Integrative Analysis of Transcriptome, Proteome, and Phosphoproteome Reveals Potential Roles of Photosynthesis Antenna Proteins in Response to Brassinosteroids Signaling in Maize
Abstract
:1. Introduction
2. Results
2.1. Genome-Wide Expression Changes in mRNA, Protein Expression, and Phosphoproteins in Response to Brassinosteroid Treatment
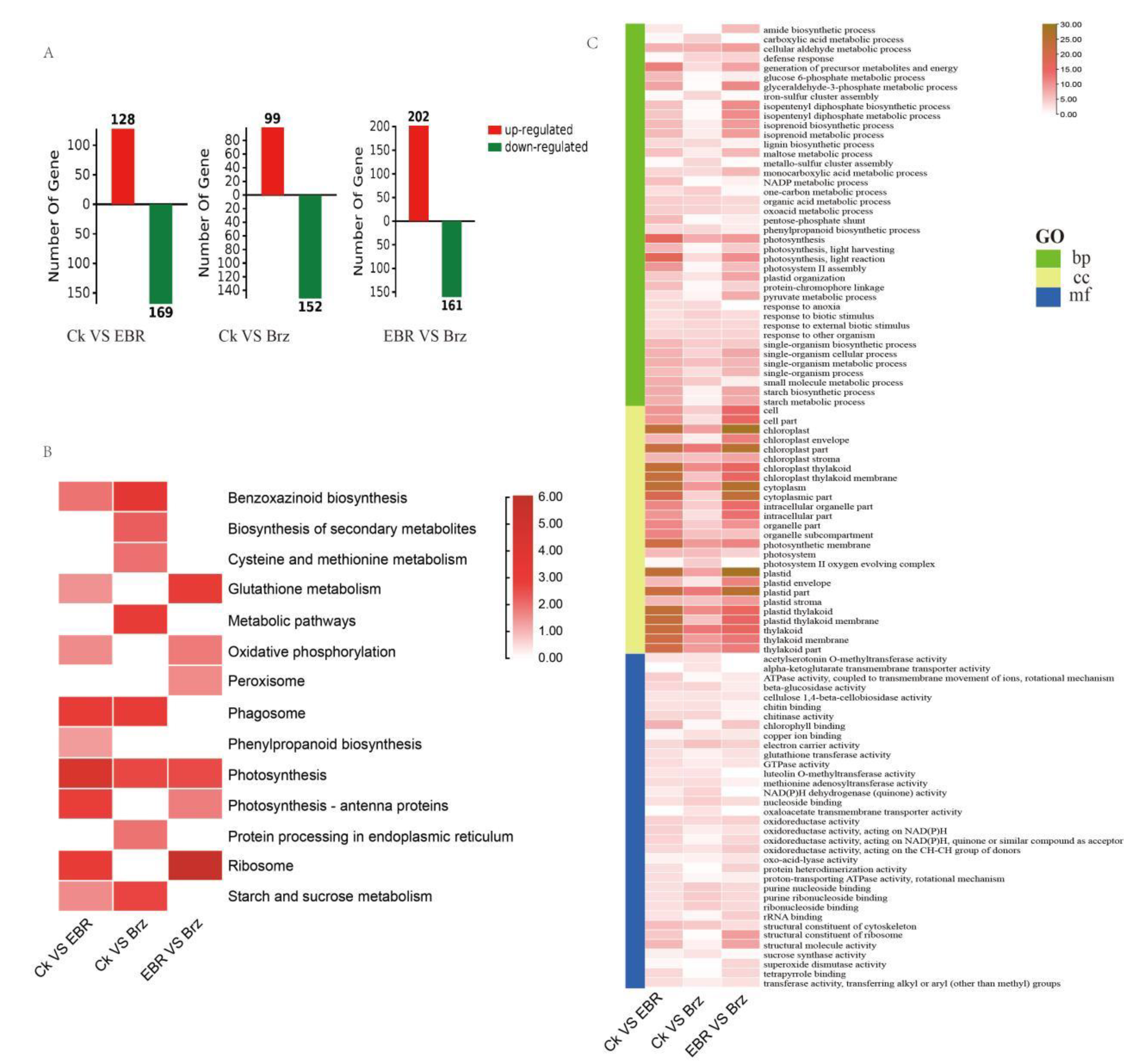
2.2. Transcriptome Differences in Mo17 in Response to BR and Brz Treatments
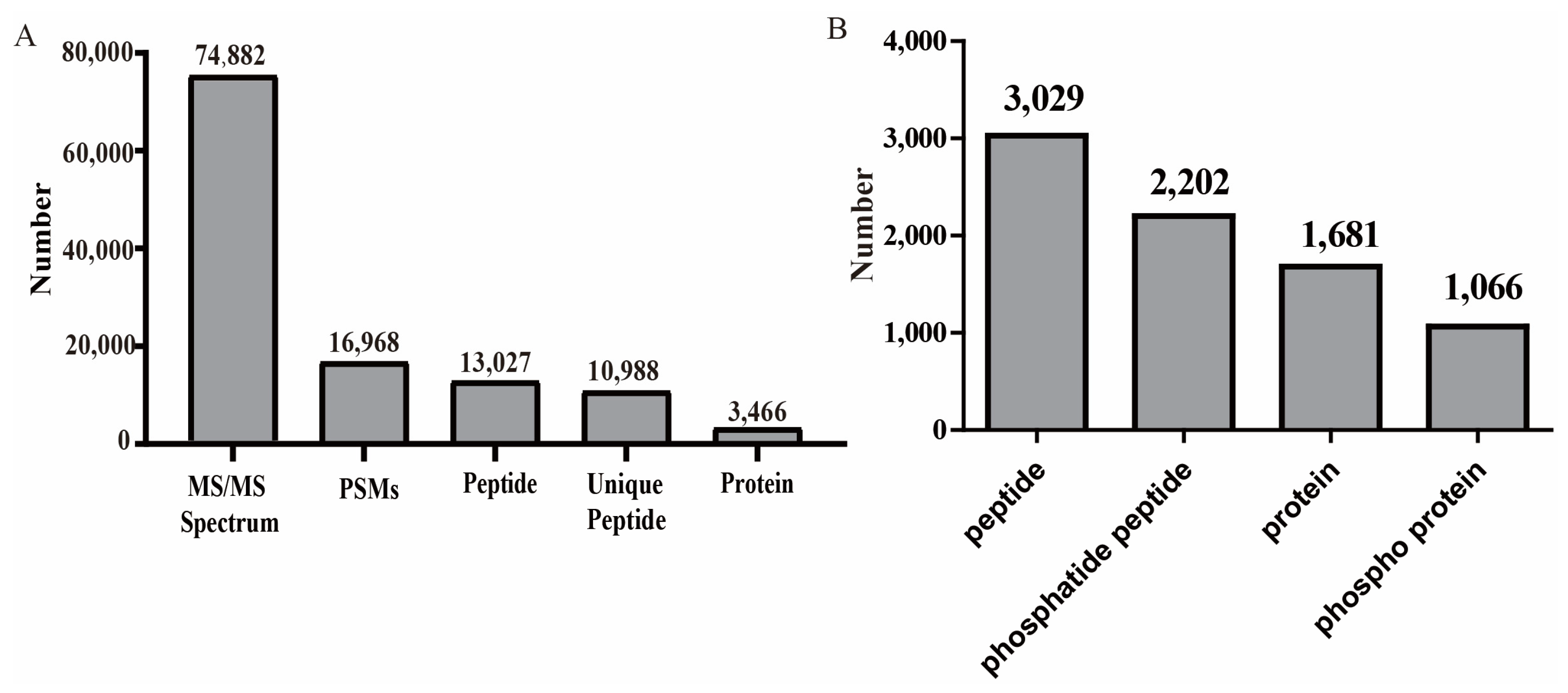
2.3. Proteome Changes in Mo17 in Response to BR and Brz Treatments
2.4. Phosphoproteomic Changes in Mo17 in Response to BR and Brz Treatments
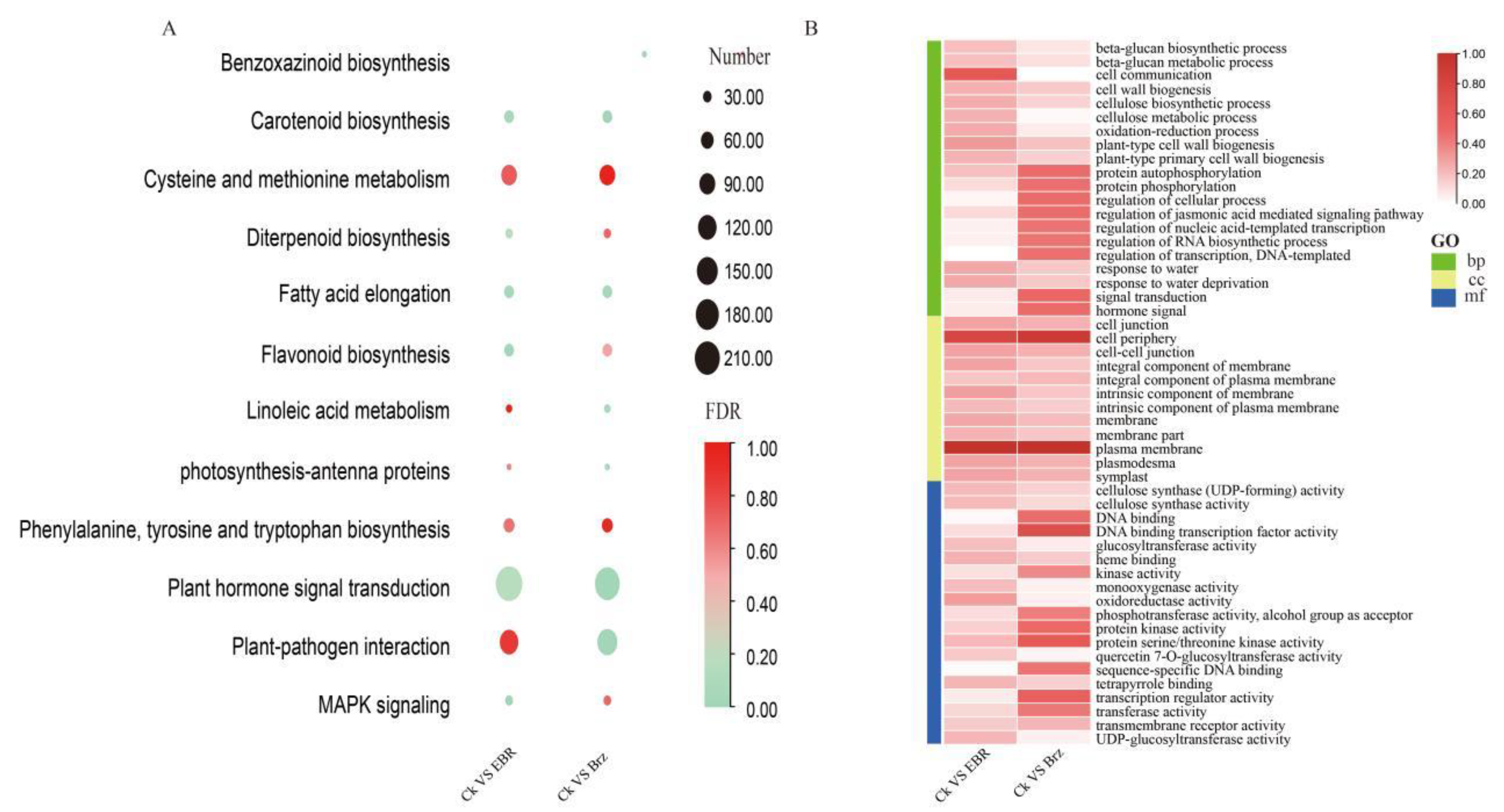
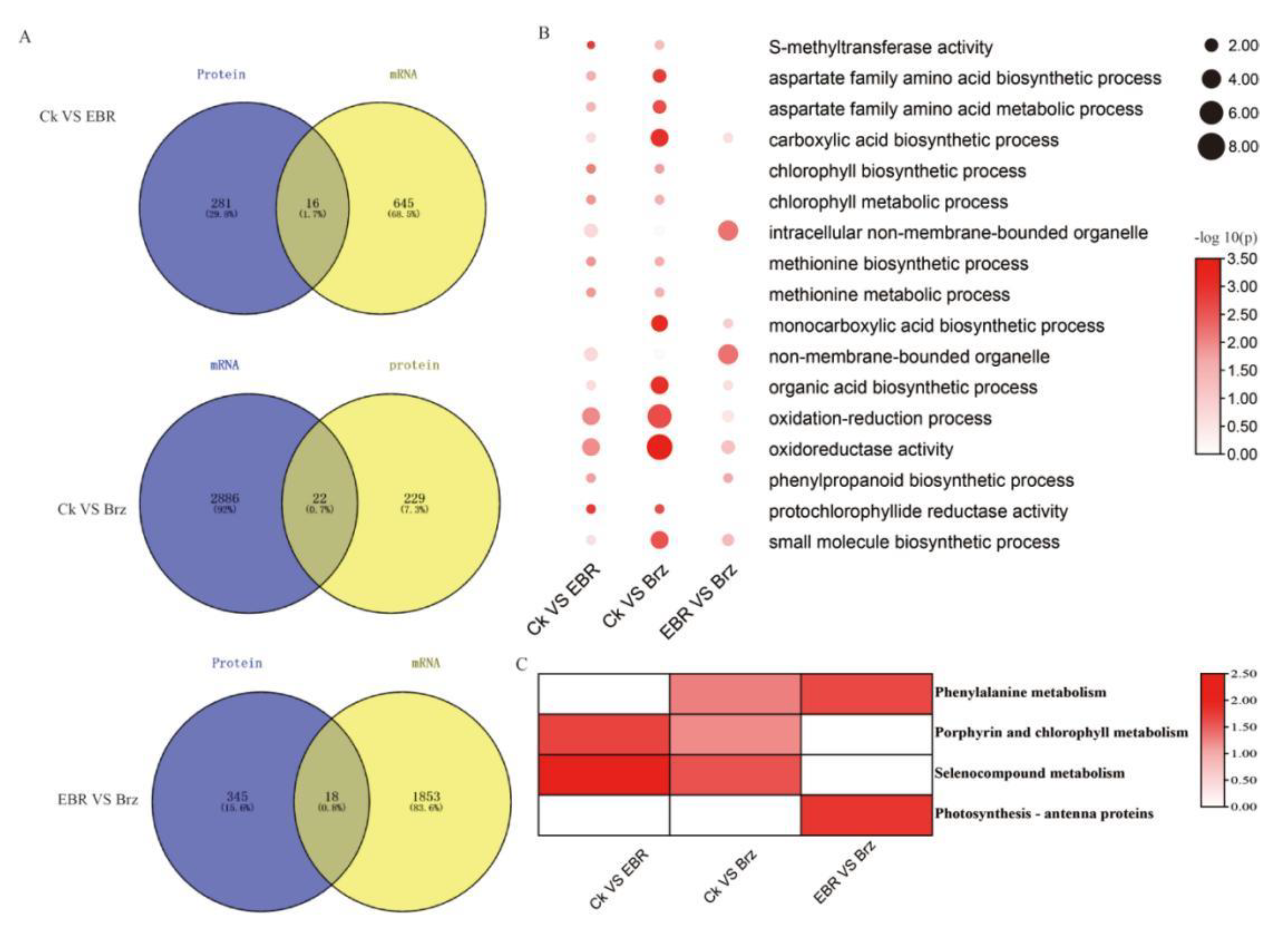
2.5. Combined Transcriptome and Proteome Analyses of the Differences in the EBR and Brz Signaling Pathway in Maize Leaves
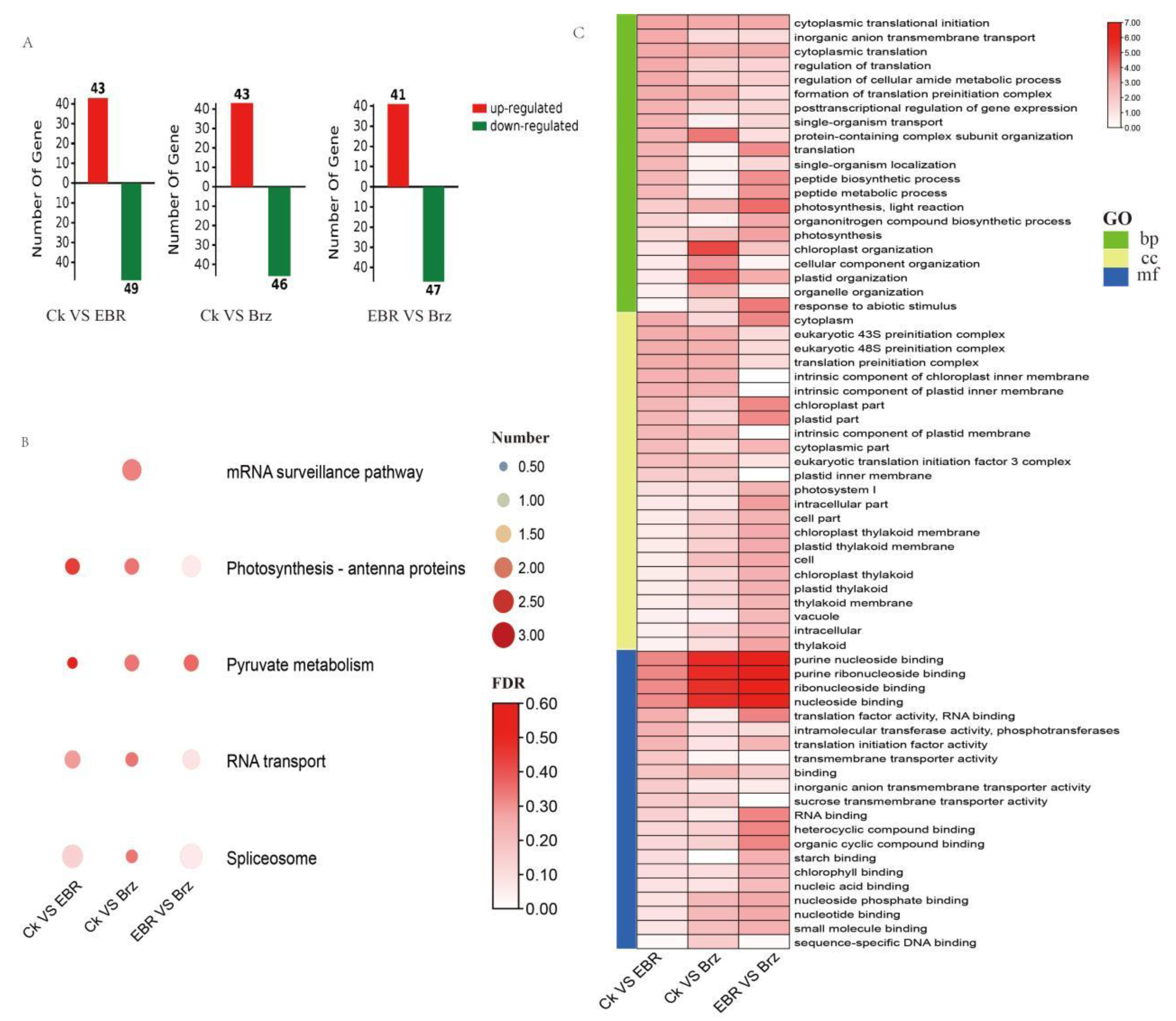
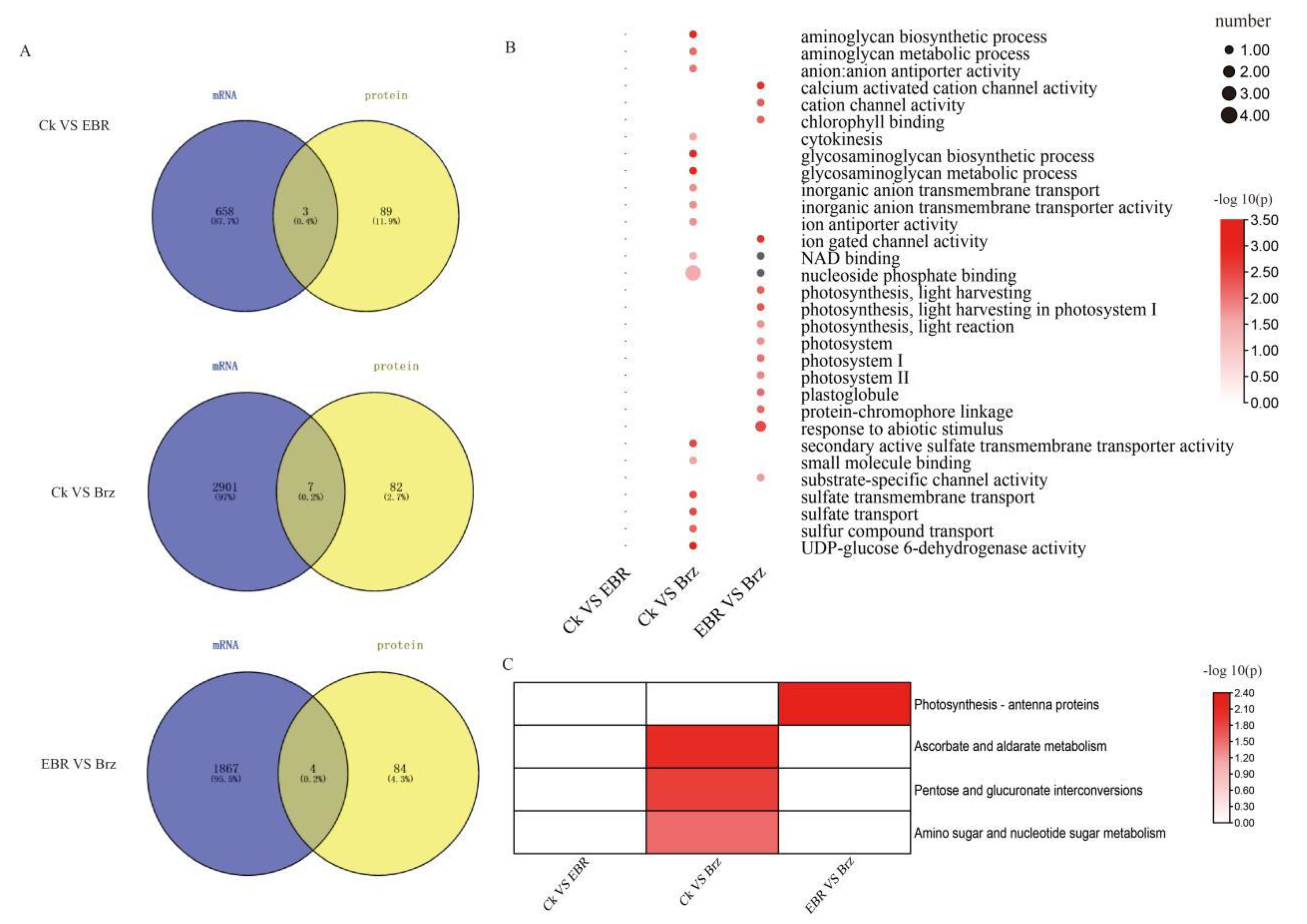
2.6. Combined Transcriptome and Phosphoproteome Analyses of the Differences in the EBR and Brz Signaling Pathway in Maize Leaves
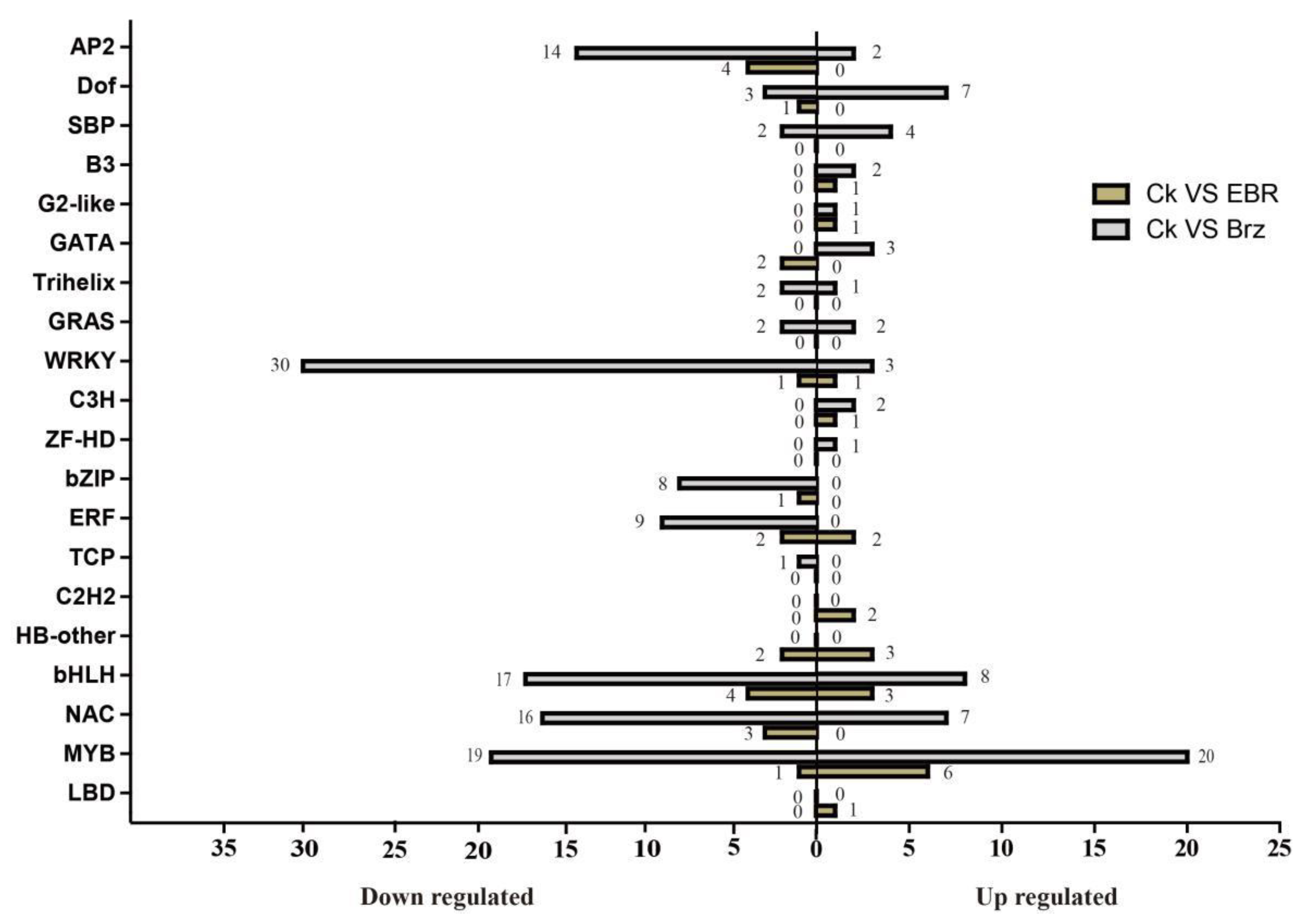
2.7. BR Signal-Responsive Transcription Factors (TFs)
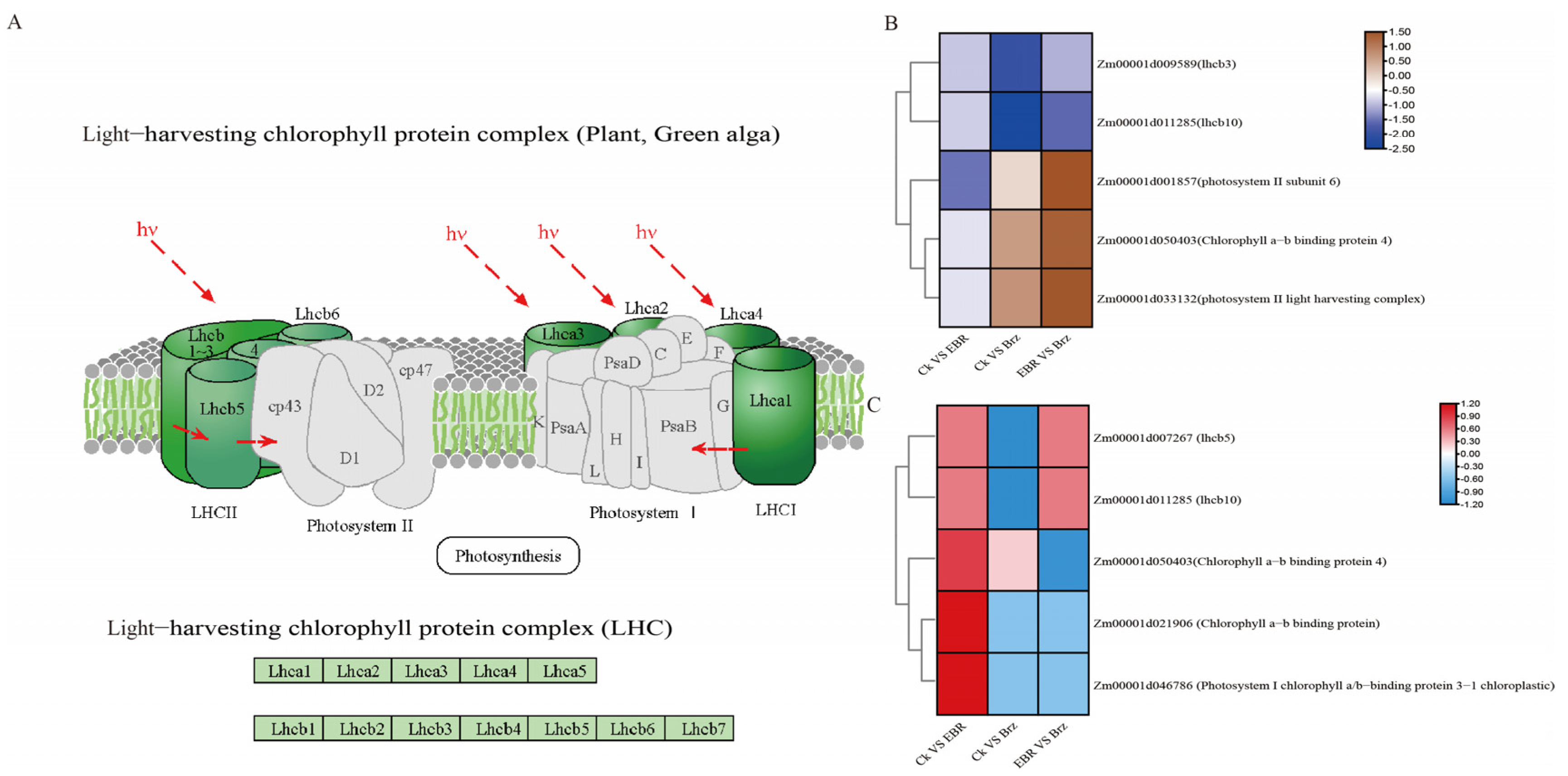
2.8. Analysis of Photosynthesis Antenna Protein Responses to BR Signaling
3. Discussion
3.1. The Transcriptome Analysis Did Not Fully Match the Proteome and Phosphoproteome Analysis
3.2. Role of TFs in Maize Responses to BR Signaling
3.3. Response of Photosynthesis Antenna Proteins to BR Signaling
