Four Invasive Plant Species in Southwest Saudi Arabia Have Variable Effects on Soil Dynamics
Abstract
:1. Introduction
2. Results
2.1. Comparison between Invaded and Native Soil Samples
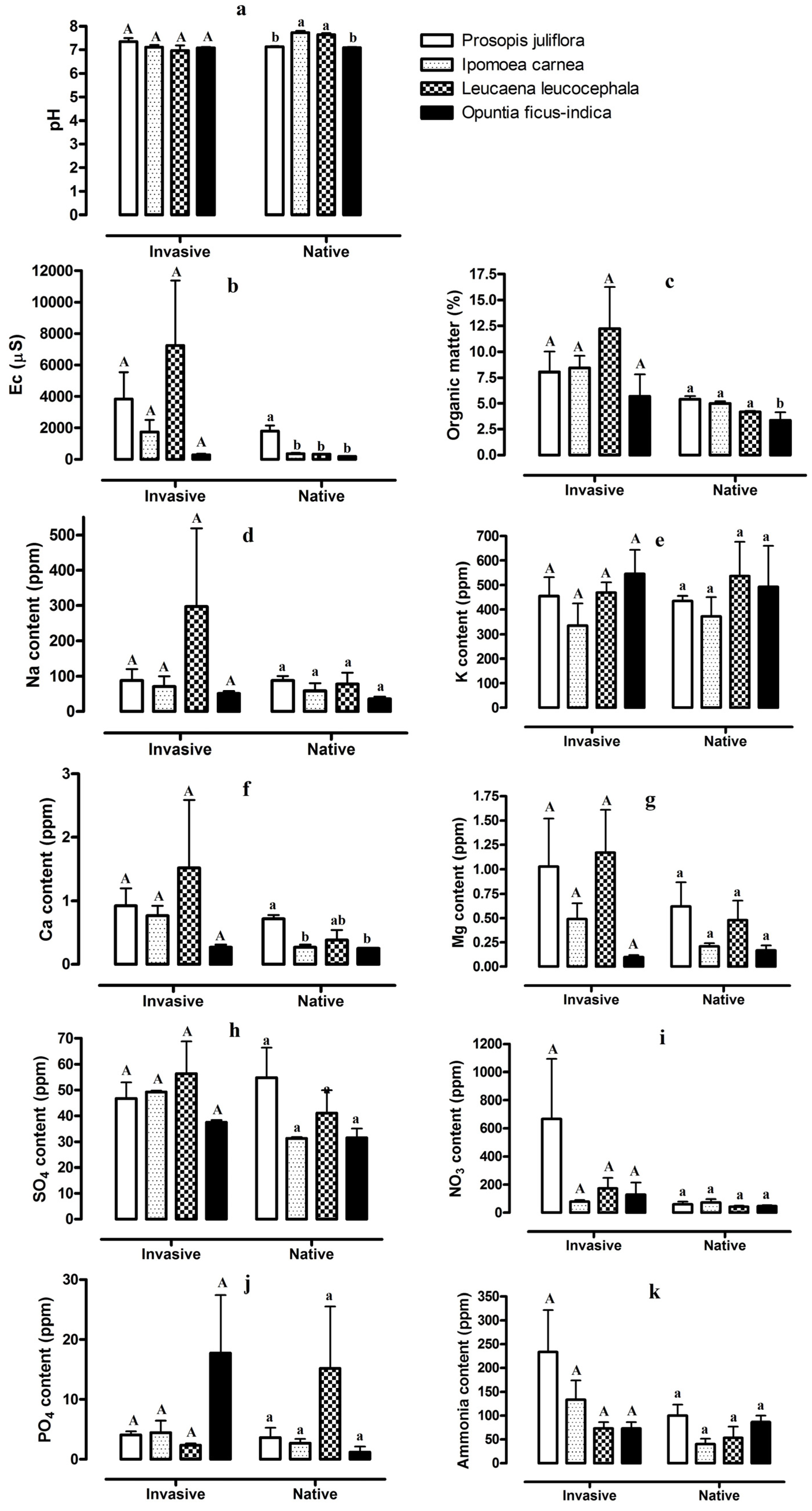
2.2. Soil Properties, Ion Concentrations, and Microelement Concentrations for the Four Invasive Plant Species and Adjacent Stands of Native Vegetation
3. Discussion
4. Materials and Methods
4.1. Species Selection
4.2. Field Sampling
4.3. Soil Analyses
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mack, R.N. The motivation for importing potentially invasive plant species: A primal urge. In Proceedings of the VI International Rangeland Congress, International Rangeland Congress, Aitkenvale, QLD, Australia, 19–23 July 1999; Volume 2, pp. 557–562. [Google Scholar]
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology; John Wiley & Sons: New York, NY, USA, 2013; p. 444. [Google Scholar]
- Czortek, P.; Królak, E.; Borkowska, L.; Bielecka, A. Impacts of soil properties and functional diversity on the performance of invasive plant species Solidago canadensis L. on post-agricultural wastelands. Sci. Total Environ. 2020, 729, 139077. [Google Scholar] [CrossRef]
- Mack, R.N. Assessing the Extent, Status, and Dynamism of Plant Invasions: Current and Emerging Approaches. Invasive Species in a Changing World; Island Press: Washington, WA, USA, 2000; pp. 141–168. [Google Scholar]
- Davis, M.A. Biotic globalization: Does competition from introduced species threaten biodiversity? Bioscience 2003, 53, 481–489. [Google Scholar] [CrossRef]
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of alien plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 2008, 157, 131–140. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Gibbons, S.M.; Lekberg, Y.; Mummey, D.L.; Sangwan, N.; Ramsey, P.W.; Gilbert, J.A. Invasive plants rapidly reshape soil properties in a grassland ecosystem. MSystems 2017, 2, e00178-16. [Google Scholar] [CrossRef]
- Jamil, M.D.; Waheed, M.; Akhtar, S.; Bangash, N.; Chaudhari, S.K.; Majeed, M.; Hussain, M.; Ali, K.; Jones, D.A. Invasive plants diversity, ecological status, and distribution pattern in relation to edaphic factors in different habitat types of district Mandi Bahauddin, Punjab, Pakistan. Sustainability 2022, 14, 13312. [Google Scholar] [CrossRef]
- Clements, D.R.; Upadhyaya, M.K.; Joshi, S.; Shrestha, A. Global Plant Invasions; Springer: Cham, Switzerland, 2022; p. 381. [Google Scholar]
