Soil Fertility Improvement with Mixtures of Wood Ash and Biogas Digestates Enhances Leaf Photosynthesis and Extends the Growth Period for Deciduous Trees
Abstract
1. Introduction
2. Materials and Methods
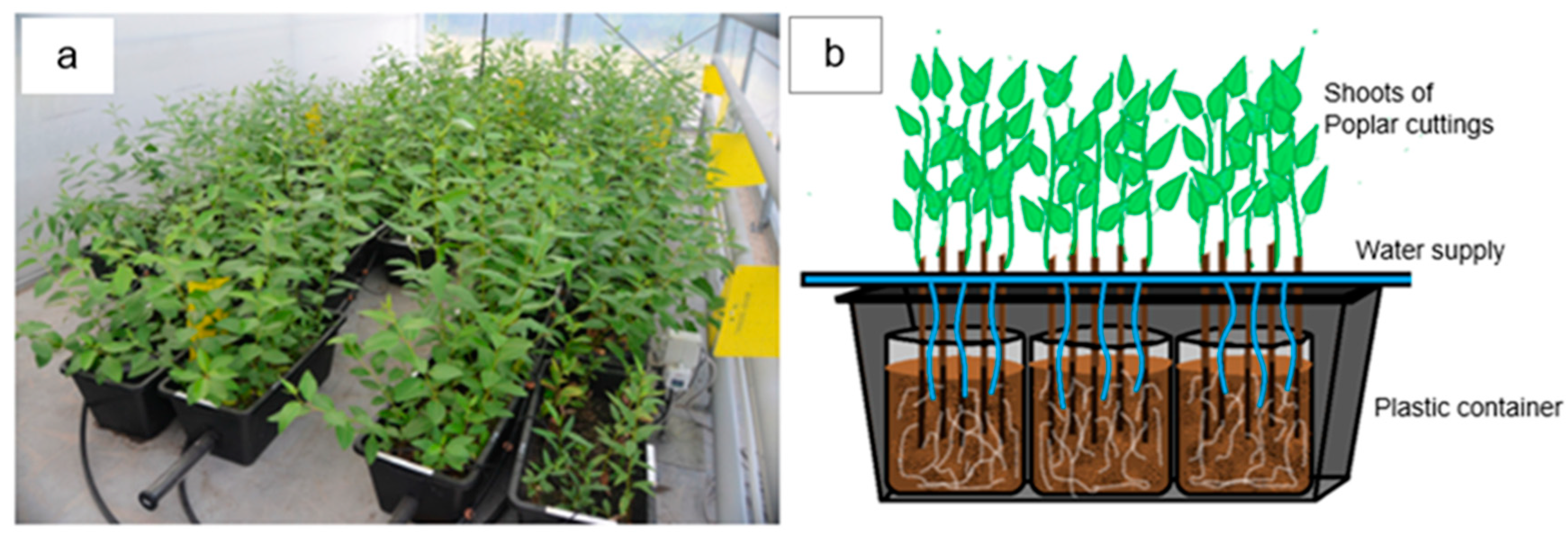
2.1. Experimental Design
2.2. Data Collection
2.3. Data Analyses
3. Results
4. Discussion
4.1. Choosing between Foreign and Local Poplar Clones
4.2. The Potential of Digestate and Wood Ash Mixtures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primary Substrate | Ash Origin | Mixture Ratios | PhCaCl2 | P Total (mg/g) | K (mg/g) | Ca (mg/g) | Mg (mg/g) | S (mg/g) | N (mg/g) |
|---|---|---|---|---|---|---|---|---|---|
| Pig manure digestate | 0:1 | 7.0 | 7.3 | 19.2 | 28.8 | 13.2 | 22.1 | 470.1 | |
| Cattle manure digestate | 0:1 | 7.6 | 5.4 | 13.9 | 18.3 | 5.7 | 18.35 | 456.0 | |
| a | 1:0 | 12.5 | 8.9 | 35.1 | 135.6 | 25.1 | 25.0 | 0.5 | |
| b | 1:0 | 12.3 | 8.0 | 114.2 | 290.2 | 78.7 | 52.0 | 0.3 | |
| Pig manure Digestate | a | 1:1 | 9.3 | 8.1 | 38.7 | 154.1 | 37.9 | 43.2 | 11.3 |
| 2:1 | 9.5 | 6.9 | 45.3 | 147.7 | 32.4 | 42.6 | 8.5 | ||
| 3:1 | 9.8 | 7.6 | 54.7 | 189.2 | 44.9 | 51.8 | 6.0 | ||
| 4:1 | 9.7 | 7.6 | 56.8 | 198.1 | 47.2 | 59.3 | 3.4 | ||
| b | 1:1 | 9.6 | 7.5 | 62.1 | 187.2 | 40.3 | 27.9 | 10.9 | |
| 2:1 | 10.2 | 8.0 | 71.5 | 212.7 | 50.0 | 40.9 | 7.9 | ||
| 3:1 | 11.5 | 7.6 | 84.4 | 258.6 | 65.5 | 44.6 | 4.9 | ||
| 4:1 | 12.3 | 7.8 | 89.7 | 256.6 | 67.3 | 48.8 | 3.8 | ||
| Cattle manure Digestate | a | 1:1 | NA | NA | NA | NA | NA | NA | NA |
| 2:1 | NA | NA | NA | NA | NA | NA | NA | ||
| 3:1 | NA | NA | NA | NA | NA | NA | NA | ||
| 4:1 | NA | NA | NA | NA | NA | NA | NA | ||
| b | 1:1 | 9.6 | 6.6 | 75.5 | 191.3 | 40.2 | 32.3 | 10.3 | |
| 2:1 | 10.3 | 7.1 | 80.7 | 228.7 | 53.0 | 37.8 | 5.4 | ||
| 3:1 | 11.8 | 7.4 | 90.9 | 236.3 | 57.5 | 43.7 | 4.1 | ||
| 4:1 | 12.3 | 7.8 | 90.5 | 250.7 | 65.9 | 49.5 | 3.2 | ||
| Forest soil | Control | 4.3 | 0.3 | 4.4 | 9.64 | 0.9 | 3.4 | 92.5 | |
Appendix B
| Clone | Primary Substrate | Ash Origin | Mixture Ratios | pHCaCl2 | P Total (mg/g) | K (mg/g) | Ca (mg/g) | Mg (mg/g) | N (mg/g) | C (mg/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| AUCE | Pig manure digestate | a | 1:1 | 3.6 | 0.2 | 5.1 | 18.6 | 6.8 | 3.5 | 76.7 |
| 2:1 | 3.8 | 0.2 | 7.4 | 19.3 | 8.1 | 2.2 | 64.2 | |||
| 3:1 | 4.8 | 0.3 | 5.6 | 27.8 | 10.4 | 2.4 | 67.0 | |||
| 4:1 | 3.6 | 0.2 | 5.5 | 19.2 | 6.6 | 2.4 | 62.7 | |||
| b | 1:1 | 3.6 | 0.4 | 5.8 | 22.2 | 7.5 | 3.5 | 80.6 | ||
| 2:1 | 5.5 | 0.3 | 6.8 | 39.1 | 12.2 | 2.1 | 60.0 | |||
