Nitrogen Fertilization in a Faba Bean–Wheat Intercropping System Can Alleviate the Autotoxic Effects in Faba Bean
Abstract
:1. Introduction
2. Results
2.1. Effect of the Water Extract of Faba Bean Roots on Faba Bean Seed Germination
2.2. Effect of Water Extract of Faba Bean Stems on Faba Bean Seed Germination
2.3. Effect of Water Extract of Faba Bean Leaves on Seed Germination of Faba Bean
2.4. Effect of the Water Extract of Faba Bean Rhizosphere Soil on Faba Bean Seed Germination
2.5. Types and Content of Phenolic Acids in the Water Extract of Faba Bean Roots, Stems, Leaves, and Rhizosphere Soil
2.6. Effects of Exogenous Phenolic Acids on the Germination of Faba Bean Seeds
2.7. Effects of Nitrogen Application and Intercropping on Phenolic Acid Content in the Roots, Stems, Leaves, and Rhizosphere Soil of Faba Bean
2.8. Effects of Nitrogen Application and Intercropping on the Dry Weight of the Faba Bean
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Preparation of the Water Extract from Faba Bean Plant and Rhizosphere Soil
4.3. Determination of the Biological Activity of Faba Bean Seed Germination
4.3.1. Determination of the Water Extract of Various Parts of the Faba Bean on the Germination of Faba Bean Seeds
4.3.2. Determination of the Effect of Phenolic Acids on the Germination of Faba Bean Seeds
- (1)
- Germination energy (GE)
- (2)
- Germination rate (GR)
- (3)
- Germination index (GI)where Gt is the number of germinated seeds on day t, and Dt is the number of days of germination.
- (4)
- response index (RI)C: CK; T: treatment
4.4. Determination of Phenolic Acid Composition and Content
4.5. Field Test
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Plant-Soil Feedbacks and Soil Sickness: From Mechanisms to Application in Agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- Guo, Y.; Lv, J.; Zhao, Q.; Dong, Y.; Dong, K. Cinnamic Acid Increased the Incidence of Fusarium Wilt by Increasing the Pathogenicity of Fusarium oxysporum and Reducing the Physiological and Biochemical Resistance of Faba Bean, Which Was Alleviated by Intercropping with Wheat. Front. Plant Sci. 2020, 11, 608389. [Google Scholar] [CrossRef] [PubMed]
- Blok, W.J.; Bollen, G.J. The role of autotoxins from root residues of the previous crop in the replant disease of asparagus. Neth. J. Plant Pathol. 1993, 99, 29–40. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, Y.; Zhu, W.; Tang, J.; Hu, S.; Zhou, T.; Chen, X. Baicalin Released from Scutellaria baicalensis Induces Autotoxicity and Promotes Soilborn Pathogens. J. Chem. Ecol. 2010, 36, 329. [Google Scholar] [CrossRef]
