Design of an Herbal Preparation Composed by a Combination of Ruscus aculeatus L. and Vitis vinifera L. Extracts, Magnolol and Diosmetin to Address Chronic Venous Diseases through an Anti-Inflammatory Effect and AP-1 Modulation
Abstract
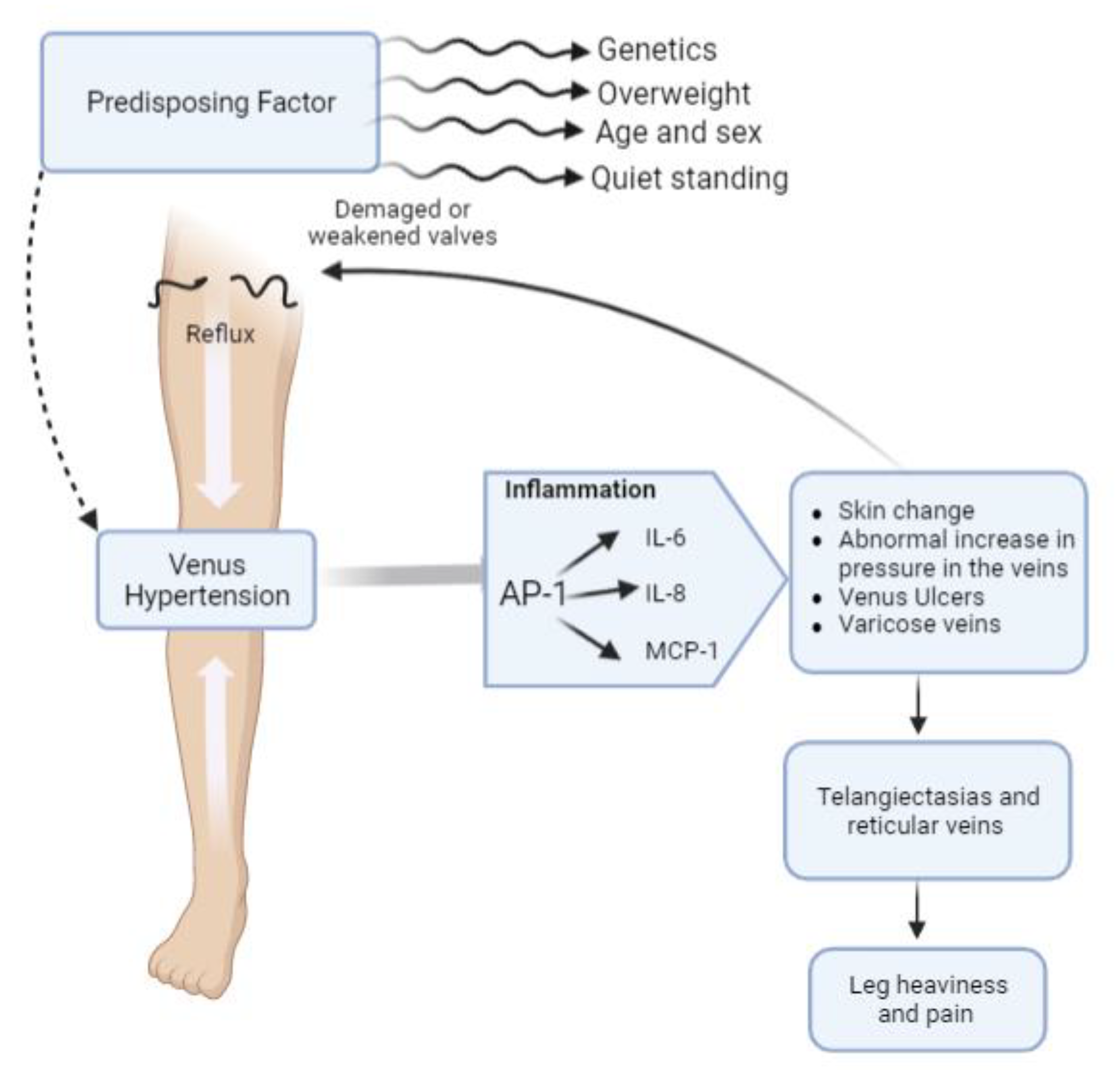
:1. Introduction
2. Results and Discussion
2.1. Extracts Characterization
2.2. Cytotoxicity Assays
2.3. Anti-Inflammatory Activity
2.4. Modulation of NF-κB and AP-1
3. Materials and Methods
3.1. Reagents and Materials
3.2. Extracts and Formulations Preparation
3.3. Quantitative Analysis of Ruscogenin and Procyanidin B1
3.4. Cell Culture
3.5. Cell Viability Assay
3.6. Competitive ELISA Assays
3.7. NF-κB Translocation Analysis
3.8. RNA Isolation and Quantitative Real-Time-PCR (qRT-PCR)
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Robertson, L.; Evans, C.A.; Fowkes, F. Epidemiology of chronic venous disease. Phlebology 2008, 23, 103–111. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Report of the WHO Informal Working Group on Cystic and Alveolar Echninococcosis Surveillance, Prevention and Control, with the Participation of the Food and Agriculture Organization of the United Nations and the World Organisation for Animal Health, 22–23 June 2011; Department of Control of Neglected Tropical Diseases, WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Ligi, D.; Croce, L.; Mannello, F. Chronic venous disorders: The dangerous, the good, and the diverse. Int. J. Mol. Sci. 2018, 19, 2544. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, B.N.; Andraska, E.A.; Obi, A.T.; Wakefield, T.W. Pathophysiology of varicose veins. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 460–467. [Google Scholar] [CrossRef]
- Nakano, L.C.U.; Cacione, D.G.; Baptista-Silva, J.C.C.; Flumignan, R.L.G. Treatment for telangiectasias and reticular veins. Cochrane Database Syst. Rev. 2021, 10, CD012723. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Álvarez-Mon, M.A.; Chaowen, C.; Ruiz-Grande, F.; Pekarek, L.; Monserrat, J.; Asúnsolo, A.; García-Honduvilla, N.; et al. Understanding Chronic Venous Disease: A Critical Overview of Its Pathophysiology and Medical Management. J. Clin. Med. 2021, 10, 3239. [Google Scholar] [CrossRef]
- Eschrich, J.; Meyer, R.; Kuk, H.; Wagner, A.H.; Noppeney, T.; Debus, S.; Hecker, M.; Korff, T. Varicose remodeling of veins is suppressed by 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor. J. Am. Heart Assoc. 2016, 5, e002405. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Arrigo, A.-P.; Currie, R.W. Heat shock treatment suppresses angiotensin II-induced activation of NF-κB pathway and heart inflammation: A role for IKK depletion by heat shock? Am. J. Physiol.-Heart C. 2004, 287, H1104–H1114. [Google Scholar] [CrossRef]
- Zolotukhin, I.; Golovanova, O.; Efremova, O.; Golovina, V.; Seliverstov, E. Monocyte chemoattractant protein 1 plasma concentration in blood from varicose veins decreases under venoactive drug treatment. Int. Angiol. 2022, 41, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 1993, 268, 14553–14556. [Google Scholar] [CrossRef] [PubMed]