| 3:1 | 4.2 | 0.2 | 6.5 | 22.7 | 8.3 | 2.3 | 69.8 | |||
| 4:1 | 3.5 | 0.2 | 5.9 | 17.6 | 6.7 | 2.4 | 67.4 | |||
| Cattle manure digestate | a | 1:1 | 3.5 | 0.3 | 4.7 | 22.5 | 7.2 | 2.9 | 76.0 | |
| 2:1 | 5.9 | 0.5 | 9.4 | 63.0 | 20.8 | 1.9 | 45.9 | |||
| 3:1 | 3.5 | 0.1 | 3.7 | 14.0 | 4.8 | 2.0 | 53.6 | |||
| 4:1 | 5.9 | 0.2 | 4.2 | 15.6 | 5.9 | 2.0 | 49.0 | |||
| b | 1:1 | 3.5 | 0.1 | 3.9 | 11.6 | 4.5 | 2.4 | 57.2 | ||
| 2:1 | 3.9 | 0.3 | 5.2 | 17.9 | 6.7 | 2.4 | 60.4 | |||
| 3:1 | 3.9 | 0.2 | 3.7 | 17.0 | 6.0 | 2.5 | 70.0 | |||
| 4:1 | 3.7 | 0.2 | 6.2 | 16.7 | 6.4 | 2.1 | 52.5 | |||
| Control | 4.1 | 0.4 | 4.1 | 0.4 | 3.5 | 0.2 | 3.4 | |||
| OP42 | Pig manure digestate | a | 1:1 | 4.1 | 0.2 | 4.9 | 23.6 | 8.8 | 3.3 | 87.6 |
| 2:2 | 3.3 | 0.2 | 5.1 | 18.1 | 6.6 | 2.2 | 65.9 | |||
| 3:1 | 3.6 | 0.2 | 4.6 | 15.5 | 5.8 | 2.0 | 55.7 | |||
| 4:1 | 3.6 | 0.2 | 5.0 | 14.0 | 5.6 | 2.1 | 58.7 | |||
| b | 1:1 | 3.6 | 0.3 | 4.7 | 18.6 | 6.5 | 3.5 | 77.3 | ||
| 2:1 | 3.6 | 0.2 | 5.7 | 16.4 | 6.2 | 2.4 | 68.8 | |||
| 3:1 | 6.7 | 0.6 | 11.0 | 10.4 | 29.4 | 2.9 | 84.4 | |||
| 4:1 | 3.8 | 0.2 | 6.6 | 14.8 | 6.6 | 2.0 | 52.2 | |||
| Cattle manure digestate | a | 1:1 | 4.0 | 0.3 | 4.6 | 25.0 | 9.6 | 2.3 | 61.3 | |
| 2:1 | 4.7 | 0.3 | 5.7 | 36.0 | 13.7 | 2.2 | 57.9 | |||
| 3:1 | 3.5 | 0.2 | 4.3 | 16.7 | 7.4 | 2.8 | 76.4 | |||
| 4:1 | 3.5 | 0.2 | 3.7 | 15.6 | 5.5 | 1.7 | 47.4 | |||
| b | 1:1 | 3.6 | 0.1 | 4.1 | 15.5 | 5.9 | 3.2 | 93.1 | ||
| 2:1 | 3.6 | 0.1 | 4.1 | 14.0 | 5.7 | 2.1 | 57.8 | |||
| 3:1 | 3.7 | 0.5 | 11.1 | 67.9 | 20.1 | 3.0 | 81.8 | |||
| 4:1 | 4.9 | 0.2 | 7.9 | 34.3 | 10.7 | 1.7 | 42.8 | |||
| Control | 4.1 | 0.5 | 4.1 | 0.5 | 3.4 | 0.2 | 3.5 | |||
Appendix C
| Clone | Primary Substrate | Ash Origin | Mixture Ratios | C (g/kg) | N (g/kg) | P Total (g/kg) |
|---|---|---|---|---|---|---|
| AUCE | Pig manure digestate | a | 1:1 | 467.9 | 13.9 | 7.0 |
| 2:1 | 470.5 | 14.2 | 5.0 | |||
| 3:1 | 466.6 | 11.6 | 5.3 | |||
| 4:1 | 467.4 | 12.6 | 4.5 | |||
| b | 1:1 | 470.7 | 13.5 | 5.9 | ||
| 2:1 | 465.5 | 17.7 | 5.9 | |||
| 3:1 | 472.7 | 19.2 | 4.4 | |||
| 4:1 | 486.0 | 16.5 | 3.3 | |||
| Cattle manure digestate | a | 1:1 | 469.9 | 18.5 | 6.0 | |
| 2:1 | 472.2 | 14.7 | 5.1 | |||
| 3:1 | 469.7 | 12.6 | 4.8 | |||
| 4:1 | 474.5 | 12.3 | 3.7 | |||
| b | 1:1 | 471.3 | 12.0 | 4.9 | ||
| 2:1 | 468.2 | 14.3 | 5.3 | |||
| 3:1 | 469.1 | 21.5 | 5.0 | |||
| 4:1 | 472.6 | 13.2 | 3.8 | |||
| OP42 | Pig manure digestate | a | 1:1 | 483.9 | 12.4 | 4.6 |
| 2:2 | 483.9 | 13.5 | 4.3 | |||
| 3:1 | 477.5 | 14.5 | 4.0 | |||
