Ethnopharmacological Value and Biological Activities via Antioxidant and Anti-Protein Denaturation Activity of Morinda lucida Benth and Momordica charantia L. Leaves Extracts from Benin
Abstract
1. Introduction
2. Results
2.1. Ethnopharmacological Survey of Medicinal Use of M. charantia and M. lucida
2.1.1. Socio-Cultural Characteristics of Respondents
2.1.2. Knowledge of Plant Material
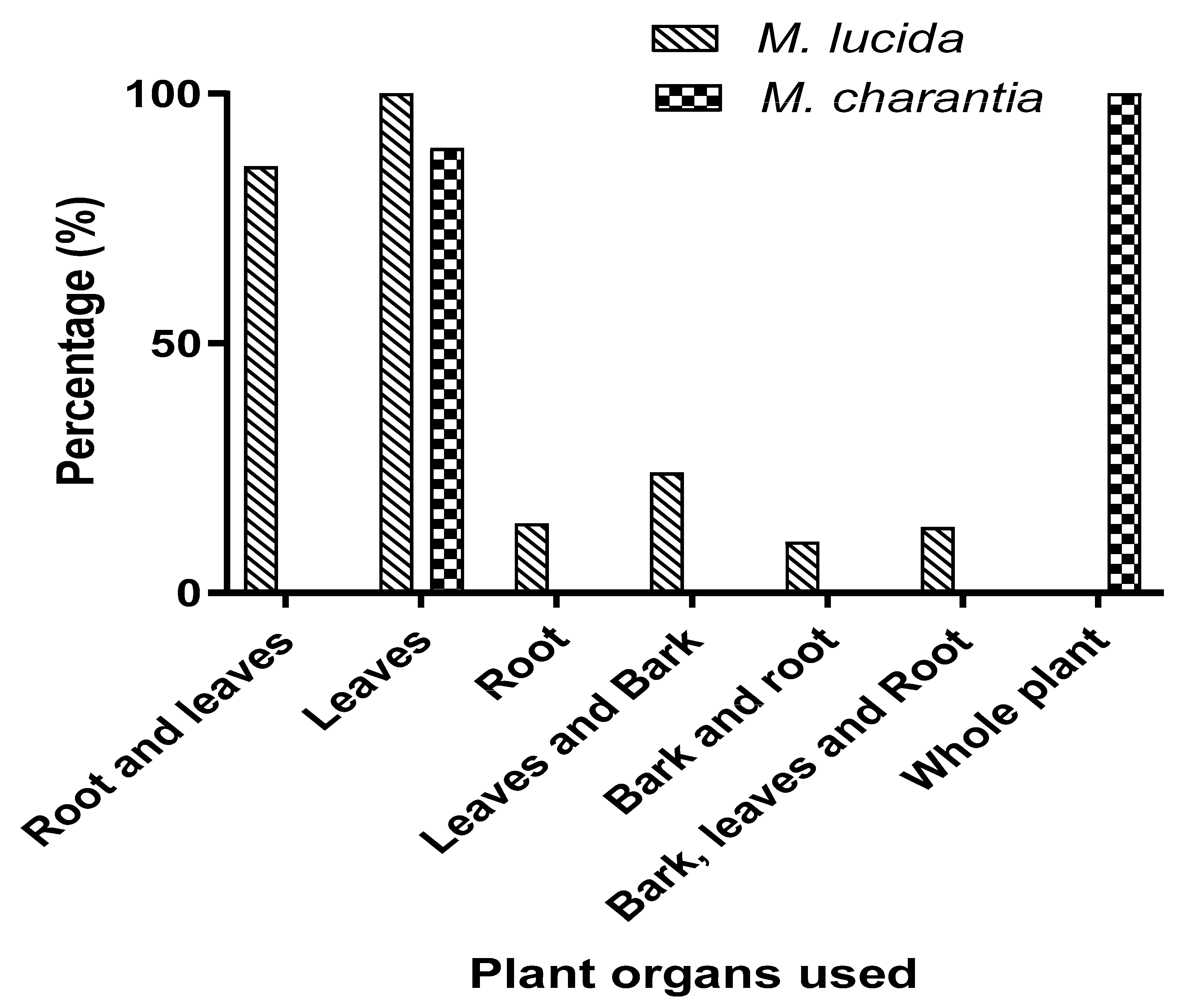
2.1.3. Ethnopharmacological Data Related to the Use of the Two Species
2.2. Antioxidant Activity of M. charantia and M. lucida Extracts
2.2.1. ABTS Method
2.2.2. Ferric Reducing Antioxidant Power (FRAP)
2.3. Cyclic Voltammetry Analysis of M. charantia and M. lucida Extracts
2.4. Anti-Inflammatory Activity of M. charantia and M. lucida Extracts
2.5. Gas Chromatography Coupled with Mass Spectrometry (GC-MS)
3. Discussion
4. Materials and Methods
4.1. Ethnopharmacological Study Area
4.2. Ethnopharmacology Survey
4.3. Biological Activity
4.3.1. Chemicals
4.3.2. Plant Material
4.3.3. Preparation of Plants Extracts
4.3.4. Antioxidant Activity of Extracts by the ABTS Essay
4.3.5. Ferric Reducing Antioxidant Power (FRAP) Essay
4.3.6. Evaluation of the Reversible Effects of the Antioxidant Activity of M. charantia and M. lucida Extracts by the Cyclic Voltammetry Technique
4.3.7. Anti-Inflammatory Activity by Inhibiting Protein Denaturation
4.4. GC-MS Analysis
4.5. Data Processing and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahlberg, A.C.; Trygger, S.B. Indigenous medicine and primary health care: The importance of lay knowledge and use of medicinal plants in rural South Africa. Hum. Ecol. 2009, 37, 79–94. [Google Scholar] [CrossRef]
- Ndhlovu, P.T.; Omotayo, A.O.; Otang-Mbeng, W.; Aremu, A.O. Ethnobotanical review of plants used for the management and treatment of childhood diseases and well-being in South Africa. S. Afr. J. Bot. 2021, 137, 197–215. [Google Scholar] [CrossRef]
- Popoola, T.D.; Segun, P.A.; Ekuadzi, E.; Dickson, R.A.; Awotona, O.R.; Nahar, L.; Sarker, S.D.; Fatokun, A.A. West African medicinal plants and their constituent compounds as treatments for viral infections, including SARS-CoV-2/COVID-19. DARU J. Pharm. Sci. 2022, 30, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. Diversity of use and local knowledge of wild and cultivated plants in the Eastern Cape province, South Africa. J. Ethnobiol. Ethnomed. 2017, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Akoègninou, A.; van der Burg, W.J.; van der Maesen, L.J.G. Flore Analytique du Bénin; Backhuys Publishers: Leiden, The Netherlands, 2006; 1034p. [Google Scholar]
- Chokki, M.; Cudalbeanu, M.; Zongo, C.; Dah-Nouvlessounon, D.; Otilia Ghinea, I.; Furdui, B.; Raclea, R.; Savadogo, A.; Baba-Moussa, L.; Avamescu, S.M.; et al. Exploring Antioxidant and Enzymes (A-Amylase and B-Glucosidase) Inhibitory Activity of Morinda lucida and Momordica charantia leaves from Benin. Foods 2020, 9, 434. [Google Scholar] [CrossRef]
- Saxena, M.; Prabhu, S.V.; Mohseen, M.; Pal, A.K.; Alarifi, S.; Gautam, N.; Palanivel, H. Antidiabetic Effect of Tamarindus indica and Momordica charantia and Downregulation of TET-1 Gene Expression by Saroglitazar in Glucose Feed Adipocytes and Their Involvement in the Type 2 Diabetes-Associated Inflammation In Vitro. BioMed Res. Int. 2022, 2022, 9565136. [Google Scholar] [CrossRef]
- Çiçek, S.S. Momordica charantia L. Diabetes-Related Bioactivities, Quality Control, and Safety Considerations. Front. Pharmacol. 2022, 13, 904643. [Google Scholar] [CrossRef]
