Abstract
Sclareol, a diterpene, has a wide range of physiological effects on plants, such as antimicrobial activity; disease resistance against pathogens; and the expression of genes encoding proteins involved in metabolism, transport, and phytohormone biosynthesis and signaling. Exogenous sclareol reduces the content of chlorophyll in Arabidopsis leaves. However, the endogenous compounds responsible for sclareol-induced chlorophyll reduction remain unknown. The phytosterols campesterol and stigmasterol were identified as compounds that reduce the content of chlorophyll in sclareol-treated Arabidopsis plants. The exogenous application of campesterol or stigmasterol dose-dependently reduced the content of chlorophyll in Arabidopsis leaves. Exogenously-applied sclareol enhanced the endogenous contents of campesterol and stigmasterol and the accumulation of transcripts for phytosterol biosynthetic genes. These results suggest that the phytosterols campesterol and stigmasterol, the production of which is enhanced in response to sclareol, contribute to reductions in chlorophyll content in Arabidopsis leaves.
Keywords:
sclareol; Arabidopsis; chlorosis; chlorophyll reduction; phytosterols; campesterol; stigmasterol 1. Introduction
Disease control strategies that utilize a plant’s natural resistance to pathogens are attractive to researchers because of the potential to reduce environmental impact. Some of the main materials used in disease control techniques are disease-resistance-inducing compounds, characterized by the capability to induce resistance to a broad range of pathogens without targeted antimicrobial activity. Many natural and synthetic compounds have been identified as disease-resistance-inducing compounds [1,2,3,4], among which the diterpene sclareol is attractive due to its physiological activities.
Pharmacological studies show that sclareol exhibits antimicrobial activity against plant pathogenic bacteria and fungi [5,6,7,8,9]. The exogenous application of sclareol induces the expression of genes encoding proteins involved in disease resistance, metabolism, transport, and phytohormone biosynthesis and signaling in Nicotiana spp. and Arabidopsis (Arabidopsis thaliana) [10,11,12]. Sclareol also induces resistance to a bacterial pathogen and plant-parasitic nematode in tobacco (Nicotiana tabacum), tomato (Solanum lycopersicum), and Arabidopsis plants without antibacterial or nematicidal activity [13,14]. While sclareol is produced in only a limited number of plant species, such as clary sage (Salvia sclarea) and Nicotiana species [15,16,17], it has been suggested that an ethylene signaling pathway mediates sclareol-induced disease resistance not only in tobacco, but also in Arabidopsis [13,14], a plant that does not produce sclareol. These findings indicate that a mechanism for sclareol-responsive signal transduction exists in plants.
Our research group recently reported that when exogenous sclareol was applied to Arabidopsis plants, the leaves developed chlorosis-like symptoms that were accompanied by a reduction in chlorophyll content [18]. However, whether such symptoms are related to sclareol-induced disease resistance remains unclear. As the first step to clarify the physiological meaning of sclareol-induced chlorosis-like symptoms, we tested the assumption that in response to exogenously-applied sclareol, Arabidopsis plants produce endogenous substances that decrease the content of chlorophyll. Herein, we identified and isolated phytosterols as chlorophyll-content-reducing substances.
2. Results and Discussion
2.1. Isolation and Identification of Chlorophyll-Content-Reducing Substances
First, we examined whether chlorophyll-content-reducing substances could be extracted with organic solvents. Because our preliminary experiments showed that in response to exogenous sclareol, four-week-old Arabidopsis plants exhibited an increase in chlorophyll reduction compared to eight-week-old plants, the growth stage used in our previous study [18], four-week-old plants were used in this study. We compared two common solvents, acetone and methanol, for extraction. When an acetone extract was applied to Arabidopsis plants, a significant reduction in chlorophyll was reproducibly observed. Such a reduction was not observed with the methanol extraction, although the cause is unknown. Therefore, we proceeded with an acetone extraction system in further experiments. To confirm whether chlorophyll-content-reducing substances exist in sclareol-untreated plants, we treated plants with 0.1% methanol—the solvent used to dissolve sclareol—which we characterized as the “mock treatment”. These samples were also subjected to the acetone extraction system in the same way. Chlorophyll content was lower in leaves treated with the acetone extract from sclareol-treated plants than in leaves treated with the acetone extract from the mock treatment (Supplementary Figure S1). As a negative control, we also measured chlorophyll content in plants that were treated with only 0.1% methanol and not the acetone extract from sclareol-treated or mock-treated plants. There was no difference in chlorophyll content between those in 0.1% methanol and the mock treatment. These results suggest that endogenous substances that are involved in sclareol-induced chlorophyll reduction are acetone-extractable. Hereafter, we used a 0.1% methanol treatment as the negative control.
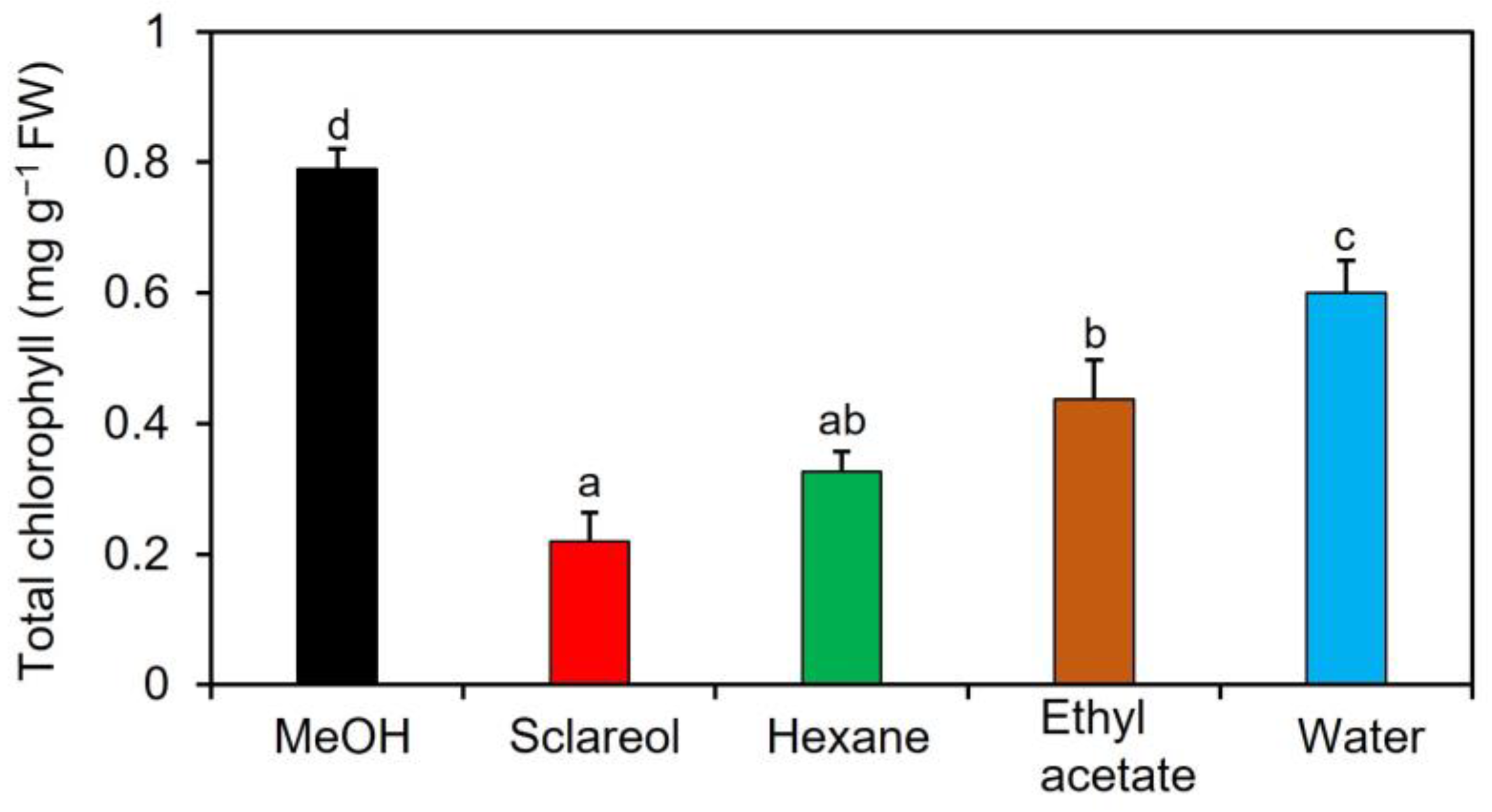
To purify and isolate acetone-extractable substances, we performed a large-scale extraction with three different solvents: ethyl acetate, hexane, and water. The content of chlorophyll was lower in all other fractions than in 0.1% methanol (Figure 1).