4. Materials and Methods
4.1. Plant Materials and Hormone Induction Procedure
4.2. Transcriptome Library Construction, Sequencing, and Annotation
4.3. Proteome Sequencing and Data Analysis
4.4. Phosphoprotein Sequencing and Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godfray, H.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 5967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covshoff, S.; Hibberd, J.M. Integrating C4 photosynthesis into C3 crops to increase yield potential. Curr. Opin. Biotechnol. 2012, 23, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Kefauver, S.; Araus, J.L.; Muller, O.; Rascher, U.; Flood, P.J.; Lawson, T. Measuring the dynamic photosynthome. Ann. Bot. 2018, 122, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, B.R.; Vicente, R.; Molero, G.; Serret, M.D.; Maydup, M.L.; Araus, J.L. New avenues for increasing yield and stability in C3 cereals: Exploring ear photosynthesis. Curr. Opin. Plant Biol. 2020, 56, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Crafts, B.S. Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol. 2004, 134, 1460–1470. [Google Scholar] [CrossRef] [Green Version]
- Kurek, I.; Chang, T.K.; Bertain, S.M.; Madrigal, A.; Liu, L.; Lassner, M.W.; Zhu, G. Enhanced Thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 2007, 19, 3230–3241. [Google Scholar] [CrossRef] [Green Version]
