A Comprehensive Review on Cannabis sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities
Abstract
:1. Introduction
2. Generalities about Cannabis sativa L.
2.1. Plant Nomenclature and Synonyms
2.2. Description and Botanical Aspect
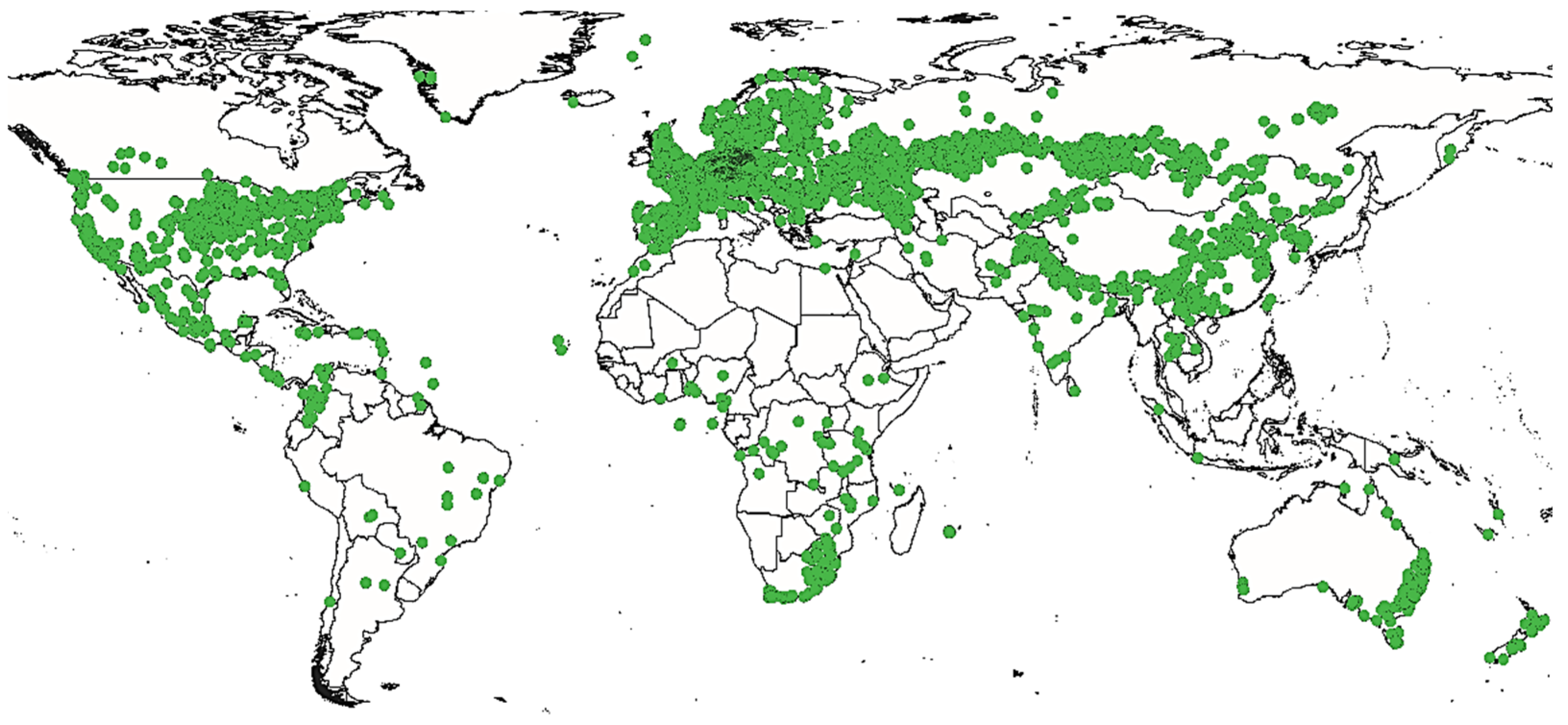
2.3. Geographic Distribution and History
3. Methodology
4. Results and Discussion
4.1. Traditional Uses of Cannabis sativa L.
4.2. Chemical Composition of Cannabis sativa L.
4.3. Molecular Docking Studies of Cannabis sativa L.
4.3.1. Pesticidal Activity
4.3.2. Antimalarial and Anti-Leishmania Activities
4.3.3. Antiviral Activity
4.3.4. Anti-Inflammatory Activity
4.3.5. Anticancer Activity
4.3.6. Antiepileptic Activity
4.3.7. Neuroprotective Activity
4.3.8. Dermocosmetic Activities
4.4. Biological Activities of Cannabis sativa L.
4.4.1. Antioxidant Activity
4.4.2. Antimicrobial Activity
4.4.3. Insecticidal Activity
4.4.4. Anticoagulant Activity
4.4.5. Antidiabetic Activity
4.4.6. Anticancer Activity
4.4.7. Anti-Inflammatory and Analgesic Activities
4.4.8. Neuroprotective Activity
4.4.9. Antiepileptic and Anticonvulsant Activities
4.4.10. Dermocosmetic Activity
4.5. Drugs Based on Cannabis sativa L.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McPartland, J.M.; Hegman, W.; Long, T. Cannabis in Asia: Its center of origin and early cultivation, based on a synthesis of subfossil pollen and archaeobotanical studies. Veg. Hist. Archaeobot. 2019, 28, 691–702. [Google Scholar] [CrossRef]
- Anderson, L.C. Leaf variation among Cannabis species from a controlled garden. Bot. Mus. Leafl. Harv. Univ. 1980, 28, 61–69. [Google Scholar] [CrossRef]
- McPartland, J.M. Cannabis systematics at the levels of family, genus, and species. Cannabis Cannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef] [Green Version]
- McPartland, J.M.; Small, E. A classification of endangered high-THC cannabis (Cannabis sativa subsp. indica) domesticates and their wild relatives. PhytoKeys 2020, 144, 81. [Google Scholar] [CrossRef] [Green Version]
- Clarke, R.; Merlin, M. Cannabis: Evolution and Ethnobotany; University of California Press: Berkeley, CA, USA, 2016. [Google Scholar]
- Gedik, G.; Avinc, O. Hemp fiber as a sustainable raw material source for textile industry: Can we use its potential for more eco-friendly production? In Sustainability in the Textile and Apparel Industries; Springer: Berlin/Heidelberg, Germany, 2020; pp. 87–109. [Google Scholar]
- Gomez, F.P.; Hu, J.; Clarke, M.A. Cannabis as a Feedstock for the Production of Chemicals, Fuels, and Materials: A Review of Relevant Studies To Date. Energy Fuels 2021, 35, 5538–5557. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef]
- Fleming, M.P.; Clarke, R.C. Physical evidence for the antiquity of Cannabis sativa L. J. Int. Hemp Assoc. 1998, 5, 80–95. [Google Scholar]
- Lowe, H.; Steele, B.; Bryant, J.; Toyang, N.; Ngwa, W. Non-cannabinoid metabolites of Cannabis sativa L. with therapeutic potential. Plants 2021, 10, 400. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Giupponi, L.; Leoni, V.; Carrer, M.; Ceciliani, G.; Sala, S.; Panseri, S.; Pavlovic, R.; Giorgi, A. Overview on Italian hemp production chain, related productive and commercial activities and legislative framework. Ital. J. Agron. 2020, 15, 194–205. [Google Scholar] [CrossRef]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Shoyama, Y.; Morimoto, S. Phytocannabinoids in Cannabis sativa: Recent studies on biosynthetic enzymes. Chem. Biodivers. 2007, 4, 1649–1663. [Google Scholar] [CrossRef]
- Brenneisen, R. Chemistry and analysis of phytocannabinoids and other Cannabis constituents. In Marijuana and the Cannabinoids; Humana Press: Clifton, NJ, USA, 2007; pp. 17–49. [Google Scholar]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S. Cannabinoids for medical use: A systematic review and meta-analysis. Jama 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [Green Version]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, L.B.; Salama, A.K. A review of cancer immunotherapy toxicity. CA A Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [Green Version]
- Pollio, A. The name of Cannabis: A short guide for nonbotanists. Cannabis Cannabinoid Res. 2016, 1, 234–238. [Google Scholar] [CrossRef] [Green Version]
- Bruneau, D. Le Cannabis sativa: Une plante psychotrope ayant des intérêtsthérapeutiques. Ph.D. Thesis, University of Rennes, Rennes, France, 2016. [Google Scholar]
- Russo, E.B. History of cannabis and its preparations in saga, science, and sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Wujastyk, D. Cannabis in Traditional Indian Herbal Medicine; Ayurveda at the Crossroads of Care and Cure, Centro de Historia del Alêmm-Mar, Universidade Nova de Lisboa: Lisbon, Portugal, 2002; pp. 45–73. [Google Scholar]
- Hazekamp, A. Cannabis Review; Department of Plant Metobolomics, Leiden University: Leiden, The Netherlands, 2008; Volume 2009. [Google Scholar]
- Farag, S.; Kayser, O. The cannabis plant: Botanical aspects. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–12. [Google Scholar]
- Schultes, R.E.; Klein, W.M.; Plowman, T.; Lockwood, T.E. Cannabis: An example of taxonomic neglect. In Cannabis and Culture; Rubin, V., Ed.; De Gruyter Mouton: Berlin, NY, USA, 1975; pp. 21–38. [Google Scholar]
- Pacifico, D.; Miselli, F.; Carboni, A.; Moschella, A.; Mandolino, G. Time course of cannabinoid accumulation and chemotype development during the growth of Cannabis sativa L. Euphytica 2008, 160, 231–240. [Google Scholar] [CrossRef]
- Small, E.; Cronquist, A. A practical and natural taxonomy for Cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]
- Clarke, R.C.; Merlin, M.D.; Small, E. Evolution and classification of Cannabis sativa (Marijuana, Hemp) in relation to human utilization. Bot. Rev. 2015, 81, 189–294. [Google Scholar]
- Amaducci, S.; Zatta, A.; Raffanini, M.; Venturi, G. Characterisation of hemp (Cannabis sativa L.) roots under different growing conditions. Plant Soil 2008, 313, 227–235. [Google Scholar] [CrossRef]
- Amaducci, S.; Zatta, A.; Pelatti, F.; Venturi, G. Influence of agronomic factors on yield and quality of hemp (Cannabis sativa L.) fibre and implication for an innovative production system. Field Crops Res. 2008, 107, 161–169. [Google Scholar] [CrossRef]
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Plant density and nitrogen fertilization affect agronomic performance of industrial hemp (Cannabis sativa L.) in Mediterranean environment. Ind. Crops Prod. 2017, 100, 246–254. [Google Scholar] [CrossRef]
- Bouloc, P. Le Chanvre Industriel: Production et Utilisations; France Agricole Editions: Paris, France, 2006. [Google Scholar]
- Anwar, F.; Latif, S.; Ashraf, M. Analytical characterization of hemp (Cannabis sativa) seed oil from different agro-ecological zones of Pakistan. J. Am. Oil Chem. Soc. 2006, 83, 323–329. [Google Scholar] [CrossRef]
- Bouloc, P. Hemp: Industrial Production and Uses; CABI: Wallingford, UK, 2013. [Google Scholar]
- Schilling, S.; Melzer, R.; McCabe, P.F. Cannabis sativa. Curr. Biol. 2020, 30, R8–R9. [Google Scholar] [CrossRef]
- Lynch, R.C.; Vergara, D.; Tittes, S.; White, K.; Schwartz, C.; Gibbs, M.J.; Ruthenburg, T.C.; DeCesare, K.; Land, D.P.; Kane, N.C. Genomic and chemical diversity in Cannabis. Crit. Rev. Plant Sci. 2016, 35, 349–363. [Google Scholar] [CrossRef] [Green Version]
- Newton, D.E. Marijuana: A Reference Handbook; Abc-Clio: Santa Barbara, CA, USA, 2013. [Google Scholar]
- Botineau, M. Botanique Systématique et Appliquée des Plantes à Fleurs; Tec & doc: Paris, France, 2010. [Google Scholar]
- Richard, D.; Senon, J.-L. Le Cannabis; Presses universitaires de France: Paris, France, 2010. [Google Scholar]
- Strzelczyk, M.; Lochynska, M.; Chudy, M. Systematics and botanical characteristics of industrial hemp Cannabis sativa L. J. Nat. Fibers 2021, 19, 5804–5826. [Google Scholar] [CrossRef]
- Radosevich, S.R.; Holt, J.S.; Ghersa, C. Weed Ecology: Implications for Management; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Citterio, S.; Santagostino, A.; Fumagalli, P.; Prato, N.; Ranalli, P.; Sgorbati, S. Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil 2003, 256, 243–252. [Google Scholar] [CrossRef]
- Magnusson, K.; Svennerstedt, B. Influence of temperature on the water retting process of hemp (Cannabis sativa L.) cultivated under Swedish climate conditions. J. Ind. Hemp 2007, 12, 3–17. [Google Scholar] [CrossRef]
- Matthieu, M.L. Les Cannabinoïdes Dans La Prise en Charge des Patients Sous Anticancereux et Antiretroviraux: Connaissances Actuelles et Perspectives D’avenir en France; Faculty of Pharmacy, University of Lille: Lille, France, 2015. [Google Scholar]
- Li, H.-L. An archaeological and historical account of cannabis in China. Econ. Bot. 1974, 28, 437–448. [Google Scholar] [CrossRef]
- Hill, B. Legalized Marijuana: Canada Comes Round to the Wisdom of Ages, Ancient-Origins. Available online: https://www.ancient-origins.net/history/cannabis-journey-through-ages-003084 (accessed on 6 March 2023).