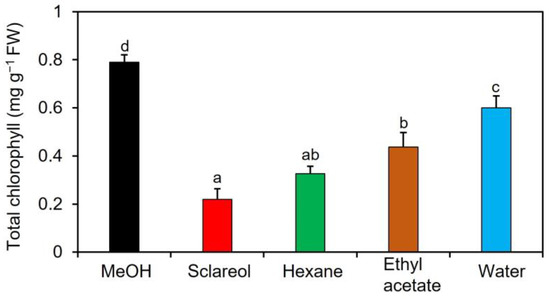
Figure 1.
Effects of fractions obtained from an acetone extract of sclareol-treated plants on the accumulation of chlorophyll in Arabidopsis leaves. Sclareol-treated Arabidopsis plants were extracted with acetone. The extract was divided into hexane-, ethyl acetate-, and water-soluble fractions and applied to Arabidopsis plants by soaking their roots in a solution containing each fraction for 48 h. The leaves of the treated plants were collected and the contents of total chlorophyll, represented as the sum of chlorophyll a and b, were measured. As a negative control, 0.1% methanol (MeOH) was used, as previously described in this section. We used 100 μM sclareol as a positive control. Values are the means ± standard deviation of three biological replicates. Different letters indicate significant differences among treatments (Tukey’s test, p < 0.05).
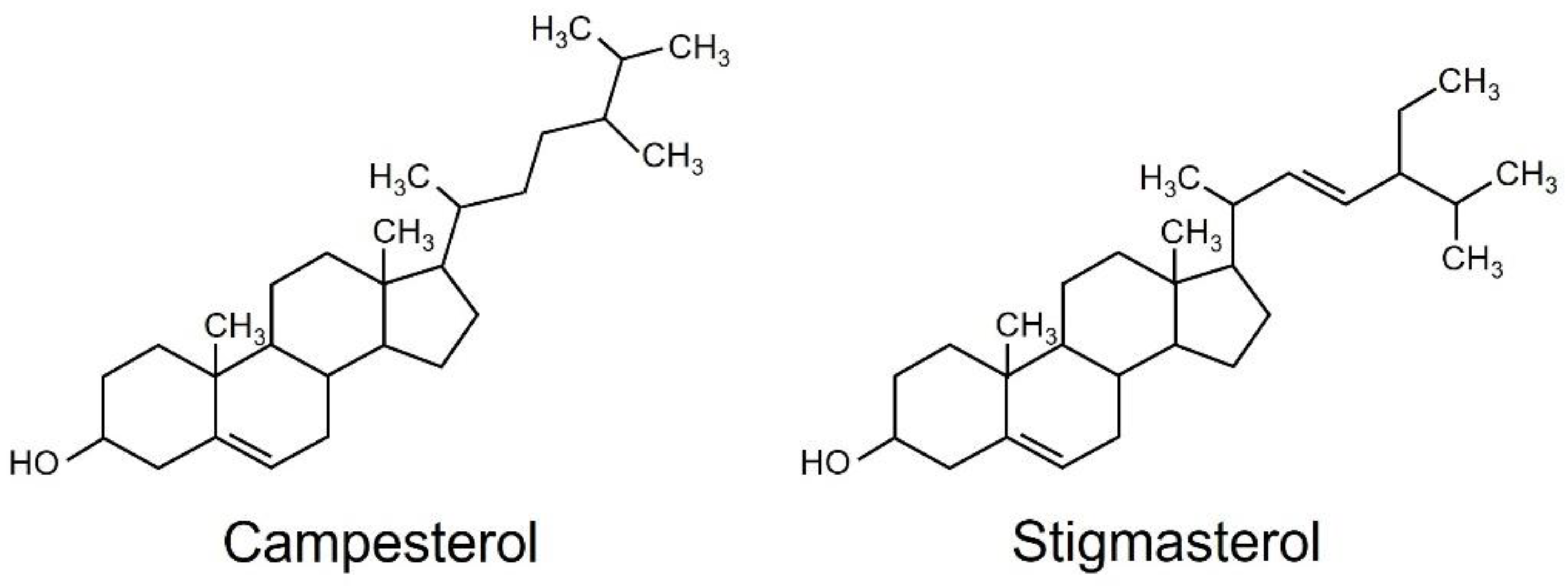
The hexane-soluble fraction exhibited slightly higher activity for reducing the content of chlorophyll than the ethyl acetate-soluble fraction. Therefore, we focused on the hexane-soluble fraction for further purification. The hexane-soluble fraction was fractionated by chromatography on a silica gel column followed by a C18-based solid-phase extraction (SPE) column. The active fractions obtained by SPE were subjected to preparative thin-layer chromatography (TLC) or silica gel column chromatography (Supplementary Figure S2). Activity to reduce the content of chlorophyll was detected in two fractions: compound one was obtained from TLC, and compound two from silica gel column chromatography. These two active fractions were collected and purified. Resonances in 1H- and 13C-nuclear magnetic resonance (NMR) spectra for compounds 1 and 2 were assigned to campesterol and stigmasterol, respectively (Figure 2). Campesterol and stigmasterol are phytosterols that belong to isoprenoids, the largest class of natural compounds which play essential roles in plant growth and development, such as seed germination, architecture, and reproduction [19].
