Phytochemical Composition and Biological Activities of Extracts from Early, Mature, and Germinated Somatic Embryos of Cotyledon orbiculata L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Somatic Embryogenesis (SE)
2.2. Phytochemical Analysis
2.2.1. Extract Preparation
2.2.2. Estimation of Total Phenolics Content (TPC) and Flavonoids Content (TFC)
2.2.3. Chemical Characterization
2.3. Biological Activities of C. orbiculata SoEs Extracts
2.3.1. Antioxidant Assay
2.3.2. Enzyme Inhibition Assay
2.4. Statistical Analysis
3. Results
3.1. Somatic Embryogenesis (SE)
3.1.1. Influence of Auxins on SE in C. orbiculata
3.1.2. Effect of Cytokinins Plus 25 µM 2,4-D on SE in C. orbiculata
3.1.3. Effect of 6-BA and GA3 on Conversion of C. orbiculata SoEs
3.2. Phytochemical Analysis
3.3. Antioxidant Abilities
3.4. Enzyme Inhibition Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amabeoku, G.J.; Green, I.; Kabatende, J. Anticonvulsant activity of Cotyledon Orbiculata L. (Crassulaceae) leaf extract in mice. J. Ethnopharmacol. 2007, 112, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Ndhlala, A.R.; Fawole, O.A.; Light, M.E.; Finnie, J.F.; Van Staden, J. In vitro pharmacological evaluation and phenolic content of ten South African medicinal plants used as anthelmintics. S. Afr. J. Bot. 2010, 76, 558–566. [Google Scholar] [CrossRef]
- Maroyi, A. A review of botany, medicinal uses, phytochemistry and biological activities of Cotyledon Orbiculata. J. Pharm. Sci. Res. 2019, 11, 3491–3496. [Google Scholar]
- Makhafola, T.J.; Mbele, M.; Yacqub-Usman, K.; Hendren, A.; Haigh, D.B.; Blackley, Z.; Meyer, M.; Mongan, N.P.; Bates, D.O.; Dlamini, Z. Apoptosis in Cancer Cells Is Induced by Alternative Splicing of hnRNPA2/B1 Through Splicing of Bcl-x, a Mechanism that Can Be Stimulated by an Extract of the South African Medicinal Plant, Cotyledon orbiculata. Front. Oncol. 2020, 10, 547392. [Google Scholar] [CrossRef] [PubMed]
- Amabeoku, G.J.; Kabatende, J. Antinociceptive and anti-inflammatory activities of leaf methanol extract of Cotyledon Orbiculata L. (Crassulaceae). Adv. Pharmacol. Sci. 2012, 2012, 862625. [Google Scholar]
- Ondua, M.; Njoya, E.M.; Abdalla, M.A.; McGaw, L.J. Anti-inflammatory and antioxidant properties of leaf extracts of eleven South African medicinal plants used traditionally to treat inflammation. J. Ethnopharmacol. 2019, 234, 27–35. [Google Scholar] [CrossRef]
- Kumari, A.; Baskaran, P.; Van Staden, J. In vitro propagation and antibacterial activity in Cotyledon Orbiculata: A valuable medicinal plant. Plant Cell. Tissue Organ Cult. 2016, 124, 97–104. [Google Scholar] [CrossRef]
- Molefe, N.I.; Tsotetsi, A.M.; Ashafa, A.O.T.; Thekisoe, O.M.M. In Vitro anthelmintic activity of Cotyledon orbiculata, Hermannia depressa and Nicotiana glauca extracts against parasitic gastrointestinal nematodes of livestock. J. Med. Plant Res. 2013, 7, 536–542. [Google Scholar]