- Kočová, M.; Holá, D.; Wilhelmová, N.; Rothová, O. The influence of low-temperature on the photochemical activity of chloroplasts and activity of antioxidant enzymes in maize leaves. Biol. Plant. 2009, 53, 475–483. [Google Scholar] [CrossRef]
- Kutík, J.; Holá, D.; Koová, M.; Rothová, O.; Haisel, D.; Wilhelmová, N.; Tichá, I. Ultrastructure and Dimensions of Chloroplasts in Leaves of Three Maize (Zea mays L.) Inbred Lines and their F1 Hybrids Grown Under Moderate Chilling Stress. Photosynthetica 2004, 42, 447–455. [Google Scholar] [CrossRef]
- Blum, A. Heterosis, stress, and the environment: A possible road map towards the general improvement of crop yield. J. Exp. Bot. 2013, 64, 4829–4837. [Google Scholar] [CrossRef] [Green Version]
- Smith, H. Phytochromes and light signal perception by plants—An emerging synthesis. Nature 2000, 407, 585–591. [Google Scholar] [CrossRef]
- Sawers, R.J.; Sheehan, M.J.; Brutnell, T.P. Cereal phytochromes: Targets of selection, targets for manipulation? Trends Plant Sci. 2005, 10, 138–143. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 2019, 135, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Lu, Q.; Wang, J.; Wang, L.; Xiang, F.; Liu, Z. MiR160 and its target genes ARF10, ARF16 and ARF17 modulate hypocotyl elongation in a light, BRZ, or PAC-dependent manner in Arabidopsis: miR160 promotes hypocotyl elongation. Plant Sci. 2021, 303, 110686. [Google Scholar] [CrossRef] [PubMed]