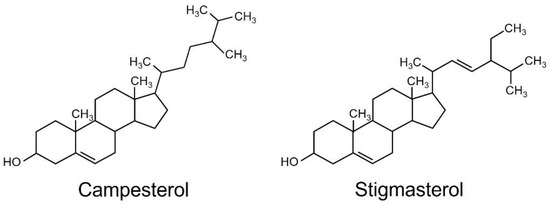
Figure 2.
Structures of campesterol and stigmasterol.
2.2. Effects of Campesterol and Stigmasterol on Chlorophyll Reductions
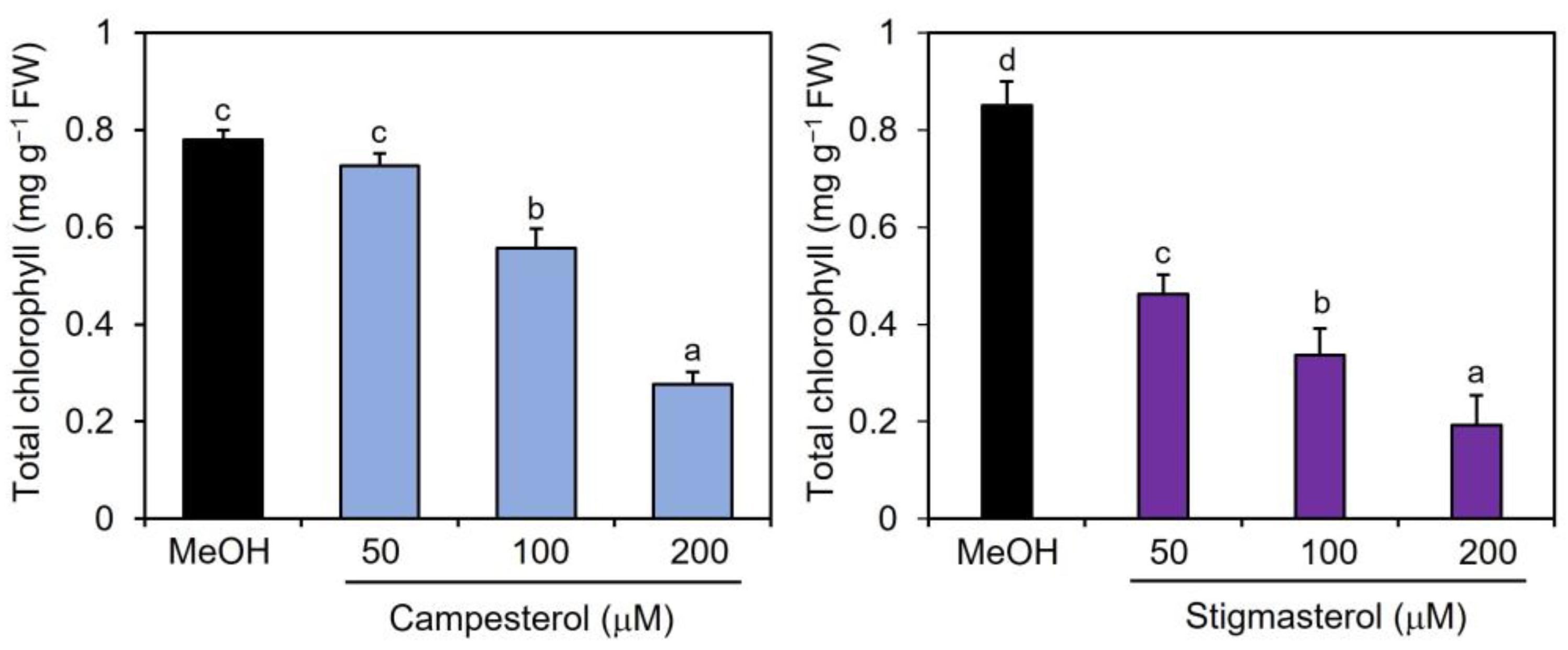
To examine the effects of campesterol and stigmasterol on chlorophyll reduction, Col-0 plants were treated by immersing their roots in a solution containing various concentrations (50, 100, and 200 μM) of campesterol or stigmasterol for 48 h, after which the chlorophyll contents of the treated plant leaves were measured. The effects of campesterol on chlorophyll reductions were detected at 100 μM or higher (Figure 3). Stigmasterol induced dose-dependent reductions in chlorophyll content. These results suggest that exogenously applied campesterol and stigmasterol reduced the chlorophyll content in Arabidopsis leaves.
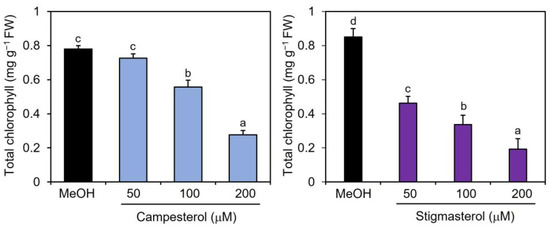
Figure 3.
Effects of campesterol and stigmasterol on the accumulation of chlorophyll in Arabidopsis leaves. Arabidopsis plants were treated by soaking their roots in a solution containing various concentrations of campesterol or stigmasterol for 48 h. The leaves of the treated plants were collected and the total chlorophyll content, represented as the sum of chlorophyll a and b, was measured. As a negative control, 0.1% methanol (MeOH) was used. Values are the means ± standard deviation of three biological replicates. Different letters indicate significant differences among treatments (Tukey’s test, p < 0.05). The experiment was repeated three times with similar results.