- Anderson, L.A.P.; Schultz, R.A.; Kellerman, T.S.; Kotzé, S.M.; Prozesky, L.; Erasmus, G.L.; Labuschagne, L. Isolation and characterization of and some observations on poisoning by bufadienolides from Cotyledon orbiculata L. var. orbiculata. Onderstepoort J. Vet. 1985, 52, 21–24. [Google Scholar]
- Steyn, P.S.; van Heerden, F.R.; Vleggaar, R.; Anderson, L.A.P. Bufadienolide glycosides of the Crassulaceae. Structure and stereochemistry of orbicusides A–C, novel toxic metabolites of Cotyledon orbiculata. J. Chem. Soc. Perk. Trans. 1 1986, 1633–1636. [Google Scholar] [CrossRef]
- Semenya, S.S.; Potgieter, M.J.; Erasmus, L.J.C. Indigenous plant species used by Bapedi healers to treat sexually transmitted infections: Their distribution, harvesting, conservation and threats. S. Afr. J. Bot. 2013, 87, 66–75. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, D.H.; Sivanesan, I. Micropropagation of Ajuga species: A mini review. Biotechnol. Lett. 2017, 39, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, D.H.; Saini, R.K.; Gopal, J.; Keum, Y.-S.; Sivanesan, I. Micropropagation and Quantification of Bioactive Compounds in Mertensia maritima (L.) Gray. Int. J. Mol. Sci. 2019, 20, 2141. [Google Scholar] [CrossRef] [PubMed]
- Kalaipandian, S.; Mu, Z.; Kong, E.Y.Y.; Biddle, J.; Cave, R.; Bazrafshan, A.; Wijayabandara, K.; Beveridge, F.C.; Nguyen, Q.; Adkins, S.W. Cloning Coconut via Somatic Embryogenesis: A Review of the Current Status and Future Prospects. Plants 2021, 10, 2050. [Google Scholar] [CrossRef] [PubMed]
- Kessel-Domini, A.; Pérez-Brito, D.; Guzmán-Antonio, A.; Barredo-Pool, F.A.; Mijangos-Cortés, J.O.; Iglesias-Andreu, L.G.; Cortés-Velázquez, A.; Canto-Flick, A.; Avilés-Viñas, S.A.; Rodríguez-Llanes, Y.; et al. Indirect Somatic Embryogenesis: An Efficient and Genetically Reliable Clonal Propagation System for Ananas comosus L. Merr. Hybrid “MD2”. Agriculture 2022, 12, 713. [Google Scholar] [CrossRef]
- Hadi, A.; Singh, S.; Rafiq, S.; Nawchoo, I.A.; Wagay, N.A.; Mahmoud, E.A.; El-Ansary, D.O.; Sharma, H.; Casini, R.; Yessoufou, K.; et al. In Vitro Propagation of Aconitum violaceum Jacq. ex Stapf through Seed Culture and Somatic Embryogenesis. Horticulturae 2022, 8, 599. [Google Scholar] [CrossRef]
- Olah, R.; Turcsan, M.; Olah, K.; Farkas, E.; Deak, T.; Jahnke, G.; Sardy, D.A.N. Somatic Embryogenesis: A Tool for Fast and Reliable Virus and Viroid Elimination for Grapevine and other Plant Species. Horticulturae 2022, 8, 508. [Google Scholar] [CrossRef]
- Martínez, M.T.; Suárez, S.; Moncaleán, P.; Corredoira, E. Cryopreservation of Holm Oak Embryogenic Cultures for Long-Term Conservation and Assessment of Polyploid Stability. Plants 2022, 11, 1266. [Google Scholar] [CrossRef]