- Assaeed, A.M.; Alharthi, A.S.; Abd-ElGawad, A.M. Impacts of Nicotiana glauca Graham invasion on the vegetation composition and soil: A case study of Taif, western Saudi Arabia. Plants 2021, 10, 2587. [Google Scholar] [CrossRef] [PubMed]
- Unger, I.M.; Kremer, R.J.; Veum, K.S.; Goyne, K.W. Immediate and long-term effects of invasive plant species on soil characteristics. Soil Ecol. Lett. 2022, 4, 276–288. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G.; Kourtev, P.; Huang, W. Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecol. Appl. 2001, 11, 1287–1300. [Google Scholar] [CrossRef]
- Weidenhammer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef]
- Qu, T.; Du, X.; Peng, Y.; Guo, W.; Zhao, C.; Losapio, G. Invasive species allelopathy decreases plant growth and soil microbial activity. PLoS ONE 2021, 16, e0246685. [Google Scholar] [CrossRef] [PubMed]
- Coykendall, K.E.; Houseman, G.R. Lespedeza cuneata invasion alters soils facilitating its own growth. Biol. Invasions 2014, 16, 1735–1742. [Google Scholar] [CrossRef]
- Anning, A.K.; Gyamfi, B.; Effah, A.T. Broussonetia papyrifera controls nutrient return to soil to facilitate its invasion in a tropical forest of Ghana. J. Plant Ecol. 2018, 11, 909–918. [Google Scholar] [CrossRef]
- Wang, W.; Sardans, J.; Wang, C.; Asensio, D.; Bartrons, M.; Peñuelas, J. Species-specific impacts of invasive plant success on vertical profiles of soil carbon accumulation and nutrient retention in the Minjiang River tidal estuarine wetlands of China. Soil Syst. 2018, 2, 5. [Google Scholar] [CrossRef]
- Maass, J.M.; Balvanera, P.; Castillo, A.; Daily, G.C.; Mooney, H.A.; Ehrlich, P.; Quesada, M.; Miranda, A.; Jaramillo, V.J.; García-Oliva, F.; et al. Ecosystem services of tropical dry forests: Insights from long-term ecological and social research on the Pacific Coast of Mexico. Ecol. Soc. 2005, 10. [Google Scholar] [CrossRef]
- Vasquez-Valderrama, M.; González-M, R.; López-Camacho, R.; Baptiste, M.P.; Salgado-Negret, B. Impact of invasive species on soil hydraulic properties: Importance of functional traits. Biol. Invasions 2020, 22, 1849–1863. [Google Scholar] [CrossRef]
- Liao, C.; Peng, R.; Luo, Y.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Vitousek, P.M. Resource-use efficiency and plant invasion in low-resource systems. Nature 2007, 446, 1079–1081. [Google Scholar] [CrossRef]
- González, A.L.; Kominoski, J.S.; Danger, M.; Ishida, S.; Iwai, N.; Rubach, A. Can ecological stoichiometry help explain patterns of biological invasions? Oikos 2010, 119, 779–790. [Google Scholar] [CrossRef]