- Abel, E.L. Marihuana: The First Twelve Thousand Years; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A review of natural fibers used in biocomposites: Plant, animal and regenerated cellulose fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Turner, C.E.; Elsohly, M.A.; Boeren, E.G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980, 43, 169–234. [Google Scholar] [CrossRef]
- Veiga, P. Oncology and Infectious Diseases in Ancient Egypt: The Ebers Papyrus’ Treatise on Tumours 857–877 and the Cases Found in Ancient Egyptian Human Material; University of Manchester: Manchester, UK, 2009. [Google Scholar]
- Dawson, W.R. Studies in the Egyptian Medical Texts—III. J. Egypt. Archaeol. 1934, 20, 41–46. [Google Scholar] [CrossRef]
- Faulkner, R.O. The Ancient Egyptian Pyramid Texts; Aris & Phillips: Wiltshire, England, 1969. [Google Scholar]
- Ferrara, M.S. Peak-experience and the entheogenic use of cannabis in world religions. J. Psychedelic Stud. 2021, 4, 179–191. [Google Scholar] [CrossRef]
- Gately, I. Tobacco: A Cultural History of How an Exotic Plant Seduced Civilization; Open Road and Grove Atlantic: New York, NY, USA, 2007. [Google Scholar]
- Holmes, W.H. Prehistoric Textile Art of Eastern United States; Independently published: Washington, DC, USA, 1896; Volume 13, p. 44. [Google Scholar]
- Deitch, R. Hemp: American History Revisited: The Plant with a Divided History; Algora Publishing: New York, NY, USA, 2003. [Google Scholar]
- Chouvy, P.-A. Cannabis cultivation in the world: Heritages, trends and challenges. EchoGéo 2019, 48, 21. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.-H.; Hu, H.-R.; Du, G.-H.; Deng, G.; Yang, Y. Ethnobotanical research on origin, cultivation, distribution and utilization of hemp (Cannabis sativa L.) in China. Indian J. Tradit. Knowl. 2017, 16, 235–242. [Google Scholar]
- Singh, R.; Upadhyay, S.K.; Rani, A.; Kumar, P.; Sharma, P.; Sharma, I.; Singh, C.; Chauhan, N.; Kumar, M. Ethnobotanical study of weed flora at district Ambala, Haryana, India: Comprehensive medicinal and pharmacological aspects of plant resources. Int. J. Pharm. Res. 2020, 12, 1941–1956. [Google Scholar]
- El Khomsi, M.; Dandani, Y.; Chaachouay, N.; Hmouni, D. Ethnobotanical study of plants used for medicinal, cosmetic, and food purposes in the region of Moulay Yacoub, Northeast of Morocco. J. Pharm. Pharmacogn. Res. 2022, 10, 13–29. [Google Scholar] [CrossRef]
- Klauke, A.L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Rahmatullah, M.; Mollik, M.A.H.; Azam, A.; Islam, M.R.; Chowdhury, M.A.M.; Jahan, R.; Chowdhury, M.H.; Rahman, T. Ethnobotanical survey of the Santal tribe residing in Thakurgaon District, Bangladesh. Am. Eurasian J. Sustain. Agric. 2009, 3, 889–898. [Google Scholar]
- Mawla, F.; Khatoon, S.; Rehana, F.; Jahan, S.; Shelley, M.M.R.; Hossain, S.; Haq, W.M.; Rahman, S.; Debnath, K.; Rahmatullah, M. Ethnomedicinal plants of folk medicinal practitioners in four villages of Natore and Rajshahi districts, Bangladesh. Am. Eur. J. Sustain. Agric. 2012, 6, 406–416. [Google Scholar]
- Ahmed, M.; Azam, K.; Nur, M. Traditional knowledge and formulations of medicinal plants used by the traditional medical practitioners of Bangladesh to treat schizophrenia like psychosis. Schizophr. Res. Treat. 2014, 2014, 679810. [Google Scholar] [CrossRef] [Green Version]
- Rahmatullah, M.; Mollik, M.A.H.; Khatun, M.A.; Jahan, R.; Chowdhury, A.R.; Seraj, S.; Hossain, M.S.; Nasrin, D.; Khatun, Z. A survey on the use of medicinal plants by folk medicinal practitioners in five villages of Boalia sub-district, Rajshahi district, Bangladesh. Adv. Nat. Appl. Sci. 2010, 4, 39–44. [Google Scholar]
- Benkhnigue, O.; Zidane, L.; Fadli, M.; Elyacoubi, H.; Rochdi, A.; Douira, A. Etude ethnobotanique des plantes médicinales dans la région de Mechraâ Bel Ksiri (Région du Gharb du Maroc). Acta Botánica Barcinonensia 2010, 53, 191–216. [Google Scholar]
- Kona, S.; Rahman, A. Inventory of medicinal plants at Mahadebpur upazila of Naogaon district, Bangladesh. Appl. Ecol. Environ. Sci. 2016, 4, 75–83. [Google Scholar]
- Rahmatullah, M.; Azam, M.N.K.; Rahman, M.M.; Seraj, S.; Mahal, M.J.; Mou, S.M.; Nasrin, D.; Khatun, Z.; Islam, F.; Chowdhury, M.H. A survey of medicinal plants used by Garo and non-Garo traditional medicinal practitioners in two villages of Tangail district, Bangladesh. Am. Eurasian J. Sustain. Agric. 2011, 5, 350–357. [Google Scholar]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017, 2, 210–216. [Google Scholar] [CrossRef]
- Bouarfa, M.; Lebtar, S.; Boukhira, S.; Bousta, D. An ethnobotanical and ethnopharmacological survey of Cannabis sativa of Taounate Region in Northern Morocco. Int. J. Pharm. Sci. Rev. Res 2020, 64, 116–122. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Carus, M.; Sarmento, L. The European Hemp Industry: Cultivation, processing and applications for fibres, shivs, seeds and flowers. Eur. Ind. Hemp Assoc. 2016, 5, 1–9. [Google Scholar]
- Turner, B.D.; Sloan, S.W.; Currell, G.R. Novel remediation of per-and polyfluoroalkyl substances (PFASs) from contaminated groundwater using Cannabis sativa L.(hemp) protein powder. Chemosphere 2019, 229, 22–31. [Google Scholar] [CrossRef]
- Cosarca, S.; Rosca, I.; Coman, A.; Bota, M.C.N.; Tanase, C. Effect of treatment with saline solution (NaCl) on rape plants in presence of the hemp shives. Rev. Chim. 2017, 68, 1843–1846. [Google Scholar] [CrossRef]
- Hussain, W.; Ullah, M.; Dastagir, G.; Badshah, L. Quantitative ethnobotanical appraisal of medicinal plants used by inhabitants of lower Kurram, Kurram agency, Pakistan. Avicenna J. Phytomed. 2018, 8, 313. [Google Scholar]
- Knapp, A.A.; Lee, D.C.; Borodovsky, J.T.; Auty, S.G.; Gabrielli, J.; Budney, A.J. Emerging trends in cannabis administration among adolescent cannabis users. J. Adolesc. Health 2019, 64, 487–493. [Google Scholar] [CrossRef]
- Chopra, I.; Chopra, R.N. The use of the cannabis drugs in India. Bull. Narc. 1957, 9, 4–29. [Google Scholar]
- Wood, T.B.; Spivey, W.N.; Easterfield, T.H. III.—Cannabinol. Part I. J. Chem. Soc. Trans. 1899, 75, 20–36. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.A.; ElSohly, H.N.; ElKashoury, E.A.; ElSohly, M.A. Fatty acids of cannabis seeds. Phytochem. Anal. 1996, 7, 279–283. [Google Scholar] [CrossRef]
- Babiker, E.E.; Uslu, N.; Al Juhaimi, F.; Ahmed, I.A.M.; Ghafoor, K.; Özcan, M.M.; Almusallam, I.A. Effect of roasting on antioxidative properties, polyphenol profile and fatty acids composition of hemp (Cannabis sativa L.) seeds. LWT 2021, 139, 110537. [Google Scholar] [CrossRef]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect οf genotype and growing year on the nutritional, phytochemical, and antioxidant properties of industrial hemp (Cannabis sativa L.) seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef] [Green Version]
- Moccia, S.; Siano, F.; Russo, G.L.; Volpe, M.G.; La Cara, F.; Pacifico, S.; Piccolella, S.; Picariello, G. Antiproliferative and antioxidant effect of polar hemp extracts (Cannabis sativa L., Fedora cv.) in human colorectal cell lines. Int. J. Food Sci. Nutr. 2020, 71, 410–423. [Google Scholar] [CrossRef]
- Stambouli, H.; El Bouri, A.; Bouayoun, T.; Bellimam, A. Caractérisation de l’huile de graines de Cannabis sativa L. cultivé au nord du Maroc. Ann. Toxicol. Anal. 2006, 18, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Aiello, A.; Pizzolongo, F.; Scognamiglio, G.; Romano, A.; Masi, P.; Romano, R. Effects of supercritical and liquid carbon dioxide extraction on hemp (Cannabis sativa L.) seed oil. Int. J. Food Sci. Technol. 2020, 55, 2472–2480. [Google Scholar] [CrossRef]
- Nagy, D.U.; Cianfaglione, K.; Maggi, F.; Sut, S.; Dall’Acqua, S. Chemical characterization of leaves, male and female flowers from spontaneous Cannabis (Cannabis sativa L.) growing in Hungary. Chem. Biodivers. 2019, 16, e1800562. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive effect of Cannabis sativa L. herb extracts on skin cells and assessment of cannabinoid-based hydrogels properties. Molecules 2021, 26, 802. [Google Scholar] [CrossRef]
- Fasakin, O.W.; Oboh, G.; Ademosun, A.O.; Lawal, A.O. The modulatory effects of alkaloid extracts of Cannabis sativa, Datura stramonium, Nicotiana tabacum and male Carica papaya on neurotransmitter, neurotrophic and neuroinflammatory systems linked to anxiety and depression. Inflammopharmacology 2022, 30, 2447–2476. [Google Scholar] [CrossRef]
- Guo, T.; Liu, Q.; Hou, P.; Li, F.; Guo, S.; Song, W.; Zhang, H.; Liu, X.; Zhang, S.; Zhang, J. Stilbenoids and cannabinoids from the leaves of Cannabis sativa f. sativa with potential reverse cholesterol transport activity. Food Funct. 2018, 9, 6608–6617. [Google Scholar] [CrossRef]
- Laznik, Ž.; Košir, I.J.; Košmelj, K.; Murovec, J.; Jagodič, A.; Trdan, S.; Ačko, D.K.; Flajšman, M. Effect of Cannabis sativa L. root, leaf and inflorescence ethanol extracts on the chemotrophic response of entomopathogenic nematodes. Plant Soil 2020, 455, 367–379. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V.; Pistelli, L.; Flamini, G.; Bassolino, L.; Fulvio, F.; Montanari, M.; Paris, R. Essential Oil of Cannabis sativa L: Comparison of Yield and Chemical Composition of 11 Hemp Genotypes. Molecules 2021, 26, 4080. [Google Scholar] [CrossRef]
- Gunjević, V.; Grillo, G.; Carnaroglio, D.; Binello, A.; Barge, A.; Cravotto, G. Selective recovery of terpenes, polyphenols and cannabinoids from Cannabis sativa L. inflorescences under microwaves. Ind. Crops Prod. 2021, 162, 113247. [Google Scholar] [CrossRef]
- Vanhoenacker, G.; Van Rompaey, P.; De Keukeleire, D.; Sandra, P. Chemotaxonomic features associated with flavonoids of cannabinoid-free cannabis (Cannabis sativa subsp. sativa L.) in relation to hops (Humulus lupulus L.). Nat. Prod. Lett. 2010, 16, 57–63. [Google Scholar] [CrossRef]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical composition and antioxidant activity of thyme, hemp and coriander extracts: A comparison study of maceration, Soxhlet, UAE and RSLDE techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef]