2.3. Effects of Sclareol on Accumulation of Campesterol and Stigmasterol, and Expression of Phytosterol Biosynthetic Genes
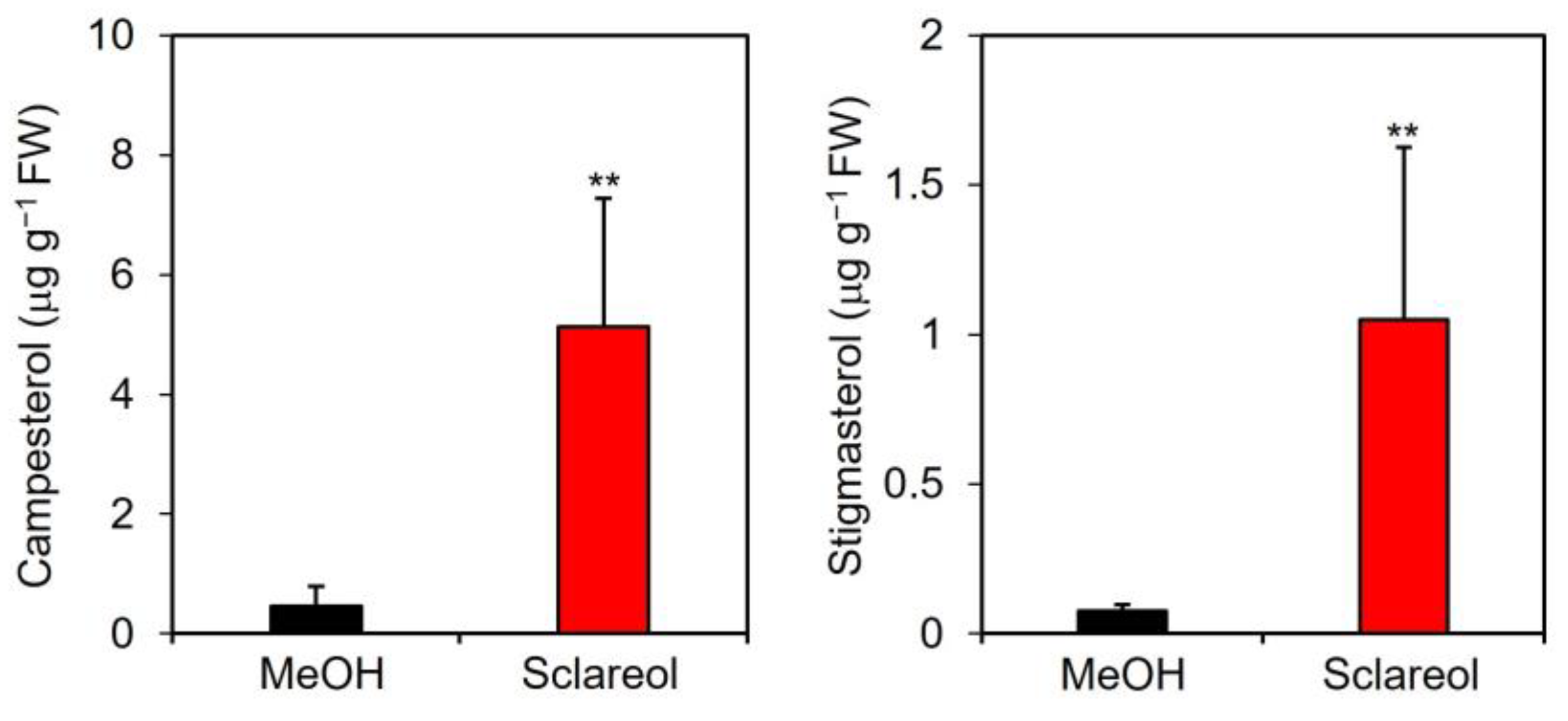
A quantitative analysis showed that endogenous levels of campesterol and stigmasterol were approximately 11- and 15-fold higher, respectively, in sclareol-treated leaves than in methanol-treated leaves (Figure 4).
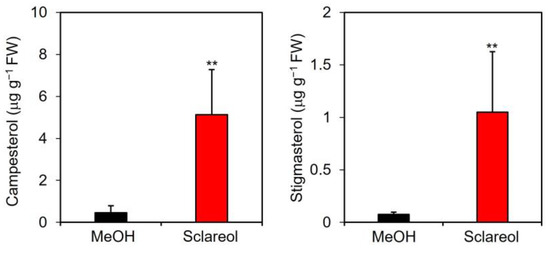
Figure 4.
Effects of exogenous sclareol on the accumulation of campesterol and stigmasterol in Arabidopsis leaves. Arabidopsis plants were treated by soaking their roots in a solution containing 100 μM sclareol for 48 h. The leaves of the treated plants were collected and subjected to the measurement of campesterol and stigmasterol. As a control, 0.1% methanol (MeOH) was used. Values are the means ± standard deviation of three biological replicates. Asterisks denote significant differences from the 0.1% methanol sample (t-test, ** p < 0.01). The experiment was repeated three times with similar results.
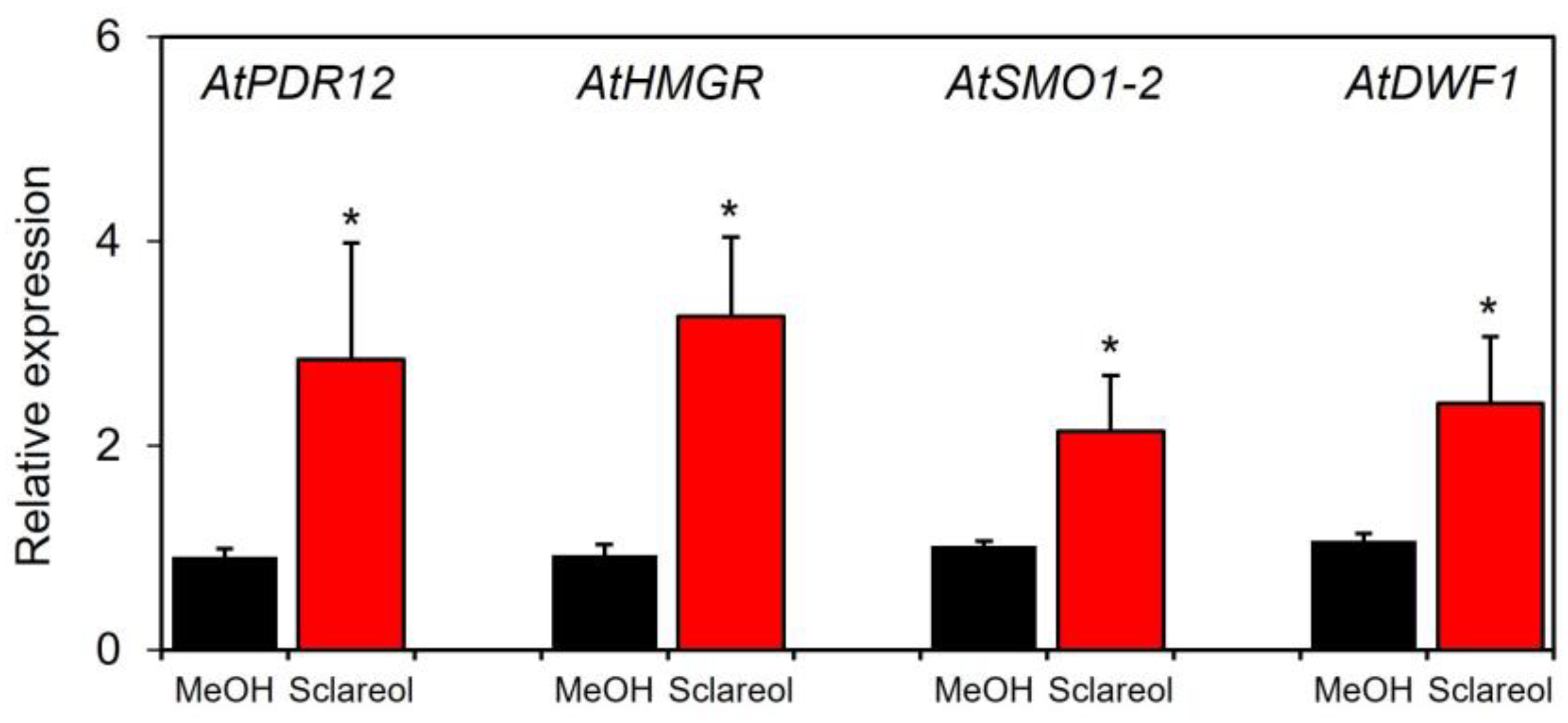
To examine whether sclareol-induced accumulation of campesterol and stigmasterol was associated with the induction of phytosterol biosynthetic genes, induction kinetics in sclareol-treated leaves were assessed for 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), reductase (HMGR), sterol 4α-methyl oxidase1 (SMO1), and sterol C-24 reductase (DWF1). HMGR catalyzes the conversion of HMG-CoA to mevalonate, a precursor of phytosterols [20]. SMO1 and DWF1 are involved in the biosynthesis of phytosterols, including campesterol and stigmasterol [21,22,23]. We also used AtPDR12, a sclareol-responsive gene [10,13], as a positive control to confirm the functionality of the experimental system. The treatment with sclareol enhanced transcripts for AtHMGR, AtSMO1-2, and AtDWF1 in Col-0 leaves (Figure 5). Sclareol-induced accumulation of AtPDR12 transcripts was also observed.