- Matzek, V. Superior performance and nutrient-use efficiency of invasive plants over non-invasive congeners in a resource-limited environment. Biol. Invasions 2011, 13, 3005–3014. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol. 2012, 160, 1741–1761. [Google Scholar] [CrossRef] [PubMed]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.G.; Stander, E.K. Habitat function in urban riparian zones. Urban Ecosyst. Ecol. 2010, 55, 103–118. [Google Scholar]
- Elgersma, K.J.; Ehrenfeld, J.G.; Yu, S.; Vor, T. Legacy effects overwhelm the short-term effects of exotic plant invasion and restoration on soil microbial community structure, enzyme activities, and nitrogen cycling. Oecologia 2011, 167, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.M.; Pyšek, P.; Rejmanek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Davis, M.A.; Chew, M.K.; Hobbs, R.J.; Lugo, A.E.; Ewel, J.J.; Vermeij, G.J.; Brown, J.H.; Rosenzweig, M.L.; Gardener, M.R.; Carroll, S.P.; et al. Don’t judge species on their origins. Nature 2011, 474, 153–154. [Google Scholar] [CrossRef]
- Reaser, J.K.; Simpson, A.; Guala, G.F.; Morisette, J.T.; Fuller, P. Envisioning a national invasive species information framework. Biol. Invasions 2020, 22, 21–36. [Google Scholar] [CrossRef]
- Vujanović, D.; Losapio, G.; Milić, S.; Milić, D. The Impact of multiple species invasion on soil and plant communities increases with invasive species co-occurrence. Front. Plant Sci. 2022, 13, 875824. [Google Scholar] [CrossRef]
- Jamin, J.; Diehl, D.; Meyer, M.; Davis, J.; Schaumann, G.E. Physio-chemical soil properties affected by invasive plants in southwest Germany (Rhineland-Palatinate)—A case study. Soil Syst. 2022, 6, 93. [Google Scholar] [CrossRef]
- Thomas, J.; El-Sheikh, M.A.; Alfarhan, A.H.; Alatar, A.A.; Sivadasan, M.; Basahi, M.; Al-Obaid, S.; Rajakrishnan, R. Impact of alien invasive species on habitats and species richness in Saudi Arabia. J. Arid Environ. 2016, 127, 53–65. [Google Scholar] [CrossRef]
- Lehan, N.E.; Murphy, J.R.; Thorburn, L.P.; Bradley, B.A. Accidental introductions are an important source of invasive plants in the continental United States. Am. J. Bot. 2013, 100, 1287–1293. [Google Scholar] [CrossRef]
- Alfaran, A.H.; Thomas, J.; Rajakrishnan, R. Impact assessment and management of invasive species in plant diversity centers and agricultural fields in Saudi Arabia. In Invasive Alien Species: Observations and Issues from Around the World. Volume 2: Issues and Invasions in Asia and the Pacific Region; Pullaiah, T., Ielmini, M.R., Eds.; John Wiley & Sons: New York, NY, USA, 2021; pp. 207–225. [Google Scholar]
- Al-Sodany, Y.M. A new record to the flora of Saudi Arabia: Ipomoea carnea Jacq., Convolvulaceae. World J. Res. Rev. 2016, 3, 25–30. [Google Scholar]