- Elkins, A.C.; Deseo, M.A.; Rochfort, S.; Ezernieks, V.; Spangenberg, G. Development of a validated method for the qualitative and quantitative analysis of cannabinoids in plant biomass and medicinal cannabis resin extracts obtained by super-critical fluid extraction. J. Chromatogr. B 2019, 1109, 76–83. [Google Scholar] [CrossRef]
- Stambouli, H.; El Bouri, A.; Bouayoun, T. Évolution de la teneur en Δ9-THC dans les saisies de résines de cannabis au Maroc de 2005 à 2014. Toxicol. Anal. et Clin. 2016, 28, 146–152. [Google Scholar] [CrossRef]
- ElSohly, M.A. Marijuana and the Cannabinoids; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Taaifi, Y.; Benmoumen, A.; Belhaj, K.; Aazza, S.; Abid, M.; Azeroual, E.; Elamrani, A.; Mansouri, F.; Serghini Caid, H. Seed composition of non-industrial hemp (Cannabis sativa L.) varieties from four regions in northern Morocco. Int. J. Food Sci. Technol. 2021, 56, 5931–5947. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Tubaro, F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crops Prod. 2012, 36, 401–404. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure− activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Marzorati, S.; Friscione, D.; Picchi, E.; Verotta, L. Cannabidiol from inflorescences of Cannabis sativa L.: Green extraction and purification processes. Ind. Crops Prod. 2020, 155, 112816. [Google Scholar] [CrossRef]
- Stambouli, H.; El Bouri, A.; Bouayoun, T.; El Karni, N.; Naciri, Z.; Johar, A.; Saoura, A.; Saidi, S. Expérimentation de la culture de chanvre industriel à fibres au Maroc. Ann. Toxicol. Anal. 2011, 23, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Jang, E.; Kim, H.; Jang, S.; Lee, J.; Baeck, S.; In, S.; Kim, E.; Kim, Y.-U.; Han, E. Concentrations of THC, CBD, and CBN in commercial hemp seeds and hempseed oil sold in Korea. Forensic Sci. Int. 2020, 306, 110064. [Google Scholar] [CrossRef]
- Deferne, J.-L.; Pate, D.W. Hemp seed oil: A source of valuable essential fatty acids. J. Int. Hemp. Assoc. 1996, 3, 1–7. [Google Scholar]
- Orhan, İ.; Küsmenoǧlu, Ş.; Şener, B. GC-MS analysis of the seed oil of Cannabis sativa L. cultivated in Turkey. Gazi Univ. Eczaci. Fak. Derg. 2000, 17, 79–81. [Google Scholar]
- Cunningham, S.A.; Summerhayes, B.; Westoby, M. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol. Monogr. 1999, 69, 569–588. [Google Scholar] [CrossRef]
- Chik, S.C.; Or, T.C.; Luo, D.; Yang, C.L.; Lau, A.S. Pharmacological effects of active compounds on neurodegenerative disease with gastrodia and uncaria decoction, a commonly used poststroke decoction. Sci. World J. 2013, 2013, 896873. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef]
- Pate, D.W. Chemical ecology of Cannabis. J. Int. Hemp. Assoc. 1994, 2, 32–37. [Google Scholar]
- Yang, R.; Berthold, E.C.; McCurdy, C.R.; da Silva Benevenute, S.; Brym, Z.T.; Freeman, J.H. Development of cannabinoids in flowers of industrial hemp (Cannabis sativa L.): A pilot study. J. Agric. Food Chem. 2020, 68, 6058–6064. [Google Scholar] [CrossRef]
- Sakakibara, I.; Ikeya, Y.; Hayashi, K.; Mitsuhashi, H. Three phenyldihydronaphthalene lignanamides from fruits of Cannabis sativa. Phytochemistry 1992, 31, 3219–3223. [Google Scholar] [CrossRef]
- Lesma, G.; Consonni, R.; Gambaro, V.; Remuzzi, C.; Roda, G.; Silvani, A.; Vece, V.; Visconti, G. Cannabinoid-free Cannabis sativa L. grown in the Po valley: Evaluation of fatty acid profile, antioxidant capacity and metabolic content. Nat. Prod. Res. 2014, 28, 1801–1807. [Google Scholar] [CrossRef]
- Slatkin, D.J.; Doorenbos, N.J.; Harris, L.S.; Masoud, A.N.; Quimby, M.W.; Schiff, P.L. Chemical constituents of Cannabis sativa L. root. J. Pharm. Sci. 1971, 60, 1891–1892. [Google Scholar] [CrossRef]
- Keller, A.; Leupin, M.; Mediavilla, V.; Wintermantel, E. Influence of the growth stage of industrial hemp on chemical and physical properties of the fibres. Ind. Crops Prod. 2001, 13, 35–48. [Google Scholar] [CrossRef]
- Kushima, H.; Shoyama, Y.; Nishioka, I. Cannabis. Xii. Variations of cannabinoid contents in several strains of Cannabis sativa L. with leaf-age, season and sex. Chem. Pharm. Bull. 1980, 28, 594–598. [Google Scholar] [CrossRef] [Green Version]
- Mechqoq, H.; El Yaagoubi, M.; Momchilova, S.; Msanda, F.; El Aouad, N. Comparative study on yields and quality parameters of argan oils extracted by conventional and green extraction techniques. Grain Oil Sci. Technol. 2021, 4, 125–130. [Google Scholar] [CrossRef]
- Copeland, L.O.; McDonald, M.F. Principles of Seed Science and Technology; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Morris, G.M.; Lim-Wilby, M. Molecular docking. In Molecular Modeling of Proteins; Springer: Berlin/Heidelberg, Germany, 2008; pp. 365–382. [Google Scholar]
- Hourfane, S.; Mechqoq, H.; Errajouani, F.; Rocha, J.M.; El Aouad, N. In Vitro and In Silico Evaluations of Boswellia carterii Resin Dermocosmetic Activities. Cosmetics 2022, 9, 131. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Sousa, S.F.; Fernandes, P.A.; Ramos, M.J. Protein–ligand docking: Current status and future challenges. Proteins Struct. Funct. Bioinform. 2006, 65, 15–26. [Google Scholar] [CrossRef]
- Chen, G.; Seukep, A.J.; Guo, M. Recent advances in molecular docking for the research and discovery of potential marine drugs. Mar. Drugs 2020, 18, 545. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. EADock: Docking of small molecules into protein active sites with a multiobjective evolutionary optimization. Proteins Struct. Funct. Bioinform. 2007, 67, 1010–1025. [Google Scholar] [CrossRef]
- Muegge, I.; Rarey, M. Small molecule docking and scoring. Rev. Comput. Chem. 2001, 17, 1–60. [Google Scholar]
- Karimi, I.; Yousofvand, N.; Hussein, B.A. In vitro cholinesterase inhibitory action of Cannabis sativa L. Cannabaceae and in silico study of its selected phytocompounds. Silico Pharmacol. 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Nasreen, N.; Niaz, S.; Khan, A.; Zaman, M.A.; Ayaz, S.; Naeem, H.; Khan, N.; Elgorban, A.M. The potential of Allium sativum and Cannabis sativa extracts for anti-tick activities against Rhipicephalus (Boophilus) microplus. Exp. Appl. Acarol. 2020, 82, 281–294. [Google Scholar] [CrossRef]
- Quan, P.M.; Toan, T.Q.; Hung, N.P.; Nam, P.H.; Kiet, N.T.; Ha, N.X.; Le, D.T.T.; An, T.N.T.; Show, P.L.; Thi, H.H.P. Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development. Open Chem. 2021, 19, 1244–1250. [Google Scholar] [CrossRef]
- Ogungbe, I.V.; Erwin, W.R.; Setzer, W.N. Antileishmanial phytochemical phenolics: Molecular docking to potential protein targets. J. Mol. Graph. Model. 2014, 48, 105–117. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Kumar, A.; Misra, N. On the inhibition of COVID-19 protease by Indian herbal plants: An in silico investigation. arXiv 2020, arXiv:2004.03411. [Google Scholar]
- Akhtar, A.; Hussain, W.; Rasool, N. Probing the pharmacological binding properties, and reactivity of selective phytochemicals as potential HIV-1 protease inhibitors. Univ. Sci. 2019, 24, 441–464. [Google Scholar] [CrossRef]
- Nouadi, B.; Ezaouine, A.; El Messal, M.; Blaghen, M.; Bennis, F.; Chegdani, F. Prediction of anti-COVID 19 therapeutic power of medicinal Moroccan plants using molecular docking. Bioinform. Biol. Insights 2021, 15, 11779322211009199. [Google Scholar] [CrossRef]
- Ngo, S.T.; Quynh Anh Pham, N.; Thi Le, L.; Pham, D.-H.; Vu, V.V. Computational determination of potential inhibitors of SARS-CoV-2 main protease. J. Chem. Inf. Model. 2020, 60, 5771–5780. [Google Scholar] [CrossRef]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Kepel, B.J.; Idroes, R.; Effendi, Y.; Sakib, S.A.; Emran, T.B. Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and spike (S) glycoprotein inhibitors: A molecular docking study. Scientifica 2020, 2020, 6307457. [Google Scholar] [CrossRef]
- Ma, H.; Xu, F.; Liu, C.; Seeram, N.P. A Network Pharmacology Approach to Identify Potential Molecular Targets for Cannabidiol’s Anti-Inflammatory Activity. Cannabis Cannabinoid Res. 2021, 6, 288–299. [Google Scholar] [CrossRef]
- TÜZÜN, B. Investigation of the molecules obtained from marijuana: Computational study of spectral, structural and docking. J. Phys. Theor. Chem. (IAU Iran) 2020, 16, 59–74. [Google Scholar]
- Baroi, S.; Saha, A.; Bachar, R.; Bachar, S.C. Cannabinoid as potential aromatase inhibitor through molecular modeling and screening for anti-cancer activity. Dhaka Univ. J. Pharm. Sci. 2020, 19, 47–58. [Google Scholar] [CrossRef]
- Metibemu, D.S. 3D-QSAR and Molecular Docking Approaches for the Identification of Phyto-Inhibitors of Hsp90. LIANBS 2022, 11, 3871–3886. [Google Scholar]
- Adeniran, O.Y.; Ayorinde, O.; Boboye, S.O. Virtual high-throughput screening (VHTS), three-dimensional quantitative structure-activity and relationship (3D-QSAR) and molecular docking studies of novel phyto-inhibtors of topoisomerase II alpha. GSC Biol. Pharm. Sci. 2021, 15, 072–082. [Google Scholar] [CrossRef]
- Zaka, M.; Sehgal, S.A.; Shafique, S.; Abbasi, B.H. Comparative in silico analyses of Cannabis sativa, Prunella vulgaris and Withania somnifera compounds elucidating the medicinal properties against rheumatoid arthritis. J. Mol. Graph. Model. 2017, 74, 296–304. [Google Scholar] [CrossRef]
- Bouchentouf, S.; Ghalem, S.; Allali, H.; Missoum, N. Investigating the Inhibition of 5-LO Enzyme by Main Cannabinoids Contained in Cannabis safiva and Seized Resin Cannabis by Using Molecular Modeling. Insights Enzym. Res. 2017, 1, 10. [Google Scholar]