- Ogweno, J.O.; Song, X.S.; Shi, K.; Hu, W.H.; Mao, W.H.; Zhou, Y.H.; Yu, J.Q.; Nogue´s, S. Brassinosteroids Alleviate Heat-Induced Inhibition of Photosynthesis by Increasing Carboxylation Efficiency and Enhancing Antioxidant Systems in Lycopersicon esculentum. J. Plant Growth Regul. 2008, 27, 49–57. [Google Scholar] [CrossRef]
- Yu, H.Q.; Sun, F.A.; Feng, W.Q.; Lu, F.Z.; Li, W.C.; Fu, F.L. The BES1/BZR1 transcription factors regulate growth, development and stress resistance in plants. Yi Chuan 2019, 20, 206–214. [Google Scholar]
- Yu, H.Q.; Feng, W.Q.; Sun, F.A.; Zhang, Y.Y.; Qu, J.T.; Liu, B.L.; Lu, F.Z.; Yang, L.; Fu, F.L.; Li, W.C. Cloning and characterization of BES1/BZR1 transcription factor genes in maize. J. Plant Growth Regul. 2018, 86, 235–249. [Google Scholar] [CrossRef]
- Feng, W.Q.; Liu, Y.; Cao, Y.; Zhao, Y.R.; Zhang, H.W.J.; Sun, F.A.; Yang, Q.Q.; Li, W.C.; Lu, Y.L.; Zhang, X.C.; et al. Maize ZmBES1/BZR1-3 and-9 transcription factors negatively regulate drought tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2022, 23, 6025. [Google Scholar] [CrossRef]
- Sun, F.A.; Ding, L.; Feng, W.; Cao, Y.; Lu, F.; Yang, Q.; Li, W.; Lu, Y.; Nitzan, S.; Fu, F. Maize transcription factor ZmBES1/BZR1-5 positively regulates kernel size. J. Exp. Bot. 2021, 72, 1714–1726. [Google Scholar] [CrossRef]
- Sun, F.A.; Yu, H.Q.; Qu, J.T.; Cao, Y.; Ding, L.; Feng, W.Q.; Khalid, M.H.B.; Li, W.C.; Fu, F.L. Maize ZmBES1/BZR1-5 decreases ABA sensitivity and confers tolerance to osmotic stress in transgenic Arabidopsis. Int. J. Mol. Sci. 2020, 21, 996. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.A.; Palayam, M.; Shabek, N. Structure of maize BZR1-type β-amylase BAM8 provides new insights into its noncatalytic adaptation. J. struct. biol. 2022, 214, 107885. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Milliken, A.L. Photosynthesis-beyond the leaf. New Phytol. 2023, 238, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, G.C.; Klimov, V.V.; Baranov, S.V.; Kozlov, Y.N.; DasGupta, J.; Tyryshkin, A. The origin of atmospheric oxygen on Earth: The innovation of oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 2001, 98, 2170–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodson, J.D. Chloroplast quality control—balancing energy production and stress. New Phytol. 2016, 212, 36–41. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Yuan, Q.; Feng, Y. Structure and Function of the Photosystem Supercomplexes. Front. Plant Sci. 2018, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Blankenship, R.E. On the interface of light-harvesting antenna complexes and reaction centers in oxygenic photosynthesis. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 148079. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, R.E.; Chen, M. Spectral expansion and antenna reduction can enhance photosynthesis for energy production. Curr. Opin. Chem. Biol. 2013, 17, 457–461. [Google Scholar] [CrossRef]
- Hashimoto, H.; Sugisaki, M.; Yoshizawa, M. Ultrafast time-resolved vibrational spectroscopies of carotenoids in photosynthesis. Biochim. Biophys. Acta 2015, 1847, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, H.; Uragami, C.; Yukihira, N.; Gardiner, A.T.; Cogdell, R.J. Understanding/unravelling carotenoid excited singlet states. J. R. Soc. Interface 2018, 15, 20180026. [Google Scholar] [CrossRef]
- Bode, S.; Quentmeier, C.C.; Liao, P.N.; Hafi, N.; Barros, T.; Wilk, L.; Bittner, F.; Walla, P.J. On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc. Natl. Acad. Sci. USA 2009, 106, 12311–12316. [Google Scholar] [CrossRef] [Green Version]
- Alton, P.B. Retrieval of seasonal Rubisco-limited photosynthetic capacity at global FLUXNET sites from hyperspectral satellite remote sensing: Impact on carbon modelling. Agric. For. Meteorol. 2017, 232, 74–88. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.; Hasan, S.A.; Hayat, S.; Hayat, Q.; Yadav, S.; Fariduddin, Q.; Ahmad, A. A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ. Exp. Bot. 2008, 62, 153–159. [Google Scholar] [CrossRef]
- Hayat, S.; Ali, B.; Hasan, S.A. A Ahmad Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ. Exp. Bot. 2007, 60, 33–41. [Google Scholar] [CrossRef]
- Hasan, S.A.; Hayat, S.; Ali, B.; Ahmad, A. 28-Homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidants. Environ. Pollut. 2008, 151, 60–66. [Google Scholar] [CrossRef]
- Swamy, K.N.; Rao, S.S.R. Influence of 28-Homobrassinolide on Growth, Photosynthesis Metabolite and Essential Oil Content of Geranium [Pelargonium graveolens (L.) Herit]. Am. J. Plant Physiol. 