| 4:1 | 487.6 | 10.1 | 2.2 | |||
| b | 1:1 | 489.0 | 12.9 | 4.2 | ||
| 2:1 | 480.2 | 11.2 | 3.2 | |||
| 3:1 | 488.8 | 27.4 | 3.7 | |||
| 4:1 | 479.7 | 10.8 | 2.2 | |||
| Cattle manure digestate | a | 1:1 | 483.2 | 20.6 | 5.0 | |
| 2:1 | 485.6 | 15.1 | 4.3 | |||
| 3:1 | 482.5 | 13.2 | 4.0 | |||
| 4:1 | 487.4 | 12.9 | 3.4 | |||
| b | 1:1 | 487.7 | 11.2 | 3.0 | ||
| 2:1 | 484.8 | 11.5 | 3.5 | |||
| 3:1 | 480.1 | 12.0 | 1.9 | |||
| 4:1 | 489.4 | 24.3 | 2.7 |
References
- Purnomo, C.; Respito, A.; Sitanggang, E.; Mulyono, P. Slow Release Fertilizer Preparation from Sugar Cane Industrial Waste. Environ. Technol. Innov. 2018, 10, 275–280. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Wangu, M.M.; Dubois, T.; Ekesi, S.; et al. Low-Cost Technology for Recycling a Agro-Industrial Waste into Nutrient-Rich Organic Fertilizer Using Black Soldier Fly. Waste Manag. 2021, 119, 183–194. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, S.K. Exploring the Feasibility of Thermal Digestion Process: A Novel Technique, for the Rapid Treatment and Reuse of Solid Organic Waste as Organic Fertilizer. J. Clean. Prod. 2021, 318, 128600. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the Circular Economy: An Analysis of 114 Definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Du, C.; Abdullah, J.J.; Greetham, D.; Fu, D.; Yu, M.; Ren, L.; Li, S.; Lu, D. Valorization of Food Waste into Biofertiliser and Its Field Application. J. Clean. Prod. 2018, 187, 273–284. [Google Scholar] [CrossRef]
- Karim, A.A.; Kumar, M.; Mohapatra, S.; Singh, S.K. Nutrient Rich Biomass and Effluent Sludge Wastes Co-Utilization for Production of Biochar Fertilizer through Different Thermal Treatments. J. Clean. Prod. 2019, 228, 570–579. [Google Scholar] [CrossRef]
- Rehl, T.; Müller, J. Life Cycle Assessment of Biogas Digestate Processing Technologies. Resour. Conserv. Recycl. 2011, 56, 92–104. [Google Scholar] [CrossRef]
- Torrijos, M. State of Development of Biogas Production in Europe. Procedia Environ. Sci. 2016, 35, 881–889. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Degrève, J.; Helsen, L.; Lievens, B.; Willems, K.; Van Impe, J.; Dewil, R. Anaerobic Digestion in Global Bio-Energy Production: Potential and Research Challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive Characterization of the Liquid Fraction of Digestates from Full-Scale Anaerobic Co-Digestion. Waste Manag. 2017, 59, 118–128. [Google Scholar] [CrossRef]
- Mystkowski, E. Poferment z Biogazowni Rolniczej Nawozem Dla Rolnictwa. E-Biul. Cent. Doradz. Rol. W Brwinowie Kukurydza 2015, 1, 52–56. [Google Scholar]