- Gao, J.; Li, T.; Chen, D.; Gu, H.; Mao, X. Identification and molecular docking of antioxidant peptides from hemp seed protein hydrolysates. Lwt 2021, 147, 111453. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Aluko, R.E. Kinetics and molecular docking studies of the inhibitions of angiotensin converting enzyme and renin activities by hemp seed (Cannabis sativa L.) peptides. J. Agric. Food Chem. 2014, 62, 4135–4144. [Google Scholar] [CrossRef]
- Ngamsuk, S.; Huang, T.-C.; Hsu, J.-L. ACE inhibitory activity and molecular docking of gac seed protein hydrolysate purified by HILIC and RP-HPLC. Molecules 2020, 25, 4635. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.; Xiao, W.; Zhu, J.-B. Investigation on the active ingredient and mechanism of Cannabis sativa L. for treating epilepsy based on network pharmacology. Biotechnol. Biotechnol. Equip. 2021, 35, 994–1009. [Google Scholar] [CrossRef]
- di Giacomo, V.; Chiavaroli, A.; Recinella, L.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Ronci, M.; Leone, S.; Brunetti, L. Antioxidant and neuroprotective effects induced by cannabidiol and cannabigerol in rat CTX-TNA2 astrocytes and isolated cortexes. Int. J. Mol. Sci. 2020, 21, 3575. [Google Scholar] [CrossRef]
- Sarkar, I.; Sen, G.; Bhattacharya, M.; Bhattacharyya, S.; Sen, A. In silico inquest reveals the efficacy of Cannabis in the treatment of post-Covid-19 related neurodegeneration. J. Biomol. Struct. Dyn. 2022, 40, 8030–8039. [Google Scholar] [CrossRef]
- Onoda, T.; Li, W.; Sasaki, T.; Miyake, M.; Higai, K.; Koike, K. Identification and evaluation of magnolol and chrysophanol as the principle protein tyrosine phosphatase-1B inhibitory compounds in a Kampo medicine, Masiningan. J. Ethnopharmacol. 2016, 186, 84–90. [Google Scholar] [CrossRef]
- Kazemi, F.; Karimi, I.; Yousofvand, N. Molecular docking study of lignanamides from Cannabis sativa against P-glycoprotein. Silico Pharmacol. 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Holgado, M.; Martín-Banderas, L.; Álvarez-Fuentes, J.; Fernández-Arévalo, M. Neuroprotective effect of cannabinoids nanoplatforms in neurodegenerative diseases. J. Drug Deliv. Sci. Technol. 2017, 42, 84–93. [Google Scholar] [CrossRef]
- Marsh, D.T.; Smid, S.D. Cannabis Phytochemicals: A Review of Phytocannabinoid Chemistry and Bioactivity as Neuroprotective Agents. Aust. J. Chem. 2020, 74, 388–404. [Google Scholar] [CrossRef]
- Dreno, B.; Araviiskaia, E.; Berardesca, E.; Bieber, T.; Hawk, J.; Sanchez-Viera, M.; Wolkenstein, P. The science of dermocosmetics and its role in dermatology. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1409–1417. [Google Scholar] [CrossRef]
- Kim, J.K.; Heo, H.-Y.; Park, S.; Kim, H.; Oh, J.J.; Sohn, E.-H.; Jung, S.-H.; Lee, K. Characterization of Phenethyl Cinnamamide Compounds from Hemp Seed and Determination of Their Melanogenesis Inhibitory Activity. ACS Omega 2021, 6, 31945–31954. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Kietthanakorn, B.O.; Ruksiriwanich, W.; Chaikul, P.; Boonpisuttinant, K.; Sainakham, M.; Manosroi, W.; Tangjai, T.; Manosroi, J. Pharmaceutical and cosmeceutical biological activities of hemp (Cannabis sativa L. var. sativa) leaf and seed extracts. Chiang Mai J. Sci. 2019, 46, 180–195. [Google Scholar]
- Yan, X.; Tang, J.; dos Santos Passos, C.; Nurisso, A.; Simoes-Pires, C.A.; Ji, M.; Lou, H.; Fan, P. Characterization of lignanamides from hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J. Agric. Food Chem. 2015, 63, 10611–10619. [Google Scholar] [CrossRef]
- Teh, S.-S.; Bekhit, A.E.-D.A.; Carne, A.; Birch, J. Antioxidant and ACE-inhibitory activities of hemp (Cannabis sativa L.) protein hydrolysates produced by the proteases AFP, HT, Pro-G, actinidin and zingibain. Food Chem. 2016, 203, 199–206. [Google Scholar] [CrossRef]
- Frassinetti, S.; Gabriele, M.; Moccia, E.; Longo, V.; Di Gioia, D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. Lwt 2020, 124, 109149. [Google Scholar] [CrossRef]
- Anjum, M. 35. Evaluation of antimicrobial activity and ethnobotanical study of Cannabis sativa L. Pure Appl. Biol. (PAB) 2018, 7, 706–713. [Google Scholar]
- Coetzee, C.; Levendal, R.-A.; Van de Venter, M.; Frost, C. Anticoagulant effects of a Cannabis extract in an obese rat model. Phytomedicine 2007, 14, 333–337. [Google Scholar] [CrossRef]
- Al Khoury, A.; Sleiman, R.; Atoui, A.; Hindieh, P.; Maroun, R.G.; Bailly, J.-D.; El Khoury, A. Antifungal and anti-aflatoxigenic properties of organs of Cannabis sativa L.: Relation to phenolic content and antioxidant capacities. Arch. Microbiol. 2021, 203, 4485–4492. [Google Scholar] [CrossRef]
- Rossi, P.; Cappelli, A.; Marinelli, O.; Valzano, M.; Pavoni, L.; Bonacucina, G.; Petrelli, R.; Pompei, P.; Mazzara, E.; Ricci, I. Mosquitocidal and anti-inflammatory properties of the essential oils obtained from monoecious, male, and female inflorescences of hemp (Cannabis sativa L.) and their encapsulation in nanoemulsions. Molecules 2020, 25, 3451. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Mechqoq, H.; Hourfane, S.; Yaagoubi, M.E.; Hamdaoui, A.E.; Msanda, F.; Almeida, J.R.G.d.S.; Rocha, J.M.; Aouad, N.E. Phytochemical Screening, and In Vitro Evaluation of the Antioxidant and Dermocosmetic Activities of Four Moroccan Plants: Halimium antiatlanticum, Adenocarpus artemisiifolius, Pistacia lentiscus and Leonotis nepetifolia. Cosmetics 2022, 9, 94. [Google Scholar] [CrossRef]
- Mirzamohammad, E.; Alirezalu, A.; Alirezalu, K.; Norozi, A.; Ansari, A. Improvement of the antioxidant activity, phytochemicals, and cannabinoid compounds of Cannabis sativa by salicylic acid elicitor. Food Sci. Nutr. 2021, 9, 6873–6881. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Pérez-Jiménez, J. What contribution is beer to the intake of antioxidants in the diet. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2009; pp. 441–448. [Google Scholar]
- Shahidi, F.; Janitha, P.; Wanasundara, P. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Smeriglio, A.; Alloisio, S.; Raimondo, F.M.; Denaro, M.; Xiao, J.; Cornara, L.; Trombetta, D. Essential oil of Citrus lumia Risso: Phytochemical profile, antioxidant properties and activity on the central nervous system. Food Chem. Toxicol. 2018, 119, 407–416. [Google Scholar] [CrossRef]
- Novak, J.; Zitterl-Eglseer, K.; Deans, S.G.; Franz, C.M. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001, 16, 259–262. [Google Scholar] [CrossRef]
- El Hamdaoui, A.; Msanda, F.; Boubaker, H.; Leach, D.; Bombarda, I.; Vanloot, P.; El Aouad, N.; Abbad, A.; Boudyach, E.; Achemchem, F. Essential oil composition, antioxidant and antibacterial activities of wild and cultivated Lavandula mairei Humbert. Biochem. Syst. Ecol. 2018, 76, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- El Yaagoubi, M.; Mechqoq, H.; Ortiz, S.; Cavaleiro, C.; Lecsö-Bornet, M.; Pereira, C.G.; Rodrigues, M.J.; Custódio, L.; El Mousadik, A.; Picot, L. Chemical Composition and Biological Screening of the Essential Oils of Micromeria macrosiphon and M. arganietorum (Lamiaceae). Chem. Biodivers. 2021, 18, e2100653. [Google Scholar] [CrossRef]
- Xiong, W.-T.; Gu, L.; Wang, C.; Sun, H.-X.; Liu, X. Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J. Ethnopharmacol. 2013, 150, 935–945. [Google Scholar] [CrossRef]
- Agarwal, P.; Gupta, R. Alpha-amylase inhibition can treat diabetes mellitus. Res. Rev. J. Med. Health Sci. 2016, 5, 1–8. [Google Scholar]
- Kajaria, D.; Tripathi, J.; Tripathi, Y.B.; Tiwari, S. In-vitro α amylase and glycosidase inhibitory effect of ethanolic extract of antiasthmatic drug—Shirishadi. J. Adv. Pharm. Technol. Res. 2013, 4, 206. [Google Scholar] [CrossRef]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef] [Green Version]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J. Biol. Chem. 2006, 281, 39480–39491. [Google Scholar] [CrossRef] [Green Version]
- Lukhele, S.T.; Motadi, L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Altern. Med. 2016, 16, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H. Cannabidiol Enhances the Inhibitory Effects of Δ9-Tetrahydrocannabinol on Human Glioblastoma Cell Proliferation and SurvivalCannabinoid Synergy Inhibits Glioblastoma Cell Growth. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Cianchi, F.; Papucci, L.; Schiavone, N.; Lulli, M.; Magnelli, L.; Vinci, M.C.; Messerini, L.; Manera, C.; Ronconi, E.; Romagnani, P. Cannabinoid receptor activation induces apoptosis through tumor necrosis factor α–mediated ceramide de novo synthesis in colon cancer cells. Clin. Cancer Res. 2008, 14, 7691–7700. [Google Scholar] [CrossRef] [Green Version]
- McKallip, R.J.; Lombard, C.; Fisher, M.; Martin, B.R.; Ryu, S.; Grant, S.; Nagarkatti, P.S.; Nagarkatti, M. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood J. Am. Soc. Hematol. 2002, 100, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.L.; Hill, D.S.; McKee, C.S.; Hernandez-Tiedra, S.; Lorente, M.; Lopez-Valero, I.; Anagnostou, M.E.; Babatunde, F.; Corazzari, M.; Redfern, C.P. Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J. Investig. Dermatol. 2015, 135, 1629–1637. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef] [Green Version]
- Galanti, G.; Fisher, T.; Kventsel, I.; Shoham, J.; Gallily, R.; Mechoulam, R.; Lavie, G.; Amariglio, N.; Rechavi, G.; Toren, A. Δ9-Tetrahydrocannabinol inhibits cell cycle progression by downregulation of E2F1 in human glioblastoma multiforme cells. Acta Oncol. 2008, 47, 1062–1070. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; del Pulgar, T.G.; Izquierdo, M.; Guzmán, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef]
- McAllister, S.D.; Murase, R.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Allison, J.; Almanza, C.; Pakdel, A.; Lee, J.; Limbad, C. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat. 2011, 129, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS ONE 2013, 8, e76918. [Google Scholar] [CrossRef] [Green Version]
- Klein, T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005, 5, 400–411. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [Green Version]