2008, 3, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Khallal, S.M.; Hathout, T.A.; Ashour, A.A.; Kerrit, A.A. Brassinolide and salicylic acid induced growth, biochemical activities and productivity of maize plants grown under salt stress. Res. J. Agric. Biol. Sci. 2009, 5, 380–390. [Google Scholar]
- Piñol, R.; Simón, E. Effect of 24-Epibrassinolide on Chlorophyll Fluorescence and Photosynthetic CO2 Assimilation in Vicia faba Plants Treated with the Photosynthesis-Inhibiting Herbicide Terbutryn. J. Plant Growth Regul. 2009, 28, 97–105. [Google Scholar] [CrossRef]
- Jiang, Y.P.; Cheng, F.; Zhou, Y.H.; Xia, X.J.; Mao, W.H.; Shi, K.; Chen, Z.; Yu, J.Q. Cellular glutathione redox homeostasis plays an important role in the brassinosteroid-induced increase in CO2 assimilation in Cucumis sativus. New Phytol. 2012, 194, 932–943. [Google Scholar] [CrossRef]
- Li, X.J.; Guo, X.; Zhou, Y.H.; Shi, K.; Zhou, J.; Yu, J.Q.; Xia, X.J. Overexpression of a brassinosteroid biosynthetic gene Dwarf enhances photosynthetic capacity through activation of Calvin cycle enzymes in tomato. BMC Plant Biol. 2016, 28, 33. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.J.; Dhanasekaran, S.M.; Petralia, F.; Pan, J.; Song, X.; Hu, Y.; Veiga, L.F.; Reva, B.; Lih, T.M.; Chang, H.Y. Integrated Proteogenomic Characterization of Clear Cell Renal Cell Carcinoma. Cell 2019, 179, 964–983. [Google Scholar] [CrossRef] [Green Version]