- Koszel, M.; Lorencowicz, E. Agricultural Use of Biogas Digestate as a Replacement Fertilizers. Agric. Agric. Sci. Procedia 2015, 7, 119–124. [Google Scholar] [CrossRef]
- Kolář, L.; Kužel, S.; Peterka, J.; Borová-Batt, J. Utilisation of Waste from Digesters for Biogas Production. In Biofuel’s Engineering Process Technology; dos S. Bernardes, M.A., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Odlare, M.; Pell, M.; Svensson, K. Changes in Soil Chemical and Microbiological Properties during 4 Years of Application of Various Organic Residues. Waste Manag. 2008, 28, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.; Roß, C.-L.; Hoffmann, M.; Muskolus, A.; Ellmer, F.; Kautz, T. The Chemical Composition of Biogas Digestates Determines Their Effect on Soil Microbial Activity. Agriculture 2020, 10, 244. [Google Scholar] [CrossRef]
- Mata-Álvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Almansa, X.F.; Peces, M.; Astals, S. A Critical Review on Anaerobic Co-Digestion Achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on Research Achievements of Biogas from Anaerobic Digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Rouez, M.; Crest, M.; Steyer, J.-P.; Delgenès, J.-P.; Escudie, R.R. Food Waste Valorization via Anaerobic Processes: A Review. Rev. Environ. Sci. Biotechnol. 2016, 15, 499–547. [Google Scholar] [CrossRef]
- Viancelli, A.; Michelon, W.; Elmahdy, E.M. Current Efforts for the Production and Use of Biogas Around the World: Technological Challenges, Alternative Sources, Future Developments. In Improving Biogas Production; Treichel, H., Fongaro, G., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 277–287. [Google Scholar]
- Gissén, C.; Prade, T.; Kreuger, E.; Nges, I.A.; Rosenqvist, H.; Svensson, S.-E.; Lantz, M.; Mattsson, J.E.; Börjesson, P.; Björnsson, L. Comparing Energy Crops for Biogas Production—Yields, Energy Input and Costs in Cultivation Using Digestate and Mineral Fertilisation. Biomass Bioenergy 2014, 64, 199–210. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas Upgrading and Utilization: Current Status and Perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Zervos, A.; Adib, R. REN21 Renewables 2018 Global Status Report; REN21 Secretariat: Paris, France, 2018; p. 325. ISBN 978-3-9818911-3-3. [Google Scholar]
- Risberg, K.; Cederlund, H.; Pell, M.; Arthurson, V.; Schnürer, A. Comparative Characterization of Digestate versus Pig Slurry and Cow Manure—Chemical Composition and Effects on Soil Microbial Activity. Waste Manag. 2017, 61, 529–538. [Google Scholar] [CrossRef]