- Dang, S.; Zhang, L.; Han, S.; Qi, L. Agrobacterium-Mediated Genetic Transformation of Larix kaempferi (Lamb.) Carr. Embryogenic Cell Suspension Cultures and Expression Analysis of Exogenous Genes. Forests 2022, 13, 1436. [Google Scholar] [CrossRef]
- Manokari, M.; Latha, R.; Priyadharshini, S.; Shekhawat, M.S. Short-term cold storage of encapsulated somatic embryos and retrieval of plantlets in grey orchid (emopenVanda tessellataemclose (Roxb.) Hook. ex G.Don). Plant Cell Tissue Organ Cult. 2021, 144, 171–183. [Google Scholar] [CrossRef]
- Lema-Rumińska, J.; Kulus, D.; Tymoszuk, A.; Varejão, J.M.T.B.; Bahcevandziev, K. Profile of Secondary Metabolites and Genetic Stability Analysis in New Lines of Echinacea purpurea (L.) Moench Micropropagated via Somatic Embryogenesis. Ind. Crops Prod. 2019, 142, 111851. [Google Scholar] [CrossRef]
- Von Arnold, S.; Sabala, I.; Bozhkov, P.; Dyachok, J.; Filonova, L. Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult. 2002, 69, 233–249. [Google Scholar] [CrossRef]
- Marimuthu, K.; Subbaraya, U.; Suthanthiram, B.; Marimuthu, S.S. Molecular analysis of somatic embryogenesis through proteomic approach and optimization of protocol in recalcitrant Musa spp. Physiol. Plant 2019, 167, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Lim, M.Y.; Jeong, B.R. Somatic embryogenesis and plant regeneration from leaf and petiole explants of Campanula punctata Lam. Var. rubriflora Makino. Plant Cell Tissue Organ Cult. 2011, 107, 365–369. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Y.; Jeong, B.R. Enhanced Somatic Embryo Induction of a Tree Peony, Paeonia ostii ‘Fengdan’, by a Combination of 6-benzylaminopurine (BA) and 1-naphthylacetic Acid (NAA). Plants 2020, 9, 3. [Google Scholar] [CrossRef]
- Simonović, A.D.; M. Trifunović-Momčilov, M.; Filipović, B.K.; Marković, M.P.; Bogdanović, M.D.; Subotić, A.R. Somatic Embryogenesis in Centaurium erythraea Rafn—Current Status and Perspectives: A Review. Plants 2021, 10, 70. [Google Scholar] [CrossRef]
- Ahmed, A.B.A.; Amar, D.I.; Taha, R.M. Indirect Regeneration and Somatic Embryogenesis from Leaf and Stem Explants of Crassula ovata (Mill.) Druce–An Ornamental Medicinal Plant. Int. J. Biol. Biomol. Agricult. Food Biotechnol. Eng. 2014, 8, 1274–1277. [Google Scholar]
- Chao, C.; Guilan, W.; Limin, T.; Ruisheng, C. Embryoid induction and regeneration in callus of Kalanchoe blossfeldiana. Acta Hortic. Sin. 2004, 31, 249–252. [Google Scholar]
- Song, M.J.; Park, Y.G. Somatic Embryogenesis and Plant Regeneration from Stem Tissues of Orostachys japonicus A. Berger. J. Plant Biotechnol. 2007, 34, 181–187. [Google Scholar] [CrossRef]
- Seo, C.-S.; Shin, H.-K. Liquid Chromatography Tandem Mass Spectrometry for the Simultaneous Quantification of Eleven Phytochemical Constituents in Traditional Korean Medicine, Sogunjung Decoction. Processes 2021, 9, 153. [Google Scholar] [CrossRef]