- Shaltout, K.H.; Al-Sodany, Y.M.; Eid, E.M. The biology of Egyptian woody perennials-2. Ipomoea Carnea Jacq. Ass. Univ. Bull. Environ. Res. 2006, 9, 75–91. [Google Scholar]
- Waring, B.G.; Alvarez-Cansino, L.; Barry, K.E.; Becklund, K.K.; Dale, S.; Gei, M.G.; Keller, A.B.; Lopez, O.R.; Markesteijn, L.; Mangan, S.; et al. Pervasive and strong effects of plants on soil chemistry: A meta-analysis of individual plant ‘Zinke’ effects. Proc. R. Soc. B. 2015, 282, 20151001. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Skočajić, D.; Nešić, M.L. Invasive Species: Routes of Introduction, Establishment, and Expansion; Springer: Berlin/Heidelberg, Germany, 2021; pp. 571–582. [Google Scholar]
- Gaertner, M.; Den Breeyen, A.; Hui, C.; Richardson, D.M. Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: A meta-analysis. Prog. Phys. Geogr. 2009, 33, 319–338. [Google Scholar] [CrossRef]
- Joshi, C.; De Leeuw, J.; van Duren, I.C. Remote sensing and GIS applications for mapping and spatial modelling of invasive species. Proc. ISPRS 2004, 35, B7. [Google Scholar]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Godoy, O.; Alonso, A.; Gallardo, A.; Saldaña, A. What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta-analysis. Ecol. Lett. 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Ashton, I.W.; Hyatt, L.A.; Howe, K.M.; Gurevitch, J.; Lerdau, M.T. Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous forest. Ecol. Appl. 2005, 15, 1263–1272. [Google Scholar] [CrossRef]
- Feng, Y.L.; Lei, Y.B.; Wang, R.F.; Callaway, R.M.; Valiente-Banuet, A.; Inderjit; Li, Y.P.; Zheng, Y.L. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc. Natl. Acad. Sci. USA 2009, 106, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.G. Implications of invasive species for belowground community and nutrient processes. Weed Technol. 2004, 18, 1232–1235. [Google Scholar] [CrossRef]
- Rout, M.E.; Callaway, R.M. An invasive plant paradox. Science 2009, 324, 734. [Google Scholar] [CrossRef] [PubMed]
- Novak, S.J. The role of evolution in the invasion process. Proc. Natl. Acad. Sci. USA 2007, 104, 3671–3672. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-Y.; Julien, M.H.; Fatemi, M.; Girod, C.; van Klinken, R.; Gross, C.L.; Novak, S.J. Phenotypic divergence during the invasion of Pyla canescens in Australia and France: Evidence for selection-driven evolution. Ecol. Lett. 2010, 13, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Sax, D.F.; Stachowicz, J.J.; Gaines, S.D. (Eds.) Species Invasions: Insights into Ecology, Evolution and Biogeography; Sinauer: Sunderland, MA, USA, 2005. [Google Scholar]