- Manning, M.W.; Cassis, L.A.; Daugherty, A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II–induced atherosclerosis and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. 2003, 23, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Gohel, M.S.; Davies, A.H. Pharmacological agents in the treatment of venous disease: An update of the available evidence. Curr. Vasc. Pharmacol. 2009, 7, 303–308. [Google Scholar] [CrossRef]
- Wang, J.; An, F.S.; Zhang, W.; Gong, L.; Wei, S.J.; Qin, W.D.; Wang, X.P.; Zhao, Y.X.; Zhang, Y.; Zhang, C. Inhibition of c-Jun N-terminal kinase attenuates low shear stress-induced atherogenesis in apolipoprotein E-deficient mice. Mol. Med. 2011, 17, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Antignani, P. Medical Treatment of Chronic Venous Disease. SM J. Pharmacol. Ther. 2017, 3, 1015. [Google Scholar]
- Ibegbuna, V.; Nicolaides, A.; Sowade, O.; Leon, M.; Geroulakos, G. Venous elasticity after treatment with Daflon 500 mg. Angiology 1997, 48, 45–49. [Google Scholar] [CrossRef] [PubMed]
- das Graças, M.C.; Cyrino, F.Z.; de Carvalho, J.J.; Blanc-Guillemaud, V.; Bouskela, E. Protective effects of micronized purified flavonoid fraction (MPFF) on a novel experimental model of chronic venous hypertension. Eur. J. Vasc. Endovasc. 2018, 55, 694–702. [Google Scholar] [CrossRef]
- Martinez-Zapata, M.J.; Vernooij, R.W.; Uriona Tuma, S.M.; Stein, A.T.; Moreno, R.M.; Vargas, E.; Capellà, D.; Bonfill Cosp, X. Phlebotonics for venous insufficiency. Cochrane Database Syst. Rev. 2016, 4, CD003229, Erratum in: Cochrane Database Syst. Rev. 2020, 11, CD003229. [Google Scholar]
- Rybak, Z. Management of lower-limb venous symptoms: What the guidelines tell us. Medicographia 2015, 37, 50–55. [Google Scholar]
- Feldo, M.; Wójciak-Kosior, M.; Sowa, I.; Kocki, J.; Bogucki, J.; Zubilewicz, T.; Kęsik, J.; Bogucka-Kocka, A. Effect of diosmin administration in patients with chronic venous disorders on selected factors affecting angiogenesis. Molecules 2019, 24, 3316. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, Z.; Huang, X.; Shi, W.; Zhang, R.; Chen, M.; Huang, H.; Wu, L. Insights on the multifunctional activities of magnolol. Biomed. Res. Int. 2019, 2019, 1847130. [Google Scholar] [CrossRef] [Green Version]
- Kuk, H.; Arnold, C.; Meyer, R.; Hecker, M.; Korff, T. Magnolol inhibits venous remodeling in mice. Sci. Rep. 2017, 7, 17820. [Google Scholar] [CrossRef] [Green Version]
- Bogucka–Kocka, A.; Woźniak, M.; Feldo, M.; Kocki, J.; Szewczyk, K. Diosmin–isolation techniques, determination in plant material and pharmaceutical formulations, and clinical use. Nat. Prod. Commun. 2013, 8, 1934578X1300800435. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xu, L.; Guo, S.; Wang, Z.; Jiang, L.; Wang, F.; Zhang, J.; Liu, B. Profiling and comparison of the metabolites of diosmetin and diosmin in rat urine, plasma and feces using UHPLC-LTQ-Orbitrap MSn. J. Chromatogr. B 2019, 1124, 58–71. [Google Scholar] [CrossRef]