- Wood, C.; Duparc, N.; Leblanc, V.; Cunin-Roy, C. L’hypnose et la douleur. Médecine Clin. Pour Les Pédiatres 2004, 11, 40–44. [Google Scholar]
- Munch, G. Le Cannabis, Les Deux Versants: Drogue et Médicament; Université de Lorraine: Lorraine, France, 2015. [Google Scholar]
- Jeannin, C. Évaluation et Prise en Charge de la Douleur Chez le Lapin de Compagnie: Comment les Optimiser en L’état Actuel des Connaissances? Master Thesis, University of Liege, Liege, Belgium, 2020. [Google Scholar]
- Li, J.; Wang, G.; Qin, Y.; Zhang, X.; Wang, H.-F.; Liu, H.-W.; Zhu, L.-J.; Yao, X.-S. Neuroprotective constituents from the aerial parts of Cannabis sativa L. subsp. sativa. RSC Adv. 2020, 10, 32043–32049. [Google Scholar] [CrossRef]
- Landucci, E.; Mazzantini, C.; Lana, D.; Davolio, P.L.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Neuroprotective effects of cannabidiol but not Δ9-Tetrahydrocannabinol in rat hippocampal slices exposed to oxygen-glucose deprivation: Studies with Cannabis extracts and selected cannabinoids. Int. J. Mol. Sci. 2021, 22, 9773. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Savani, C.; Steardo Jr, L.; De Filippis, D.; Cottone, P.; Iuvone, T.; Cuomo, V.; Steardo, L. Cannabidiol in vivo blunts β-amyloid induced neuroinflammation by suppressing IL-1β and iNOS expression. Br. J. Pharmacol. 2007, 151, 1272–1279. [Google Scholar] [CrossRef] [Green Version]
- Perez, M.; Benitez, S.U.; Cartarozzi, L.P.; Del Bel, E.; Guimaraes, F.S.; Oliveira, A.L. Neuroprotection and reduction of glial reaction by cannabidiol treatment after sciatic nerve transection in neonatal rats. Eur. J. Neurosci. 2013, 38, 3424–3434. [Google Scholar] [CrossRef]
- Friedman, L.K.; Wongvravit, J.P. Anticonvulsant and neuroprotective effects of cannabidiol during the juvenile period. J. Neuropathol. Exp. Neurol. 2018, 77, 904–919. [Google Scholar] [CrossRef]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef]
- Farrelly, A.M.; Vlachou, S.; Grintzalis, K. Efficacy of phytocannabinoids in epilepsy treatment: Novel approaches and recent advances. Int. J. Environ. Res. Public Health 2021, 18, 3993. [Google Scholar] [CrossRef]
- Stone, N.L.; Murphy, A.J.; England, T.J.; O’Sullivan, S.E. A systematic review of minor phytocannabinoids with promising neuroprotective potential. Br. J. Pharmacol. 2020, 177, 4330–4352. [Google Scholar] [CrossRef]
- Dunn, L.B.; Damesyn, M.; Moore, A.A.; Reuben, D.B.; Greendale, G.A. Does estrogen prevent skin aging?: Results from the first National Health and Nutrition Examination Survey (NHANES I). Arch. Dermatol. 1997, 133, 339–342. [Google Scholar] [CrossRef]
- Gaisey, J.; Narouze, S.N. Dronabinol (Marinol®). In Cannabinoids and Pain; Springer: Berlin/Heidelberg, Germany, 2021; pp. 105–107. [Google Scholar]
- Abuhasira, R.; Shbiro, L.; Landschaft, Y. Medical use of cannabis and cannabinoids containing products–Regulations in Europe and North America. Eur. J. Intern. Med. 2018, 49, 2–6. [Google Scholar] [CrossRef]
- Babson, K.A.; Sottile, J.; Morabito, D. Cannabis, cannabinoids, and sleep: A review of the literature. Curr. Psychiatry Rep. 2017, 19, 1–12. [Google Scholar] [CrossRef]
- Sekar, K.; Pack, A. Epidiolex as adjunct therapy for treatment of refractory epilepsy: A comprehensive review with a focus on adverse effects. F1000Research 2019, 8, 8. [Google Scholar] [CrossRef] [Green Version]

| Plant Part | Traditional Use | Preparation | Administration | Reference |
|---|---|---|---|---|
| Seed | Nutrition | Powder | Oral | [59] |
| Seed | Narcotic, painkiller, treat nausea and vomiting, stimulate appetite in AIDS patients, hepatitis C, anxiety, seizure, muscle relaxants, anticancer and weight control | ND | ND | [60] |
| Seed | Hair fortification | Powder | External | [61] |
| Seed | Analgesic, antiarthritic and antirheumatic | Oil | External | [62] |
| Leaf | Eczema | Powder | External | [61] |
| Leaf | Bloating, cough, mucus | Leaf juice | Oral | [63] |
| Leaf | Central nervous system (CNS) depressant, gout, arthritic pain | Powder | Oral | [64] |
| Leaf | Schizophrenia-like psychotic problems | Oil | External | [65] |
| Leaf | Gastric disorders | ND | ND | [66] |
| Leaf | Skin and subcutaneous tissue disorders, circulatory system and blood disorders | Juice, paste or powder | ND | [62] |
| Inflorescence | Sedative, dysentery, diarrhea and appetite loss | ND | Oral | [62] |
| Stem | Firewood or torch wood | Raw | ND | [62] |
| Stem | Construction, materials, dress, papermaking, making ropes | Raw | ND | [5] |
| Seed, flower | Hair care | ND | ND | [67] |
| Leaf, inflorescence | Soporific, abortifacient | ND | ND | [68] |
| Leaf, root | Cancer, hypertension, antidote to poison, itch, rheumatoid arthritis | ND | ND | [66] |
| Root | Fever | Maceration | External | [69] |
| Root | Gout, arthritis | Boiled roots | External (cataplasm) | [70] |
| Root | Joint pain | Decoction | External (cataplasm) | [70] |
| Root | Skin burns | Raw or decoction mixed with butter | External (topical) | [70] |
| Root | Inflammation | Boiled roots, decoction | External (cataplasm) | [70] |
| Root | Childbirth, postpartum, hemorrhage | Decoction | Oral | [70] |
| Root | Erysipelas, toxins and infections | Pulverized and mixed with wine | Oral/external | [70] |
| Whole plant | Pain, gastric disorders, diabetes, scars and asthma | ND | ND | [71] |
| Plant Part | Extraction Method (Type of Extract) | Class of Metabolites | Compounds (Quantities) | Reference |
|---|---|---|---|---|
| Seed | Maceration (hexane) | Fatty acids | Linoleic acid (47.06%) Oleic acid (43.20%) Palmitic acid (4.88%) Stearic acid (3.32%) | [80] |
| Seed | Soxhlet (petroleum benzine) | Fatty acids | Linoleic acid (58.41 ± 0.04%) α-Linolenic Acid (16.26 ± 0.03%) Oleic acid (16.05 ± 0.02%) Palmitic acid (5.59 ± 0.12%) Stearic acid (2.46 ± 0.01%) | [81] |
| Seed | Soxhlet (ethanol–water 80:20) | Polyphenols | Gallic acid (12.9 ± 18.28 mg/100 g) (+)-Catechin (5.995 ± 5.23 mg/100 g) 1,2-Dihydroxybenzene (5.155 ± 4.59 mg/100 g) 3,4-Dihydroxybenzoic acid (4.89 ± 4.68 mg/100 g) Syringic acid (3.795 ± 1.99 mg/100 g) Caffeic acid (2.475 ± 3.53 mg/100 g) Quercetin (2.08 ± 3.36 mg/100 g) Rutin trihydrate (0.915 ± 1.15 mg/100 g) Isorhamnetin (0.765 ± 0.89 mg/100 g) trans-Ferulic acid (0.685 ± 0.61 mg/100 g) Apigenin-7-glucoside (0.55 ± 0.38 mg/100 g) Naringenin (0.255 ± 0.33 mg/100 g) trans-Cinnamic acid (0.19 ± 0.24 mg/100 g) Resveratrol (0.17 ± 0.24 mg/100 g) p-Coumaric acid (0.165 ± 0.17 mg/100 g) | [81] |
| Seed | Ultrasound-assisted extraction (ethanol) | Polyphenols | Cannabisin A (105.1 ± 54 mg/100 g DW) N-trans-caffeoyltyramine (49 ± 34.2 mg/100 g DW) Cinnamic acid (3.75 ± 3.55 mg/100 g DW) p-hydroxybenzoic acid (2.1 ± 0.9 mg/100 g DW) Protocatechuic acid (1 ± 0.6 mg/100 g DW) | [82] |
| Seed | Maceration (methanol–water 80:20) | Polyphenols | Quercetin-O-glucoside (ND) N-trans-caffeoyltyramine (ND) Rutin (ND) | [83] |
| Seed | Maceration (methanol–water 80:20) | Polyphenols | Cannabisin A, B, C, D, E, F, G, I, M, N, O (ND) | [83] |
| Seed | Soxhlet (hexane) | Tocopherols | γ-tocopherol (426 mg/kg) δ-tocopherol (33 mg/kg) α-tocopherol (13 mg/kg) β-tocopherol (2 mg/kg) | [84] |
| Seed | Ultrasound-assisted extraction (ethanol) | Tocopherols | γ-tocopherol (7.95 ± 3.35 mg/100 g DW) δ-tocopherol (0.95 ± 0.35 mg/100 g DW) | [82] |
| Seed | Maceration (hexane) | Phytosterols | β-Sitosterol (90.75 ± 0.42%) Campesterol (6.20 ± 0.00%) Stigmasterol (2.88 ± 0.17%) | [85] |
| Seed | Soxhlet (hexane) | Phytosterols | β-sitosterol (68.0%) Campesterol (17.1%) Δ-5-avenasterol (7.8%) Stigmasterol (3.8%) Δ-5,25-stigmastadienol (1.1%) | [84] |
| Seed | Ultrasound-assisted extraction (ethanol) | Carotenoids | Lutein (2.45 ± 0.95 mg/100 g DW) β-Carotene (0.5 ± 0.3 mg/100 g DW) | [82] |
| Seed | Supercritical fluid extraction (CO2) | Aldehydes | Hexanal (39.57 ± 0.91 mg/kg) Octadienal (10.29 ± 3.18 mg/kg) Heptadienal (9.38 ± 1.41 mg/kg) Nonenal (8.77 ± 1.27 mg/kg) Nonanal (8.34 ± 1.28 mg/kg) | [85] |
| Seed | Supercritical fluid extraction (CO2) | Alcohols | Hexanol (30.66 ± 0.95 mg/kg) | [85] |
| Seed | Maceration (hexane) | Hydrocarbons | Dodecane (112.5 ± 2.06 mg/kg) Tetradecane (69.0 ± 1.40 mg/kg) 1.3-Di-tert-butylbenzene (46.6 ± 2.25 mg/kg) | [85] |
| Leaf | Hydrodistillation | Terpenes | (E)-Caryophyllene (28.3 ± 4.1%) α-Humulene (9.3 ± 1.1%) β-Selinene (4.7 ± 0.9%) Caryophyllene oxide (4.3 ± 0.9%) α-Selinene (3.1± 0.6%) | [86] |
| Leaf | Ultrasound-assisted extraction (methanol) | Polyphenols | Apigenin C-(hexoside-O-rhamnoside) (0.83 mg/g) Luteolin C-(hexoside-O-rhamnoside) (0.67 mg/g) Luteolin di-C-hexoside (0.60 mg/g) Luteolin glucuronide(0.60 mg/g) Apigenin di-C-hexoside (0.54 mg/g) | [86] |
| Leaf | Ultrasound-assisted extraction (methanol) | Cannabinoids | CBD (11.2 ± 1.9%) CBDV (0.8 ± 0.2%) THC (0.7 ± 0.2%) CBC (0.5 ± 0.1%) | [86] |
| Leaf | Ultrasound-assisted extraction (water–ethanol 20:80) | Cannabinoids | Cannabidiol acid (150.00 ± 16.84 mg/g DW) Cannabidiol (31.00 ± 2.86 mg/g DW) THCA (6.50 ± 0.52mg/g DW) Cannabigerolic acid (6.30 ± 0.52 mg/g DW) Δ9-THC (4.00 ± 0.34 mg/g DW) | [87] |
| Leaf | Maceration (ethanol–acetic acid 90:10) | Alkaloids | Cannabisativine (410.30 μg/g) Cannabimine C (376.12 μg/g) Anhydrocannabisativine (218.11 μg/g) Aconitine (160.43 μg/g) Boldine (103.41 μg/g) Strychnine (72.63 μg/g) | [88] |
| Leaf | Vacuum liquid chromatography (ethanol–water 95:05) | Stilbenoids | Canniprene (ND); Combretastatin B-2 (ND) α,α′-dihydro-3,4′,5-trihydroxy-4,5′-diisopentenylstilbene (ND) | [89] |
| Flower | Hydrodistillation | Terpenes | (E)-Caryophyllene (28.5 ± 3.1%) α-Humulene (9.2 ± 1.7%); β-Selinene (4.3 ± 0.8%); α-Selinene (2.9 ± 0.6%) Caryophyllene oxide (2.3 ± 0.5%); α-trans-Bergamotene (1.9 ± 0.4%) | [86] |
| Flower | Ultrasound-assisted extraction (methanol) | Polyphenols | Quercetin di-C-hexoside (2.55 mg/g) Luteolin C-hexoside-2″-O-hexoside (1.01 mg/g) Apigenin di-C-hexoside (0.68 mg/g) Apigenin C-(hexoside-O-rhamnoside) (0.51 mg/g) | [86] |