- Pitman, R.M. Wood Ash Use in Forestry—A Review of the Environmental Impacts. For. Int. J. For. Res. 2006, 79, 563–588. [Google Scholar] [CrossRef]
- Quimet, R.; Moore, J.-D. Effects of Fertilization and Liming on Tree Growth, Vitality and Nutrient Status in Boreal Balsam Fir Stands. For. Ecol. Manag. 2015, 345, 39–49. [Google Scholar] [CrossRef]
- Neimane, S.; Celma, S.; Zuševica, A.; Lazdiņa, D.; Ievinsh, G. The Effect of Wood Ash Application on Growth, Leaf Morphological and Physiological Traits of Trees Planted in a Cutaway Peatland. Mires Peat 2021, 12. [Google Scholar] [CrossRef]
- Kouřimská, L.; Poustková, I.; Babička, B. The Use of Digestate as a Replacement of Mineral Fertilizers for Vegetables Growing. Sci. Agric. Bohem. 2012, 43, 121–126. [Google Scholar] [CrossRef]
- Tampio, E.; Salo, T.; Rintala, J. Agronomic Characteristics of Five Different Urban Waste Digestates. Environ. Manag. 2016, 169, 293–302. [Google Scholar] [CrossRef]
- Sogn, T.; Dragicevic, I.; Linjordet, R.; Krogstad, T.; Eijsink, V.G.H.; Eich-Greatorex, S. Recycling of Biogas Digestates in Plant Production: NPK Fertilizer Value and Risk of Leaching. Int. J. Recycl. Org. Waste Agric. 2018, 7, 49–58. [Google Scholar] [CrossRef]
- Guilayn, F.; Jimenez, J.; Rouez, M.; Crest, M.; Patureau, D. Digestate Mechanical Separation: Efficiency Profiles Based on Anaerobic Digestion Feedstock and Equipment Choice. Bioresour. Technol. 2019, 274, 180–189. [Google Scholar] [CrossRef]
- Ren, T.; Yu, X.; Liao, J.; Du, Y.; Zhu, Y.; Jin, L.; Wang, B.; Xu, H.; Xiao, W.; Chen, H.Y.H.; et al. Application of Biogas Slurry Rather than Biochar Increases Soil Microbial Functional Gene Signal Intensity and Diversity in a Poplar Plantation. Soil Biol. Biochem. 2020, 146, 107825. [Google Scholar] [CrossRef]
- Holm, B.; Heinsoo, K. Biogas Digestate Suitability for the Fertilisation of Young Salix Plants. Balt. For. 2014, 20, 263–271. [Google Scholar]
- R Core Team. A Language and Environment; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix, Version 0.92; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Harrell, F., Jr. Hmisc: Harrell Miscellaneous. In R Package Version 4.7-0; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Šēnhofa, S.; Lazdiņa, D.; Zeps, M. Winter Frost Damage and Its Links to Early Growth and Survival in a Poplar Clone Collection. For. Wood Process. 2021, 36, 70–76. [Google Scholar] [CrossRef]
