Comparison of In Vitro Antimelanoma and Antimicrobial Activity of 2,3-Indolo-betulinic Acid and Its Glycine Conjugates
Abstract
1. Introduction
2. Results
2.1. Chemical Synthesis of the Compounds
2.2. Cell Viability Assay
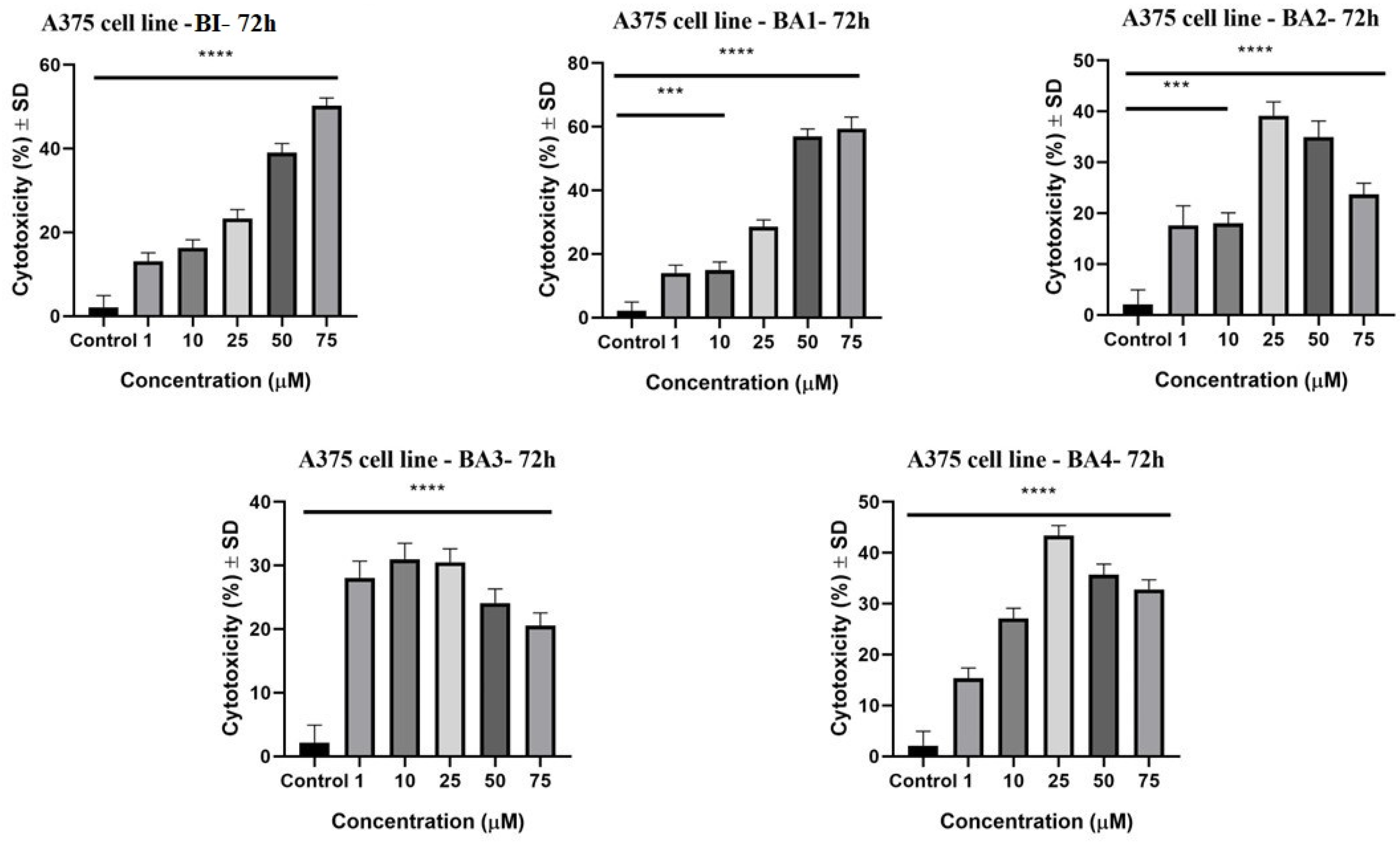
2.3. Evaluation of the Cytotoxic Potential by Lactate Dehydrogenase (LDH) Assay
2.4. Anti-Migratory Activity Evaluation Using the Scratch Assay Method
2.5. Antimicrobial Activity Assays
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Instruments
4.1.2. Synthesis
4.2. Cell Culture
4.3. Cellular Viability
4.4. Evaluation of the Cytotoxic Potential by Lactate Dehydrogenase (LDH) Assay
4.5. Anti-Migratory Potential—Scratch Assay Method
4.6. Antimicrobial Activity Assays
4.6.1. Kirby Bauer Disk Diffusion Method
4.6.2. Broth Dilution Method for Determination of MIC and MBC/MFC
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddiqui, A.J.; Jahan, S.; Singh, R.; Saxena, J.; Ashraf, S.A.; Khan, A.; Choudhary, R.K.; Balakrishnan, S.; Badraoui, R.; Bardakci, F.; et al. Review Article Plants in Anticancer Drug Discovery: From Molecular Mechanism to Chemoprevention. BioMed Res. Int. 2022, 2022, 5425485. [Google Scholar] [CrossRef]
- Majolo, F.; de Oliveira Becker Delwing, L.K.; Marmitt, D.J.; Bustamante-Filho, I.C.; Goettert, M.I. Medicinal Plants and Bioactive Natural Compounds for Cancer Treatment: Important Advances for Drug Discovery. Phytochem. Lett. 2019, 31, 196–207. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Chikezie, P.C. Herbal Medicine: Yesterday, Today and Tomorrow. Altern. Integr. Med. 2015, 4, 195. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants-Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules 2018, 23, 2300. [Google Scholar] [CrossRef]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulin Is a Potent Anti-Tumor Agent That Is Enhanced by Cholesterol. PLoS ONE 2009, 4, e1. [Google Scholar] [CrossRef]
- Saaby, L.; Jäger, A.K.; Moesby, L.; Hansen, E.W.; Christensen, S.B. Isolation of Immunomodulatory Triterpene Acids from a Standardized Rose Hip Powder (Rosa canina L.). Phytother. Res. 2011, 25, 195–201. [Google Scholar] [CrossRef]
- Prasad, S.; Kalra, N.; Shukla, Y. Hepatoprotective Effects of Lupeol and Mango Pulp Extract of Carcinogen Induced Alteration in Swiss Albino Mice. Mol. Nutr. Food Res. 2007, 51, 352–359. [Google Scholar] [CrossRef]
- Huang, L.; Guan, T.; Qian, Y.; Huang, M.; Tang, X.; Li, Y.; Sun, H. Anti-Inflammatory Effects of Maslinic Acid, a Natural Triterpene, in Cultured Cortical Astrocytes via Suppression of Nuclear Factor-Kappa B. Eur. J. Pharmacol. 2011, 672, 169–174. [Google Scholar] [CrossRef]
- Pavel, I.Z.; Danciu, C.; Oprean, C.; Dehelean, C.A.; Muntean, D.; Csuk, R.; Muntean, D.M. In Vitro Evaluation of the Antimicrobial Ability and Cytotoxicity on Two Melanoma Cell Lines of a Benzylamide Derivative of Maslinic Acid. Anal. Cell. Pathol. 2016, 2016, 2787623. [Google Scholar] [CrossRef]
- Ghiulai, R.; Roşca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macaşoi, I.; Olariu, T.; Dehelean, C.; Creţu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef]
- Prodea, A.; Mioc, A.; Banciu, C.; Trandafirescu, C.; Milan, A.; Racoviceanu, R.; Ghiulai, R.; Mioc, M.; Soica, C. The Role of Cyclodextrins in the Design and Development of Triterpene-Based Therapeutic Agents. Int. J. Mol. Sci. 2022, 23, 736. [Google Scholar] [CrossRef]
- Xu, H.; Ji, L.; Yu, C.; Chen, Q.; Ge, Q.; Lu, Y. MiR-423-5p Regulates Cells Apoptosis and Extracellular Matrix Degradation via Nucleotide-Binding, Leucine-Rich Repeat Containing X1 (NLRX1) in Interleukin 1 Beta (IL-1β)-Induced Human Nucleus Pulposus Cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e922497-1. [Google Scholar] [CrossRef]