- Zhang, Q. Potential role of allelopathy in the soil and the decomposing root of Chinese-fir replant woodland. Plant Soil 1993, 151, 205–210. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Nakamura, K.; Okuda, N. Involvement of an autotoxic compound in asparagus decline. J. Plant Physiol. 2018, 224–225, 49–55. [Google Scholar] [CrossRef]
- Singh, H. Autotoxicity: Concept, Organisms, and Ecological Significance. Crit. Rev. Plant Sci. 1999, 18, 757–772. [Google Scholar] [CrossRef]
- Wu, B.; Long, Q.; Gao, Y.; Wang, Z.; Shao, T.; Liu, Y.; Li, Y.; Ding, W. Comprehensive characterization of a time-course transcriptional response induced by autotoxins in Panax ginseng using RNA-Seq. BMC Genom. 2015, 16, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wang, Y.; Li, B.; Zhang, J. Self allelopathic effect of water extract of Elsholtzia chinensis on its seed germination. North. Hortic. 2016, 366, 62–66. [Google Scholar]
- Wang, L.; Chen, J.; Li, J.; Li, J.; Chen, C.; Ye, C.; Luo, J. Study on Liquefaction Induced Autotoxicity of Water Extract from Oil flax. Chin. J. Oil Crops 2019, 41, 445–454. [Google Scholar]
- Baerson, S.R.; Dayan, F.E.; Rimando, A.M.; Nanayakkara, N.P.D.; Liu, C.-J.; Schroder, J.; Fishbein, M.; Pan, Z.; Kagan, I.A.; Pratt, L.H.; et al. A functional genomics investigation of allelochemical biosynthesis in Sorghum bicolor root hairs. J. Biol. Chem. 2008, 283, 3231–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, X.; Li, Z.H.; Wang, Q.; Pan, C.D.; Jiang, D.A.; Wang, G.G. Autotoxicity and allelopathy of 3,4-dihydroxyacetophenone isolated from Picea schrenkiana needles. Molecules 2011, 16, 8874–8893. [Google Scholar] [CrossRef]
- Min, L.; Xingfu, Y.; Li, M.; Lu, M.; Rong, S. Allelopathic inhibition of phenolic acids on germination of Wolfberry (Lycium barbarum Linn.). Acta Ecol. Sin. 2020, 40, 2072–2079. [Google Scholar]
- Liang, W.; Chen, L.; Duan, W.; Li, Y.; Li, S.; Yu, Y. Effects of phenolic acids on seed germination, seedling growth and physiological characteristics of Pinus koraiensis. J. Ecol. 1989, 8, 1583–1592. [Google Scholar]
- Homulle, Z.; George, T.S.; Karley, A.J. Root traits with team benefits: Understanding belowground interactions in intercropping systems. Plant Soil 2022, 471, 1–26. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wang, Y.P.; Yang, J.S.; Liu, D. A study on the rotation of crops among Panax quinquefolium, Perilla frutescens and Coix lacryma-jobi. Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2005, 30, 12–15. [Google Scholar]
- Wang, L.G.; Ye, C.L.; Chen, J.; Li, J.J.; Luo, J.J. Effects of oil flax/wheat intercropping and oil flax wheat rotation on soil physical and chemical properties and flax growth. China Agric. Sci. Technol. Bull. 2021, 23, 161–171. [Google Scholar] [CrossRef]