- Gilliam, F.S. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J. Ecol. 2006, 94, 1176–1191. [Google Scholar] [CrossRef]
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Ostertag, R.; Verville, J.H. Fertilization with nitrogen and phosphorus increases abundance of non-native species in Hawaiian montane forests. Plant Ecol. 2002, 162, 77–90. [Google Scholar] [CrossRef]
- Laungani, R.; Knops, J.M. Species-driven changes in nitrogen cycling can provide a mechanism for plant invasions. Proc. Natl. Acad. Sci. USA 2009, 106, 12400–12405. [Google Scholar] [CrossRef]
- Kueffer, C. Reduced risk for positive soil-feedback on seedling regeneration by invasive trees on a very nutrient-poor soil in Seychelles. Biol. Invasions 2010, 12, 97–102. [Google Scholar] [CrossRef]
- Duda, J.J.; Freeman, D.C.; Emlen, J.M.; Belnap, J.; Kitchen, S.G.; Zak, J.C.; Sobek, E.; Tracy, M.; Montante, J. Differences in native soil ecology associated with invasion of the exotic annual chenopod, Halogeton glomeratus. Biol. Fertil. Soils 2003, 38, 72–77. [Google Scholar] [CrossRef]
- Rodgers, V.L.; Wolfe, B.E.; Werden, L.K.; Finzi, A.C. The invasive species Alliaria petiolata (garlic mustard) increases soil nutrient availability in northern hardwood-conifer forests. Oecologia 2008, 157, 459–471. [Google Scholar] [CrossRef]
- Li, H.; Meng, Z.; Dang, X.; Yang, P. Soil Properties under artificial mixed forests in the desert-yellow river coastal transition zone, China. Forests 2022, 13, 1174. [Google Scholar] [CrossRef]
- Odum, W.E. Comparative ecology of tidal freshwater and salt marshes. Annu. Rev. Ecol. Syst. 1988, 19, 147–176. [Google Scholar] [CrossRef]
- Windham, L.; Weis, J.S.; Weis, P. Patterns and processes of mercury release from leaves of two dominant salt marsh macrophytes, Phragmites australis and Spartina alterniflora. Estuaries 2001, 24, 787–795. [Google Scholar] [CrossRef]
- Ermakov, V.V.; Korobova, E.M.; Degtyarev, A.P.; Tyutikov, S.F.; Karpova, E.A.; Petrunina, N.S. Impact of natural and man-made factors on migration of heavy metals in the Ardon River basin (North Ossetia). J. Soils Sediments 2016, 16, 1253–1266. [Google Scholar] [CrossRef]
- Chai, M.; Shi, F.; Li, R.; Shen, X. Heavy metal contamination and ecological risk in Spartina alterniflora marsh in intertidal sediments of Bohai Bay, China. Mar. Pollut. Bull. 2014, 84, 115–124. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Liu, J.; Hong, H.; Yan, C. The influence of flavonoid amendment on the absorption of cadmium in Avicennia marina roots. Ecotoxicol. Environ. Saf. 2015, 120, 1–6. [Google Scholar] [CrossRef]