- Manu, K.A.; Kuttan, G. Ursolic Acid Induces Apoptosis by Activating P53 and Caspase-3 Gene Expressions and Suppressing NF-ΚB Mediated Activation of Bcl-2 in B16F-10 Melanoma Cells. Int. Immunopharmacol. 2008, 8, 974–981. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, Q.; Xiang, H.; Liu, M.; Zhang, Q.; Xue, W.; Song, B.; Yang, S. Antiproliferative Activity and Apoptosis-Inducing Mechanism of Constituents from Toona Sinensis on Human Cancer Cells. Cancer Cell Int. 2013, 13, 1. [Google Scholar] [CrossRef]
- Gheorgheosu, D.; Duicu, O.; Dehelean, C.; Soica, C.; Muntean, D. Betulinic Acid as a Potent and Complex Antitumor Phytochemical: A Minireview. Anticancer. Agents Med. Chem. 2014, 14, 936–945. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Hmadi, R.; Kareh, M.; Tohme, R.; Darwiche, N. Cell Death Mechanisms of Plant-Derived Anticancer Drugs: Beyond Apoptosis. Apoptosis 2015, 20, 1531–1562. [Google Scholar] [CrossRef]
- Ali-Seyed, M.; Jantan, I.; Vijayaraghavan, K.; Bukhari, S.N.A. Betulinic Acid: Recent Advances in Chemical Modifications, Effective Delivery, and Molecular Mechanisms of a Promising Anticancer Therapy. Chem. Biol. Drug Des. 2016, 87, 517–536. [Google Scholar] [CrossRef]
- Coricovac, D.; Dehelean, C.A.; Pinzaru, I.; Mioc, A.; Aburel, O.M.; Macasoi, I.; Draghici, G.A.; Petean, C.; Soica, C.; Boruga, M.; et al. Assessment of Betulinic Acid Cytotoxicity and Mitochondrial Metabolism Impairment in a Human Melanoma Cell Line. Int. J. Mol. Sci. 2021, 22, 4870. [Google Scholar] [CrossRef]
- Sarek, J.; Kvasnica, M.; Vlk, M.; Urban, M.; Dzubak, P.; Hajduch, M. The Potential of Triterpenoids in the Treatment of Melanoma. Res. Melanoma Glimpse Curr. Dir. Future Trends 2011, 1, 125–158. [Google Scholar] [CrossRef]
- Honda, T.; Rounds, B.V.; Bore, L.; Finlay, H.J.; Favaloro, F.G.; Suh, N.; Wang, Y.; Sporn, M.B.; Gribble, G.W. Synthetic Oleanane and Ursane Triterpenoids with Modified Rings A and C: A Series of Highly Active Inhibitors of Nitric Oxide Production in Mouse Macrophages. J. Med. Chem. 2000, 43, 4233–4246. [Google Scholar] [CrossRef]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and Rexinoids as Multifunctional Agents for the Prevention and Treatment of Cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef]
- Ikeda, T.; Sporn, M.; Honda, T.; Gribble, G.W.; Kufe, D. The Novel Triterpenoid CDDO and Its Derivatives Induce Apoptosis by Disruption of Intracellular Redox Balance. Cancer Res. 2003, 63, 5551–5558. [Google Scholar]
- Wang, Y.-Y.; Yang, Y.-X.; Zhe, H.; He, Z.-X.; Zhou, S.-F. Bardoxolone Methyl (CDDO-Me) as a Therapeutic Agent: An Update on Its Pharmacokinetic and Pharmacodynamic Properties. Drug Des. Devel. Ther. 2014, 2075–2088. [Google Scholar]
- Borella, R.; Forti, L.; Gibellini, L.; De Gaetano, A.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Synthesis and Anticancer Activity of CDDO and CDDO-Me, Two Derivatives of Natural Triterpenoids. Molecules 2019, 24, 4097. [Google Scholar] [CrossRef]
- Lombrea, A.; Scurtu, A.D.; Avram, S.; Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Dehelean, C.A.; Soica, C.; Danciu, C. Anticancer Potential of Betulonic Acid Derivatives. Int. J. Mol. Sci. 2021, 22, 3676. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Zhang, Y.-X.; Sun, Y.-R.; Zhang, D. Qualitative and Quantitative Methods of Betulonic Acid in Fruits of Liquidambar Formosana. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2005, 30, 1168–1170. [Google Scholar]