- Pliura, A.; Suchockas, V.; Sarsekova, D.; Gudynaitė, V. Genotypic Variation and Heritability of Growth and Adaptive Traits, and Adaptation of Young Poplar Hybrids at Northern Margins of Natural Distribution of Populus Nigra in Europe. Biomass Bioenergy 2014, 70, 513–529. [Google Scholar] [CrossRef]
- Šēnhofa, S.; Neimane, U.; Grava, A.; Sisenis, L.; Lazdiņa, D.; Jansons, Ā. Juvenil Growth and Frost Damages of Poplar Clone OP42 in Latvia. Agron. Res. 2017, 15, 2113–2125. [Google Scholar] [CrossRef]
- Gudynaitė-Franckevičienė, V.; Pliura, A.; Suchockas, V. Ecogenetic Plasticity and Genetic Variation in Populus Hybrids under the Impact of Simulated Climate Change Related Stressors. Balt. For. 2020, 26, 157–169. [Google Scholar] [CrossRef]
- Čakša, L.; Šēnhofa, S.; Šņepsts, G.; Elferts, D.; Liepa, L.; Jansons, Ā. Effect of Stem Snapping on Aspen Timber Assortment Recovery in Hemiboreal Forests. Forests 2021, 12, 28. [Google Scholar] [CrossRef]
- Adler, A.; Karacic, A.; Rönnberg Wästljung, A.-C.; Johansson, U.; Liepins, K.; Gradeckas, A.; Christersson, L. Variation of Growth and Phenology Traits in Poplars Planted in Clonal Trials in Northern Europe—Implications for Breeding. Bioenergy Res. 2021, 14, 426–444. [Google Scholar] [CrossRef]
- Schreiber, S.G.; Hamann, A.; Hacke, U.G.; Thomas, B.R. Sixteen Years of Winter Stress: An Assessment of Cold Hardiness, Growth Performance and Survival of Hybrid Poplar Clones at a Boreal Planting Site. Plant Cell Environ. 2012, 36, 419–428. [Google Scholar] [CrossRef]
- Kimball, B.A.; Kobayashi, K.; Bindi, M. Responses of Agricultural Crops to Free-Air CO2 Enrichment. Adv. Agron. 2002, 77, 293–368. [Google Scholar] [CrossRef]
- Poorter, H.; Villar, R. The Fate of Acquired Carbon in Plants: Chemical Composition and Construction Costs. In Plant Resource Allocation; Grace, J., Bazzaz, F.A., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 39–72. [Google Scholar]
- Meziane, D.; Shipley, B. Interacting Determinants of Specific Leaf Area in 22 Herbaceous Species: Effects of Irradiance and Nutrient Availability. Plant Cell Environ. 1999, 22, 447–459. [Google Scholar] [CrossRef]
- Gong, H.; Cui, Q.; Gao, J. Latitudinal, Soil and Climate Effects on Key Leaf Traits in Northeastern China. Glob. Ecol. Conserv. 2020, 22, e00904. [Google Scholar] [CrossRef]
- de Carvalho Gonçalves, J.F.; dos Santos Junior, U.M.; da Silva, E.A. Evaluation of a Portable Chlorophyll Meter to Estimate Chlorophyll Concentrations in Leaves of Tropical Wood Species from Amazonian Forest. Hoehnea 2008, 35, 185–188. [Google Scholar] [CrossRef]