- De Combarieu, E.; Falzoni, M.; Fuzzati, N.; Gattesco, F.; Giori, A.; Lovati, M.; Pace, R. Identification of Ruscus steroidal saponins by HPLC-MS analysis. Fitoterapia 2002, 73, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Morazzoni, P.; Vanzani, P.; Santinello, S.; Gucciardi, A.; Zennaro, L.; Miotto, G.; Ursini, F. Grape seeds proanthocyanidins: Advanced technological preparation and analytical characterization. Antioxidants 2021, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.A.; Hodges, A.E.; Guthrie, J.R.; O'Brien, B.M.; Robaugh, D.; Clark, A.P.; Harris, R.K.; Algaier, J.W.; Smith, C.S. Comparison of proanthocyanidins in commercial antioxidants: Grape seed and pine bark extracts. J. Agric. Food Chem. 2007, 55, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive constituents: An update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Jain, S.; Bhardwaj, A.; Nagpal, R.; Puniya, M.; Tomar, R.; Singh, V.; Parkash, O.; Prasad, G.; Marotta, F. Biological and medicinal properties of grapes and their bioactive constituents: An update. J. Med. Food 2009, 12, 473–484. [Google Scholar] [CrossRef]
- Weiss, R.F. Butcher Brooms, Herbal Medicine; Beaconsfield Publisher: Beaconsfield, UK, 1998. [Google Scholar]
- De Freitas, V.A.P.; Glories, Y. Concentration and compositional changes of procyanidins in grape seeds and skin of white Vitis vinífera varieties. J. Agric. Food Chem. 2020, 28, 7378–7386. [Google Scholar]
- Balica, G.; Vostinaru, O.; Tamas, M.; Crisan, G.; Mogosan, C. Anti-inflammatory effect of the crude steroidal saponin from the rhizomes of Ruscus aculeatus L.(Ruscaceae) in two rat models of acute inflammation. J. Food Agric. Environ. 2013, 11, 106–108. [Google Scholar]
- Aghbali, A.; Hosseini, S.V.; Delazar, A.; Gharavi, N.K.; Shahneh, F.Z.; Orangi, M.; Bandehagh, A.; Baradaran, B. Induction of apoptosis by grape seed extract (Vitis vinifera) in oral squamous cell carcinoma. Bosn. J. Basic Med. Sci. 2013, 13, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertin, R.; Chen, Z.; Marin, R.; Donati, M.; Feltrinelli, A.; Montopoli, M.; Zambon, S.; Manzato, E.; Froldi, G. Activity of myricetin and other plant-derived polyhydroxyl compounds in human LDL and human vascular endothelial cells against oxidative stress. Biomed. Pharmacother. 2016, 82, 472–478. [Google Scholar] [CrossRef]
- Mansilha, A.; Sousa, J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int. J. Mol. Sci. 2018, 19, 1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rus, H.; Vlaicu, R.; Niculescu, F. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis 1996, 127, 263–271. [Google Scholar] [CrossRef]
- Wisithphrom, K.; Windsor, L.J. The effects of tumor necrosis factor-α, interleukin-1β, interleukin-6, and transforming growth factor-β1 on pulp fibroblast mediated collagen degradation. J. Endod. 2006, 32, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Takahashi, T.; Kapahi, P.; Delhase, M.; Chen, Y.; Makris, C.; Rothwarf, D.; Baud, V.; Natoli, G.; Guido, F. Oxidative stress and gene expression: The AP-1 and NF-κB connections. Biofactors 2001, 15, 87–89. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Abate, C.; Patel, L.; Rauscher, F.J., III; Curran, T. Redox regulation of fos and jun DNA-binding activity in vitro. Science 1990, 249, 1157–1161. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar]