- Mutai, C.; Abatis, D.; Vagias, C.; Moreau, D.; Roussakis, C.; Roussis, V. Cytotoxic Lupane-Type Triterpenoids from Acacia Mellifera. Phytochemistry 2004, 65, 1159–1164. [Google Scholar] [CrossRef]
- Chiang, Y.-M.; Chang, J.-Y.; Kuo, C.-C.; Chang, C.-Y.; Kuo, Y.-H. Cytotoxic Triterpenes from the Aerial Roots of Ficus Microcarpa. Phytochemistry 2005, 66, 495–501. [Google Scholar] [CrossRef]
- Kim, D.S.H.L.; Chen, Z.; van Nguyen, T.; Pezzuto, J.M.; Qiu, S.; Lu, Z.-Z. A Concise Semi-Synthetic Approach to Betulinic Acid from Betulin. Synth. Commun. 1997, 27, 1607–1612. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Boreko, E.I.; Nigmatullina, L.R.; Tret′yakova, E.V.; Pavlova, N.I.; Baltina, L.A.; Nikolaeva, S.N.; Savinova, O.V.; Eremin, V.F.; Galin, F.Z.; et al. Synthesis and Antiviral Activity of Betulonic Acid Amides and Conjugates with Amino Acids. Russ. J. Bioorganic Chem. 2004, 30, 80–88. [Google Scholar] [CrossRef]
- Pavlova, N.I.; Savinova, O.V.; Nikolaeva, S.N.; Boreko, E.I.; Flekhter, O.B. Antiviral Activity of Betulin, Betulinic and Betulonic Acids against Some Enveloped and Non-Enveloped Viruses. Fitoterapia 2003, 74, 489–492. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Tolstikov, G.A.; Medvedeva, N.I.; Utkina, T.M.; Kartashova, O.L. Synthesis, Modification, and Antimicrobial Activity of the N-Methylpiperazinyl Amides of Triterpenic Acids. Russ. J. Bioorganic Chem. 2010, 36, 383–389. [Google Scholar] [CrossRef]
- Haque, S.; Nawrot, D.A.; Alakurtti, S.; Ghemtio, L.; Yli-Kauhaluoma, J.; Tammela, P. Screening and Characterisation of Antimicrobial Properties of Semisynthetic Betulin Derivatives. PLoS ONE 2014, 9, e102696. [Google Scholar] [CrossRef]
- Dinh Ngoc, T.; Moons, N.; Kim, Y.; De Borggraeve, W.; Mashentseva, A.; Andrei, G.; Snoeck, R.; Balzarini, J.; Dehaen, W. Synthesis of Triterpenoid Triazine Derivatives from Allobetulone and Betulonic Acid with Biological Activities. Bioorg. Med. Chem. 2014, 22, 3292–3300. [Google Scholar] [CrossRef]
- Hsu, C.L.; Fang, S.C.; Huang, H.W.; Yen, G.C. Anti-Inflammatory Effects of Triterpenes and Steroid Compounds Isolated from the Stem Bark of Hiptage Benghalensis. J. Funct. Foods 2015, 12, 420–427. [Google Scholar] [CrossRef]
- Vasilevsky, S.F.; Govdi, A.I.; Shults, E.E.; Shakirov, M.M.; Sorokina, I.V.; Tolstikova, T.G.; Baev, D.S.; Tolstikov, G.A.; Alabugin, I.V. Efficient Synthesis of the First Betulonic Acid-Acetylene Hybrids and Their Hepatoprotective and Anti-Inflammatory Activity. Bioorg. Med. Chem. 2009, 17, 5164–5169. [Google Scholar] [CrossRef]
- Sorokina, I.V.; Tolstikova, T.G.; Zhukova, N.A.; Petrenko, N.I.; Schults, E.E.; Uzenkova, N.V.; Grek, O.R.; Pozdnyakova, S.V.; Tolstikov, G.A. Betulonic Acid and Derivatives, a New Group of Agents Reducing Side Effects of Cytostatics. Dokl. Biol. Sci. 2004, 399, 434–437. [Google Scholar] [CrossRef]
- Semenov, D.E.; Zhukova, N.A.; Ivanova, E.P.; Sorokina, I.V.; Baiev, D.S.; Nepomnyashchikh, G.I.; Tolstikova, T.G.; Biryukova, M.S. Hepatoprotective Properties of Betulonic Acid Amide and Heptral in Toxic Liver Injury Induced by Carbon Tetrachloride in Combination with Ethanol. Bull. Exp. Biol. Med. 2015, 158, 336–341. [Google Scholar] [CrossRef]
- Anikina, L.V.; Tolmacheva, I.A.; Vikharev, Y.B.; Grishko, V.V. The Immunotropic Activity of Lupane and Oleanane 2,3-Seco-Triterpenoids. Russ. J. Bioorg. Chem. 2010, 36, 240–244. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Medvedeva, N.I.; Lopatina, T.V.; Apryshko, G.N.; Pugacheva, R.B.; Yavorskaya, N.P.; Golubeva, I.S.; Tolstikov, G.A. Synthesis and the Antineoplastic Activity of Imidazolides of Betulonic Acid. Russ. J. Bioorg. Chem. 2015, 41, 305–314. [Google Scholar] [CrossRef]