- Selvi, F.; Carrari, E.; Colzi, I.; Coppi, A.; Gonnelli, C. Responses of serpentine plants to pine invasion: Vegetation diversity and nickel accumulation in species with contrasting adaptive strategies. Sci. Total Environ. 2017, 595, 72–80. [Google Scholar] [CrossRef]
- Li, J.; Du, Z.; Zou, C.B.; Dai, Z.; Du, D.; Yan, C. The mutual restraint effect between the expansion of Alternanthera philoxeroides (Mart.) Griseb and cadmium mobility in aquatic environment. Ecotoxicol. Environ. Saf. 2018, 148, 237–243. [Google Scholar] [CrossRef]
- El-Bakatoushi, R.; Alframawy, A.M.; Tammam, A.; Youssef, D.; El-Sadek, L. Molecular and physiological mechanisms of heavy metal tolerance in Atriplex halimus. Int. J. Phytoremed. 2015, 17, 789–800. [Google Scholar] [CrossRef]
- Ellili, A.; Rabier, J.; Prudent, P.; Salducci, M.D.; Heckenroth, A.; Lachaâl, M.; Laffont-Schwob, I. Decision-making criteria for plant-species selection for phytostabilization: Issues of biodiversity and functionality. J. Environ. Manag. 2017, 201, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.A.; Sommers, L. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 539–579. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (No. 939); US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Berghage, R.D.; Krauskopf, D.M.; Warncke, D.D.; Widders, I. Micronutrient testing of plant growth media: Extractant identification and evaluation. Commun. Soil Sci. Plant Anal. 1987, 18, 1089–1109. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Warman, P.R. Comparison of three digestion methods for the recovery of 17 plant essential nutrients and trace elements from six composts. Compos. Sci. Util. 2002, 10, 197–203. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989; p. 1191. [Google Scholar]

| Invasive | Native | t-Test | p-Values | |
|---|---|---|---|---|
| Soil properties | ||||
| pH | 7.35 ± 0.15 | 7.13 ± 0.03 | 1.960 | 0.234 |
| EC μS | 3837.67 ± 1712.79 | 1797.67 ± 357.34 | 1.359 | 0.308 |
| OM % | 8.03 ± 1.99 | 5.39 ± 0.31 | 1.726 | 0.259 |
| Ions (cations and anions) | ||||
| Na⁺ ppm | 88.09 ± 33.55 | 87.95 ± 12.63 | 0.001 | 0.982 |
| K⁺ ppm | 454.52 ± 77.13 | 435.58 ± 20.53 | 0.056 | 0.824 |
| Ca2+ ppm | 0.92 ± 0.28 | 0.72 ± 0.06 | 0.477 | 0.528 |
| Mg2+ ppm | 1.03 ± 0.49 | 0.62 ± 0.25 | 0.551 | 0.499 |
| SO42− ppm | 46.68 ± 6.28 | 54.89 ± 11.58 | 0.389 | 0.567 |
| NO3− ppm | 666.67 ± 425.57 | 60.00 ± 20.00 | 2.028 | 0.228 |
| PO43− ppm | 4.08 ± 0.60 | 3.59 ± 1.66 | 0.078 | 0.794 |