- Park, H.-Y.; Kim, K.-S.; Ak, G.; Zengin, G.; Cziáky, Z.; Jekő, J.; Adaikalam, K.; Song, K.; Kim, D.-H.; Sivanesan, I. Establishment of a Rapid Micropropagation System for Kaempferia parviflora Wall. Ex Baker: Phytochemical Analysis of Leaf Extracts and Evaluation of Biological Activities. Plants 2021, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, K.W.; Sivanesan, I. Influence of auxins on somatic embryogenesis in Haworthia retusa Duval. Biologia 2018, 74, 25–33. [Google Scholar] [CrossRef]
- Kim, D.H.; Sivanesan, I. Somatic embryogenesis in Hosta minor (Baker) Nakai. Propag. Ornam. Plants 2019, 19, 24–29. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R. Sideritis galatica Bornm.: A source of multifunctional agents for the management of oxidative damage, Alzheimer’s’s and diabetes mellitus. J. Funct. Foods 2014, 11, 538–547. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Diuzheva, A.; Gunes, E.; Jeko, J.; Cziaky, Z.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Characterization of phytochemical components of Ferula halophile extracts using HPLC-MS/MS and their pharmacological potentials: A multifunctional insight. J. Pharm. Biomed. Anal. 2018, 160, 374–382. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Faisal, M.; Seob, P.K.; Kang, K.W.; Sivanesan, I. In Vitro Propagation of Cremastra appendiculata var. variabilis by Asymbiotic Seed Germination. Horticulturae 2022, 8, 926. [Google Scholar]
- Nic-Can, G.I.; Loyola-Vargas, V.M. The role of the auxins during somatic embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 171–182. ISBN 9783319337050. [Google Scholar]
- Yan, R.; Sun, Y.; Sun, H. Current status and future perspectives of somatic embryogenesis in Lilium. Plant Cell Tissue Organ Cult. 2020, 143, 229–240. [Google Scholar] [CrossRef]
- Wójcik, A.M.; Wójcikowska, B.; Gaj, M.D. Current perspectives on the auxin-mediated genetic network that controls the induction of somatic embryogenesis in plants. Int. J. Mol. Sci. 2020, 21, 1333. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, D.; Lü, W.; Zhang, X.; Ma, M.; Liu, G.; Jiang, J.; Li, C. Somatic embryogenesis and plant regeneration in Betula platyphalla. J. For. Res. 2020, 3, 937–944. [Google Scholar] [CrossRef]
- Tao, J.; Chen, S.G.; Qin, C.Y.; Li, Q.M.; Cai, J.F.; Sun, C.; Wang, W.; Weng, Y. Somatic embryogenesis in mature zygotic embryos of Picea pungens. Sci. Rep. 2021, 11, 19072. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Naderi, R.; Tohidfar, M.; Yoosefzadeh-Najafabadi, M. Development of support vector machine-based model and comparative analysis with artificial neural network for modeling the plant tissue culture procedures: Effect of plant growth regulators on somatic embryogenesis of chrysanthemum, as a case study. Plant Methods 2020, 16, 112. [Google Scholar] [CrossRef]