- Borkova, L.; Adamek, R.; Kalina, P.; Drašar, P.; Dzubak, P.; Gurska, S.; Rehulka, J.; Hajduch, M.; Urban, M.; Sarek, J. Synthesis and Cytotoxic Activity of Triterpenoid Thiazoles Derived from Allobetulin, Methyl Betulonate, Methyl Oleanonate, and Oleanonic Acid. ChemMedChem 2017, 12, 390–398. [Google Scholar] [CrossRef]
- Ledeţi, I.; Avram, Ş.; Bercean, V.; Vlase, G.; Vlase, T.; Ledeţi, A.; Zupko, I.; Mioc, M.; Şuta, L.M.; Şoica, C.; et al. Solid-State Characterization and Biological Activity of Betulonic Acid Derivatives. Molecules 2015, 20, 22691–22702. [Google Scholar] [CrossRef]
- Yang, S.J.; Liu, M.C.; Zhao, Q.; Hu, D.Y.; Xue, W.; Yang, S. Synthesis and Biological Evaluation of Betulonic Acid Derivatives as Antitumor Agents. Eur. J. Med. Chem. 2015, 96, 58–65. [Google Scholar] [CrossRef]
- Kerru, N.; Bhaskaruni, S.V.H.S.; Gummidi, L.; Maddila, S.N.; Maddila, S.; Jonnalagadda, S.B. Recent Advances in Heterogeneous Catalysts for the Synthesis of Imidazole Derivatives. Synth. Commun. 2019, 49, 2437–2459. [Google Scholar] [CrossRef]
- Kalaria, P.N.; Karad, S.C.; Raval, D.K. A Review on Diverse Heterocyclic Compounds as the Privileged Scaffolds in Antimalarial Drug Discovery. Eur. J. Med. Chem. 2018, 158, 917–936. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Apryshko, G.N.; Kukovinets, O.S.; Kazakova, O.B. Synthesis and Cytotoxicity of Indole Derivatives of Betulin, Erythrodiol, and Uvaol. Russ. J. Bioorganic Chem. 2018, 44, 322–329. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, N.; Aggarwal, P.; Sanna, V.K.; Singh, A.T.; Jaggi, M.; Joshi, N.; Sharma, P.K.; Irchhaiya, R.; Burman, A.C. Synthesis and Cytotoxic Activity of Heterocyclic Ring-Substituted Betulinic Acid Derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 5058–5062. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Haque, A.; Brazeau, D.; Amin, A.R. Perspectives on Natural Compounds in Chemoprevention and Treatment of Cancer: An Update with New Promising Compounds. Eur. J. Cancer 2021, 149, 165–183. [Google Scholar] [CrossRef]
- Hanson, J.R. The Pentacyclic Triterpenes. Pentacyclic Triterpenes Promis. Agents Cancer 2011, 1–11. [Google Scholar]
- Ghiulai, R.; Avram, S.; Stoian, D.; Pavel, I.Z.; Coricovac, D.; Oprean, C.; Vlase, L.; Farcas, C.; Mioc, M.; Minda, D.; et al. Lemon Balm Extracts Prevent Breast Cancer Progression in Vitro and in Ovo on Chorioallantoic Membrane Assay. Evid. Based Complement. Altern. Med. 2020, 2020, 6489159. [Google Scholar] [CrossRef]
- Banerjee, S.; Bose, S.; Mandal, S.C.; Dawn, S.; Sahoo, U.; Ramadan, M.A.; Mandal, S.K. Pharmacological Property of Pentacyclic Triterpenoids. Egypt. J. Chem. 2019, 62, 13–35. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Hossain, M.T.; Rahaman, M.A.; Rahman, P.; Hasan, M.S.; Das, R.C.; Khan, M.K.; Sikder, M.H.; Alam, M.; Uddin, M.J.; et al. Molecular Pharmacology and Therapeutic Advances of the Pentacyclic Triterpene Lupeol. Phytomedicine 2022, 99, 154012. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Abeni, D.; Boyle, P.; Melchi, C.F. Meta-Analysis of Risk Factors for Cutaneous Melanoma: I. Common and Atypical Naevi. Eur. J. Cancer 2005, 41, 28–44. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Zanetti, R.; Masini, C.; Boyle, P.; Melchi, C.F. Meta-Analysis of Risk Factors for Cutaneous Melanoma: III. Family History, Actinic Damage and Phenotypic Factors. Eur. J. Cancer 2005, 41, 2040–2059. [Google Scholar] [CrossRef]
- MacKie, R.M.; Hauschild, A.; Eggermont, A.M.M. Epidemiology of Invasive Cutaneous Melanoma. Ann. Oncol. 2009, 20, vi1–vi7. [Google Scholar] [CrossRef]