| Ammonia (NH4+) ppm | 233.33 ± 88.19 | 100.00 ± 23.09 | 2.139 | 0.217 |
| Microelements | ||||
| Mn ppm | 172.92 ± 122.92 | 270.83 ± 90.60 | 0.411 | 0.556 |
| Zn mg/L | 43.54 ± 3.15 | 67.92 ± 8.90 | 6.665 | 0.061 |
| Fe ppm | 10,445.83 ± 3732.17 | 22,385.42 ± 7700.57 | 1.947 | 0.235 |
| Cr ppm | 4.99 ± 0.47 | 12.05 ± 0.90 | 48.472 | 0.002 |
| Co ppm | 4.25 ± 0.48 | 8.91 ± 0.42 | 53.902 | 0.002 |
| Cu ppm | 5.42 ± 1.63 | 12.50 ± 0.63 | 16.514 | 0.015 |
| Ni ppm | 9.69 ± 1.31 | 79.58 ± 9.42 | 54.015 | 0.002 |
| Invasive | Native | t-Test | p-Values | |
|---|---|---|---|---|
| Soil properties | ||||
| pH | 7.11 ± 0.09 | 7.73 ± 0.07 | 28.782 | 0.006 |
| EC μS | 1736.67 ± 777.63 | 357.33 ± 63.34 | 3.126 | 0.152 |
| OM % | 8.44 ± 1.17 | 4.99 ± 0.20 | 8.393 | 0.044 |
| Ions (cations and anions) | ||||
| Na⁺ ppm | 70.61 ± 28.87 | 58.42 ± 21.24 | 0.116 | 0.751 |
| K⁺ ppm | 334.65 ± 90.64 | 372.31 ± 79.34 | 0.098 | 0.770 |
| Ca2+ ppm | 0.77 ± 0.15 | 0.27 ± 0.04 | 10.843 | 0.030 |
| Mg2+ ppm | 0.49 ± 0.16 | 0.21 ± 0.03 | 2.770 | 0.171 |
| SO42− ppm | 49.25 ± 0.52 | 31.27 ± 0.61 | 511.249 | 0.001 |
| NO3− ppm | 80.00 ± 11.55 | 73.33 ± 24.04 | 0.063 | 0.815 |
| PO43− ppm | 4.42 ± 2.04 | 2.67 ± 0.73 | 0.651 | 0.465 |
| Ammonia (NH4+) ppm | 133.33 ± 40.55 | 40.00 ± 11.55 | 4.900 | 0.091 |
| Microelements | ||||
| Mn ppm | 229.17 ± 182.30 | 269.58 ± 126.90 | 0.033 | 0.864 |
| Zn mg/L | 55.42 ± 11.59 | 45.00 ± 17.14 | 0.253 | 0.641 |
| Fe ppm | 10,564.58 ± 2597.91 | 16,856.25 ± 9276.55 | 0.427 | 0.549 |
| Cr ppm | 9.96 ± 6.17 | 12.97 ± 7.86 | 0.091 | 0.778 |
| Co ppm | 7.52 ± 2.82 | 9.76 ± 4.41 | 0.183 | 0.691 |
| Cu ppm | 10.21 ± 6.01 | 19.38 ± 9.57 | 0.658 | 0.463 |
| Ni ppm | 35.69 ± 25.95 | 66.25 ± 32.37 | 0.543 | 0.502 |
| Invasive | Native | t-Test | p-Values | |
|---|---|---|---|---|
| Soil properties | ||||
| pH | 6.97 ± 0.22 | 7.64 ± 0.07 | 8.894 | 0.041 |
| EC μS | 7250.00 ± 4145.68 | 324.00 ± 12.22 | 2.791 | 0.170 |
| OM % | 12.22 ± 4.06 | 4.19 ± 0.09 | 3.901 | 0.119 |
| Ions (cations and anions) | ||||
| Na⁺ ppm | 297.22 ± 221.53 | 77.89 ± 31.77 | 0.960 | 0.383 |
| K⁺ ppm | 468.29 ± 41.84 | 536.73 ± 139.35 | 0.221 | 0.663 |
| Ca2+ ppm | 1.52 ± 1.07 | 0.38 ± 0.16 | 1.096 | 0.354 |
| Mg2+ ppm | 1.17 ± 0.44 | 0.48 ± 0.20 | 2.029 | 0.227 |
| SO42− ppm | 56.42 ± 12.45 | 41.07 ± 8.84 | 1.010 | 0.372 |
| NO3− ppm | 173.33 ± 75.13 | 43.33 ± 8.82 | 2.953 | 0.161 |
| PO43− ppm | 2.33 ± 0.30 | 15.20 ± 10.33 | 1.551 | 0.281 |
| Ammonia (NH4+) ppm | 73.33 ± 13.33 | 53.33 ± 24.04 | 0.529 | 0.507 |
| Microelements | ||||
| Mn ppm | 73.33 ± 13.33 | 53.33 ± 24.04 | 0.529 | 0.507 |