- Liu, X.H. Research on Allelopathy and Autotoxicity Abatement Technology of Cotton in Xinjiang. Master’s Thesis, Tarim University, Alar, China, 2022. [Google Scholar] [CrossRef]
- Wang, Y.Y. Study on Autotoxicity and Its Mitigation Measures in Continuous Cropping Obstacles of Millet. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2019. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, J. Research progress on nitrogen uptake and utilization in plants. Chin. Agric. Sci. Bull. 2010, 26, 75–78. [Google Scholar]
- Mur, L.A.; Simpson, C.; Kumari, A.; Gupta, A.K.; Gupta, K.J. Moving nitrogen to the centre of plant defence against pathogens. Ann. Bot. 2017, 119, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Guo, Y.; Li, Y.; Yang, W.; Dong, Y. Intercropping of wheat alleviates the adverse effects of phenolic acids on faba bean. Front. Plant Sci. 2022, 13, 997768. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Han, X.; Wu, J.; Zhang, L.; Wang, J.; Wang-Pruski, G. Specific response mechanism to autotoxicity in melon (Cucumis melo L.) root revealed by physiological analyses combined with transcriptome profiling. Ecotoxicol. Environ. Saf. 2020, 200, 110779. [Google Scholar] [CrossRef]
- Xin, A.; Li, X.; Jin, H.; Yang, X.; Zhao, R.; Liu, J.; Qin, B. The accumulation of reactive oxygen species in root tips caused by autotoxic allelochemicals—A significant factor for replant problem of Angelica sinensis (Oliv.) Diels. Ind. Crops Prod. 2019, 138, 111432. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Liu, Q.; Zhang, Y.; Li, X.; Li, H.; Li, W. Phase changes of continuous cropping obstacles in strawberry (Fragaria× ananassa Duch.) production. Appl. Soil Ecol. 2020, 155, 103626. [Google Scholar] [CrossRef]
- Jiao, X.; Du, J.; Gao, W. Autotoxicity and promoting: Dual effects of root litter on American ginseng growth. Shengtai Xuebao/Acta Ecol. Sin. 2012, 32, 3128–3135. [Google Scholar]
- Yu, J.Q.; Shou, S.Y.; Qian, Y.R.; Zhu, Z.J.; Hu, W.H. Autotoxic potential of cucurbit crops. Plant Soil 2000, 223, 149–153. [Google Scholar] [CrossRef]
- Hassan, M.M.; Daffalla, H.M.; Yagoub, S.O.; Osman, M.G.; Gani, M.E.A.; Babiker, A.G.E. Allelopathic effects of some botanical extracts on germination and seedling growth of Sorghum bicolor L. J. Agric. Technol. 2012, 8, 1423–1469. [Google Scholar]
- Dong, F.H.; Liu, Y.; Jing, M.J.; Li, H.B.; Hu, Y.X. Allelopathic effect of Xanthium spinosum on seeds of Triticum aestivum and Medicago sativa. Arid Zone Res. 2014, 3, 530–535. [Google Scholar]
- Ferreira, P.J.; Zonetti, P.D.C.; Albrecht, A.J.P.; Rosset, I.G.; Silva, A.F.M.; Albrecht, L.P.; Vieira, A.H.; Paulert, R. Conyza sumatrensis allelopathy effect on Bidens pilosa (Asteraceae) seed germination. Bot. Sci. 2020, 98, 348–354. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Han, X.R.; Yang, J.F.; Liang, C.H.; Zhan, X.M. Autotoxicity of peanut and identification of phytotoxic substances in rhizosphere soil. Allelopath. J. 2013, 31, 297. [Google Scholar]