- Mouawad, R.; Sebert, M.; Michels, J.; Bloch, J.; Spano, J.-P.; Khayat, D. Treatment for Metastatic Malignant Melanoma: Old Drugs and New Strategies. Crit. Rev. Oncol. Hematol. 2010, 74, 27–39. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Das, P.J.; Pal, P.; Mazumder, B. Topical Delivery of Paclitaxel for Treatment of Skin Cancer. Drug Dev. Ind. Pharm. 2016, 42, 1482–1494. [Google Scholar] [CrossRef]
- Aydoğmuş-Öztürk, F.; Jahan, H.; Öztürk, M.; Günaydın, K.; Choudhary, M.I. Preclinical Study of the Medicinal Plants for the Treatment of Malignant Melanoma. Mol. Biol. Rep. 2020, 47, 5975–5983. [Google Scholar] [CrossRef]
- Wu, P.; Tu, B.; Liang, J.; Guo, S.; Cao, N.; Chen, S.; Luo, Z.; Li, J.; Zheng, W.; Tang, X.; et al. Synthesis and Biological Evaluation of Pentacyclic Triterpenoid Derivatives as Potential Novel Antibacterial Agents. Bioorg. Chem. 2021, 109, 104692. [Google Scholar] [CrossRef]
- Wang, C.-M.; Chen, H.-T.; Wu, Z.-Y.; Jhan, Y.-L.; Shyu, C.-L.; Chou, C.-H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia Scholaris. Molecules 2016, 21, 139. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Khalitova, R.R.; Nedopekina, D.A.; Gubaidullin, R.R. Antimicrobial Properties of Amine- and Guanidine-Functionalized Derivatives of Betulinic, Ursolic and Oleanolic Acids: Synthesis and Structure/Activity Evaluation. Steroids 2020, 154, 108530. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Brunel, J.M.; Khusnutdinova, E.F.; Negrel, S.; Giniyatullina, G.V.; Lopatina, T.V.; Petrova, A.V. A-Ring-Modified Triterpenoids and Their Spermidine–Aldimines with Strong Antibacterial Activity. Molbank 2019, 2019, M1078. [Google Scholar] [CrossRef]
- Khlebnicova, T.S.; Piven, Y.A.; Baranovsky, A.V.; Lakhvich, F.A.; Shishkina, S.V.; Zicāne, D.; Tetere, Z.; Rāviņa, I.; Kumpiņš, V.; Rijkure, I. Synthesis of Novel Lupane Triterpenoid-Indazolone Hybrids with Oxime Ester Linkage. Steroids 2017, 117, 77–89. [Google Scholar] [CrossRef]
- Levdanskii, V.A.; Levdanskii, A.V.; Kuznetsov, B.N. Sulfonation of Betulinic Acid by Sulfamic Acid. Chem. Nat. Compd. 2015, 51, 894–896. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Thu, H.N.T.; Tu, A.L.T.; Thanh, T.N.; Thi, C.B.; Babkov, D.A.; Kazakova, O.B. Structural Modifications of 2,3-Indolobetulinic Acid: Design and Synthesis of Highly Potent α-Glucosidase Inhibitors. Bioorg. Chem. 2019, 88, 102957. [Google Scholar] [CrossRef]
- Mukherjee, R.; Kumar, V.; Srivastava, S.K.; Agarwal, S.K.; Burman, A.C. Betulinic Acid Derivatives as Anticancer Agents: Structure Activity Relationship. Anticancer. Agents Med. Chem. 2008, 6, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Wunder, M.B.; Norris, D.A.; Shellman, Y.G. A Simple Protocol for Using a LDH-Based Cytotoxicity Assay to Assess the Effects of Death and Growth Inhibition at the Same Time. PLoS ONE 2011, 6, e26908. [Google Scholar] [CrossRef] [PubMed]
- Barreto Vianna, D.R.; Gotardi, J.; Baggio Gnoatto, S.C.; Pilger, D.A. Natural and Semisynthetic Pentacyclic Triterpenes for Chronic Myeloid Leukemia Therapy: Reality, Challenges and Perspectives. ChemMedChem 2021, 16, 1835–1860. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Dong, W.; Guo, Q.; Li, X.; Huang, L. The Importance of Indole and Azaindole Scaffold in the Development of Antitumor Agents. Eur. J. Med. Chem. 2020, 203, 112506. [Google Scholar] [CrossRef]
- Khwaza, V.; Mlala, S.; Oyedeji, O.O.; Aderibigbe, B.A. Pentacyclic Triterpenoids with Nitrogen-Containing Heterocyclic Moiety, Privileged Hybrids in Anticancer Drug Discovery. Molecules 2021, 26, 2401. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Dmitrenko, V.V. Pitfalls of the MTT Assay: Direct and off-Target Effects of Inhibitors Can Result in over/Underestimation of Cell Viability. Gene 2015, 574, 193–203. [Google Scholar] [CrossRef]