- Yang, Y.S.; Wu, N.Y.; Kornelius, E.; Huang, C.N.; Yang, N.C. A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Hypoglycemic Efficacy of the mcIRBP-19-Containing Momordica charantia L. Fruit Extracts in the Type 2 Diabetic Subjects. Food Nutr. Res. 2022, 66, 3685. [Google Scholar] [CrossRef]
- Oladeji, O.S.; Oluyori, A.P.; Dada, A.O. Antiplasmodial activity of Morinda lucida Benth. Leaf and bark extracts against Plasmodium berghei infected mice. Saudi. J. Biol. Sci. 2022, 29, 2475–2482. [Google Scholar] [CrossRef]
- Oyetayo, F.L.; Oseni, O.A.; Akinlolu, O.S.; Momodu, D.U. Antidiabetic, Antilipidemic and Antioxidant Properties of Aqueous Extracts of Morinda lucida and Nauclea latifolia Leaves in Alloxan Induced Rats. Bioint. Res. Appl. Chem. 2021, 11, 11602–11615. [Google Scholar] [CrossRef]
- Abdulkareem, A.O.; Igunnu, A.; Adefoluke, A.A.; Olatunji, L.A. Leaf extract of Morinda lucida improves pancreatic beta-cell function in alloxan-induced diabetic rats. Egypt. J. Basic Appl. Sci. 2019, 6, 73–81. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Bai, W. Characterization of Momordica charantia L. Polysaccharide and its Protective Effect on Pancreatic Cells Injury in STZ-Induced Diabetic Mice. Int. J. Biol. Macromol. 2018, 115, 45–52. [Google Scholar] [CrossRef]
- Kothari, V.; Galdo, J.A.; Suresh, T.; Mathews, S.T. Hypoglycemic agents and potential anti-inflammatory activity. J. Inflamm. Res. 2016, 9, 27–38. [Google Scholar] [CrossRef]
- Montane, J.; Lisa-Cadavez, L.; Anna, N.A. Stress and the inflammatory process: A major cause of pancreatic cell death in type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014, 7, 25–34. [Google Scholar]
- Babizhayev, M.A.; Strokov, I.A.; Nosikov, V.V.; Savel’yeva, E.L.; Sitnikov, V.F.; Yegorov, Y.E.; Lankin, V.Z. The Role of Oxidative Stress in Diabetic Neuropathy: Generation of Free Radical Species in the Glycation Reaction and Gene Polymorphisms Encoding Antioxidant Enzymes to Genetic Susceptibility to Diabetic Neuropathy in Population of Type I Diabetic Patients. Cell Biochem. Biophys. 2015, 71, 1425–1443. [Google Scholar] [CrossRef]
- Pham, T.N.; Nguyen, X.T.; Phan, T.D.; Le, T.D.; Nguyen, T.B.T.; Hoang, T.P.L.; Bach, L.G. Anti-arthritic activity and phytochemical composition of “Cao Khai” (Aqueous extracts of Coptosapelta flavescens Korth). Heliyon 2022, 8, e08933. [Google Scholar] [CrossRef]
- Rehman, Z.S.I.; Rahmatullah, Q.; Inayat, U.R.; Muazzam, A.K.; Mohamed, M.A.; Elshaer, D.A.; Al Farraj, M.S.E.; Muhammad, Y.; Shazia, S.; Ghazala, N.; et al. Ethnogynaecological Knowledge of Traditional Medicinal Plants Used by the Indigenous Communities of North Waziristan, Pakistan. Evid.-Based Compl. Alt. Med. 2022, 2022, 6528264. [Google Scholar] [CrossRef]
- Rekka, R.; Murugesh, S.; Prabakaran, R.; Tiruchengode, N.D. Plants used by malayali tribes in ethnogynaecological disorders in yercaud hills, southern eastern ghats, salem district, Tamil nadu. Reporter 2013, 3, 190–192. [Google Scholar]
- Kiringe, J.W. Ecological and anthropological threats to ethno-medicinal plant resources and their utilization in Maasai communal ranches in the Amboseli region of Kenya. Ethnobot. Res. Appl. 2005, 3, 231–242. [Google Scholar] [CrossRef]
- Gnagne, A.S.; Camara, D.; Fofie, N.B.Y.; Bene, K.; Zirihi, G.N. Étude ethnobotanique des plantes médicinales utilisées dans le traitement du diabète dans le Département de Zouénoula (Côte d’Ivoire). J. Appl. Biosci. 2017, 113, 11257–11266. [Google Scholar] [CrossRef]
- Dassou, H.G.; Ogni, C.A.; Yédomonhan, H.; Adomou, A.C.; Tossou, M.; Dougnon, J.T.; Akoègninou, A. Diversité, usages vétérinaires et vulnérabilité des plantes médicinales au Nord-Bénin. Int. J. Biol. Chem. Sci. 2014, 8, 189–210. [Google Scholar] [CrossRef]