| Flower | Ultrasound-assisted extraction (methanol) | Cannabinoids | CBD (24.9 ± 3.9%) THC (1.4 ± 0.3%) CBDV (1.4 ± 0.3%) CBTC (0.9 ± 0.2%) | [86] |
| Inflorescence | Hydrodistillation | Terpenes | Transcaryophyllene (38.2 ± 1.7%) Nerolidol (12.7 ± 1.2%) α-pinene (11.8 ± 0.4%) β-pinene (3.4 ± 0.3%) Cedrol (2.2 ± 0.0%) Myrcene (1.7 ± 0.5%) α-bisabolol (0.6 ± 0.1%) γ-terpinene (0.5 ± 0.3%) Camphene (0.2 ± 0.0%) α-humulene (0.2 ± 0.0%) α-terpinene (0.1 ± 0.0%) Menthol (0.1 ± 0.0%) | [90] |
| Inflorescence | Hydrodistillation | Terpenes | β-caryophyllene (14.4 ± 0.89%) Caryophyllene oxide (7.0 ± 1.06%) α-humulene (5.3 ± 0.10%) Selina-3,7(11)-diene (3.4 ± 0.33%) α-pinene (3.0 ± 0.04%) Myrcene (2.6 ± 0.16%) 14-hydroxy-9-epi-(E) -caryophyllene (2.5 ± 0.12%) Humulene oxide II (2.4 ± 0.24%) Caryophylla-4(14),8(5)-dien-5-ol (1.4 ± 0.15%) β-selinene (1.2 ± 0,07%) α-bisabolol (1.1 ± 0.13%) Selin-6-en-4-ol (0.8 ± 0.23%) | [91] |
| Inflorescence | Maceration (methanol) | Cannabinoids | CBDA (9.515 ± 1.085%) tCBD (8.695 ± 0.955%) tTHC (0.545 ± 0.075%) CBGA (0.535 ± 0.365%) CBG (0.535 ± 0.355%) tCBG (0.490 ± 0.080%) THCA (0.490 ± 0.080%) CBD (0.345 ± 0.005%) Δ9-THC (0.080 ± 0.000%) Δ8-THC (0.045 ± 0.005%) | [90] |
| Inflorescence | Microwave-assisted hydrodistillation | Cannabinoids | THCA (0.66 ± 0.04%) THC (0.34 ± 0.02%) CBDA (0.05 ± 0.005%) | [92] |
| Leaf and flower | Soxhlet (methanol–water 75:25) | Flavonoids | Luteolin-O-β-D-glucuronide (3.75 ± 0.75 mg/g DW) Apigenin-O-β-D-glucuronide (1.25 ± 0.25 mg/g DW) Vitexin (1.25 ± 0.25 mg/g DW) | [93] |
| Leaf and inflorescence | Hydrodistillation | Terpenes | (E)-Caryophyllene (28%) Caryophyllene oxide (15%) Humulene (13%) β-Myrcene (11%) α-Pinene (8%) | [94] |
| Leaf and inflorescence | Hydrodistillation | Phenolic compounds | Naringenin (706 µg/mL) Naringin (83 µg/mL) Catechin (60 µg/mL) Epicatechin (56 µg/mL) | [94] |
| Leaf and stem | Rapid solid–liquid dynamic extraction (ethanol) | Terpenes | Caryophyllene (52.78 ± 2.61%) Humulene (13.49 ± 0.14%) Linalool (9.42 ± 0.24%) α-bergamotene (6.14 ± 0.51%) cis-β-farnesene (3.54 ± 0.42%) Aromadendrene (2.86 ± 0.12%) | [95] |
| Leaf and stem | Rapid solid–liquid dynamic extraction (ethanol) | Polyphenols | Luteolin (304.37 ± 1.10 µg/g DW) Ferulic acid (247.77 ± 0.64 µg/g DW) Gallic acid (52.29 ± 0.98 µg/g DW) Apigenin (51.43 ± 0.48 µg/g DW) p-OH-benzoic acid (47.70 ± 0.75 µg/g DW) Rosmarinic acid (27.09 ± 0.85 µg/g DW) | [95] |
| Resin | Supercritical fluid (CO2) | Cannabinoids | CBD (72.12 μg/mL) THC (48.02 μg/mL) CBC (4.78 μg/mL) CBDA (2.34 μg/mL) CBN (0.40 μg/mL) | [96] |
| Resin | Ultrasound-assisted extraction (ethanol) | Cannabinoids | Δ9-THC > 20% | [97] |
| Enzyme | Ligand | Docking Tool | Score (kcal/mol) | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Name | ID (pdb = p / Unipr = up) | Class of Protein | Biological Activity | Name | Class of Metabolite | |||
| Acetylcholine esterase (ACHE) | 1EVE (p) | Hydrolase | Pesticidal | Cannabioxepane | Cannabinoids | AutoDock vina | –10.4 | [127] |
| Δ-9-THCA | Cannabinoids | –10.3 | ||||||
| Δ-8-THC | Cannabinoids | –10.1 | ||||||
| CBN | Cannabinoids | –10.1 | ||||||
| CBT | Cannabinoids | –9.8 | ||||||
| CBD | Cannabinoids | –9.8 | ||||||
| CBL | Cannabinoids | –9.6 | ||||||
| Isocannabispiradienone | Cannabinoids | –9.4 | ||||||
| CBC | Cannabinoids | –9.4 | ||||||
| CBCA | Cannabinoids | –9.3 | ||||||
| CBGA | Cannabinoids | –9.2 | ||||||
| Cannabispiradienone | Terpenes | –9.2 | ||||||
| Cannabispirol | Polyphenols | –9.2 | ||||||
| Butyrylcholinesterase (BCHE) | 1P0I (p) | Hydrolase | Pesticidal | Cannabioxepane | Cannabinoids | AutoDock vina | –9.8 | [127] |
| CBL | Cannabinoids | –8.9 | ||||||
| CBN | Cannabinoids | –8.8 | ||||||
| CBT | Cannabinoids | –8.7 | ||||||
| Δ-8-THC | Cannabinoids | –8.7 | ||||||
| CBL | Cannabinoids | –8.4 | ||||||
| Isocannabispiradienone | Cannabinoids | –8.3 | ||||||
| CBD | Cannabinoids | –8.2 | ||||||
| Cannabispiradienone | Terpenes | –8.2 | ||||||
| Cannabispiran | Polyphenols | –8.2 | ||||||
| Acetylcholine esterase (ACHE) | 1EVE (p) | Hydrolase | Pesticidal | CBD | Cannabinoids | AutoDock vina | −14.38 | [128] |
| CBN | Cannabinoids | −13.91 | ||||||
| Δ-9-THC | Cannabinoids | −13.82 | ||||||
| Plasmodium falciparum dihydrofolate reductase-thymidinesynthase (pfdhfr-ts) | 1J3I (p) | Lyase | Antimalarial | 7-oxo-9a-hydroxyhexahydrocannabinol | Cannabinoids | AutoDock vina | −9.40 | [129] |
| 10ar-hydroxyhexahydrocannabinol | Cannabinoids | −9.20 | ||||||
| 10-oxo-delta6a,10a-tetrahydrocannabinol | Cannabinoids | −9.20 | ||||||
| 8-oxo-delta9-tetrahydrocannabinol | Cannabinoids | −9.10 | ||||||
| 10a-hydroxyhexahydrocannabinol | Cannabinoids | −9.10 | ||||||
| Leishmania major pteridine reductase 1 (lmajptr1) | 1E7W, 1W0C, 2BF7 and 3H4V (p) | Oxido-reductase | Anti-leishmania | 4,5,4’5’-dimethylenedioxy-3,3’-dimethoxy-7,7’-epoxylignan | Polyphenols | Molegro virtual docker | −35.01 | [130] |
| Cannflavin A | Flavonoids | −34.42 | ||||||
| Leishmania mexicana glycerol-3-phosphate dehydrogenase (lmexgpdh) | 1EVZ, 1M66, 1N1E and 1N1G (p) | Oxido-reductase | Anti-leishmania | 4-terpenylcannabinolate | Cannabinoids | Molegro virtual docker | −35.90 | [130] |
| 3’-o-methyldiplacone | Flavonoids | −35.44 | ||||||
| 4’-o-methyldiplacone | Flavonoids | −34.18 | ||||||
| Sophoronol E | Flavonoids | −33.89 | ||||||
| Leishmania major methionyl-transynthetase (lmajmetrs) | 3KFL (p) | Ligase | Anti-leishmania | 4,6-dibenzoyl-2-[phenylhydroxymethyl]-3(2h)-benzofuranone | Polyphenols | Molegro virtual docker | −38.81 | [130] |
| Leishmania donovani cyclophilin (ldoncyp) | 2HAQ and 3EOV (p) | Isomerase | Anti-leishmania | 3’-o-methyldiplacone | Flavonoids | Molegro virtual docker | −30.23 | [130] |
| Leishmania major oligopeptidase b (lmajopb) | 2XE4 (p) | Protease | Anti-leishmania | 3-methoxycitrunobin-4-methyl ether | Polyphenols | Molegro virtual docker | −30.74 | [130] |
| 4’-o-methylglycyrrhisoflavone | Flavonoids | −30.47 | ||||||
| Leishmania major uridine 2p-glucose pyrophosphorylase (lmajugpase) | 2OEF and 2OEG (p) | Transferase | Anti-leishmania | 4’,6-dihydroxy-2-[phenylmethylene]-3(2h)-benzofuranone | Polyphenols | Molegro virtual docker | −33.94 | [130] |
| Leishmania major n-myristoyl-transferase (lmajnmt) | 2WSA, 3H5Z and 4A30 (p) | Transferase | Anti-leishmania | Diplacone | Flavonoids | Molegro virtual docker | −32.43 | [130] |
| Trans-4-isopentenyl-3,5,2,4 -tetrahydroxystilbene | Polyphenols | −30.45 | ||||||
| Leishmania infantum nicotinamidase (linfpnc1) | 3R2J (p) | Hydrolase | Anti-leishmania | 5,8-dihydroxy-1-hydroxymethylnaphtho-[2,3-c] furan-4,9-dione | Polyphenols | Molegro virtual docker | −23.45 | [130] |
| Umckalin | Polyphenols | −21.85 | ||||||
| Scoparone | Flavonoids | −21.77 | ||||||
| Leishmania major dihydroorotate dehydrogenase (lmajdhodh) | 3GYE, 3MHU and 3MJY (p) | Oxido-reductase | Anti-leishmania | Aristolignin | Flavonoids | Molegro virtual docker | −31.21 | [130] |
| Crotaorixin | Flavonoids | −31.84 | ||||||
| Mammea B/BA | Polyphenols | −29.09 | ||||||
| Leishmania mexicana pyruvate kinase (lmexpyk) | 1PKL, 3HQP and 3PP7 (p) | Protein kinase | Anti-leishmania | Kusunokinin | Polyphenols | Molegro virtual docker | −31.19 | [130] |
| Leishmania major phosphodiesterase 1 (lmajpde1) | 2R8Q (p) | Hydrolase | Anti-leishmania | Machaeriol B | Cannabinoids | Molegro virtual docker | −28.97 | [130] |
| Leishmania major tyrosyl-trna synthetase (lmajtyrrs) | 3P0H and 3P0J (p) | Ligase | Anti-leishmania | Bractein triacetate | Polyphenols | Molegro virtual docker | −33.08 | [130] |
| COVID-19 protease | 6LU7 (p) | Protease | Antiviral | CBD | Cannabinoids | AutoDock vina | −7.10 | [131] |
| Hiv-1 protease | ND | Protease | Antiviral | Cannflavin | Flavonoids | AutoDock vina | −9.70 | [132] |
| Human angiotensin-converting enzyme (ACE2) | 1R4L (p) | Protease | Antiviral | THC | Cannabinoids | AutoDock vina | −9.2 | [133] |
| 2019-ncov spike protein s2 subunit | 6LXT (p) | Surface glycoprotein | Antiviral | THC | Cannabinoids | AutoDock vina | −4.2 | [133] |
| SARS-CoV-2 mpro | ND | Protease | Antiviral | Cannabisin A | Lignanamide | AutoDock vina | −12.76 | [134] |
| 3c-like protease (C) | 6LU7 (p) | Protease | Antiviral | Hesperidin | Flavonoids | AutoDock vina | −8.3 | [135] |
| Nabiximols | Cannabinoids | −8 | ||||||
| Spike glycoprotein (S) | 6VXX (p) | Surface glycoprotein | Antiviral | Hesperidin | Flavonoids | AutoDock vina | −10.4 | [135] |
| Nabiximols | Cannabinoids | −10.2 | ||||||
| Inhibitor of nuclear factor kappa-b kinase subunit β (IKKbeta) | 3BRT (p) | Inhibitor kappa kinase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −7.99 | [136] |
| Mitogen-activated protein kinase 14 | 4IDT (p) | Map kinase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −7.35 | [136] |
| Cellular tumor antigen p53 | IAIE (p) | Tumor suppressor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −6.08 | [136] |
| Nf-kappa-b inhibitor alpha | 1IKN (p) | Nfkb inhibitor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.82 | [136] |
| Tnf receptor-associated factor 6 | 1IB6 (p) | Traf protein | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.74 | [136] |
| Transcription factor p65 | 1NFI (p) | Nfkb inhibitor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.66 | [136] |
| Epidermal growth factor receptor | 1MOX (p) | Epidermal growth factor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.64 | [136] |
| Nf-kappa-b essential modulator | 3BRV (p) | Nfkb inhibitor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.36 | [136] |
| Rac-alpha serine/threonine-protein kinase | 1UNQ (p) | Transferase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.34 | [136] |
| Mitogen-activated protein kinase 3 | 2O2V (p) | Map kinase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.34 | [136] |