- Liebscher, G.; Vanchangiri, K.; Mueller, T.; Feige, K.; Cavalleri, J.-M.; Paschke, R. In Vitro Anticancer Activity of Betulinic Acid and Derivatives Thereof on Equine Melanoma Cell Lines from Grey Horses and Invivo Safety Assessment of the Compound NVX-207 in Two Horses. Chem. Biol. Interact. 2016, 246, 20–29. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Cabaj, J.; Bąk, W.; Bargieł, J.; Grabarska, A.; Góralczyk, A.; Łuszczki, J.J. Additive Interactions between Betulinic Acid and Two Taxanes in In Vitro Tests against Four Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 9641. [Google Scholar] [CrossRef]
- Jeong, H.J.; Chai, H.B.; Park, S.Y.; Kim, D.S.H.L. Preparation of Amino Acid Conjugates of Betulinic Acid with Activity against Human Melanoma. Bioorg. Med. Chem. Lett. 1999, 9, 1201–1204. [Google Scholar] [CrossRef]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In Vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 107. [Google Scholar] [CrossRef]
- Coricovac, D.; Pînzaru, I.; Avram, Ș.; Macașoi, I.; Șoica, C.; Dehelean, C. In Vitro and In Ovo Assessment of Betulinic Acid Antimelanoma Effect. Timisoara Med. J. 2020, 2020, 1. [Google Scholar] [CrossRef]
- Liu, M.-C.; Yang, S.-J.; Jin, L.-H.; Hu, D.-Y.; Xue, W.; Song, B.-A.; Yang, S. Synthesis and Cytotoxicity of Novel Ursolic Acid Derivatives Containing an Acyl Piperazine Moiety. Eur. J. Med. Chem. 2012, 58, 128–135. [Google Scholar] [CrossRef]
- Yang, S.; Liang, N.; Li, H.; Xue, W.; Hu, D.; Jin, L.; Zhao, Q.; Yang, S. Design, Synthesis and Biological Evaluation of Novel Betulinic Acid Derivatives. Chem. Cent. J. 2012, 6, 141. [Google Scholar] [CrossRef]
- Tian, T.; Liu, X.; Lee, E.-S.; Sun, J.; Feng, Z.; Zhao, L.; Zhao, C. Synthesis of Novel Oleanolic Acid and Ursolic Acid in C-28 Position Derivatives as Potential Anticancer Agents. Arch. Pharm. Res. 2017, 40, 458–468. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, C.; Shi, J.; Hou, X.; Feng, B.; Zhao, L. Design, Synthesis, and Biofunctional Evaluation of Novel Pentacyclic Triterpenes Bearing O-[4-(1-Piperazinyl)-4-Oxo-Butyryl Moiety as Antiproliferative Agents. Bioorg. Med. Chem. Lett. 2015, 25, 4500–4504. [Google Scholar] [CrossRef]
- V Patel, R.; Won Park, S. An Evolving Role of Piperazine Moieties in Drug Design and Discovery. Mini Rev. Med. Chem. 2013, 13, 1579–1601. [Google Scholar] [CrossRef]
- Giniyatullina, G.V.; Kazakova, O.B. Synthesis and Cytotoxicity of Lupane Mono- and Bis-Piperazinylamides. Chem. Nat. Compd. 2021, 57, 698–705. [Google Scholar] [CrossRef]
- Ciorîță, A.; Suciu, M.; Macavei, S.; Kacso, I.; Lung, I.; Soran, M.-L.; Pârvu, M. Green Synthesis of Ag-MnO(2) Nanoparticles Using Chelidonium Majus and Vinca Minor Extracts and Their In Vitro Cytotoxicity. Molecules 2020, 25, 819. [Google Scholar] [CrossRef]
- Ghițu, A.; Schwiebs, A.; Radeke, H.H.; Avram, S.; Zupko, I.; Bor, A.; Pavel, I.Z.; Dehelean, C.A.; Oprean, C.; Bojin, F. A Comprehensive Assessment of Apigenin as an Antiproliferative, Proapoptotic, Antiangiogenic and Immunomodulatory Phytocompound. Nutrients 2019, 11, 858. [Google Scholar] [CrossRef]
- Pacheco, A.G.; Alcântara, A.F.C.; Abreu, V.G.C.; Corrêa, G.M. Relationships between Chemical Structure and Activity of Triterpenes against Gram-Positive and Gram-Negative Bacteria. In A Search Antibacterials Agents; InTech: Rijeka, Croatia, 2012; pp. 1–24. [Google Scholar]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of Oxidative Stress on the Antibacterial Activity of Betulin, Betulinic Acid and Ursolic Acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef]