- Trotter, R.; Logan, M. Informant consensus: A new approach for identifying potentially effective medicinal plants. In Plants Indigenous Medicine and Diet: Biobehavioural Approaches; Etkin, N.L., Ed.; Redgrave Publishers: Bedfort Hills, NY, USA, 1986; pp. 91–112. [Google Scholar]
- Cantwell, M.; Nie, X.; Zong, R.J.; Yamaguchi, M. Progress in New Crops. In Asian Vegetables: Selected Fruit and Leafy Types; ASHS Press: Arlington, TX, USA, 1996; pp. 488–495. [Google Scholar]
- Cefalu, W.T.; Ye, J.; Wang, Z.Q. Efficacy of dietary supplementation with botanicals on carbohydrate metabolism in humans. Endocr. Metab. Immune Disord. Drugs Target 2008, 8, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Adewole, K.E.; Attah, A.F.; Adebayo, J.O. Morinda lucida Benth (Rubiaceae): A review of its ethnomedicine, phytochemistry and pharmacology. J. Ethnopharmacol. 2021, 276, 114055. [Google Scholar] [CrossRef] [PubMed]
- Ndam, L.M.; Mih, A.M.; Fongod, A.G.N.; Tening, A.S.; Tonjock, R.K.; Enang, J.E.; Fujii, Y. Phytochemical screening of the bioactive compounds in twenty (20) Cameroonian medicinal plants. Int. J. Cur. Microbiol. Appl. Sci. 2014, 3, 768–778. [Google Scholar]
- Kazeem, M.I.; Adamson, J.O.; Ogunwande, I.A. Modes of Inhibition of α-Amylase and α-Glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Res. Int. 2013, 2013, 527570. [Google Scholar] [CrossRef] [PubMed]
- Lakouéténé, D.P.B.; Ndolngar, G.; Berké, B.; Moyen, J.M.; Komba, E.K.; Zinga, I.; Silla-Millogo-Rasolodimby, J.; Vincendeau, P.; Syssa-Magalé, J.L.; Nacoulma-ouedrago, O.G.; et al. Enquête ethnobotanique des plantes utilisées dans le traitement du paludisme à Bangui. Bull. Soc. Pharm. 2009, 148, 123–138. [Google Scholar]
- Bla, K.B.; Trébissou, J.N.D.; Bidié, A.P.; Assi, Y.J.; Zirihi-Guede, N.; Djaman, A.J. Étude ethnopharmacologique des plantes antipaludiques utilisées chez les Baoulé-N’Gban de Toumodi dans le Centre de la Côte d’Ivoire. J. Appl. Biosci. 2015, 85, 7775–7783. [Google Scholar] [CrossRef][Green Version]
- Ladoh-Yemeda, C.F.; Vandi, D.; Dibong, S.D.; Mpondo, E.; Wansi, J.D.; Betti, J.L.; Choula, F.; Ndongo, D.; Tomedi, M.; Eyango, M. Étude ethnobotanique des plantes médicinales commercialisées dans les marchés de la ville de Douala, Cameroun. J. Appl. Biosci. 2016, 99, 9450–9468. [Google Scholar] [CrossRef]
- Ouattara, D. Contribution à l’Inventaire des Plantes Médicinales Significatives Utilisées dans la Région de Divo (Sud Forestier de la Côte-d’Ivoire) et à la Diagnose du Poivrier de Guinée: Xylopia aethiopica (Dunal) A. Rich. (Annonaceae). Ph.D. Thesis, Université de Cocody, Abidjan, Côte-d’Ivoire, 2006. [Google Scholar]
- Wang, R.; Ding, S.; Zhao, D.; Wang, Z.; Wu, J.; Hu, X. Effect of dehydration methods on antioxidant activities, phenolic contents, cyclic nucleotides, and volatiles of jujube fruits. Food. Sci. Biotechnol. 2016, 25, 137–143. [Google Scholar] [CrossRef]
- Zeb, A. Phenolic Antioxidants in Edible Oils. In Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Springer: Cham, Switzerland, 2021; pp. 239–280. [Google Scholar] [CrossRef]
- Haider, M.H.; Hadi, H.I.; Ibraheem, O.A. Antimicrobial activity and spectral chemical analysis of methanolic leaves extract of Adiantos Capillus-Veneris using GC–MS and FT-IR spectroscopy. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 369–385. [Google Scholar]
- Jasso de Rodríguez, D.; García-Hernández, L.C.; Rocha-Guzmán, N.E.; Moreno-Jiménez, M.R.; Rodríguez-García, R.; Díaz-Jiménez, M.L.V.; Carrillo-Lomelí, D.A. Psacalium paucicapitatum has in vitro antibacterial activity. Ind. Crops Prod. 2017, 107, 489–498. [Google Scholar] [CrossRef]