- Niazian, M.; Sadat-Noori, S.A.; Abdipour, M.; Tohidfar, M.; Mortazavian, S.M.M. Image Processing and Artificial Neural Network-Based Models to Measure and Predict Physical Properties of Embryogenic Callus and Number of Somatic Embryos in Ajowan (Trachyspermum ammi (L.) Sprague). Vitr. Cell. Dev. Biol. Plant 2018, 54, 54–68. [Google Scholar] [CrossRef]
- Khajuria, A.K.; Hano, C.; Bisht, N.S. Somatic Embryogenesis and Plant Regeneration in Viola canescens Wall. Ex. Roxb.: An Endangered Himalayan Herb. Plants 2021, 10, 761. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, A.; Qin, M.; Qin, X.; Yang, S.; Su, S.; Sun, Y.; Zhang, L. Direct and indirect somatic embryogenesis induction in Camellia oleifera Abel. Front. Plant Sci. 2021, 12, 644389. [Google Scholar] [CrossRef]
- Almeida, N.V.; Rivas, E.B.; Cardoso, J.C. Somatic embryogenesis from flower tepals of Hippeastrum aiming regeneration of virus-free plants. Plant Sci. 2022, 317, 111191. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Habashi, A.A.; Emadpour, M.; Kazemi, N. Recovery of virus-free Almond (Prunus dulcis) cultivars by somatic embryogenesis from meristem undergone thermotherapy. Sci. Rep. 2022, 12, 14948. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Marasek-Ciolakowska, A. Micropropagation of Tulip via Somatic Embryogenesis. Agronomy 2020, 10, 1857. [Google Scholar] [CrossRef]
- Deepa, A.V.; Anju, M.; Thomas, T.D. The applications of TDZ in medicinal plant tissue culture. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Springer: Singapore, 2018; pp. 297–316. [Google Scholar]
- Maruyama, T.E.; Ueno, S.; Mori, H.; Kaneeda, T.; Moriguchi, Y. Factors Influencing Somatic Embryo Maturation in Sugi (Japanese Cedar, Cryptomeria japonica (Thunb. ex L.f.) D. Don). Plants 2021, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Hosseini, M.; Vahdati, K.; Leslie, C.A. Germination of Persian Walnut Somatic Embryos and Evaluation of their Genetic Stability by ISSR Fingerprinting and Flow Cytometry. HortScience 2019, 54, 1576–1580. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S. A review on health benefits of phenolics derived from dietary spices. Curr. Res. Food Sci. 2022, 5, 1508–1523. [Google Scholar] [CrossRef] [PubMed]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Myint, K.Z.; Yu, Q.; Qing, J.; Zhu, S.; Shen, J.; Xia, Y. Botanic antimicrobial agents, their antioxidant properties, application and safety issue. Food Packag. Shelf Life 2022, 34, 100924. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.L.; Lima, E.T.G.; Silva, J.C.; Medeiros, M.A.; Pinheiro, E.B.F. Rhamnetin: A review of its pharmacology and toxicity. J. Pharm. Pharmacol. 2022, 74, 793–799. [Google Scholar] [CrossRef]