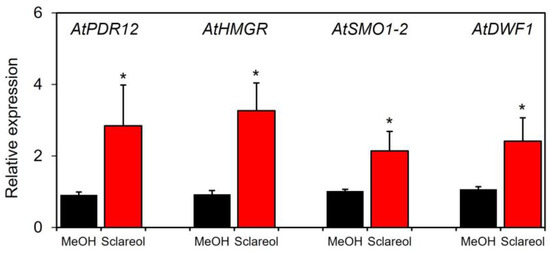
Figure 5.
Effects of exogenous sclareol on the expression of phytosterol biosynthetic genes in Arabidopsis leaves. Arabidopsis plants were treated by soaking their roots in a solution containing 100 μM sclareol for 48 h. The leaves of the treated plants were collected and subjected to a quantitative real-time PCR analysis. As a control, 0.1% methanol (MeOH) was used. Values are the means ± standard deviation of three biological replicates. Asterisks denote significant differences from the negative control (0.1% methanol) sample (t-test, * p < 0.05). The experiment was repeated three times with similar results.
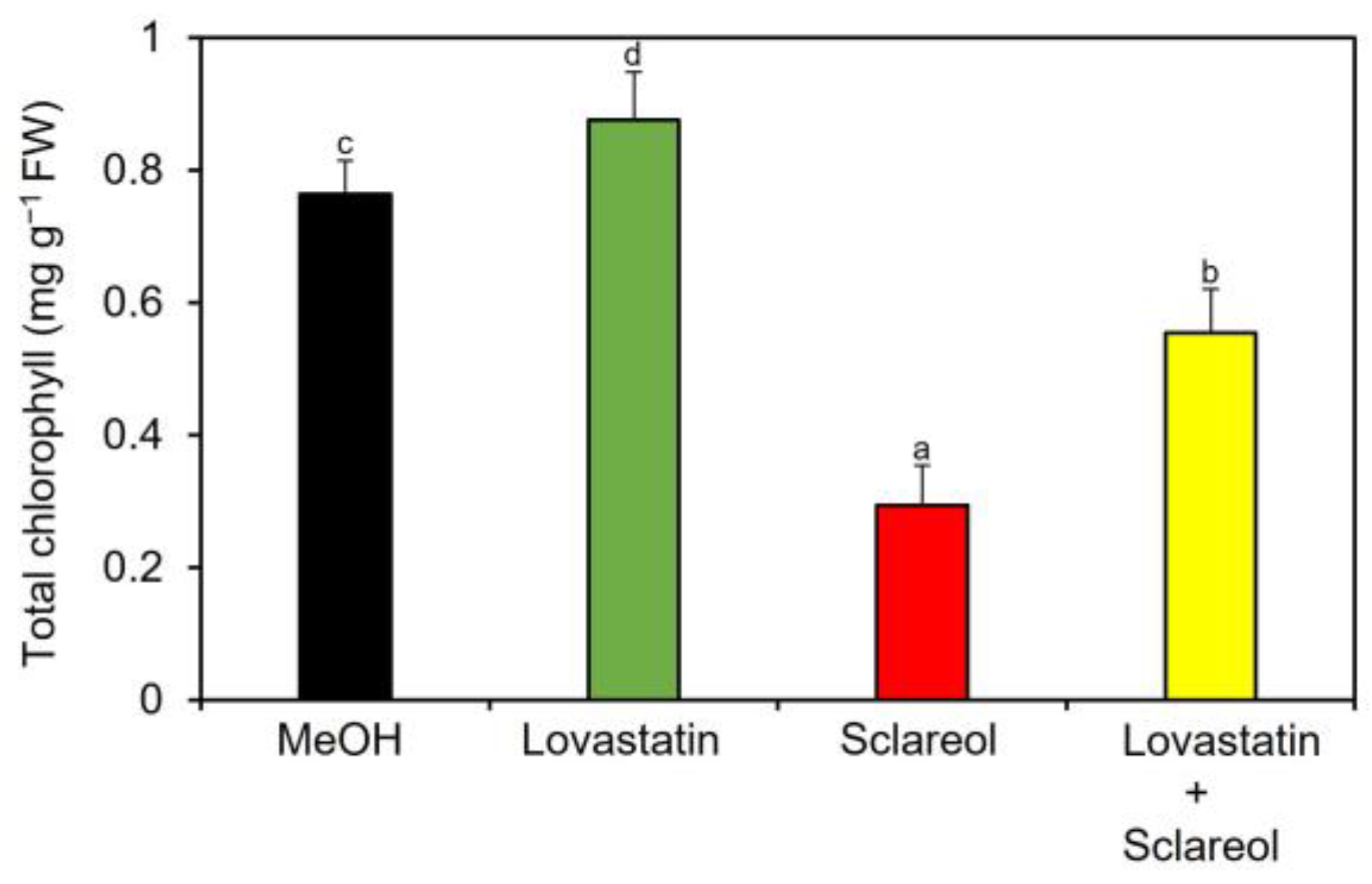
To examine whether phytosterols are involved in sclareol-mediated reduction of chlorophyll, the effect of lovastatin, an inhibitor of HMGR, on chlorophyll reduction in the presence of sclareol was tested. When lovastatin was only applied to plants, an increase in chlorophyll content was observed (Figure 6; MeOH vs. lovastatin), which is consistent with previous findings showing that lovastatin increases chlorophyll content when applied to Arabidopsis seedlings [24]. Sclareol-induced reduction in chlorophyll content was partially restored by lovastatin (Figure 6; sclareol vs. lovastatin + sclareol).