- Singariya, P.; Kumar, P.; Mourya, K.K. Isolation of new steroids of Kala Dhaman grass (Cenchrus setigerus) and evaluation of their bioactivity. Braz. Arch. Biol. Technol. 2014, 57, 62–66. [Google Scholar] [CrossRef]
- Keller, A.C.; Ma, J.; Kavalier, A.; He, K.; Brillantes, A.M.B.; Kennelly, E.J. Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro. Phytomedicine 2011, 19, 32–37. [Google Scholar] [CrossRef]
- Cimanga, R.K.; Tona, G.L.; Mesia, G.K.; Kambu, O.K.; Bakana, D.P.; Kalenda, P.D.T.; Penge, A.O.; Muyembe, J.J.T.; Totté, J.; Pieters, L.; et al. Bioassay-Guided Isolation of Antimalarial Triterpenoid Acids from the Leaves of Morinda lucida. Pharmaceut. Biol. 2006, 44, 677–681. [Google Scholar] [CrossRef]
- Vasudevan, U.M.; Lee, E.Y. Flavonoids, terpenoids, and polyketide antibiotics: Role of glycosylation and biocatalytic tactics in engineering glycosylation. Biotechnol. Adv. 2020, 41, 107550. [Google Scholar] [CrossRef]
- Raja, S.N.; Haythornthwaite, J.A.; Pappagallo, M.; Clark, M.R.; Travison, T.G.; Sabeen, S.; Royall, R.M.; Max, M.B. Opioids versus antidepressants in postherpetic neuralgia: A randomized, placebo-controlled trial. Neurology 2002, 59, 1015–1021. [Google Scholar] [CrossRef]
- Lee, S.Y.; Wong, W.F.; Dong, J.; Cheng, K.K. Momordica charantia Suppresses Inflammation and Glycolysis in Lipopolysaccharide-Activated RAW264.7 Macrophages. Molecules 2020, 25, 3783. [Google Scholar] [CrossRef]
- Ayertey, F.; Ofori-Attah, E.; Antwi, S.; Amoa-Bosompem, M.; Djameh, G.; Lartey, N.L.; Okine, L.K. Anti-inflammatory activity and mechanism of ethanolic leaf extract of Morinda lucida Benth. J. Tradit. Complement. Med. 2020, 11, 249–258. [Google Scholar] [CrossRef]
- Cao, T.Q.; Phong, N.V.; Kim, J.H.; Gao, D.; Anh, H.L.T.; Ngo, V.D. Inhibitory Effects of Cucurbitane-Type Triterpenoids from Momordica charantia Fruit on Lipopolysaccharide-Stimulated Pro-Inflammatory Cytokine Production in Bone Marrow-Derived Dendritic Cells. Molecules. 2021, 26, 4444. [Google Scholar] [CrossRef]
- Fischer, P.; Gomes, G.C.M.; Etcheverry, B.F.; Rios, N.V.; Chaves, P.E.E.; Sotelo, E.C.; Campos, D.N.; Zuravski, L.; Escobar, T.A.; Machado, M.M. Momordica charantia extract modulates inflammatory response in human lymphocytes via suppression of TNF-α. Ars Pharm. 2022, 63, 320–334. [Google Scholar] [CrossRef]
- Boyer, F. Stress oxydant et pathologie diabétique: Impact de l’hyperglycémie et de l’albumine glyquée sur les cellules cardiaques et adipeuses. In Médecine Humaine et Pathologie; Université de la Réunion: Réunion, France, 2016. [Google Scholar]
- Sibel, A.; Özkan, A.; Bengi, U.; Hassan, Y.; Aboul-Enein, A. Analysis of Pharmaceuticals and Biological Fluids Using Modern Electroanalytical Techniques. Crit. Rev. Anal. Chem. 2003, 33, 155–181. [Google Scholar] [CrossRef]
- Melinda, D.; Adrian, S.; Cristiana, R.; Andrei, F.D.; Monica, F. Bioelectrochemical evaluation of plant extracts and gold nanozyme-based sensors for total antioxidant capacity determination. Bioelectrochemistry 2019, 129, 124–134. [Google Scholar] [CrossRef]
- Ana, P.; Lima, W.T.P.; dos Santos, P.; Edson, N.; Eduardo, M.; Richter, M.; Rodrigo, A.A.; Munoz, A. Critical evaluation of voltammetric techniques for antioxidant capacity and activity: Presence of alumina on glassy-carbon electrodes alters the results, Electrochim. Acta 2020, 358, 136925. [Google Scholar] [CrossRef]
- David, M.; Şerban, A.; Popa, C.V.; Florescu, M. Nanoparticle-based label-free sensor for screening the relative antioxidant capacity of hydro-soluble plant extracts. Sensors 2009, 19, 590. [Google Scholar] [CrossRef]
- Karyakina, E.E.; Vokhmyanina, D.V.; Sizova, N.V.; Sabitov, A.N.; Borisova, A.V.; Sazontova, T.G.; Arkhipenko, Y.V.; Tkachuk, V.A.; Zolotov, Y.A.; Karyakin, A.A. Kinetic approach for evaluation of total antioxidant activity. Talanta 2009, 80, 749–753. [Google Scholar] [CrossRef]