- Li, Q.Q. Studies on Autotoxicity of Adzuki Bean Extracts from Different Organs and Rhizosphere Soil. Master’s Thesis, Northwest University of Agriculture and Forestry Science and Technology, Xianyang, China, 2013. [Google Scholar]
- Bruce Williamson, G.; Richardson, D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181–187. [Google Scholar] [CrossRef]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Reinhardt, C.F.; Bezuidenhout, S.R. Growth stage of Cyperus esculentus influences its allelopathic effect on ectomycorrhizal and higher plant species. J. Crop Prod. 2001, 4, 323–333. [Google Scholar] [CrossRef]
- Hao, W.Y.; Ren, L.X.; Ran, W.; Shen, Q.R. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. Niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Ye, S.F.; Zhou, Y.H.; Sun, Y.; Zou, L.Y.; Yu, J.Q. Cinnamic acid causes oxidative stress in cucumber roots, and promotes incidence of Fusarium wilt. Environ. Exp. Bot. 2006, 56, 255–262. [Google Scholar] [CrossRef]
- Einhellig, F.A. Mechanism of Action of Allelochemicals in Allelopathy; ACS Publications: Washington, DC, USA, 1995. [Google Scholar]
- Turk, M.A.; Tawaha, A.M. Allelopathic effect of black mustard (Brassica nigra L.) on germination and growth of wild oat (Avena fatua L.). Crop Prot. 2003, 22, 673–677. [Google Scholar] [CrossRef]
- Safari, H.; Tavili, A.; Saberi, M. Allelopathic effects of Thymus kotschyanus on seed germination and initial growth of Bromus tomentellus and Trifolium repens. Front. Agric. China 2010, 4, 475–480. [Google Scholar] [CrossRef]
- Dai, C.C.; Chen, Y.; Wang, X.X.; Li, P.D. Effects of intercropping of peanut with the medicinal plant Atractylodes lancea on soil microecology and peanut yield in subtropical China. Agrofor. Syst. 2013, 87, 417–426. [Google Scholar] [CrossRef]
- He, Z.; Mao, R.; Dong, J.E.; Liang, Z.; Zhang, H.; Liu, L. Remediation of deterioration in microbial structure in continuous Pinellia ternata cropping soil by crop rotation. Can. J. Microbiol. 2019, 65, 282–295. [Google Scholar] [CrossRef]
- Hua, C.; Wang, Y.; Xie, Z.; Guo, Z.; Zhang, Y.; Qiu, Y.; Wang, L. Effects of intercropping on rhizosphere soil microorganisms and root exudates of Lanzhou lily (Lilium davidii var. unicolor). Sci. Cold Arid Reg. 2018, 10, 159–168. [Google Scholar]
- Jin, X.; Wang, Z.; Wu, F.; Li, X.; Zhou, X. Litter Mixing Alters Microbial Decomposer Community to Accelerate Tomato Root Litter Decomposition. Microbiol. Spectr. 2022, 10, e00186-22. [Google Scholar] [CrossRef]