- Rauf, A.; Jehan, N. Natural products as a potential enzyme inhibitors from medicinal plants. In Enzyme Inhibitors and Activators; InTech: Rijeka, Croatia, 2017; pp. 165–177. [Google Scholar]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C.K. Validation of medicinal herbs for anti-tyrosinase potential. J. Herb. Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Food-derived non-phenolic α-amylase and α-glucosidase inhibitors for controlling starch digestion rate and guiding diabetes-friendly recipes. LWT—Food Sci. Technol. 2022, 153, 112455. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, Y.; Li, X.; Wang, S.; Wang, Z. Recent advance on carbamate-based cholinesterase inhibitors as potential multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 2022, 240, 114606. [Google Scholar] [CrossRef]
- Xie, L.P.; Chen, Q.X.; Huang, H.; Wang, H.Z.; Zhang, R.Q. Inhibitory effects of some flavonoids on the activity of mushroom tyrosinase. Biochemistry 2003, 68, 487–491. [Google Scholar] [PubMed]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Miao, M. Inhibition of α-amylase by polyphenolic compounds: Substrate digestion, binding interactions and nutritional intervention. Trends Food Sci. Technol. 2020, 104, 190–207. [Google Scholar] [CrossRef]

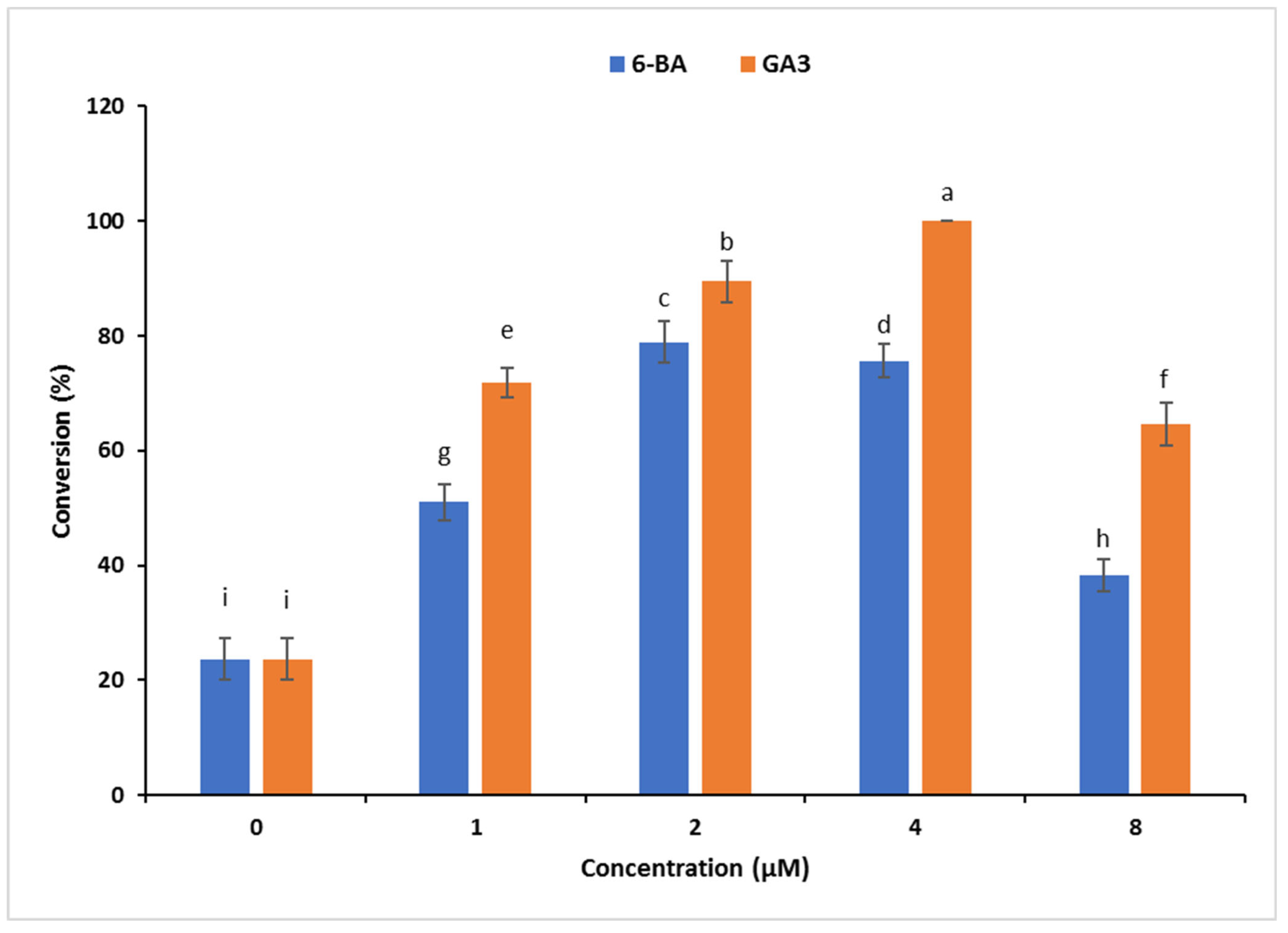

| Auxin | Auxin Conc. (µM) | SoE Induction (%) | Number of SoEs per Explant | ||
|---|---|---|---|---|---|
| Control | 0 | 0.0 ± 0.0 k | 0.0 ± 0.0 j | ||
| 2,4-D | 5 | 0.0 ± 0.0 k | 0.0 ± 0.0 j | ||
| 10 | 0.0 ± 0.0 k | 0.0 ± 0.0 j | |||
| 15 | 23.1 ± 4.2 g | 5.4 ± 1.0 e | |||
| 20 | 32.7 ± 4.1 de | 9.8 ± 1.9 b | |||
| 25 | 60.6 ± 3.5 a | 14.9 ± 2.1 a | |||
| 30 | 43.2 ± 5.2 b | 7.6 ± 1.5 d | |||
| IAA | 5 | 0.0 ± 0.0 k | 0.0 ± 0.0 j | ||
| 10 | 0.0 ± 0.0 k | 0.0 ± 0.0 j | |||
| 15 | 10.7 ± 2.9 i | 2.9 ± 0.8 h | |||
| 20 | 34.2 ± 4.5 d | 5.3 ± 1.0 e | |||
| 25 | 20.2 ± 4.7 h | 3.6 ± 1.3 gh | |||
| 30 | 4.9 ± 1.3 j | 1.7 ± 0.7 i | |||
| IBA | 5 | 0.0 ± 0.0 k | 0.0 ± 0.0 j | ||
| 10 | 0.0 ± 0.0 k | 0.0 ± 0.0 j | |||
| 15 | 7.1 ± 1.5 j | 1.9 ± 0.8 i | |||
| 20 | 18.7 ± 2.9 h | 2.9 ± 1.1 h | |||
| 25 | 30.6 ± 3.2 e | 6.7 ± 1.6 d | |||
| 30 | 24.0 ± 3.5 g | 4.3 ± 1.0 fg | |||
| NAA | 5 | 0.0 ± 0.0 k | 0.0 ± 0.0 j | ||
| 10 | 0.0 ± 0.0 k | 0.0 ± 0.0 j | |||
| 15 | 12.7 ± 2.5 i | 3.6 ± 0.9 gh | |||
| 20 | 39.6 ± 2.5 c | 8.7 ± 1.0 c | |||
| 25 | 27.6 ± 4.6 f | 7.0 ± 1.1 d | |||
| 30 | 21.4 ± 3.1 gh | 5.1 ± 1.1 ef | |||
| ANOVA | R-square | 0.9738 | 0.9411 | ||
| Coefficient of variation | 16.93 | 26.58 | |||
| Root mean square error | 2.89 | 1.01 | |||
| F-value | p-value | F-value | p-value | ||
| Auxin type | 285.68 | 0.001 | 176.20 | 0.001 | |
| Auxin conc. | 984.44 | 0.001 | 394.14 | 0.001 | |
| Auxin type *Auxin conc. | 90.29 | 0.001 | 37.81 | 0.001 | |
| Cytokinin Type | Cytokinin Conc. (µM) | SoE Induction (%) | Number of SoEs per Explant | ||