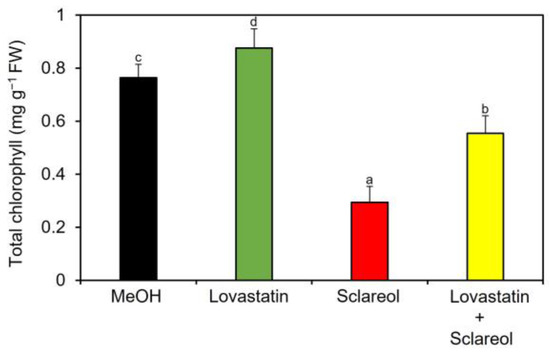
Figure 6.
Effect of lovastatin on sclareol-induced reduction in chlorophyll. Arabidopsis plants were treated by soaking their roots in a solution containing 100 μM lovastatin, 100 μM sclareol, or a 50:50 mixture of lovastatin and sclareol for 48 h. The leaves of the treated plants were collected and the contents of total chlorophyll, as represented as the sum of chlorophyll a and b, were measured. As a control, 0.1% methanol (MeOH) was used. Values are the means ± standard deviation of three biological replicates. Different letters indicate significant differences among treatments (Tukey’s test, p < 0.05). The experiment was repeated three times with similar results.
Collectively, these results show that the production of stigmasterol and campesterol in Arabidopsis leaves is enhanced by the up-regulated expression of phytosterol biosynthetic genes, such as AtHMGR, AtSMO1-2, and AtDWF1, in response to sclareol, suggesting that accumulated campesterol and stigmasterol contribute to a reduction in chlorophyll content. These results are consistent with previous findings showing that Arabidopsis mutants with mutations in phytosterol homeostasis genes had elevated levels of phytosterols, including campesterol-related and stigmasterol-related compounds, and showed early senescence [25,26].
During solvent–solvent fractionation after the acetone extraction of sclareol-treated plants, we detected chlorophyll-content-reducing activity in ethyl acetate- and water-soluble fractions, suggesting that substances other than campesterol and stigmasterol are also involved in chlorophyll reductions. To identify these active substances, future studies are warranted.
3. Materials and Methods
3.1. Plant Materials
Arabidopsis Columbia (Col-0) was used in this study. Arabidopsis plants were grown in a hydroponic system. Seeds were sown on half-strength Murashige and Skoog medium (Wako Pure Chemical, Osaka, Japan) supplemented with 0.8% agar and grown under a cycle of 8 h of light and 16 h of dark with a photon flux density of 120 μ mol m−2 s−1 at 22 °C. Ten-day-old seedlings were grown on a sheet of a polyethylene raft through holes (1 cm in diameter) floating over a 1500-fold diluted liquid fertilizer (Hyponex). The fertilizer was changed weekly, and their roots were soaked in the fertilizer under the light conditions described above. Unless otherwise stated, four-week-old plants were used.
3.2. Extraction, Fractionation, and Purification of Active Substances
3.2.1. Small-Scale Extraction
The roots of four-week-old Arabidopsis Col-0 wild-type plants (10 g fresh weight) were immersed in a solution containing 100 μM sclareol for 48 h, the minimum concentration and sufficient time needed to decrease the content of chlorophyll in this plant species [18]. As the mock treatment, we also treated plants with 0.1% methanol in the same way. The treated plants were extracted by soaking the plants in five volumes (50 mL) of cold 80% (v/v) acetone or cold 80% (v/v) methanol at 4 °C for one week. After filtration, each extract was evaporated to dryness, and the remaining residue was dissolved in 20 mL of 0.1% methanol. The methanol solution was applied to Col-0 plants according to the procedure described in Section 3.3. The chlorophyll content of treated plant leaves was subsequently measured.
3.2.2. Large-Scale Extraction
The plant extracts of sclareol-treated Arabidopsis plants (500 g fresh weight) were extracted by soaking the plants in five volumes of cold 80% (v/v) acetone at 4 °C for one week. After the removal of acetone under a vacuum using a rotary evaporator, the residual aqueous layer was partitioned with hexane followed by ethyl acetate. The hexane and ethyl acetate layers were regarded as the hexane- and ethyl acetate-soluble fractions, respectively. The remaining aqueous layer was regarded as the water-soluble fraction. The hexane layer (472 mg) was separated on a column (53 mm in diameter, 550 mm in length) of silica gel (Wakogel C-200, FUJIFILM Wako Pure Chemical, Osaka, Japan) eluted with increasingly higher concentrations of ethyl acetate in hexane. Activity for reducing chlorophyll content was detected in a fraction eluted with 30% (v/v) ethyl acetate in hexane and fractions eluted with 40 to 50% (v/v) ethyl acetate in hexane. The fraction eluted with 30% (v/v) ethyl acetate in hexane was collected and separated on a C18-based solid phase extraction (SPE) cartridge column (Waters) eluted with increasingly higher concentrations of methanol in water, starting with 0% methanol and ending with 100% methanol. A fraction eluted in 100% methanol was separated on a column of silica gel eluted with increasingly higher concentrations of chloroform in methanol to obtain two active fractions. One active fraction was further purified by preparative TLC (silica gel 60 F254, Merck, Rahway, NJ, USA) using 100% chloroform to yield compound 1 (1.1 mg), and another fraction was separated on a column of silica gel eluted with 10% (v/v) chloroform in methanol to yield compound 2 (2 mg).
All fractions obtained were evaporated, dissolved in methanol, diluted to appropriate concentrations with water, and used in the treatment of Arabidopsis plants to measure activity-reducing chlorophyll content.
3.3. Chemical Treatments
Sclareol was purchased from Sigma-Aldrich (St. Louis, MO, USA). Campesterol, stigmasterol, and lovastatin were purchased from Tokyo Chemical Industry (Tokyo, Japan). Sclareol, campesterol, stigmasterol, and lovastatin were dissolved in methanol and diluted in water to various concentrations. Methanol concentrations did not exceed 0.1% (v/v) in any experiment.