- Assogbadjo, A.E.; Codjia, J.T.C.; Sinsin, B.; Ekue, M.R.M.; Mensah, G.A. Importance of rodents as a human food source in Benin. Belg. J. Zool. 2005, 135, 11–15. [Google Scholar]
- INSAE. RGPH2 Cahier des Villages et Quartiers de Ville Département de l’OUEME, Bénin; INSAE: Cotonou, Benin, 2004; 22p. [Google Scholar]
- Assogbadjo, A.E.; Glèlè Kakaï, R.; Vodouhê, F.G.; Djagoun, C.A.M.S.; Codjia, J.T.C.; Sinsin, B. Biodiversity and socioeconomic factors supporting farmers’ choice of wild edible trees in the agroforestry systems of Benin (West Africa). For. Pol. Econom. 2012, 14, 41–49. [Google Scholar] [CrossRef]
- Dagnelie, P. Statistique Théorique et Appliquée. Tome 2: Inférences Statistiques à Une et Deux Dimensions; De Boeck et Larcier: Paris, France; Brussels, Belgium, 1998; 659p. [Google Scholar]
- Legba, B.B.; Dougnon, V.; Agbankpe, J.; Fabiyi, K.; Lougbegnon, C.; Soha, A.; Ayena, C.; Deguenon, E.; Koudokpon, H.; Baba-Moussa, L. Assessment of Anti-Salmonella Activity of Aqueous and Ethanolic Extract of Senna siamae, Used in Traditional Management of Salmonellosis in Benin. Pharmacol. Pharm. 2020, 11, 226–234. [Google Scholar] [CrossRef]
- Cudalbeanu, M.; Furdui, B.; Cârâc, G.; Barbu, V.; Iancu, A.V.; Marques, F.; Leitão, J.H.; Sousa, S.A.; Dinica, R.M. Antifungal, Antitumoral and Antioxidant Potential of the Danube Delta Nymphaea alba Extracts. Antibiotics 2019, 9, 7. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, H.Y.; Seo, W.T.; Lee, J.H.; Cho, K.M. Roasting enhances antioxidant effect of bitter melon (Momordica charantia L.) increasing in flavan-3-ol and phenolic acid contents. Food. Sci. Biotechnol. 2012, 21, 19–26. [Google Scholar] [CrossRef]
- Sangita, C.; Priyanka, C.; Protapaditya, D.; Sanjib, B. Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pac. J. Trop. Biomed. 2012, 2, S178–S180. [Google Scholar] [CrossRef]



| Diseases Group | Diseases/Symptoms | Frequency (%) | Fic | |
|---|---|---|---|---|
| M. charantia | M. lucida | |||
| Digestive system diseases | Ulcer | 9.48 | 3.64 | 0.77 |
| Stomach pain in children | - | 16.05 | ||
| Constipation | 24.08 | - | ||
| Diarrhea | 12.40 | 9.48 | ||
| Microbial and parasitic disease | Measles | 100 | - | 0.96 |
| Malaria | 62.04 | 93.43 | ||
| Genital infection | - | 10.21 | ||
| Gynaecological diseases | Sticky menstruation | 52.55 | - | 0.98 |
| Painful menstruation | - | 10.21 | ||
| Postpartum lactation problem | 8.75 | - | ||
| Primary infertility | - | 20.43 | ||
| Early menopause | - | 14.59 | ||
| Blood-related diseases | Hypertension | 16.05 | 8.02 | 0.99 |
| Diabetes | 71.53 | 56.93 | ||
| Anaemia | - | 13.13 | ||
| Others | Jaundice in newborns | 59.85 | - | |
| Dermatological problem (wounds) | 98.54 | 2.91 | ||
| Muscle pain | 29.92 | - | ||
| Inflammation | 29.92 | 10.94 | ||
| Immunity boost | 45.25 | 24.08 | ||
| Abdominal pain | - | 74.45 | ||
| Diseases/Symptoms | Type of Recipe | Solvent Used | Parts of Plants Used | Preparation Mode | Dosage | Duration of the Treatment |
|---|---|---|---|---|---|---|
| Ulcer | with Parkia biglobosa stem bark | water | leaves, root | maceration | 1 glass 1/d | 14 days |
| Stomach pain in children | only | water | root | maceration | ¼ of glass 3/d | 3 days |
| Diarrhea | with Citrus aurantiifolia (fruit) | water | leaves | trituration | 1 teaspoon 1/d | until satisfied |
| Malaria | only | water | leaves | decoction | 1 glass 3/d | 3 days |
| Genital infection | with potash and sesame leaf | water | root, leaves | maceration | 1 glass 1/d | 1 week |