|---|---|---|---|---|---|
| Control (25 µM 2,4-D) | 0 | 60.6 ± 3.5 h | 14.9 ± 2.1 gf | ||
| 6-BA | 1.2 | 66.6 ± 2.7 g | 11.8 ± 1.4 g | ||
| 2.2 | 74.8 ± 3.0 ef | 18.1 ± 2.9 e | |||
| 4.4 | 88.7 ± 4.6 c | 21.3 ± 2.7 d | |||
| 8.8 | 52.6 ± 5.8 j | 7.4 ± 1.2 h | |||
| KN | 1.2 | 72.0 ± 3.9 f | 15.9 ± 2.2 f | ||
| 2.2 | 79.3 ± 3.1 d | 22.6 ± 1.8 d | |||
| 4.4 | 92.3 ± 3.0 b | 27.0 ± 2.9 c | |||
| 8.8 | 56.9 ± 3.1 i | 8.0 ± 2.2 h | |||
| TDZ | 1.2 | 90.6 ± 2.7 bc | 29.3 ± 2.9 b | ||
| 2.2 | 97.2 ± 2.8 a | 35.8 ± 2.5 a | |||
| 4.4 | 76.9 ± 4.3 de | 20.8 ± 1.9 d | |||
| 8.8 | 44.6 ± 3.4 k | 13.6 ± 2.2 g | |||
| ANOVA | R-square | 0.9561 | 0.9356 | ||
| Coefficient of variation | 4.89 | 11.91 | |||
| Root mean square error | 3.64 | 2.29 | |||
| F-value | p-value | F-value | p-value | ||
| Cytokinin type | 31.38 | 0.001 | 181.54 | 0.001 | |
| Cytokinin conc. | 513.87 | 0.001 | 247.16 | 0.001 | |
| Cytokinin type * Cytokinin conc. | 80.74 | 0.001 | 48.55 | 0.001 | |
| Samples | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg RE/g) |
|---|---|---|
| Early somatic embryos | 21.28 ± 0.05 b | 0.97 ± 0.10 b |
| Mature somatic embryos | 21.32 ± 0.20 b | 0.95 ± 0.04 b |
| Germinated somatic embryos | 32.90 ± 0.46 a | 1.45 ± 0.04 a |
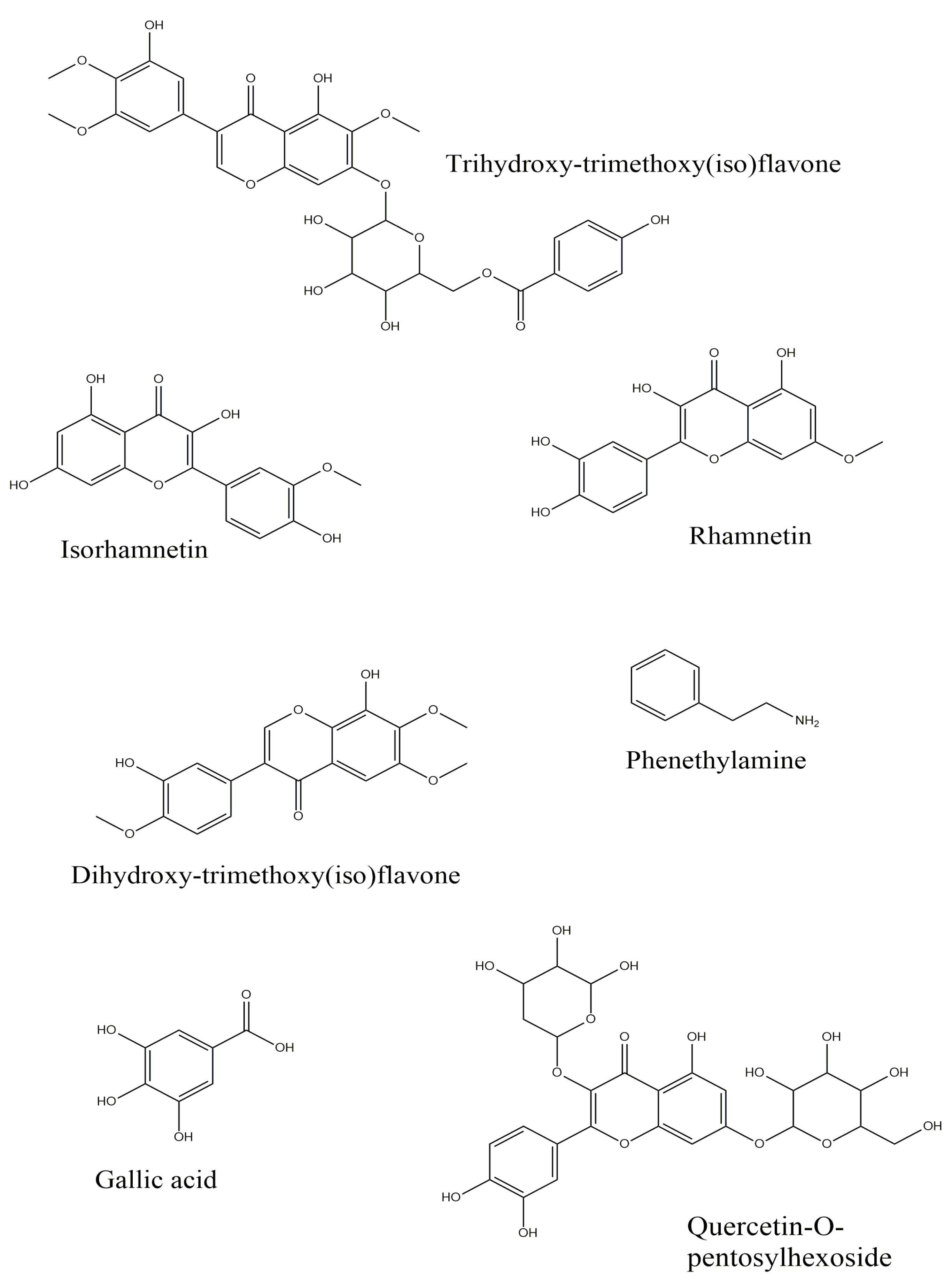
| Compounds | Early Somatic Embryo | Mature Somatic Embryo | Germinated Somatic Embryo |
|---|---|---|---|
| Trigonelline | + | + | + |
| Nicotinic acid (Niacin) | + | + | + |
| Nicotinamide | + | + | + |
| Gallic acid (3,4,5-Trihydroxybenzoic acid) 1 | + | − | + |
| Phenethylamine | + | − | − |
| Dihydroxybenzoic acid | + | + | + |
| Caffeic acid | + | + | + |
| Taxifolin (Dihydroquercetin) 1 | + | + | + |
| cis-3-[(4-hydroxy-3-Methoxyphenyl)-prop-2-enoyl]oxybutanedioic acid | + | + | + |
| Eriodictyol-O-hexoside | + | + | + |
| trans-3-[(4-hydroxy-3-methoxyphenyl) prop-2-enoyl]oxybutanedioic acid | + | + | + |
| Quercetin-O-pentosylhexoside | − | + | − |
| Luteolin-O-hexoside isomer 1 | + | + | + |
| Luteolin-O-hexoside isomer 2 | + | + | − |
| Hyperoside (Quercetin-3-O-galactoside) | + | + | + |
| Isoquercitrin (Quercetin-3-O-glucoside) 1 | + | + | + |
| Eriodictyol (3′,4′,5,7-Tetrahydroxyflavanone) 1 | + | + | + |
| Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) 1 | + | + | + |
| Naringenin (4′,5,7-Trihydroxyflavanone) 1 | + | + | + |
| Luteolin (3′,4′,5,7-Tetrahydroxyflavone) 1 | + | + | + |
| Chrysoeriol (3′-Methoxy-4′,5,7-trihydroxyflavone) | + | + | − |
| Dihydroxy-trimethoxy(iso)flavone isomer 1 | − | + | − |
| Apigenin (4′,5,7-Trihydroxyflavone) 1 | + | + | + |
| Isorhamnetin (3′-Methoxy-3,4′,5,7-tetrahydroxyflavone) 1 | − | − | + |
| Trihydroxy-trimethoxy(iso)flavone isomer I | + | + | + |
| Rhamnetin (7-Methoxy-3,3′,4′,5-tetrahydroxyflavone) | − | − | + |
| Trihydroxy-trimethoxy(iso)flavone isomer II | − | − | + |