| Poly [adp-ribose] polymerase 1 | 2COK (p) | DNA polymerase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −4.88 | [136] |
| Hypoxia-inducible factor 1-alpha | 1H2K (p) | Hypoxia inducible factor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −4.41 | [136] |
| Inhibitor of nuclear factor kappa-b kinase subunit a | 3BRT (p) | Inhibitor kappa kinase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −4.32 | [136] |
| Nuclear factor kappa-b p105 subunit | IMDI (p) | Nfkb inhibitor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −4.23 | [136] |
| G1/s-specific cyclin-d1 | 5VZU (p) | Proto-oncogene regulator | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −3.44 | [136] |
| Activator protein-1 (AP-1) | 1JUN (p) | Transcription factor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | -3.41 | [136] |
| Crystal structure of the DLC1 RhoGAP domain in liver cancer 1 | 3KUQ (p) | GTPase-activating proteins | Anticancer | CBC | Cannabinoids | Docking-server | −3.89 | [137] |
| THCV | Cannabinoids | −3.25 | ||||||
| CBGV | Cannabinoids | −3.21 | ||||||
| CBDA | Cannabinoids | −3.34 | ||||||
| Placental aromatase cytochrome p450 | 3EQM (p) | Cytochrome | Anticancer | CBDC1 | Cannabinoids | Molegro (Glide) | −9.03 | [138] |
| CBGV | Cannabinoids | −7.8 | ||||||
| CBCA | Cannabinoids | −7.73 | ||||||
| CBCVA | Cannabinoids | −7.45 | ||||||
| CBCV | Cannabinoids | −8.29 | ||||||
| CBDV | Cannabinoids | −8.34 | ||||||
| CBT | Cannabinoids | −7.86 | ||||||
| (Δ−9-THC) | Cannabinoids | −7.43 | ||||||
| CBR | Cannabinoids | −6.93 | ||||||
| Heat shock protein 90 (hsp90) | 2QG2 (p) | Chaperone protein | Anticancer | Guaiol | Terpenes | AutoDock vina | −10.80 | [139] |
| Topoisomerase II alpha | 5GWK (p) | Nuclear enzyme | Anticancer | 7-o-methylcyanidin | Flavonoids | Molegro (Glide) | −10.395 | [140] |
| Rutin | Flavonoids | −9.847 | ||||||
| Luteolin-7-o-glucoside | Flavonoids | −9.563 | ||||||
| Myricetin 7-glucoside | Flavonoids | −9.383 | ||||||
| Tumor necrosis factor-α (TNF-α) | 2E7A (p) | Tumor necrosis factor | Anticancer | Ascorbic acid | Vitamins | AutoDock vina | −5.4 | [141] |
| Linoleic acid | Terpenes | −3.8 | ||||||
| Tryptophan | Amino acids | −5.6 | ||||||
| Arachidonate 5-lypoxygenase | 5IR4 (p) | Lipoxygenase | Anticancer and anti-inflammatory | Δ-9-THC | Cannabinoids | Molecular operating environment | −4.57 | [142] |
| Δ-8-THC | Cannabinoids | −4.87 | ||||||
| CBC | Cannabinoids | −5.14 | ||||||
| CBG | Cannabinoids | −5.59 | ||||||
| CBL | Cannabinoids | −4.83 | ||||||
| CBD | Cannabinoids | −4.97 | ||||||
| Human myeloperoxidase (MPO) | 3F9P (p) | Peroxidase | Anti-inflammatory and degenerative processes | Peptide YGRDEISV | Proteins | Discovery studio | −114.6 | [143] |
| Peptide LDLVKPQ | Proteins | −82.8 | ||||||
| Angiotensin-converting enzyme (ACE) | 1O8A (p) | Protease | Hypertension | Peptide WVYY | Proteins | Accelrys discovery studio | −27.25 | [144] |
| Peptide WYT | Proteins | −21.99 | ||||||
| Renin | 2V0Z (p) | Protease | Hypertension | Peptide SVYT | Proteins | Accelrys discovery studio | −25.33 | [144] |
| Peptide WYT | Proteins | −19.12 | ||||||
| Angiotensin-converting enzyme (ACE) | 1O8A (p) | Protease | Hypertension | Peptide ALVY | Proteins | Accelrys discovery studio | −69.23 | [145] |
| Peptide LLVY | Proteins | −65.97 | ||||||
| Peptide LSTSTDVR | Proteins | −105.59 | ||||||
| Peptide LLAPHY | Proteins | −86.86 | ||||||
| Cannabinoid receptor 1 (CNR1) | P21554 (up) | G-protein coupled receptor | Epilepsy | 8b-hydroxy-δ9-trans-tetrahydrocannabinolate | Cannabinoids | Molegro (Glide) | −8.039 | [146] |
| Epilepsy | 10aa-hydroxy-10-oxo-d8-tetrahydrocannabinol | Cannabinoids | −8.52 | |||||
| Epilepsy | 10aα-hydroxyhexahydrocannabinol | Cannabinoids | −8.55 | |||||
| Epilepsy | CBNM | Cannabinoids | −8.761 | |||||
| Epilepsy | Cannabichromanone D | Cannabinoids | −8.446 | |||||
| Epilepsy | CBL | Cannabinoids | −8.336 | |||||
| Epilepsy | 5-acetoxy-6-geranyl-3-npentyl-1,4-benzoquinone | Cannabinoids | −8.571 | |||||
| Epilepsy | CBTC | Cannabinoids | −8.574 | |||||
| Androgen receptor (AR) | P10275 (up) | Nuclear receptor | Epilepsy | CBNM | Cannabinoids | Molegro (Glide) | −8.706 | [146] |
| Epilepsy | 5-acetoxy-6-geranyl-3-npentyl-1,4-benzoquinone | Cannabinoids | −8.262 | |||||
| Epilepsy | CBTC | Cannabinoids | −8.015 | |||||
| Glycogen synthase kinase-3 beta (GSK3B) | P49841 (up) | Protein kinase | Epilepsy | 8a-hydroxy-δ9-trans-tetrahydrocannabinolate | Cannabinoids | Molegro (Glide) | −6.439 | [146] |
| Epilepsy | 8b-hydroxy-δ9-trans-tetrahydrocannabinolate | Cannabinoids | −5.876 | |||||
| Epilepsy | CBL | Cannabinoids | −6.183 | |||||
| Albumin | P02768 (up) | Albumin | Epilepsy | (-)-trans-10-ethoxy-9-hydroxy-d6a(10a)-tetrahydrocannabinol | Cannabinoids | Molegro (Glide) | −6.429 | [146] |
| Epilepsy | CBNM | Cannabinoids | −6.306 | |||||
| Epilepsy | CBL | Cannabinoids | −6.057 | |||||
| Neurokinin 3 receptor (NK3R) | 1F88 (p) | Neurokinin receptor | Neuro-protective | CBD | Cannabinoids | AutoDock | −6.72 | [147] |
| CBG | Cannabinoids | −10.36 | ||||||
| Angiotensin-converting enzyme 2 (ACE2) | 6CS2 (p) | Protease | Neuro-protective | CBD | Cannabinoids | AutoDock vina | −8.9 | [148] |
| Interleukin-6 (IL6) | 1ALU (p) | Cytokine | Neuro-protective | CBD | Cannabinoids | AutoDock vina | −8.2 | [148] |
| Transmembrane protease serine 2 (TMPRSS2) | 3NPS (p) | Protease | Neuro-protective | CBN | Cannabinoids | AutoDock vina | −8.7 | [148] |
| Nrp1 protein | 7BP6 (p) | Semaphorin receptor | Neuro-protective | CBN | Cannabinoids | AutoDock vina | −8.5 | [148] |
| Tyrosine phosphatase-1b (PTP1B) | 1NWE (p) | Hydrolase | Dermo-cosmetic | Chrysophanol | Polyphenols | Charmm-based docker | −24.34 | [149] |
| P glycoprotein (P-GP) | 4Q9H (p) | GTPase | Multi-drug resistance | Cannabisin M | Lignanamide | AutoDock vina | −10.2 | [150] |
| Cannabisin N | Lignanamide | −10.2 | ||||||
| Cannabisin A | Lignanamide | −10.1 | ||||||
| Cannabisin B | Lignanamide | −10.1 | ||||||
| Cannabisin C | Lignanamide | −10.1 | ||||||
| Cannabisin D | Lignanamide | −10.1 | ||||||
| Plant Part | Extraction Method (Solvent) | Bioactive Metabolites | Biological Activity | Results | Reference |
|---|---|---|---|---|---|
| Seed | Ultrasound-assisted extraction (methanol–water 80:20) | Polyphenols | Antioxidant—DPPH Standard: (not used) | Inhibition activity = 74 ± 1% at 500 µL/mL | [83] |
| Seed | Maceration (ethanol–water 95:05) | Cannabinoids and polyphenols | Antioxidant—DPPH Standard: Ascorbic acid (IC50 = 0.012 ± 0.002 mg/mL) | IC50 = 14.39 ± 2.27 mg/mL | [155] |
| Seed | Maceration (ethanol–water 95:05) | Cannabinoids and polyphenols | Antioxidant—metal ion chelating assay Standard: EDTA (CC50 = 0.15 ± 0.002 mg/mL) | CC50 = 1.92 ± 1.05 mg/mL | [155] |
| Seed | Maceration (ethanol–water 95:05) | Cannabinoids and polyphenols | Antioxidant—lipid peroxidation inhibition Standard: α-tocopherol (IPC50 = 0.045 ± 0.002 mg/mL) | IPC50 = 92.68 ± 30.77 mg/mL | [155] |
| Seed | Maceration (ethanol–water 75:25) | Lignanamides | Antioxidant—DPPH Standard: Quercetin (IC50 = 25.5 µm) | Cannabisin M (IC50 = 69.5 µm) Cannabisin N and O (IC50 = ND) 3,3′-demethyl-heliotropamide (IC50 = 39.3 µm) | [156] |
| Seed | Maceration (ethanol–water 75:25) | Lignanamides | Antioxidant—ORAC Standard: Quercetin (IC50 = 0.40 µm) | Cannabisin M (IC50 = 6.61 µm) Cannabisin N and O (IC50 = ND) 3,3′-demethyl-heliotropamide (IC50 = 0.56 µm) | [156] |
| Seed | Maceration (ethanol–water 75:25) | Lignanamides | Antioxidant—ABTS Standard: Quercetin (IC50 = 9.19 µm) | Cannabisin M (IC50 = 74.70 µm) 3,3′-demethyl-heliotropamide (IC50 = 16.41 µm) | [156] |
| Seed oil | Acid/alkali extraction | Proteins | Antioxidant—DPPH Standard: (not used) | Alkali soluble proteins: - inhibition value = 73.33% after ht enzyme hydrolysis. Acid soluble proteins: - inhibition value = 68.67% after ht enzyme hydrolysis. | [157] |
| Seed flour | Ultrasound-assisted extraction (methanol–water 80:20) | Polyphenols | Antioxidant—DPPH Standard: (not used) | Inhibition activity = 67 ± 1% at 500 µL/mL | [83] |
| Seed oil | Ultrasound-assisted extraction (methanol–water 80:20) | Polyphenols | Antioxidant—DPPH Standard: (not used) | Inhibition activity = 22 ± 2% at 500 µL/mL | [112] |
| Seed | Maceration (ethanol–water 80:20) | Polyphenols | Antibacterial (against Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, Enterobacter aerogenes and Enterococcus faecalis) Standard: Gentamycin and vancomycin (MIC < 0.05 mg/mL) | All bacterial strains showed MIC values superior to 1 mg/mL | [158] |
| Seed | Maceration (ethanol–water 95:05) | Cannabinoids and polyphenols | Antibacterial (against Staphylococcus aureus) Standard: Erythromycin (ID = 26.67 ± 1.15 mm) | Inhibition diameter = 1.00 mm | [155] |
| Seed | Maceration (ethanol–water 80:20) | Polyphenols | Antibacterial (against Lactobacillus paracasei, Lactobacillus reuteri, Lactobacillus brevis, Lactobacillus plantarum, Bifidobacterium bifidum, Bifidobacterium longum and Bifidobacterium breve) Standard: Gentamycin and vancomycin (MIC < 0.05 mg/mL) | All bacterial strains showed MIC values superior to 1 mg/mL | [158] |
| Seed | Maceration (ethanol–water 95:05) | Terpenoid, phytocannabinoids and unsaturated fatty acids | Cytotoxicity—SRB assay Standard: (not used) | Percentages of human skin fibroblast viability 66.12 ± 7.63% at 1 mg/mL | [155] |
| Seed | Maceration (ethanol–water 95:05) | Terpenoids and flavonoids | Antiproliferative—SRB assay Standard: Doxorubicin (IC50 = 0.0042 ± 0.0025 mg/mL) | On hepg2 cell lines: IC50 = 12.07 ± 1.18 mg/mL | [155] |
| Seed oil | Ultrasound-assisted extraction (methanol–water 80:20) | Polyphenols | Antiproliferative Standard: (not used) | Reduction in caco-2 and ht-29 cell viability to less than 40% from 150 mg/mL | [83] |