| Painful menstruation | only | water | leaves, bark | decoction | 1 glass 1/d | period of menstruation |
| Primary infertility | with potash and Carica papaya leave | water | root | maceration | 1 glass 2/d | 10 days |
| Early menopause | only | palmier wine | leaves | decoction | ½ of glass 2/d | 30 days |
| Hypertension | only | water | leaves | decoction | 1 glass 1/d | 3 days |
| Diabetes | only | water | leaves | decoction or maceration | ½ of glass 3/d | until normal test |
| Anemia | with Xylopia aethiopica | ethanol | leaves | maceration | ½ of glass 1/d | 3 days |
| Dermatological affection (wounds) | with Ocimum gratissimum | water | leaves | decoction | ½ of glass 1/d | 7 days |
| Inflammation | with Cymbopogon citratus | water | leaves, bark, root | decoction | 1 glass 1/d | 3 days |
| Immunity boost | only | ethanol | leaves | maceration | ½ of glass 2/d | 3 days |
| Abdominal pain | only | water | leaves, bark | decoction | 1 glass 1/d | until satisfied |
| Diseases/Symptoms | Type of Recipe | Solvent Used | Parts of Plants Used | Preparation Mode | Dosage | Duration of the Treatment |
|---|---|---|---|---|---|---|
| Ulcer | with Vitelaria paradoxa | Coca-Cola | whole plant | maceration | ½ of glass 1/d | 7 days |
| Constipation | only | ethanol | whole plant | maceration | ½ of glass 1/d | 2 days |
| Diarrhea | with Citrus aurantiifolia (fruit) | water | leaves | trituration | 1 teaspoon 1/d | 3 days |
| Measles | only | water | whole plant | trituration | ½ of glass 1/d and pass over the body | 3 days |
| Malaria | with Annona multiflora | water | whole plant | decoction | 1 glass 3/d | 3 days |
| Sticky Menstruation | only | water | leaves | trituration | ½ of glass 1/d | 2 days before menstruation |
| Postpartum lactation problem | with unripe fruit of Carica papaya | water | whole plant | maceration | 1 glass 2/d | 2 days |
| Hypertension | only | water | whole plant | decoction | ½ of glass 1/d | 3 days |
| Diabetes | only | water | whole plant | decoction | ½ of glass 3/d | until normal test |
| Dermatological affection (wounds) | only | water | leaves | trituration | ½ of glass 1/d and pass over the body | 3 days |
| Muscle pain | only | ethanol | whole plant | maceration | ½ of glass 2/d | 3 days |
| Inflammation | with Combretum micrabtum | water | whole plant | decoction | ½ of glass 1/d | 3 days |
| Immunity boost | only | ethanol | leaves | decoction | ½ of glass 3/d | 3 days |
| Solvents/Standard | Inhibitory Rate (%) | |
|---|---|---|
| M. charantia | M. lucida | |
| Ethanol | 31.26 ± 1.32 | 48.27 ± 1.54 |
| Methanol | 3.86 ± 0.11 | 43.66 ± 2.01 |
| Ethyl Acetate | 50.13 ± 1.20 | 29.85 ± 1.86 |
| Acetone | 50.52 ± 0.88 | 4.63 ± 1.41 |
| Dichloromethane | 22.48 ± 1.45 | 43.40 ± 0.45 |
| Chloroform | 4.70 ± 1.37 | - |
| Petroleum ether | - | - |
| Water | 20.63 ± 0.28 | 13.75 ± 0.17 |
| Methanol/HCl | 42.14 ± 3.11 | 38.10 ± 2.14 |
| Ethanol/water | 34.24 ± 1.17 | 45.35 ± 1.28 |
| Methanol/HCl-PE | 43.12 ± 2.38 | 37.15 ± 2.65 |
| Methanol-EA | 12.35 ± 1.78 | 5.01 ± 1.47 |
| Ascorbic Acid | 75.23 ± 2.17 | |
| Solvents/Standard | M. charantia | M. lucida | ||
|---|---|---|---|---|
| % Inh | IC50 (mg/mL) | % Inh | IC50 (mg/mL) | |
| Ethanol | 59.46 ± 1.83 a | <0.078 | 58.80 ± 0.96 a | 0.15 ± 0.06 |
| Ethanol–water | 60.75 ± 1.07 a | <0.078 | 58.23 ± 1.26 ab | 0.21 ± 0.02 |
| Methanol | 58.98 ± 1.30 a | <0.078 | 60.24 ± 0.56 a | < 0.078 |
| Ethyl Acetate | 59.97 ± 0.064 a | <0.078 | 59.61 ± 0.60 a | 0.10 ± 0.01 |
| Acetone | 60.14 ± 1.87 a | <0.078 | 59.23 ± 0.22 a | <0.078 |
| BHT | 80.12 ± 1.02 | 22.35 ± 1.15 (µg/mL) | - | - |
| Solvents/Standard | M. charantia | M. lucida | ||
|---|---|---|---|---|