The procedure of chemical treatment, including fractions obtained from fractionation, was performed according to previous reports [16,18]. Briefly, plants were removed from the fertilizer, transferred to a glass Petri dish (9 cm in diameter) containing sterile distilled H2O, and preincubated for 24 h to diminish the influence of any stress wounds caused by the transfer. After discarding water with a pipette, plant roots were treated by gently adding a solution containing adequate concentrations of each fraction or compound for 48 h. The treated plants were harvested and used for chlorophyll measurements, gene expression analysis, and phytosterol quantification. Five to six plants were used for each treatment and regarded as one biological replicate.
For the lovastatin treatment, we used 100 μM lovastatin, 100 μM sclareol, and a mixture of 100 μM lovastatin and 100 μM sclareol in a 50:50 ratio.
3.4. Chlorophyll Measurement
Samples were extracted with cold 80% (v/v) acetone by grinding with a mortar and pestle on ice. The homogenate was centrifuged at 3000 rpm for 10 min. The supernatant was collected, and the precipitate was further extracted with cold 80% acetone. Supernatants were combined and used to measure absorbance at 646 and 663 nm with a spectrophotometer. Chlorophyll a and b contents were calculated according to the formula described by Porra et al. [27].
3.5. Structural Analyses of Active Compounds
3.5.1. NMR
1D- and 2D-NMR spectra (1H, 13C, HSQC, HMBC, COSY, and NOESY) were performed using Bruker Avance-NMR (600 and 800 MHz) spectrometers. All compounds were recorded in the solvent deuterochloroform (CDCl3).
3.5.2. TLC
TLC was performed on a precoated aluminum plate of silica gel 60 F254 (Merck) with the solvent system consisting of different ratios of chloroform and methanol. Chromatograms were visualized under ultraviolet (UV) light using a UV lamp for the localization of spots on chromatograms before and after spraying with the phosphomolybdic acid stain.
3.5.3. Gas Chromatography–Mass Spectrometry
A gas chromatography–mass spectrometry (GC–MS) analysis was performed on a GC (7890A, Agilent, Santa Clara, CA, USA) system equipped with a mass selective detector (5975C, Agilent). Separation was performed on a capillary column (HP-5MS, length of 30 m, i.d. of 0.25 mm, thickness of 0.25 μm, Agilent) with He as the carrier gas at a flow rate of 1 mL min−1. The injection mode was splitless and the injection port temperature was 280 °C. The oven temperature was set at 50 °C, increased to 300 °C at 2 °C min−1, and held at 300 °C for 5 min. The mass spectrometer was operated in the electron impact mode at 70 eV and scan mode, scanning from 50 to 500 m/z at 6.35 scan−1. The compounds isolated in the GC–MS analysis were identified by matching their mass spectra to the reference spectra of the corresponding authentic standards.
3.5.4. Silylation of Samples
Regarding the silylation of samples prior to the GC–MS analysis, 1 mg of compound was dissolved in 100 μL pyridine, and 100 μL N-Methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA; Sigma-Aldrich) was added to the pyridine solution. The mixture was heated to 60 °C for 3 h. After removal of the solvent under a nitrogen stream, the product obtained was dissolved in chloroform and injected into the GC–MS instrument.
3.5.5. Compound 1
White powder; Rf 0.33 in CHCl3-MeOH (9:1); 1H-NMR (CDCl3, 800 MHz): 5.35 (m, 1H, H6), 3.52 (m, 1H, H3), 1.01 (s, 3H, H19), 0.85 (d, J = 6.4, 3H, H26), 0.80 (d, J = 7.2, 3H, H27), 0.77 (d, J = 7.2, 3H, H28), 0.68 (s, 3H, H18) and 13C-NMR data (CDCl3, 800 MHz): 140.8, 121.7, 71.8, 20.2, 19.4, 18.7, 18.2, 15.4; GC–MS m/z 472 [M + 72]+ (C28H48O).
3.5.6. Compound 2
White powder; Rf 0.38 in CHCl3-MeOH (9:1); 1H-NMR (CDCl3, 800 MHz): 5.35 (m, 1H, H6), 5.15 (dd, J = 8.7, 5.3, 1H, H23), 5.01 (dd, J = 8.5, 5.2, 1H, H22), 3.52 (m, 1H, H3), 1.02 (d, J = 6.6, 3H, H21), 1.01 (s, 3H, H19), 0.91 (d, J = 8, 3H, H21), 0.84 (d, J = 6.5, 3H, H26), 0.81 (d, J = 7.4, 3H, H27), 0.79 (d, J = 6.6, 3H, H29), 0.69 (s, 3H, H18) and 13C-NMR data (CDCl3, 800 MHz): 140.8, 138.3, 129.3, 121.7, 71.8, 21.2, 21.1, 19.4, 18.9, 12.3, 12.1; GC–MS m/z 484 [M + 72]+ (C29H48O).
3.6. Quantification of Phytosterols
Extraction and derivatization using MSTFA of the samples were performed as previously described [28]. A GC–MS analysis of the sample was performed according to the conditions described above, except that selected ion monitoring was used for data acquisition at m/z 472 for campesterol and m/z 484 for stigmasterol.
3.7. Quantitative Real-Time PCR
The extraction of total RNA and quantitative real-time PCR using total RNA were performed in a two-step reaction using a SYBR Green kit (Bio-Rad, Hercules, CA, USA) in accordance with the procedure described by Fujimoto et al. [18]. Information on the primers used is shown in Supplementary Table S1. The expression levels of Atactin2 were used to normalize those of the target genes.
3.8. Statistical Analyses
A one-way analysis of variance followed by Tukey’s test was used to evaluate the significance of differences within all groups. Student’s t-test was employed to compare the significance of differences in the mean of two samples. Dunnett’s test was used for Supplementary Figure S2. These analyses were conducted using R version 2.13.1 (R Development Core Team, 2011).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12061282/s1, Figure S1: Effects of acetone extracts prepared from Arabidopsis plants on the accumulation of chlorophyll; Figure S2: Flow diagram for purification and isolation of chlorophyll-content-reducing substances from Arabidopsis plants; Table S1: List of primers used for the quantitative real-time PCR analysis.
Author Contributions
Conceptualization, S.S.; investigation, A.B.H., H.O. and S.S.; data curation, A.B.H. and S.S., writing—original draft preparation, A.B.H. and S.S.; writing—review and editing, A.B.H., H.O. and S.S.; project administration, S.S.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science KAKENHI Grant Numbers 16H04888 and 22H02352.
Data Availability Statement
All the data supporting the conclusions of this study are included in the manuscript.