- Hartman, K.; van der Heijden, M.G.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 1–14. [Google Scholar]
- Pramanik, M.H.R.; Nagai, M.; Asao, T.; Matsui, Y. Effects of temperature and photoperiod on phytotoxic root exudates of cucumber (Cucumis sativus) in hydroponic culture. J. Chem. Ecol. 2000, 26, 1953–1967. [Google Scholar] [CrossRef]
- Kumar, S.; Jakhar, S.R.; Dahiya, S.; Jangir, C.K.; Meena, R.S. Soil sickness and productivity from ecological aspects. J. Pharmacogn. Phytochem. 2017, 6, 827–831. [Google Scholar]
- Ju, X.; Gu, B. Current situation, problems and trends of nitrogen fertilizer application in farmland in China. J. Plant Nutr. Fertil 2014, 20, 783–795. [Google Scholar]
- Holzapfel, C.; Shahrokh, P.; Kafkewitz, D. Polyphenol oxidase activity in the roots of seedlings of Bromus (Poaceae) and other grass genera. Am. J. Bot. 2010, 97, 1195–1199. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.C.; Zhou, Y.; Chen, P.; Zhang, X.N.; Du, Q.; Yang, H.; Wang, X.N.; Yang, F.; Xiao, T.; Li, L.; et al. Maize—Legume intercropping promote N uptake through changing the root spatial distribution, legume nodulation capacity, and soil N availability. J. Integr. Agric. 2022, 21, 1755–1771. [Google Scholar]

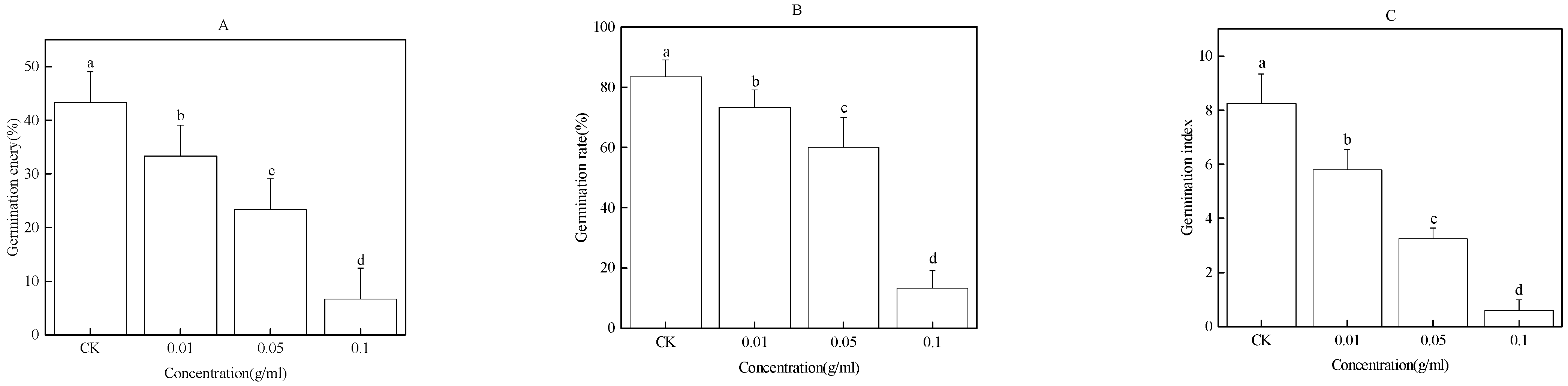
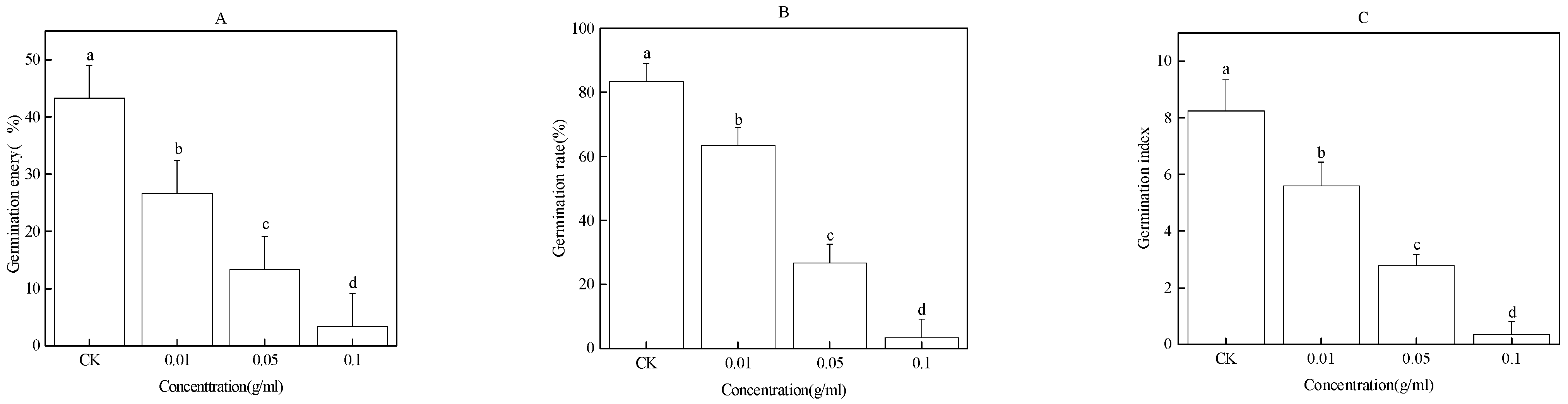
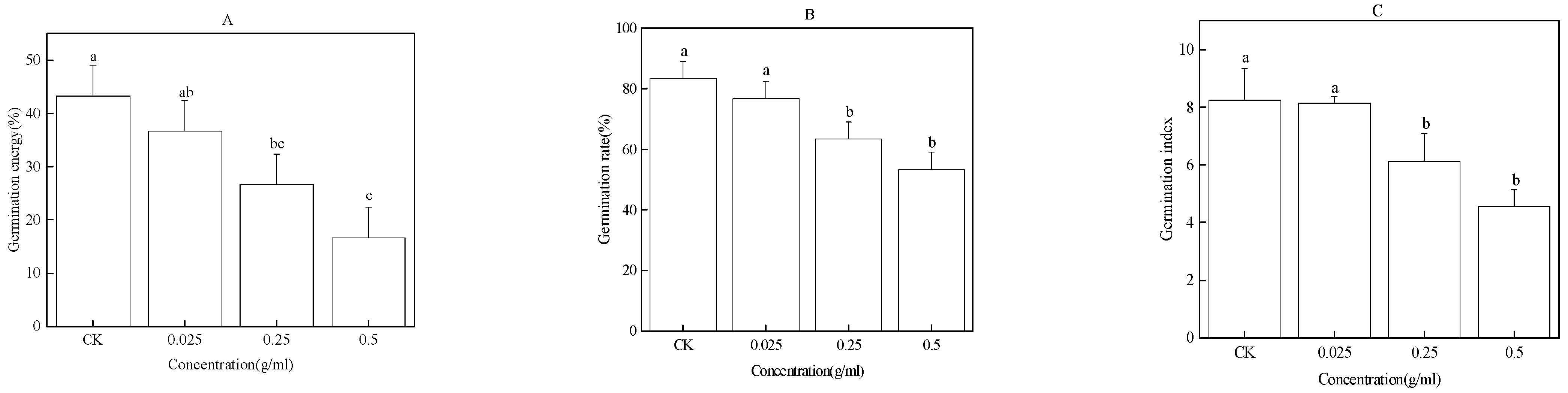
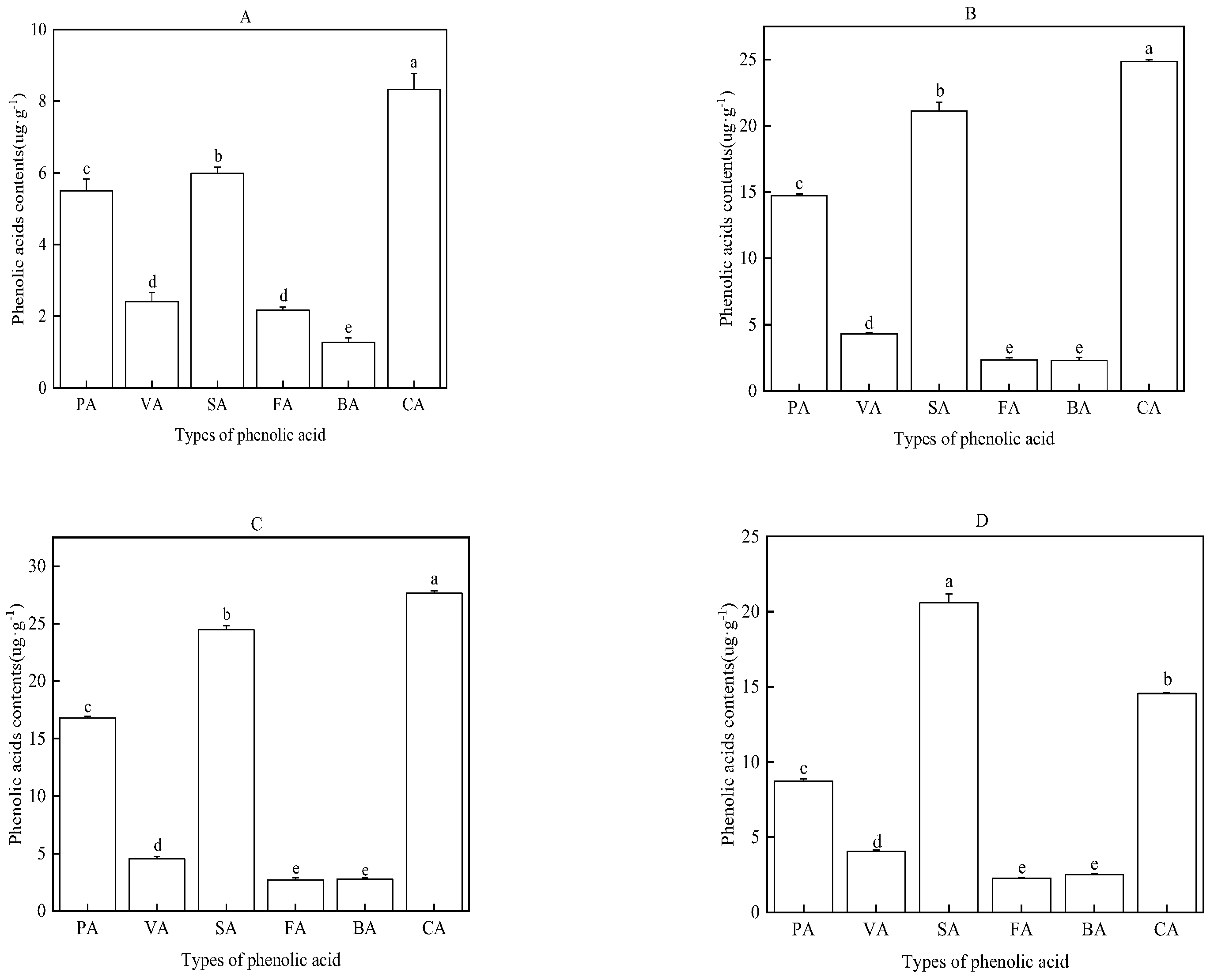
| Types of Phenolic Acids | Concentration (mg·L−1) | Germination Energy (%) | RI | Germination Rate (%) | RI | Germination Index (%) | RI |
|---|---|---|---|---|---|---|---|
| CK | 46.00 ± 5.48 a | − | 96.00 ± 5.48 a | − | 7.32 ± 0.67 a | − | |
| PA | 50 | 36.00 ± 5.48 b | −0.22 | 92.00 ± 8.37 a | −0.04 | 6.48 ± 0.67 b | −0.11 |
| 100 | 28.00 ± 4.47 c | −0.39 | 82.00 ± 8.37 b | −0.15 | 6.14 ± 0.24 bc | −0.16 | |
| 200 | 22.00 ± 4.47 cd | −0.52 | 74.00 ± 5.48 bc | −0.23 | 5.65 ± 0.42 c | −0.23 | |
| 400 | 16.00 ± 5.48 d | −0.65 | 66.00 ± 5.48 cd | −0.31 | 4.98 ± 0.56 d | −0.32 | |
| 800 | 4.00 ± 5.48 e | −0.91 | 62.00 ± 4.47 d | −0.35 | 3.80 ± 0.15 e | −0.48 | |
| CK | 46.00 ± 5.48 a | − | 96.00 ± 5.48 a | − | 7.32 ± 0.67 a | − | |
| FA | 50 | 42.00 ± 4.47 ab | −0.09 | 90.00 ± 7.07 a | −0.06 | 6.74 ± 0.54 ab | −0.08 |
| 100 | 36.00 ± 5.48 b | −0.22 | 76.00 ± 5.48 b | −0.21 | 6.41 ± 0.64 b | −0.13 | |
| 200 | 26.00 ± 5.48 c | −0.43 | 68.00 ± 4.47 c | −0.29 | 5.37 ± 0.15 c | −0.27 | |
| 400 | 8.00 ± 4.47 d | −0.83 | 64.00 ± 5.48 c | −0.33 | 3.69 ± 0.22 d | −0.50 | |
| 800 | 4.00 ± 5.48 d | −0.91 | 56.00 ± 5.48 d | −0.42 | 3.25 ± 0.62 d | −0.56 | |
| CK | 46.00 ± 5.48 a | − | 96.00 ± 5.48 a | − | 7.32 ± 0.67 a | − | |
| BA | 50 | 34.00 ± 5.48 b | −0.26 | 86.00 ± 5.48 b | −0.10 | 6.25 ± 0.62 b | −0.15 |
| 100 | 22.00 ± 4.47 c | −0.52 | 78.00 ± 8.37 c | −0.19 | 5.49 ± 0.36 c | −0.25 | |