| Seed | Maceration (ethanol–water 95:05) | Terpenoids and flavonoids | Anti-tyrosinase Standard: Kojic acid (IC50 = 0.005 ± 0.004 mg/mL) | IC50 = 0.07 ± 0.06 mg/mL | [155] |
| Seed | Maceration (ethanol–water 75:25) | Lignanamides | Acetyl choline esterase inhibition Standard: Galantamine (IC50 = 2.76 μm) | Cannabisin M, N and O (IC50 = nd) 3,3′-demethyl-heliotropamide (IC50 = 46.2 µm) | [156] |
| Leaf | Maceration (ethanol–water 95:05) | Cannabinoids and polyphenols | Antioxidant—DPPH Standard: Ascorbic acid (IC50 = 0.012 ± 0.002 mg/mL) | IC50 = 2.73 ± 0.422 mg/mL | [155] |
| Leaf | Maceration (ethanol–water 95:05) | Cannabinoids and polyphenols | Antioxidant—Metal ion chelating assay Standard: EDTA (CC50 = 0.15 ± 0.002 mg/mL) | CC50 = 0.93 ± 0.20 mg/mL | [155] |
| Leaf | Maceration (ethanol–water 95:05) | Cannabinoids and polyphenols | Antioxidant—Lipid peroxidation inhibition Standard: α-tocopherol (IPC50 = 0.045 ± 0.002 mg/mL) | IPC50 = 246.32 ± 69.38 mg/mL | [155] |
| Leaf | Ultrasound-assisted extraction (ethanol–water 80:20) | Polyphenols and flavonoids | Antioxidant—DPPH Standard: (not used) | Inhibition activity of 40% at 1000 µg/mL | [87] |
| Leaf | Maceration (acetone) | ND | Antibacterial Standard: Ampicillin (ID = 17.3 to 19.6 mm) | Inhibition diameters: Escherichia coli = 24.7 ± 1.5 mm Staphylococcus aureus = 19.6 ± 2.1 mm Pseudomonas aeruginosa = 19.0 ± 2.6 mm | [159] |
| Leaf | Maceration (chloroform) | ND | Antibacterial Standard: Ampicillin (ID = 17.3 to 19.6 mm) | Inhibition diameters: Escherichia coli = 23.0 ± 2.0 mm Staphylococcus aureus = 18.6 ± 2.08 mm Pseudomonas aeruginosa = 22.3 ± 1.52 mm | [159] |
| Leaf | Maceration (ethanol–water 60:40) | ND | Antibacterial Standard: Ampicillin (ID = 17.3 to 19.6 mm) | Inhibition diameters: Escherichia coli = 19.3 ± 1.2 mm Staphylococcus aureus = 18.6 ± 3.05 mm | [159] |
| Leaf | Maceration (ethanol–water 95:05) | Polyphenols, flavones and polyholozides | Antibacterial (against Staphylococcus mutans) Standard: Erythromycin (ID = 23 mm) | Inhibition diameter: 1.33 ± 0.58 mm | [155] |
| Leaf | Maceration (acetone) | ND | Antifungal Standard: (not used) | Inhibition diameters: Aspergillus niger = 21.3 ± 2 mm Fusarium spp. = 20 ± 2.64 mm | [159] |
| Leaf | Maceration (chloroform) | ND | Antifungal Standard: (not used) | Inhibition diameters: Aspergillus niger = 20.6 ± 1.5 mm Fusarium spp. = 18.3 ± 1.52 mm | [159] |
| Leaf | Maceration (ethanol–water 60:40) | ND | Antifungal Standard: (not used) | Inhibition diameters: Aspergillus niger = 23 ± 2 mm Fusarium spp. = 21.3 ± 3.21 mm | [159] |
| Leaf | Maceration (water) | ND | Antifungal Standard: (not used) | Inhibition diameters: Aspergillus niger = 21 ± 2.6 mm Fusarium spp. = 24.3 ± 3.51 mm | [159] |
| Leaf | Maceration (ethanol–water 95:05) | Polyphenols, flavones and polyholozides | Cytotoxicity—SRB assay Standard: (not used) | Percentages of human skin fibroblast viability 73.02 ± 3.57% at 1 mg/mL | [155] |
| Leaf | Ultrasound-assisted extraction (ethanol–water 80:20) | Polyphenols and flavonoids | Cytotoxicity Standard: (not used) | Viability of: HaCaT keratinocytes cells with 120% at 100 g/mL. BJ fibroblasts cells with 188% at 500 µg/mL. | [87] |
| Leaf | Maceration (ethanol–water 95:05) | Polyphenols, flavones and polyholozides | Antiproliferative—SRB assay Standard: Doxorubicin (IC50 = 0.0042 to 0.0274 mg/mL) | On hepg2 cell lines: IC50 = 13.17 ± 1.53mg/mL On kb cell lines: IC50 = 5.16 ± 1.66 mg/mL | [155] |
| Leaf | Maceration (ethanol–water 95:05) | Terpenoids and flavonoids | Anti-tyrosinase Standard: Kojic acid (IC50 = 0.005 ± 0.004 mg/mL) | IC50 = 0.049 ± 0.02 mg/mL | [155] |
| Leaf | Ultrasound-assisted extraction (ethanol–water 80:20) | Cannabinoids | Anti-elastase Standard (not used) | Inhibition value of 30% at 1000 µg/mL | [87] |
| Leaf | Ultrasound-assisted extraction (ethanol–water 80:20) | Cannabinoids | Anti-collagenase Standard: (not used) | Inhibition value of 80% at 1000 µg/mL | [87] |
| Leaf | Maceration (chloroform) | Cannabinoids (THC, CBD and CBN) | Anticoagulant Standard: (not used) | Inhibition values at 1 mg/mL: THC = 34.87% (IC50 = 1.79 mg/mL) CBN = 7.3% (IC50 = high value) | [160] |
| Flowers | Maceration (acetone–water 50:50) | Polyphenols | Antifungal (against Aspergillus favus) | Total inhibition at > 0.225 mg of DM/mL of medium | [161] |
| Flowers | Maceration (acetone–water 50:50) | Polyphenols | Anti-aflatoxigenic Standard: (not used) | Reduction in fungal growth by 36% at 7.2 mg DM/mL of medium | [161] |
| Inflorescences | Hydrodistillation (essential oil) | Terpenes | Insecticidal Standard: (not used) | Lethal concentration of LC50: Anopheles stephensi = 73.50 to 78.80 ppm for larvae. Anopheles gambiae = 20.13 to 67.19 ppm for pupae. | [162] |
| Inflorescences | Hydrodistillation (essential oil) | Terpenes | Cytotoxicity Standard: (not used) | IC50 values of: HaCaT cells = 2.23 ± 0.09 mg/mL Nhf a12 cells = 3.71 ± 0.2 mg/mL | [162] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) | Terpenes and polyphenols | Antioxidant—DPPH Standard: (not used) | Inhibition activity: Essential oil = 5.6 mg TE/g sample Aromatic water = 40 mg TE/g sample. | [94] |
| Aerial parts | Hydrodistillation (essential oil) | Terpenes | Antioxidant—DPPH Standard: Quercetin (IC50 = 1.1 ± 0.0 µg/mL) | IC50 = 1.6 ± 0.1 mg/mL | [163] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) | Terpenes and polyphenols | Antioxidant—FRAP Standard: (not used) | Inhibition activity: Essential oil = 57 mg TE/g sample Aromatic water = 83 mg TE/g sample. | [94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) | Terpenes and polyphenols | Antioxidant—metal chelating activity Standard: (not used) | Inhibition activity: Essential oil = 19.3 mg EDTAE/g sample Aromatic water = 3.8 mg mg EDTAE/g DW | [94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) | Terpenes and polyphenols | Antioxidant—phosphomolybdenum Standard: (not used) | Inhibition activity: Essential oil = 35.1 mmol TE/g of oil Aromatic water = 1.4 mmol TE/g DW | [94] |
| Aerial parts | Hydrodistillation (essential oil) | Terpenes | Antioxidant—reducing power assay Standard: Quercetin (EC50 = 2.3 ± 0.1 µg/mL) | EC50 = 1.8 ± 0.2 mg/mL | [163] |
| Aerial parts | Hydrodistillation (essential oil) | Terpenes | Antioxidant—β-carotene/linoleic acid Standard: Quercetin (EC50 = 0.9 ± 0.0 µg/mL) | EC50 = 0.9 ± 0.1 mg/mL | [163] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) | Terpenes and polyphenols | Antioxidant—CUPRAC Standard: (not used) | Inhibition activity: Essential oil = 141 mg TE/g sample Aromatic water = 109 mg TE/g sample. | [94] |
| Aerial parts | Hydrodistillation (essential oil) | Terpenes and polyphenols | Antibacterial (against Helicobacter pylori strains) Standard: Naringenin (MIC and MBC = 8–32 µg/mL) | MIC = 8–64 µg/mL MBC = 8–32 µg/mL | [94] |
| Aerial parts | Hydrodistillation (essential oil) | Terpenes and polyphenols | Antibacterial (against Staphylococcus aureus) Standard: (not used) | MIC = 8 mg/mL MBC = 16 mg/mL MBEC = 16–24 mg/mL | [94] |
| Aerial parts | Hydrodistillation (essential oil) | Terpenes | Antibacterial Standard: Ciprofloxacin (MIC = 0.015–1.00 mm) | Micrococcus luteus and Staphylococcus aureus: MIC = 4.7 mg/mL Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa: MIC = 1.2 mg/mL | [163] |
| Aerial parts | Hydrodistillation (essential oil) | Terpenes | Antifungal Standard: Fluconazole (MIC = 1.00 mm) | Candida albicans, Candida glabrata, Candida krusei and Candida parapsilosis: MIC = 9.5 mg/mL | [163] |
| Aerial parts | Hydrodistillation (essential oil) | Terpenes and polyphenols | Antifungal (against Candida spp. and Malassezia spp.) Standard: (not used) | MIC value > 12,460 µg/mL | [94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) | Terpenes and polyphenols | Cytotoxicity Standard: Doxorubicin (IC50 = 3.1–23.3 µg/mL) | Inhibition activity of 50%: IC50 (mda-mb-468) = 53.0 µg/mL IC50 (caco-2) = 28.7 µg/mL IC50 (mz-cha-1) = 22.3 µg/mL | [94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) | Terpenes and polyphenols | Antidiabetic Standard: (not used) | α-amylase inhibition: Essential oil = nd Aromatic water = 0.10 mmol ACAE/g extract α-glucosidase inhibition: Essential oil = 3.77 mmol ACAE/g oil Aromatic water = 0.17 mmol ACAE/g extract | [94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) | Terpenes and polyphenols | Acetyl- and butyryl-choline esterase inhibition Standard: (not used) | Acetyl-choline esterase inhibition: Essential oil = nd Aromatic water = 2.56 mg GALAE/g extract Butyryl-choline esterase inhibition: Essential oil = 3.4 mg GALAE/g oil Aromatic water = 3.48 mg GALAE/g extract | [94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) | Terpenes and polyphenols | Lipase inhibition Standard: (not used) | Inhibition activity: - essential oil = 70.14 mg OE/g oil - aromatic water = nd | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hourfane, S.; Mechqoq, H.; Bekkali, A.Y.; Rocha, J.M.; El Aouad, N. A Comprehensive Review on Cannabis sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities. Plants 2023, 12, 1245. https://doi.org/10.3390/plants12061245
Hourfane S, Mechqoq H, Bekkali AY, Rocha JM, El Aouad N. A Comprehensive Review on Cannabis sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities. Plants. 2023; 12(6):1245. https://doi.org/10.3390/plants12061245
Chicago/Turabian StyleHourfane, Sohaib, Hicham Mechqoq, Abdellah Yassine Bekkali, João Miguel Rocha, and Noureddine El Aouad. 2023. "A Comprehensive Review on Cannabis sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities" Plants 12, no. 6: 1245. https://doi.org/10.3390/plants12061245
APA StyleHourfane, S., Mechqoq, H., Bekkali, A. Y., Rocha, J. M., & El Aouad, N. (2023). A Comprehensive Review on Cannabis sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities. Plants, 12(6), 1245. https://doi.org/10.3390/plants12061245