Acknowledgments
We thank T. Fujimoto for the helpful discussion and M. Sakamoto and R. Otoh for their assistance with the gene expression analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akhter, M.D.; Nakahara, K.S.; Masuta, C. Resistance induction based on the understanding of molecular interactions between plant viruses and host plants. Viol. J. 2021, 18, 176. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yang, Y. Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol. Biochem. 2021, 165, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Iqbal, S.; Kamran, M.; El-Sabrout, A.M.; Elansary, H.O.; et al. Role of promising secondary metabolites to confer resistance against environmental stresses in crop plants: Current scenario and future perspectives. Front. Plant Sci. 2022, 13, 881032. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Guo, M.; Song, J.; Ma, Y.; Xu, Z. Signals in systemic acquired resistance of plants against microbial pathogens. Mol. Plant Biol. Rep. 2021, 48, 3747–3759. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wagner, G.J. Biosynthesis of labdenediol and sclareol in cell-free extracts from trichomes of Nicotiana glutinosa. Planta 1995, 197, 627–632. [Google Scholar] [CrossRef]
- Caniard, A.; Zerbe, P.; Legrand, S.; Cohade, A.; Valot, N.; Magnard, J.L.; Bohlmann, J.; Legendre, L. Discovery and functional characterization of two diterpene synthases for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture. BMC Plant Biol. 2012, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.A.; Carter, G.A.; Burden, R.S.; Wain, R.L. Control of rust disease by diterpenes from Nicotiana glutinosa. Nature 1975, 255, 328–329. [Google Scholar] [CrossRef]
- Kennedy, B.S.; Nielsen, M.T.; Severson, R.F.; Sisson, V.A.; Stephenson, M.K.; Jackson, D.M. Leaf surface chemicals from Nicotiana affecting germination of Peronospora tabacina (adam) sporangia. J. Chem. Ecol. 1992, 18, 1467–1479. [Google Scholar] [CrossRef]
- Jackson, D.M.; Danehower, D.A. Integrated case study: Nicotiana leaf surface components and their effects on insect pests and disease. In Plant Cuticles: An Integrated Functional Approach; Kerstiens, G., Ed.; BIOS Scientific Publishers, Ltd.: Oxford, UK, 1996; pp. 231–254. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=70621 (accessed on 1 August 2022).
- Popova, V.; Ivanova, T.; Stoyanova, A.; Nikolova, V.; Hristeva, T.; Gochev, V.; Yonchev, Y.; Nikolov, N.; Zheljazkov, V.D. Terpenoids in the essential oil and concentrated aromatic products obtained from Nicotiana glutinosa L. leaves. Molecules 2019, 25, 30. [Google Scholar] [CrossRef] [PubMed]
- Kroumova, A.B.; Artiouchine, I.; Wagner, G.J. Use of several natural products from selected Nicotiana species to prevent black shank disease in tobacco. Contrib. Tob. Nicotine Res. 2016, 27, 113–125. [Google Scholar] [CrossRef]
- Campbell, E.; Schenk, P.M.; Kazan, K.; Penninck, I.A.; Anderson, J.P.; Maclean, D.J.; Cammue, B.P.A.; Ebert, P.R.; Manners, J.M. Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance of the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis. Plant Physiol. 2003, 133, 1272–1284. [Google Scholar] [CrossRef]
- Grec, S.; Vanham, D.; De Ribaucourt, J.C.; Purnelle, B.; Boutry, M. Identification of regulatory sequence elements within the transcription promoter region of NpABC1, a gene encoding a plan ABC transporter induced by diterpenes. Plant J. 2003, 35, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, M.; Stukkens, Y.; Degand, H.; Purnelle, B.; Marchand-Brynaert, J.; Boutry, M. A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell 2001, 13, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Gomi, K.; Kaku, H.; Abe, H.; Seto, H.; Nakatsu, S.; Neya, M.; Kobayashi, M.; Nakaho, K.; Ichinose, Y.; et al. Identification of natural diterpenes that inhibit bacterial wilt disease in tobacco, tomato and Arabidopsis. Plant Cell Physiol. 2012, 53, 1432–1444. [Google Scholar] [CrossRef]
- Fujimoto, T.; Mizukubo, T.; Abe, H.; Seo, S. Sclareol induces plant resistance to root-knot nematode partially through ethylene-dependent enhancement of lignin accumulation. Mol. Plant-Microbe Interact. 2015, 28, 398–407. [Google Scholar] [CrossRef]
- Caissard, J.C.; Oliver, T.; Delbecque, C.; Palle, S.; Garry, P.P.; Audran, A.; Valot, N.; Moja, S.; Nicole, F.; Magnard, J.L.; et al. Extracellular localization of the diterpene sclareol in Clary Sage (Salvia sclarea L., Lamiaceae). PLoS ONE 2012, 7, e48253. [Google Scholar] [CrossRef]
- Fujimoto, T.; Mizukubo, T.; Abe, H.; Seo, S. Phytol, a constituent of chlorophyll, induces root-knot nematode resistance in Arabidopsis via the ethylene signaling pathway. Mol. Plant-Microbe Interact. 2021, 34, 279–285. [Google Scholar] [CrossRef]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H. Biosynthesis and the roles of plant sterols in development and stress responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef]
- Tsukagoshi, Y.; Suzuki, H.; Seki, H.; Muranaka, T.; Ohyama, K.; Fujimoto, Y. Ajuga Δ24-sterol reductase catalyzes the direct reductive conversion of 24-methylenecholesterol to campesterol. J. Biol. Chem. 2016, 291, 8189–8198. [Google Scholar] [CrossRef]
- Lange, I.; Poirier, B.C.; Herron, B.K.; Lange, B.M. Comprehensive assessment of transcriptional regulation facilitates metabolic engineering of isoprenoid accumulation in Arabidopsis. Plant Physiol. 2015, 169, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Darnet, S.; Rahier, A. Plant sterol biosynthesis: Identification of two distinct families of sterol 4α-methyl oxidases. Biochem. J. 2004, 378, 889–898. [Google Scholar] [CrossRef]
- Rahier, A. Dissecting the sterol C-4 demethylation process in higher plants. From structures and genes to catalytic mechanism. Steroids 2011, 76, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Laule, O.; Fürfholz, A.; Chang, H.S.; Zhu, T.; Wang, X.; Heifetz, P.B.; Gruissem, W.; Lange, B.M. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6866–6871. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.L.; Shimada, T.; Okazaki, Y.; Higashi, Y.; Saito, K.; Kuwata, K.; Oyama, K.; Kato, M.; Ueda, H.; Nakano, A.; et al. HIGH STEROL ESTER 1 is a key factor in plant sterol homeostasis. Nature Plants 2019, 5, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Yamaguchi, K.; Shigenobu, S.; Takahashi, H.; Murase, M.; Fukuyoshi, S.; Hara-Nishimura, I. Excess sterols disrupt plant cellular activity by inducing stress-responsive gene expression. J. Plant Res. 2020, 133, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophy. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Lytovchenko, A.; Beleggia, R.; Schauer, N.; Isaacson, T.; Leuendorf, J.E.; Hellmann, H.; Rose, J.K.C.; Fernie, A.R. Application of GC–MS for the detection of lipophilic compounds in diverse plant tissues. Plant Meth. 2009, 5, 4. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).