| 200 | 12.00 ± 4.47 d | −0.74 | 70.00 ± 7.07 d | −0.27 | 4.13 ± 0.22 d | −0.44 | |
| 400 | 6.00 ± 5.48 de | −0.87 | 62.00 ± 4.47 e | −0.35 | 3.29 ± 0.36 e | −0.55 | |
| 800 | 2.00 ± 4.47 e | −0.96 | 22.00 ± 4.47 f | −0.77 | 1.35 ± 0.36 f | −0.82 | |
| CK | 46.00 ± 5.48 a | − | 96.00 ± 5.48 a | − | 7.32 ± 0.67 a | − | |
| CA | 50 | 34.00 ± 5.48 b | −0.26 | 92.00 ± 4.47 ab | −0.04 | 6.34 ± 0.66 b | −0.13 |
| 100 | 22.00 ± 4.47 c | −0.52 | 86.00 ± 8.94 b | −0.10 | 6.20 ± 0.51 b | −0.15 | |
| 200 | 16.00 ± 5.48 cd | −0.65 | 74.00 ± 5.48 c | −0.23 | 4.74 ± 0.50 c | −0.35 | |
| 400 | 10.00 ± 0.00 de | −0.78 | 22.00 ± 4.47 d | −0.77 | 1.60 ± 0.56 d | −0.78 | |
| 800 | 6.00 ± 5.48 e | −0.87 | 6.00 ± 5.48 e | −0.94 | 0.66 ± 0.60e | −0.91 | |
| CK | 46.00 ± 5.48 a | − | 96.00 ± 5.48 a | − | 7.32 ± 0.67 a | − | |
| VA | 50 | 36.00 ± 5.48 b | −0.22 | 94.00 ± 5.48 a | −0.02 | 6.75 ± 0.92 a | −0.08 |
| 100 | 32.00 ± 4.47 b | −0.30 | 92.00 ± 4.47 a | −0.04 | 6.60 ± 0.56 a | −0.10 | |
| 200 | 24.00 ± 5.48 c | −0.48 | 80.00 ± 7.07 b | −0.17 | 5.75 ± 0.47 b | −0.21 | |
| 400 | 12.00 ± 4.47 d | −0.74 | 64.00 ± 5.48 c | −0.33 | 3.99 ± 0.38 c | −0.46 | |
| 800 | 10.00 ± 0.00 d | −0.78 | 50.00 ± 0.00 d | −0.48 | 2.72 ± 0.49 d | −0.63 | |
| CK | 46.00 ± 5.48 a | − | 96.00 ± 5.48 a | − | 7.32 ± 0.67 a | − | |
| SA | 50 | 32.00 ± 4.47 b | −0.30 | 82.00 ± 4.47 b | −0.15 | 6.21 ± 0.46 b | −0.15 |
| 100 | 24.00 ± 5.48 c | −0.48 | 62.00 ± 4.47 c | −0.35 | 5.26 ± 0.56 c | −0.28 | |
| 200 | 16.00 ± 5.48 d | −0.65 | 32.00 ± 4.47 d | −0.67 | 2.82 ± 0.39 d | −0.61 | |
| 400 | 8.00 ± 4.47 e | −0.83 | 14.00 ± 5.48 e | −0.85 | 1.14 ± 0.60 e | −0.85 | |
| 800 | 2.00 ± 4.47 e | −0.96 | 10.00 ± 0.00 e | −0.90 | 0.56 ± 0.38 e | −0.92 |
| Site of Measurement | N Level (N) | Planting Pattern (P) | Content of Phenolic Acid (μg/g) | |||||
|---|---|---|---|---|---|---|---|---|
| PA | VA | FA | BA | SA | CA | |||
| Roots | N0 | M | 9.48 ± 0.30 a | 5.42 ± 0.18 a | 3.86 ± 0.12 a | 5.48 ± 0.36 a | 9.01 ± 0.29 a | 11.31 ± 0.67 a |
| I | 8.37 ± 0.11 A* | 4.42 ± 0.35 A* | 2.39 ± 0.17 A* | 4.56 ± 0.18 A* | 8.13 ± 0.09 A* | 10.08 ± 0.35 A* | ||
| N1 | M | 9.19 ± 0.16 a | 5.16 ± 0.12 a | 2.54 ± 0.09 c | 5.01 ± 0.37 a | 8.52 ± 0.34 a | 9.58 ± 0.36 b | |
| I | 7.56 ± 0.15 B* | 3.39 ± 0.19 B* | 1.75 ± 0.13 B* | 3.66 ± 0.21 B* | 7.28 ± 0.06 B* | 8.42 ± 0.16 B* | ||
| N2 | M | 6.83 ± 0.33 c | 3.77 ± 0.07 c | 1.59 ± 0.26 d | 2.68 ± 0.16 c | 6.16 ± 0.18 c | 8.27 ± 0.38 c | |
| I | 4.68 ± 0.31 C* | 2.33 ± 0.13 C* | 1.21 ± 0.09 C* | 1.66 ± 0.23 D* | 3.58 ± 0.13 D* | 5.38 ± 0.26 D* | ||
| N3 | M | 8.75 ± 0.11 b | 4.66 ± 0.17 b | 2.85 ± 0.09 b | 3.64 ± 0.21 b | 7.42 ± 0.39 b | 9.65 ± 0.40 b | |