- Carvalho, A.R.; Martins, A.L.D.B.; Cutrim, B.D.S.; Santos, D.M.; Maia, H.S.; Da Silva, M.S.M.; Zagmignan, A.; Silva, M.R.C.; Monteiro, C.D.A.; Guilhon, G.M.S.P.; et al. Betulinic Acid Prevents the Acquisition of Ciprofloxacin-Mediated Mutagenesis in Staphylococcus Aureus. Molecules 2019, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, Oleanolic and Betulinic Acids: Antibacterial Spectra and Selectivity Indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Holmes, S.S.; Baker, G.A.; Challa, S.; Bose, H.S.; Song, Z. Ionic Derivatives of Betulinic Acid as Novel HIV-1 Protease Inhibitors. J. Enzyme Inhib. Med. Chem. 2012, 27, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Mierina, I.; Vilskersts, R.; Turks, M. Delivery Systems for Birch-Bark Triterpenoids and Their Derivatives in Anticancer Research. Curr. Med. Chem. 2020, 27, 1308–1336. [Google Scholar] [CrossRef]
- Lugiņina, J.; Linden, M.; Bazulis, M.; Kumpiņš, V.; Mishnev, A.; Popov, S.A.; Golubeva, T.S.; Waldvogel, S.R.; Shults, E.E.; Turks, M. Electrosynthesis of Stable Betulin-Derived Nitrile Oxides and Their Application in Synthesis of Cytostatic Lupane-Type Triterpenoid-Isoxazole Conjugates. Eur. J. Org. Chem. 2021, 2021, 2557–2577. [Google Scholar] [CrossRef]
- Tsepaeva, O.V.; Nemtarev, A.V.; Salikhova, T.I.; Abdullin, T.I.; Grigor`eva, L.R.; Khozyainova, S.A.; Mironov, V.F. Synthesis, Anticancer, and Antibacterial Activity of Betulinic and Betulonic Acid C-28-Triphenylphosphonium Conjugates with Variable Alkyl Linker Length. Anticancer. Agents Med. Chem. 2019, 20, 286–300. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Mustafin, A.G.; Babkov, D.A.; Sokolova, E.V.; Spasov, A.A. Evaluation of Cytotoxicity and A-glucosidase Inhibitory Activity of Amide and Polyamino-derivatives of Lupane Triterpenoids. Molecules 2020, 25, 4833. [Google Scholar] [CrossRef]
- LV15140B-Method for Purification of Betulonic Acid-Google Patents. Available online: https://patents.google.com/patent/LV15140B/en?oq=LV15140B (accessed on 3 March 2023).
- Danciu, C.; Zupko, I.; Bor, A.; Schwiebs, A.; Radeke, H.; Hancianu, M.; Cioanca, O.; Alexa, E.; Oprean, C.; Bojin, F.; et al. Botanical Therapeutics: Phytochemical Screening and Biological Assessment of Chamomile, Parsley and Celery Extracts against A375 Human Melanoma and Dendritic Cells. Int. J. Mol. Sci. 2018, 19, 3624. [Google Scholar] [CrossRef]
- Danciu, C.; Muntean, D.; Alexa, E.; Farcas, C.; Oprean, C.; Zupko, I.; Bor, A.; Minda, D.; Proks, M.; Buda, V.; et al. Phytochemical Characterization and Evaluation of the Antimicrobial, Antiproliferative and Pro-Apoptotic Potential of Ephedra Alata Decne. Hydroalcoholic Extract against the MCF-7 Breast Cancer Cell Line. Molecules 2019, 24, 13. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Prundeanu, M.; Berger, D.; Deaconu, M.; Matei, C.; Oprea, O.; Vasile, E.; Negreanu-Pîrjol, T.; Muntean, D.; Danciu, C. Properties of Salvia Officinalis L. and Thymus Serpyllum L. Extracts Free and Embedded into Mesopores of Silica and Titania Nanomaterials. Nanomaterials 2020, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Corina, D.; Bojin, F.; Ambrus, R.; Muntean, D.; Soica, C.; Paunescu, V.; Cristea, M.; Pinzaru, I.; Dehelean, C. Physico-Chemical and Biological Evaluation of Flavonols: Fisetin, Quercetin and Kaempferol Alone and Incorporated in Beta Cyclodextrins. Anti-Cancer Agents Med. Chem. 2017, 17, 615–626. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.; EUCAST-AFST. EUCAST Technical Note on the EUCAST Definitive Document EDef 7.2: Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Patel, J.B.; Bobenchik, A.M. Clinical and Laboratory Standards Institute. Perform. Stand. Antimicrob. Susceptibility Test. M 2018, 100, 148–149. [Google Scholar]