| % Inh | IC50 (mg/mL) | % Inh | IC50 (mg/mL) | |
| Ethanol | 99.13 ± 0.12 a | 1.16 ± 0.04 | 57.32 ± 1.35 a | 1.72 ± 0.23 |
| Methanol | 99.53 ± 0.08 a | 1.08 ± 0.11 | 97.67 ± 2.02 b | 1.08 ± 0.04 |
| Ethyl Acetate | 97.22 ± 1.02 a | 0.22 ± 0.01 | 93.62 ± 1.13 c | 0.47 ± 0.01 |
| Acetone | 97.02 ± 1.35 a | 0.55 ± 0.01 | 97.19 ± 0.45 b | 2.02 ± 0.01 |
| Dichloromethane | 98.95 ± 0.89 a | 0.10 ± 0.02 | 98.34 ± 0.12 b | 0.37 ± 0.14 |
| Chloroform | 97.67 ± 0.19 a | 0.38 ± 0.01 | 96.29 ± 0.89 b | 0.12 ± 0.02 |
| Water | 93.09 ± 1.17 b | 2.03 ± 0.95 | 98.13 ± 0.09 b | 0.11 ± 0.01 |
| Diclofenac | 98.30 ± 0.76 a | 13.33 ± 0.76 (µg/mL) | - | - |
| N° | Retention Time (min) | Volatile Compounds | Relative Abundance (%) | |||
|---|---|---|---|---|---|---|
| M. charantia | M. lucida | |||||
| EAC | Act | EAC | Act | |||
| 1 | 8.8 | 1, 12-Dicarbadodecarboran-2-amine, N-(4-methoxyphenyl) | 0.37 | 0.16 | 0.54 | - |
| 2 | 9.11 | Inconnu | 0.58 | - | - | - |
| 3 | 9.56 | 2-(Allyloxy)-1,5-ditert-butyl-3-chlorobenzene | 15.98 | 14.93 | 8.6 | |
| 4 | 10.51 | Dibenzoic[c,H] diazecine, 6,13-bis (2,5-dimethylphenyl)-5,6,7,12,13,14-hexahydro-1,4,8,11-tetramethyl | - | - | 0.4 | 0.42 |
| 5 | 10.73 | Harzianic acid | - | - | - | 0.3 |
| 6 | 10.81 | 4,4,7-Trimethyl-5-[(4H-1,2,4-triazol-3-ylsufanyl)acetyl]-4,5-dihydro-1H-[1,2]dithiolo [3,4-c]quinoline-1-thione | - | - | 1.39 | - |
| 7 | 10.89 | Inconnu | - | - | 9.68 | - |
| 8 | 10.92 | 2-[(5-Chloro-8-hydroxy-3-methyl-1-oxoisochroman-7-carbonyl)amino]-3-phenyl propionate | - | 2.42 | - | - |
| 9 | 12.92 | 1,3,5,7-Tetraethyl-1-butoxycyclotetrasiloxane | 0.68 | - | - | - |
| 10 | 13.67 | Trans-4,4′-Dimethoxy-β-methylchalcone | - | - | 5.34 | - |
| 11 | 13.68 | 17-Hydroxy-3,20-dioxopregna-1,4,9(11)-trien-21-yl acetate | - | 12.29 | - | 20.02 |
| 12 | 14.83 | Harzialactone | - | 0.56 | - | - |
| 13 | 14.84 | Ent-3-Acetoxy-10-hydroxy-13-iodomethyl-16-oxo-8,13-epi-17,20-dinorgibberell-1-ene-7,19-dioic acid 19,10-lactone | - | - | 0.2 | - |
| 14 | 14.89 | Inconnu | - | - | - | 0.32 |
| 15 | 16.51 | Methanethione, (2,5-dimethylphenyl)-(2,4,6-trimethylpheny)-S-oxide | 0.29 | - | - | - |
| 16 | 21.74 | 4-Methylcholesta-8,24-dien-3-ol | 0.19 | |||
| 17 | 25.96 | 17-(2-Butyl-1,3,2-dioxaborolan-4-yl) androstane-3, 11-diol | 0.48 | - | - | - |
| 18 | 26.14 | Inconnu | 1.43 | - | - | - |
| 19 | 26.16 | 2,6-Dimethyl-4-(methoxymethyl)phenol | - | 0.79 | 0.47 | 0.61 |
| 20 | 26.57 | 2-5Cyano-ethoxycarbonylmethyl)-6-methyl-4,6-bis(4-nitrophenyl)-1,6-dihydropyridin-3-3-carboxylic acid, ethyl ester | 0.43 | |||
| 21 | 27.1 | 5,6-Epoxy-7-bromocholestan-3-ol | - | - | 0.29 | - |
| 22 | 27.26 | 3-β-5-epoxy-3-α-methoxy-a-homo-5-β-cholestane | 0.41 | - | - | - |
| 23 | 27.5 | Cis-13,14-Epoxydocosanoic acid | 0.96 | 0.25 | 0.34 | 0.28 |
| 24 | 27.72 | Androst-4-ene-3,20-dione, 11,16,22-triacetoxy- | 0.24 | - | - | - |
| 25 | 29.49 | Lycopene, 3,3′,4,4′-tetradehydro-1,1′,2,2′-tetrahydro-1-hydroxy-1′-methoxy- | 0.33 | - | - | - |
| 26 | 29.54 | 2-Chloroethyl isobutyrate of terephthalate | - | - | - | 0.34 |
| 27 | 29.55 | 6,9,12,15-Docosatetraenoic acid, methyl ester | - | - | 1.14 | - |
| 28 | 29.67 | 3,5-Androstadien-17-one oxime | 0.49 | - | - | - |
| 29 | 29.71 | 2-Bromo-4,6-dimethylbenzamide | - | - | - | 0.51 |
| 30 | 29.56 | 17-Acetyl-16-hydroxy-10,17-dimethylgona-4,13-dien-3-one | - | 0.1 | - | 0.07 |
| 31 | 30.18 | Lycopene, 3,4-didehydro-1,2-dihydro-1-methoxy-,all-trans | 0.12 | - | - | - |
| 32 | 30.32 | Inconnu | - | 10.53 | 0.38 | 0.40 |
| 33 | 32.96 | Propanoic acid, 2-(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl) | 7.03 | - | 2.23 | - |
| 34 | 33.05 | Pregn-16-en-20-one, 11,18-bis (acetyloxy)-3,9-epoxy-3-methoxy | - | 0.53 | - | 0.1 |