| I | 7.29 ± 0.20 B* | 3.54 ± 0.27 B* | 1.80 ± 0.15 B* | 2.31 ± 0.26 C* | 6.38 ± 0.44 C* | 7.78 ± 0.21 C* | ||
| N level (N) | ** | ** | ** | ** | ** | ** | ||
| Planting pattern (P) | ** | ** | ** | ** | ** | ** | ||
| N × P | ** | * | ** | ns | ** | * | ||
| Site of Measurement | N Level (N) | Planting Pattern (P) | Content of Phenolic Acid (μg/g) | |||||
|---|---|---|---|---|---|---|---|---|
| PA | VA | FA | BA | SA | CA | |||
| Stems | N0 | M | 19.50 ± 0.28 a | 6.02 ± 0.18 a | 4.50 ± 0.26 a | 4.74 ± 0.18 a | 24.70 ± 0.14 a | 28.48 ± 0.32 a |
| I | 16.32 ± 0.06 A* | 4.55 ± 0.19 A* | 3.94 ± 0.18 A* | 3.88 ± 0.29 A* | 20.37 ± 0.45 A* | 23.29 ± 0.26 A* | ||
| N1 | M | 16.53 ± 0.25 c | 5.57 ± 0.34 b | 3.42 ± 0.14 b | 3.45 ± 0.24 b | 22.63 ± 0.20 b | 26.02 ± 0.82 b | |
| I | 13.45 ± 0.29 C* | 3.47 ± 0.19 B* | 2.61 ± 0.25 B* | 2.55 ± 0.11 B* | 17.60 ± 0.09 B* | 19.29 ± 0.35 B* | ||
| N2 | M | 14.71 ± 0.16 d | 4.30 ± 0.11 d | 2.35 ± 0.16 c | 2.32 ± 0.23 c | 21.11 ± 0.68 c | 24.83 ± 0.15 c | |
| I | 8.70 ± 0.19 D* | 2.33 ± 0.13 C* | 1.54 ± 0.12 C* | 1.49 ± 0.27 D* | 16.47 ± 0.25 C* | 13.92 ± 0.33 D* | ||
| N3 | M | 18.12 ± 0.19 b | 4.80 ± 0.16 c | 3.65 ± 0.19 b | 3.51 ± 0.28 b | 22.99 ± 0.24 b | 25.31 ± 0.57 bc | |
| I | 15.60 ± 0.24 B* | 3.31 ± 0.17 B* | 2.38 ± 0.16 B* | 2.07 ± 0.19 C* | 17.65 ± 0.11 B* | 18.70 ± 0.16 C* | ||
| N level (N) | ** | ** | ** | ** | ** | ** | ||
| Planting pattern (P) | ** | ** | ** | ** | ** | ** | ||
| N × P | ** | * | * | ns | ns | ** | ||
| Site of Measurement | N Level | Planting Pattern | Content of Phenolic Acid (μg/g) | |||||
|---|---|---|---|---|---|---|---|---|
| PA | VA | FA | BA | SA | CA | |||
| Leaves | N0 | M | 20.14 ± 0.53 a | 8.51 ± 0.26 a | 5.79 ± 0.20 a | 5.28 ± 0.13 a | 27.93 ± 0.33 a | 32.69 ± 0.20 a |
| I | 14.25 ± 0.16 A* | 7.26 ± 0.11 A* | 4.37 ± 0.26 A* | 4.24 ± 0.10 A* | 23.35 ± 0.34 A* | 27.60 ± 0.43 A* | ||
| N1 | M | 18.27 ± 0.23 b | 7.22 ± 0.09 b | 3.63 ± 0.32 b | 3.43 ± 0.15 b | 25.94 ± 0.35 b | 28.74 ± 0.3 c | |
| I | 11.86 ± 0.31 B* | 5.48 ± 0.10 B* | 2.62 ± 0.14 B* | 2.79 ± 0.20 B* | 20.64 ± 0.22 B* | 22.06 ± 0.35 C* | ||
| N2 | M | 16.81 ± 0.15 d | 4.55 ± 0.18 d | 2.72 ± 0.19 c | 2.78 ± 0.11 d | 24.48 ± 0.34 c | 27.66 ± 0.21 d | |
| I | 9.65 ± 0.36 C* | 2.65 ± 0.31 D* | 1.21 ± 0.09 C* | 1.42 ± 0.07 D* | 17.28 ± 0.24 D* | 20.28 ± 0.85 D* | ||
| N3 | M | 17.45 ± 0.23 c | 6.50 ± 0.12 c | 3.47 ± 0.13 b | 3.5 ± 0.11 c | 25.74 ± 0.15 b | 30.29 ± 0.22 b | |
| I | 12.35 ± 0.44 B* | 4.43 ± 0.24 C* | 2.72 ± 0.20 B* | 2.29 ± 0.16 C* | 18.58 ± 0.50 C* | 20.28 ± 0.85 B* | ||
| N level (N) | ** | ** | ** | ** | ** | ** | ||
| Planting pattern (P) | ** | ** | ** | ** | ** | ** | ||
| N × P | ** | ** | * | ** | ** | ** | ||