| Zn mg/L | 57.71 ± 10.28 | 40.63 ± 15.66 | 0.831 | 0.413 |
| Fe ppm | 23,525.00 ± 9341.35 | 17,068.75 ± 10,154.34 | 0.219 | 0.664 |
| Cr ppm | 9.60 ± 1.21 | 12.24 ± 8.09 | 0.104 | 0.763 |
| Co ppm | 7.52 ± 0.34 | 8.09 ± 4.66 | 0.015 | 0.908 |
| Cu ppm | 12.29 ± 3.61 | 16.25 ± 10.23 | 0.133 | 0.734 |
| Ni ppm | 18.52 ± 2.13 | 45.50 ± 37.03 | 0.529 | 0.507 |
| Invasive | Native | t-Test | p-Values | |
|---|---|---|---|---|
| Soil properties | ||||
| pH | 7.08 ± 0.04 | 7.09 ± 0.03 | 0.084 | 0.786 |
| EC μS | 288.60 ± 68.97 | 191.23 ± 20.50 | 1.831 | 0.247 |
| OM % | 5.68 ± 2.14 | 3.36 ± 0.80 | 1.026 | 0.368 |
| Ions (cations and anions) | ||||
| Na⁺ ppm | 51.36 ± 6.46 | 35.95 ± 6.49 | 2.828 | 0.168 |
| K⁺ ppm | 545.34 ± 98.00 | 491.97 ± 167.64 | 0.076 | 0.797 |
| Ca2+ ppm | 0.27 ± 0.04 | 0.25 ± 0.00 | 0.143 | 0.725 |
| Mg2+ ppm | 0.20 ±0.02 | 0.17 ± 0.05 | 1.690 | 0.263 |
| SO42− ppm | 37.47 ± 0.96 | 31.50 ± 3.56 | 2.616 | 0.181 |
| NO3− ppm | 126.67 ± 86.67 | 46.67 ± 6.67 | 0.847 | 0.409 |
| PO43− ppm | 17.70 ± 9.75 | 1.17 ± 0.92 | 2.848 | 0.167 |
| Ammonia (NH4+) ppm | 86.67 ± 13.33 | 73.33 ± 13.33 | 0.500 | 0.519 |
| Microelements | ||||
| Mn ppm | 312.50 ± 160.20 | 181.25 ± 76.38 | 0.547 | 0.501 |
| Zn mg/L | 81.25 ± 29.05 | 32.50 ± 6.68 | 2.674 | 0.177 |
| Fe ppm | 17,970.83 ± 5340.86 | 9552.08 ± 2189.11 | 2.127 | 0.218 |
| Cr ppm | 14.43 ± 3.84 | 33.13 ± 24.06 | 0.589 | 0.486 |
| Co ppm | 12.40 ± 0.74 | 10.10 ± 2.36 | 0.869 | 0.404 |
| Cu ppm | 18.96 ± 4.71 | 17.71 ± 4.65 | 0.036 | 0.859 |
| Ni ppm | 133.13 ± 17.51 | 52.78 ± 33.61 | 4.493 | 0.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, A.M.; Soliman, W.S.; Alomran, M.M.; Alotaibi, N.M.; Novak, S.J. Four Invasive Plant Species in Southwest Saudi Arabia Have Variable Effects on Soil Dynamics. Plants 2023, 12, 1231. https://doi.org/10.3390/plants12061231
Abbas AM, Soliman WS, Alomran MM, Alotaibi NM, Novak SJ. Four Invasive Plant Species in Southwest Saudi Arabia Have Variable Effects on Soil Dynamics. Plants. 2023; 12(6):1231. https://doi.org/10.3390/plants12061231
Chicago/Turabian StyleAbbas, Ahmed M., Wagdi S. Soliman, Maryam M. Alomran, Nahaa M. Alotaibi, and Stephen J. Novak. 2023. "Four Invasive Plant Species in Southwest Saudi Arabia Have Variable Effects on Soil Dynamics" Plants 12, no. 6: 1231. https://doi.org/10.3390/plants12061231
APA StyleAbbas, A. M., Soliman, W. S., Alomran, M. M., Alotaibi, N. M., & Novak, S. J. (2023). Four Invasive Plant Species in Southwest Saudi Arabia Have Variable Effects on Soil Dynamics. Plants, 12(6), 1231. https://doi.org/10.3390/plants12061231