| 35 | 33.38 | (2-Phenyl-1,3-dioxolan-4-yl)methyl (9E)-9-octadecenoate | 0.23 | 0.37 | - | - |
| 36 | 33.4 | Galoxolide | - | - | 2.1 | 3.58 |
| 37 | 34.36 | 3-(3-Bromophenyl)-7-chloro-10-hydroxy-3,4-dihydro-1,9 (2H, 10H)-acridinedione | 0.16 | - | - | - |
| 38 | 35.13 | 3,9,14,15-Diepoxypregn-16-en-20-one, 3,11,18-triacetoxy | 11.26 | - | - | - |
| 39 | 35.17 | 3,9,14,15-Diepoxypregn-16-en-20-one, 3,11,18-triacetoxy | - | - | - | 1.85 |
| 40 | 35.47 | 17-(1,5-Dimethylhexyl)-10,13-dimethyl-3-styryhexaderhydrocyclopenta[a]phenanthren-2-one | 0.79 | - | - | - |
| 41 | 35.63 | 5-Stigmastane-3,6-dione | - | 1.25 | - | 0.58 |
| 42 | 35.85 | 1-Heptatriacontanol | 0.68 | 2.66 | 1.06 | 0.7 |
| 43 | 36.66 | Aromadendrene | 0.59 | 10.52 | - | 0.51 |
| 44 | 37.01 | Inconnu | 6.26 | - | - | - |
| 45 | 37.02 | 6β-Hydroxyfluoxymesterone | 3.24 | 15.55 | 14.31 | |
| 46 | 37.46 | Inconnu | - | - | 2.62 | - |
| 47 | 37.83 | Ethyl iso-allocholate | 14.33 | 2.42 | 0.68 | 3.63 |
| 48 | 37.99 | 6-Hydroxyfluoxymesterone | - | 3.69 | - | - |
| 49 | 38.00 | 2,6-Bis (1,1-dimethylethyl)-4-(1-oxopropyl)phenol | 27.03 | 2.55 | 3.18 | 3.17 |
| 50 | 38.63 | Inconnu | 1.15 | - | - | - |
| 51 | 38.77 | Dermadine | 2.54 | 12.66 | 2.86 | - |
| 52 | 38.79 | 17-Ethylenedioxy-5,19-cycloandrost-6-en-3-one | - | - | - | 2.86 |
| 53 | 39.26 | (22S)-21-Acetoxy-6,11-dihydroxy-16,17-propylmethylenedioxypregna-1,4-diene-3,20-dione | 2.06 | 1.64 | 2.26 | 1.68 |
| 54 | 40.44 | Inconnu | - | 1.16 | - | 4.03 |
| 55 | 41.16 | 3,12,25-Tris(acetyloxy)cholestan-7-yl acetate | 6.05 | - | 4.49 | 4.78 |
| 56 | 41.5 | N,N’-Bis(Carbobenzyloxy)-lysine methyl (ester) | 1.13 | 0.87 | 0.84 | 22.84 |
| 57 | 42.67 | Harzianolide | 12.39 | |||
| 58 | 42.69 | Benzothiophene-2-carboxylic acid, 4,5,6,7-tetrahydro-7-hydroximino-3-[2-(4-morpholyl)-1-oxoethylamino]-, ethyl ester | 10.54 | - | 25.33 | - |
| Total | 98.83% | 99.03% | 98.30% | 96.49% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dah-Nouvlessounon, D.; Chokki, M.; Noumavo, A.D.P.; Cârâc, G.; Furdui, B.; Sina, H.; Zongo, C.; Savadogo, A.; Baba-Moussa, L.; Dinica, R.-M.; et al. Ethnopharmacological Value and Biological Activities via Antioxidant and Anti-Protein Denaturation Activity of Morinda lucida Benth and Momordica charantia L. Leaves Extracts from Benin. Plants 2023, 12, 1228. https://doi.org/10.3390/plants12061228
Dah-Nouvlessounon D, Chokki M, Noumavo ADP, Cârâc G, Furdui B, Sina H, Zongo C, Savadogo A, Baba-Moussa L, Dinica R-M, et al. Ethnopharmacological Value and Biological Activities via Antioxidant and Anti-Protein Denaturation Activity of Morinda lucida Benth and Momordica charantia L. Leaves Extracts from Benin. Plants. 2023; 12(6):1228. https://doi.org/10.3390/plants12061228
Chicago/Turabian StyleDah-Nouvlessounon, Durand, Michaelle Chokki, Agossou Damien Pacôme Noumavo, Geta Cârâc, Bianca Furdui, Haziz Sina, Cheikna Zongo, Aly Savadogo, Lamine Baba-Moussa, Rodica-Mihaela Dinica, and et al. 2023. "Ethnopharmacological Value and Biological Activities via Antioxidant and Anti-Protein Denaturation Activity of Morinda lucida Benth and Momordica charantia L. Leaves Extracts from Benin" Plants 12, no. 6: 1228. https://doi.org/10.3390/plants12061228
APA StyleDah-Nouvlessounon, D., Chokki, M., Noumavo, A. D. P., Cârâc, G., Furdui, B., Sina, H., Zongo, C., Savadogo, A., Baba-Moussa, L., Dinica, R.-M., & Baba-Moussa, F. (2023). Ethnopharmacological Value and Biological Activities via Antioxidant and Anti-Protein Denaturation Activity of Morinda lucida Benth and Momordica charantia L. Leaves Extracts from Benin. Plants, 12(6), 1228. https://doi.org/10.3390/plants12061228