| Site of Measurement | N Level | Planting Pattern (P) | Content of Phenolic Acid (μg/g) | |||||
|---|---|---|---|---|---|---|---|---|
| PA | VA | FA | BA | SA | CA | |||
| Rhizosphere soil | N0 | M | 11.38 ± 0.17 a | 5.85 ± 0.25 a | 4.55 ± 0.12 a | 4.74 ± 0.11 a | 26.48 ± 0.36 a | 21.40 ± 0.18 a |
| I | 9.35 ± 0.15 A* | 4.26 ± 0.08 A* | 3.82 ± 0.14 A* | 3.71 ± 0.21 A* | 23.30 ± 0.27 A* | 17.61 ± 0.25 A* | ||
| N1 | M | 9.76 ± 0.08 c | 5.37 ± 0.11 b | 3.51 ± 0.22 b | 3.90 ± 0.23 b | 23.79 ± 0.44 c | 16.73 ± 0.24 c | |
| I | 6.70 ± 0.20 B* | 4.03 ± 0.37 A* | 2.23 ± 0.12 B* | 2.64 ± 0.23 B* | 20.52 ± 0.25 C* | 13.92 ± 0.21 C* | ||
| N2 | M | 8.73 ± 0.14 d | 4.06 ± 0.08 d | 2.27 ± 0.06 c | 2.49 ± 0.08 c | 20.58 ± 0.60 d | 14.56 ± 0.07 d | |
| I | 5.51 ± 0.34 C* | 2.46 ± 0.18 C* | 1.25 ± 0.12 C* | 1.67 ± 0.10 C* | 15.58 ± 0.16 D* | 10.97 ± 0.24 D* | ||
| N3 | M | 10.37 ± 0.09 b | 4.83 ± 0.10 c | 3.46 ± 0.13 b | 3.87 ± 0.11 b | 24.88 ± 0.17 b | 17.64 ± 0.20 b | |
| I | 9.09 ± 0.16 B* | 3.09 ± 0.30 B* | 2.47 ± 0.26 B* | 2.52 ± 0.19 B* | 22.25 ± 0.29 B* | 15.98 ± 0.32 B* | ||
| N level (N) | ** | ** | ** | ** | ** | ** | ||
| Planting pattern (P) | ** | ** | ** | ** | ** | ** | ||
| N × P | ** | ns | ns | ns | ** | ** | ||
| N Level (N) | Planting Pattern (P) | Dry Weight of above Ground Part of the Faba Bean (g) | ||
|---|---|---|---|---|
| Branching Stage | Flowering Stage | Maturity Stage | ||
| N0 | M | 9.72 ± 0.17 b | 11.82 ± 0.85 c | 12.65 ± 0.89 d |
| I | 9.81 ± 0.38 C | 18.65 ± 0.88 C* | 19.57 ± 1.54 C* | |
| N1 | M | 10.29 ± 0.20 b | 18.63 ± 1.04 b | 19.60 ± 0.90 c |
| I | 10.83 ± 0.42 B | 25.69 ± 1.00 B* | 27.92 ± 1.49 B* | |
| N2 | M | 13.16 ± 0.65 a | 23.05 ± 1.34 a | 24.85 ± 1.12 a |
| I | 14.12 ± 0.57 A* | 27.78 ± 1.01 A* | 31.46 ± 0.81 A* | |
| N3 | M | 12.89 ± 0.82 a | 21.85 ± 1.24 a | 22.78 ± 1.61 b |
| I | 13.47 ± 0.65 A | 24.15 ± 1.77 B* | 27.10 ± 0.63 B* | |
| N level (N) | ** | ** | ** | |
| Planting pattern (P) | * | ** | ** | |
| N × P | ns | * | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cen, Z.; Zheng, Y.; Guo, Y.; Yang, S.; Dong, Y. Nitrogen Fertilization in a Faba Bean–Wheat Intercropping System Can Alleviate the Autotoxic Effects in Faba Bean. Plants 2023, 12, 1232. https://doi.org/10.3390/plants12061232
Cen Z, Zheng Y, Guo Y, Yang S, Dong Y. Nitrogen Fertilization in a Faba Bean–Wheat Intercropping System Can Alleviate the Autotoxic Effects in Faba Bean. Plants. 2023; 12(6):1232. https://doi.org/10.3390/plants12061232
Chicago/Turabian StyleCen, Zixuan, Yiran Zheng, Yuting Guo, Siyin Yang, and Yan Dong. 2023. "Nitrogen Fertilization in a Faba Bean–Wheat Intercropping System Can Alleviate the Autotoxic Effects in Faba Bean" Plants 12, no. 6: 1232. https://doi.org/10.3390/plants12061232




