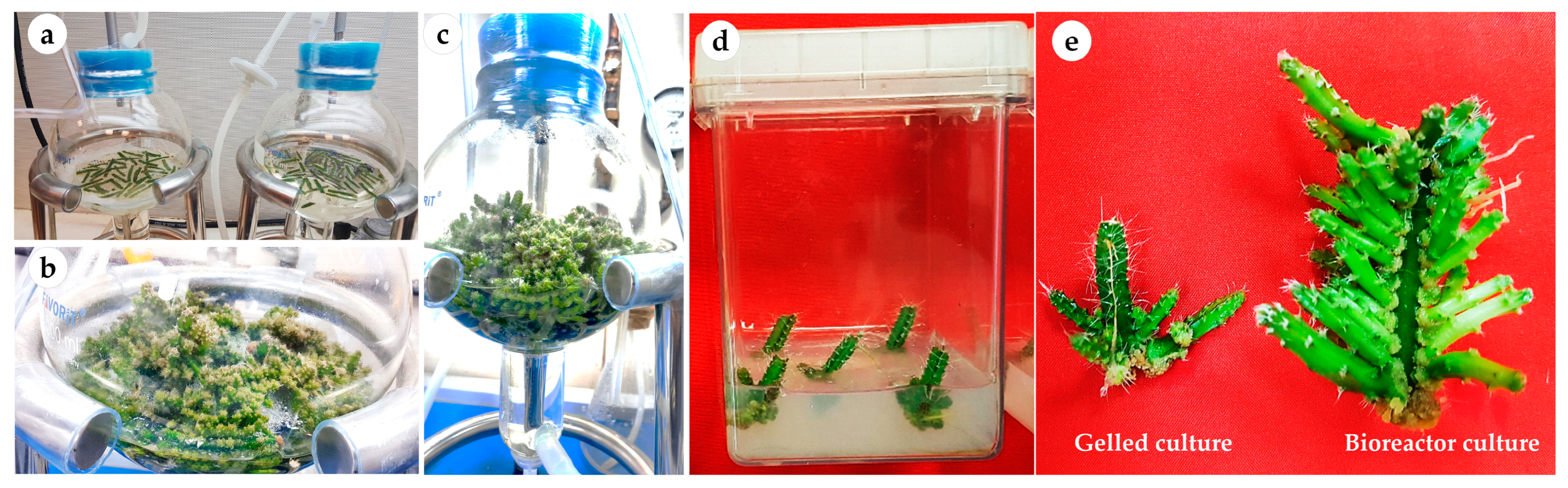


| Culture Method | Number of Cladodes /Explant | Fresh Weight /Explant (g) | Dry Weight /Explant (g) | Length of the Main Axillary Cladode (cm) | Total Chlorophyll Content (mg g−1FW) |
|---|---|---|---|---|---|
| Gelled culture | 6.7 b | 0.872 b | 0.120 b | 1.76 b | 0.255 a |
| Air-lift bioreactor (without net) | 45.9 a | 9.405 a | 0.677 a | 3.03 a | 0.222 a |
| Air-lift bioreactor (with net) | 42.8 a | 7.358 a | 0.598 a | 3.11 a | 0.253 a |
| F-value | 84.26 | 22.38 | 43.46 | 19.80 | 0.51 |
| p-value | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | 0.6232 NS |
| Growth Parameters | Non-AMF | AMF-Treated | p-Value |
|---|---|---|---|
| Number of cladodes/plant | 3.30 ± 0.236 | 4.33 ± 0.471 | 0.0533 NS |
| Length of the main cladode (cm) | 18.50 ± 0.408 | 24.83 ± 0.717 | 0.0001 * |
| Length of the main root (cm) | 8.00 ± 0.540 | 12.17 ± 0.425 | 0.0005 * |
| Fresh weight/plantlet (g) | 9.679 ± 0.056 | 14.107 ± 0.235 | <0.0001 * |
| Dry weight/plantlet (g) | 0.853 ± 0.009 | 1.073 ± 0.074 | 0.0130 * |
| Chlorophyll a (mg g−1FW) | 0.499 ± 0.060 | 0.683 ± 0.091 | 0.0304 * |
| Chlorophyll b (mg g−1FW) | 0.208 ± 0.009 | 0.198 ± 0.013 | 0.2314 NS |
| Chlorophyll a+b (mg g−1FW) | 0.707 ± 0.052 | 0.881 ± 0.090 | 0.0317 * |
| Carotenoids (mg g−1FW) | 0.129 ± 0.018 | 0.240 ± 0.030 | 0.0018 * |
| Mycorrhizal colonization (%) | 0 | 64.444 ± 2.222 | 0.000 * |
| AMF spore counts | 0 | 75.667 ± 5.507 | 0.000 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewir, Y.H.; Habib, M.M.; Alaizari, A.A.; Malik, J.A.; Al-Ali, A.M.; Al-Qarawi, A.A.; Alwahibi, M.S. Promising Application of Automated Liquid Culture System and Arbuscular Mycorrhizal Fungi for Large-Scale Micropropagation of Red Dragon Fruit. Plants 2023, 12, 1037. https://doi.org/10.3390/plants12051037
Dewir YH, Habib MM, Alaizari AA, Malik JA, Al-Ali AM, Al-Qarawi AA, Alwahibi MS. Promising Application of Automated Liquid Culture System and Arbuscular Mycorrhizal Fungi for Large-Scale Micropropagation of Red Dragon Fruit. Plants. 2023; 12(5):1037. https://doi.org/10.3390/plants12051037
Chicago/Turabian StyleDewir, Yaser Hassan, Muhammad M. Habib, Ahmed Ali Alaizari, Jahangir A. Malik, Ali Mohsen Al-Ali, AbdulAziz A. Al-Qarawi, and Mona S. Alwahibi. 2023. "Promising Application of Automated Liquid Culture System and Arbuscular Mycorrhizal Fungi for Large-Scale Micropropagation of Red Dragon Fruit" Plants 12, no. 5: 1037. https://doi.org/10.3390/plants12051037
APA StyleDewir, Y. H., Habib, M. M., Alaizari, A. A., Malik, J. A., Al-Ali, A. M., Al-Qarawi, A. A., & Alwahibi, M. S. (2023). Promising Application of Automated Liquid Culture System and Arbuscular Mycorrhizal Fungi for Large-Scale Micropropagation of Red Dragon Fruit. Plants, 12(5), 1037. https://doi.org/10.3390/plants12051037