- Navas, M.-L.; Garnier, E. Plasticity of Whole Plant and Leaf Traits in Rubia Peregrina in Response to Light, Nutrient and Water Availability. Acta Oecol. 2002, 23, 375–383. [Google Scholar] [CrossRef]
- Vazquez, E.; Benito, M.; Espejo, R.; Teutscherova, N. Effects of no-tillage and liming amendment combination on soil carbon and nitrogen mineralization. Eur. J. Soil Biol. 2019, 93, 103090. [Google Scholar] [CrossRef]
- Castro-Rodríguez, V.; García-Gutiérrez, A.; Canales, J.; Cañas, R.A.; Kirby, E.G.; Avila, C.; Cánovas, F.M. Poplar Trees for Phytoremediation of High Levels of Nitrate and Applications in Bioenergy. Plant Biotechnol. J. 2016, 14, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Mariën, B.; Papadimitriou, D.; Kotilainen, T.; Zuccarini, P.; Dox, I.; Verlinden, M.; Heinecke, T.; Mariën, J.; Willems, P.; Decoster, M.; et al. Timing Leaf Senescence: A Generalized Additive Models for Location, Scale and Shape Approach. Agric. For. Meteorol. 2022, 315, 108823. [Google Scholar] [CrossRef]
- Arend, M.; Fromm, J. Concomitant Analysis of Cambial Abscisic Acid and Cambial Growth Activity in Poplar. Trees 2013, 27, 1271–1276. [Google Scholar] [CrossRef]
- Soolanayakanahally, R.Y.; Guy, R.D.; Silim, S.N.; Drewes, E.C.; Schroeder, W.R. Enhanced Assimilation Rate and Water Use Efficiency with Latitude through Increased Photosynthetic Capacity and Internal Conductance in Balsam Poplar (Populus Balsamifera L.). Plant Cell Environ. 2009, 32, 1821–1832. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-Based Fertilizers: A Practical Approach towards Circular Economy. Biorecour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- Surendra, K.C.; Takara, D.; Hashinoto, A.G.; Khanal, S.K. Biogas as a Sustainable Energy Source for Developing Countries: Opportunities and Challenges. Renew. Sustain. Energy Rev. 2014, 31, 846–859. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Warmiński, K.; Krzyżaniak, M.; Olba–Zięty, E.; Akincza, M. Bioenergy Technologies and Biomass Potential Vary in Northern European Countries. Renew. Sustain. Energy Rev. 2020, 133, 110238. [Google Scholar] [CrossRef]
- Czekała, W. Solid Fraction of Digestate from Biogas Plant as a Material for Pellets Production. Energies 2021, 14, 5034. [Google Scholar] [CrossRef]
- Lantz, M. The Economic Performance of Combined Heat and Power from Biogas Produced from Manure in Sweden—A Comparison of Different CHP Technologies. Appl. Energy 2012, 98, 502–511. [Google Scholar] [CrossRef]