- Wayne, P.A. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts. CLSI m44-a 2004, 23. [Google Scholar]



| Compound | IC50 (μM) |
|---|---|
| BI | 19.2 ± 0.5 |
| BA1 | 5.7 ± 0.9 |
| BA2 | 13.7 ± 0.8 |
| BA3 | 10.0 ± 0.8 |
| BA4 | 19.6 ± 0.7 |
| Microbial Strains | Test Compounds | Disk Diffusion Method (Inhibition Zones in mm) | MIC (µg/mL) | MBC or MFC (µg/mL) |
|---|---|---|---|---|
| Streptococcus pyogenes ATCC 19615 | BA1 BA2 BA3 BA4 BI Levofloxacin DMSO | 9 17 16 17 13 28 9 | - 16.33 14.58 13.16 - - - | - 32.66 29.16 26.33 - - - |
| Staphylococcus aureus ATCC 25923 | BA1 BA2 BA3 BA4 BI Levofloxacin DMSO | 9 17 16 17 9 27 8 | - 32.66 29.16 26.33 - - - | - - - - - - - |
| Escherichia coli ATCC 25922 | BA1 BA2 BA3 BA4 BI Levofloxacin DMSO | 7 7 8 7 7 29 7 | - - - - - - - | - - - - - - - |
| Pseudomonas aeruginosa ATCC 27853 | BA1 BA2 BA3 BA4 BI Levofloxacin DMSO | 7 7 7 7 7 20 7 | - - - - - - - | - - - - - - - |
| Candida albicans ATCC 10231 | BA1 BA2 BA3 BA4 BI Fluconazole DMSO | 9 7 16 7 8 16 8 | - - 29.16 - - - - | - - - - - - - |
| Candida parapsilosis ATCC 22019 | BA1 BA2 BA3 BA4 BI Fluconazole DMSO | 7 7 16 7 9 17 7 | - - 29.16 - - - - | - - - - - - - |
| Numbering in the Manuscript | Structural Formula | Trivial Name | CAS Name and Number for Previously Described Compounds |
|---|---|---|---|
| BI | 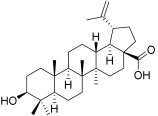 | Betulinic acid | 3β-Hydroxy-20(29)-lupaene-28-oic acid (472-15-1) |
| BA1 |  | N-(2,3-Indolo-betulinoyl)diglycylglycine | - |
| BA2 | 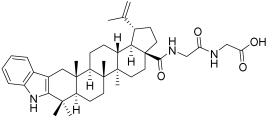 | N-(2,3-Indolo-betulinoyl)glycylglycine | - |
| BA3 | 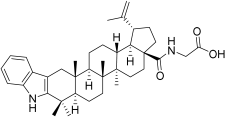 | N-(2,3-Indolo-betulinoyl)glycine | N-(28-Oxo-1′H-lupa-2,20(29)-dieno[3,2-b]indol-28-yl)glycine (905838-14-4) |
| BA4 |  | 2,3-Indolo-betulinic acid | 1’H-lupa-2,20(29)-dieno[3,2-b]indol-28-oic acid(905837-93-6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombrea, A.; Semenescu, A.-D.; Magyari-Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Muntean, D.; Dehelean, C.A.; Dinu, S.; Danciu, C. Comparison of In Vitro Antimelanoma and Antimicrobial Activity of 2,3-Indolo-betulinic Acid and Its Glycine Conjugates. Plants 2023, 12, 1253. https://doi.org/10.3390/plants12061253
Lombrea A, Semenescu A-D, Magyari-Pavel IZ, Turks M, Lugiņina J, Peipiņš U, Muntean D, Dehelean CA, Dinu S, Danciu C. Comparison of In Vitro Antimelanoma and Antimicrobial Activity of 2,3-Indolo-betulinic Acid and Its Glycine Conjugates. Plants. 2023; 12(6):1253. https://doi.org/10.3390/plants12061253
Chicago/Turabian StyleLombrea, Adelina, Alexandra-Denisa Semenescu, Ioana Zinuca Magyari-Pavel, Māris Turks, Jevgeņija Lugiņina, Uldis Peipiņš, Delia Muntean, Cristina Adriana Dehelean, Stefania Dinu, and Corina Danciu. 2023. "Comparison of In Vitro Antimelanoma and Antimicrobial Activity of 2,3-Indolo-betulinic Acid and Its Glycine Conjugates" Plants 12, no. 6: 1253. https://doi.org/10.3390/plants12061253
APA StyleLombrea, A., Semenescu, A.-D., Magyari-Pavel, I. Z., Turks, M., Lugiņina, J., Peipiņš, U., Muntean, D., Dehelean, C. A., Dinu, S., & Danciu, C. (2023). Comparison of In Vitro Antimelanoma and Antimicrobial Activity of 2,3-Indolo-betulinic Acid and Its Glycine Conjugates. Plants, 12(6), 1253. https://doi.org/10.3390/plants12061253