| Dihydroxy-dimethoxy(iso)flavone | + | + | + |
| Dihydroxy-trimethoxy(iso)flavone isomer 2 | − | + | − |
| Chrysin (5,7-Dihydroxyflavone) | + | + | + |
| Dimethoxy(iso)flavone | + | + | + |
| Galangin (3,5,7-Trihydroxyflavone) 1 | + | + | + |
| Trimethoxy(iso)flavone | + | + | + |
| Dihydroxy-methoxy(iso)flavone | + | + | + |
| Hydroxy-trimethoxy(iso)flavone | + | + | + |
| Hydroxy-methoxy(iso)flavone | + | + | + |
| Linoleamide | + | + | + |
| Oleamide | + | + | + |
| Samples | DPPH | ABTS | CUPRAC | FRAP | PBD | Chelating |
|---|---|---|---|---|---|---|
| Early somatic embryos | 2.13 ± 0.11 c | 1.59 ± 0.02 c | 1.63 ± 0.01 c | 1.03 ± 0.01 c | 2.13 ± 0.04 c | 1.93 ± 0.10 b |
| Mature somatic embryos | 2.41 ± 0.06 d | 1.68 ± 0.01 d | 1.72 ± 0.02 d | 1.01 ± 0.01 c | 2.22 ± 0.22 cd | >3 |
| Germinated somatic embryos | 0.62 ± 0.01 b | 0.83 ± 0.01 b | 0.92 ± 0.01 b | 0.55 ± 0.01 b | 1.87 ± 0.05 b | 2.04 ± 0.03 b |
| Trolox | 0.06 ± 0.01 a | 0.09 ± 0.01 a | 0.11 ± 0.01 a | 0.04 ± 0.01 a | 0.52 ± 0.02 a | nt |
| EDTA | nt | nt | nt | nt | nt | 0.02 ± 0.001 a |
| Samples | AChE | BChE | Tyrosinase | Amylase |
|---|---|---|---|---|
| Early somatic embryos | 0.83 ± 0.05 bc | 1.59 ± 0.05 c | 0.76 ± 0.01 bc | 1.32 ± 0.02 b |
| Mature somatic embryos | 0.75 ± 0.02 b | 1.28 ± 0.13 b | 0.79 ± 0.02 c | 1.51 ± 0.01 d |
| Germinated somatic embryos | 0.83 ± 0.02 c | 1.27 ± 0.23 b | 0.73 ± 0.02 b | 1.39 ± 0.03 c |
| Galantamine | 0.003 ± 0.001 a | 0.007 ± 0.002 a | nt | nt |
| Kojic acid | nt | nt | 0.08 ± 0.001 a | nt |
| Acarbose | nt | nt | nt | 0.68 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, G.; Cziáky, Z.; Jekő, J.; Kang, K.W.; Lorenzo, J.M.; Sivanesan, I. Phytochemical Composition and Biological Activities of Extracts from Early, Mature, and Germinated Somatic Embryos of Cotyledon orbiculata L. Plants 2023, 12, 1065. https://doi.org/10.3390/plants12051065
Zengin G, Cziáky Z, Jekő J, Kang KW, Lorenzo JM, Sivanesan I. Phytochemical Composition and Biological Activities of Extracts from Early, Mature, and Germinated Somatic Embryos of Cotyledon orbiculata L. Plants. 2023; 12(5):1065. https://doi.org/10.3390/plants12051065
Chicago/Turabian StyleZengin, Gokhan, Zoltán Cziáky, József Jekő, Kyung Won Kang, José Manuel Lorenzo, and Iyyakkannu Sivanesan. 2023. "Phytochemical Composition and Biological Activities of Extracts from Early, Mature, and Germinated Somatic Embryos of Cotyledon orbiculata L." Plants 12, no. 5: 1065. https://doi.org/10.3390/plants12051065
APA StyleZengin, G., Cziáky, Z., Jekő, J., Kang, K. W., Lorenzo, J. M., & Sivanesan, I. (2023). Phytochemical Composition and Biological Activities of Extracts from Early, Mature, and Germinated Somatic Embryos of Cotyledon orbiculata L. Plants, 12(5), 1065. https://doi.org/10.3390/plants12051065