| Primary Substrate of Digestate | Ash Origin | Ash and Digestate Mixture Ratio | Dry Mass of Digestate Proportion (kg ha−1) | Dry Mass of Ash Proportion (kg ha−1) | Dry Mass of Mixture Ratio (kg ha−1) | N (kg ha−1) | P2O2 (kg ha−1) | K2O (kg ha−1) |
|---|---|---|---|---|---|---|---|---|
| Control | Control | 0:0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pig manure digestate | a | 1:1 | 3750 | 3750 | 7500 | 81.5 | 55.9 | 46.6 |
| 2:1 | 3750 | 7500 | 11,250 | 88.4 | 89.6 | 80.4 | ||
| 3:1 | 3750 | 11,250 | 15,000 | 74.1 | 114.0 | 126.6 | ||
| 4:1 | 3750 | 15,000 | 18,750 | 70.5 | 146.4 | 168.2 | ||
| b | 1:1 | 3750 | 3750 | 7500 | 84.6 | 60.8 | 29.0 | |
| 2:1 | 3750 | 7500 | 11,250 | 95.3 | 77.8 | 51.0 | ||
| 3:1 | 3750 | 11,250 | 15,000 | 89.4 | 113.7 | 82.1 | ||
| 4:1 | 3750 | 15,000 | 18,750 | 63.5 | 143.2 | 106.5 | ||
| Cattle manure digestate | a | 1:1 | 3750 | 3750 | 7500 | NA | NA | NA |
| 2:1 | 3750 | 7500 | 11,250 | NA | NA | NA | ||
| 3:1 | 3750 | 11,250 | 15,000 | NA | NA | NA | ||
| 4:1 | 3750 | 15,000 | 18,750 | NA | NA | NA | ||
| b | 1:1 | 3750 | 3750 | 7500 | 77.0 | 49.1 | 56.6 | |
| 2:1 | 3750 | 7500 | 11,250 | 60.7 | 80.0 | 90.8 | ||
| 3:1 | 3750 | 11,250 | 15,000 | 61.9 | 111.1 | 136.4 | ||
| 4:1 | 3750 | 15,000 | 18,750 | 60.3 | 145.6 | 169.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuševica, A.; Adamovičs, A.; Dūmiņš, K.; Vendiņa, V.; Žīgure, S.; Lazdina, D. Soil Fertility Improvement with Mixtures of Wood Ash and Biogas Digestates Enhances Leaf Photosynthesis and Extends the Growth Period for Deciduous Trees. Plants 2023, 12, 1152. https://doi.org/10.3390/plants12051152
Zuševica A, Adamovičs A, Dūmiņš K, Vendiņa V, Žīgure S, Lazdina D. Soil Fertility Improvement with Mixtures of Wood Ash and Biogas Digestates Enhances Leaf Photosynthesis and Extends the Growth Period for Deciduous Trees. Plants. 2023; 12(5):1152. https://doi.org/10.3390/plants12051152
Chicago/Turabian StyleZuševica, Austra, Aleksandrs Adamovičs, Kārlis Dūmiņš, Viktorija Vendiņa, Sindija Žīgure, and Dagnija Lazdina. 2023. "Soil Fertility Improvement with Mixtures of Wood Ash and Biogas Digestates Enhances Leaf Photosynthesis and Extends the Growth Period for Deciduous Trees" Plants 12, no. 5: 1152. https://doi.org/10.3390/plants12051152
APA StyleZuševica, A., Adamovičs, A., Dūmiņš, K., Vendiņa, V., Žīgure, S., & Lazdina, D. (2023). Soil Fertility Improvement with Mixtures of Wood Ash and Biogas Digestates Enhances Leaf Photosynthesis and Extends the Growth Period for Deciduous Trees. Plants, 12(5), 1152. https://doi.org/10.3390/plants12051152