- Hipskind, R.A.; Büscher, D.; Nordheim, A.; Baccarini, M. Ras/MAP kinase-dependent and-independent signaling pathways target distinct ternary complex factors. Genes Dev. 1994, 8, 1803–1816. [Google Scholar] [CrossRef] [Green Version]
- Lee, F.S.; Hagler, J.; Chen, Z.J.; Maniatis, T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell 1997, 88, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-W.; Han, S.-I.; Kim, H.-H.; Lee, Z.-H. TAK1-dependent activation of AP-1 and c-Jun N-terminal kinase by receptor activator of NF-κB. BMB Rep. 2002, 35, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Finzer, P.; Soto, U.; Delius, H.; Patzelt, A.; Coy, J.F.; Poustka, A.; Zur Hausen, H.; Rosl, F. Differential transcriptional regulation of the monocyte-chemoattractant protein-1 (MCP-1) gene in tumorigenic and non-tumorigenic HPV 18 positive cells: The role of the chromatin structure and AP-1 composition. Oncogene 2000, 19, 3235–3244. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauly-Lestienne, I.; Heusler, P.; Cussac, D.; Lantoine-Adam, F.; de Almeida Cyrino, F.Z.G.; Bouskela, E. Contribution of muscarinic receptors to in vitro and in vivo effects of Ruscus extract. Microvasc. Res. 2017, 114, 1–11. [Google Scholar] [CrossRef]
- Mei, Z.; Du, L.; Liu, X.; Chen, X.; Tian, H.; Deng, Y.; Zhang, W. Diosmetin alleviated cerebral ischemia/reperfusion injury in vivo and in vitro by inhibiting oxidative stress via the SIRT1/Nrf2 signaling pathway. Food Funct. 2022, 13, 198–212. [Google Scholar] [CrossRef]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and Toxicology of Magnolol and Honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocera, R.; Eletto, D.; Santoro, V.; Parisi, V.; Bellone, M.L.; Izzo, M.; Tosco, A.; Dal Piaz, F.; Donadio, G.; De Tommasi, N. Design of an Herbal Preparation Composed by a Combination of Ruscus aculeatus L. and Vitis vinifera L. Extracts, Magnolol and Diosmetin to Address Chronic Venous Diseases through an Anti-Inflammatory Effect and AP-1 Modulation. Plants 2023, 12, 1051. https://doi.org/10.3390/plants12051051
Nocera R, Eletto D, Santoro V, Parisi V, Bellone ML, Izzo M, Tosco A, Dal Piaz F, Donadio G, De Tommasi N. Design of an Herbal Preparation Composed by a Combination of Ruscus aculeatus L. and Vitis vinifera L. Extracts, Magnolol and Diosmetin to Address Chronic Venous Diseases through an Anti-Inflammatory Effect and AP-1 Modulation. Plants. 2023; 12(5):1051. https://doi.org/10.3390/plants12051051
Chicago/Turabian StyleNocera, Raffaella, Daniela Eletto, Valentina Santoro, Valentina Parisi, Maria Laura Bellone, Marcello Izzo, Alessandra Tosco, Fabrizio Dal Piaz, Giuliana Donadio, and Nunziatina De Tommasi. 2023. "Design of an Herbal Preparation Composed by a Combination of Ruscus aculeatus L. and Vitis vinifera L. Extracts, Magnolol and Diosmetin to Address Chronic Venous Diseases through an Anti-Inflammatory Effect and AP-1 Modulation" Plants 12, no. 5: 1051. https://doi.org/10.3390/plants12051051