- Isobe, K.; Jung, H.J.; Yang, C.R.; Claxton, J.; Sandoval, P.; Burg, M.B.; Raghuram, V.; Knepper, M.A. Systems-level identification of PKA-dependent signaling in epithelial cells. Proc. Natl. Acad. Sci. USA 2017, 114, E8875–E8884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G.; Wang, T.; Korhonen, P.K.; Stroehlein, A.J.; Young, N.D.; Gasser, R.B. Dauer signalling pathway model for Haemonchus contortus. Parasites Vectors 2019, 12, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, N.; Hjorth, J.K.; Hekmat, O.; Iglesias, G.D.; Kruse, T.; Wang, C.; Wei, W.; Ke, B.; Yan, B.; Niu, Y. In vivo quantitative phosphoproteomic profiling identifies novel regulators of castration-resistant prostate cancer growth. Oncogene 2014, 34, 2764–2776. [Google Scholar] [CrossRef]
- Katsogiannou, M.; Boyer, J.B.; Valdeolivas, A.; Remy, E.; Calzone, L.; Audebert, S.; Rocchi, P.; Camoin, L.; Baudot, A. Integrative proteomic and phosphoproteomic profiling of prostate cell lines. PLoS ONE 2019, 14, e0224148. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, Q.; Zhang, B. Leveraging the complementary nature of RNA-Seq and shotgun proteomics data. Proteomics 2014, 14, 2676–2687. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, A.; Lindell, G.; Källström, B.N.; Branca, R.M.; Danielsson, K.G.; Dahlberg, M.; Larson, B.; Forshed, J.; Lehtiö, J. Tumor proteomics by multivariate analysis on individual pathway data for characterization of vulvar cancer phenotypes. Mol. Cell. Proteom. 2012, 11, M112016998. [Google Scholar] [CrossRef] [Green Version]
- Houbaert, A.; Zhang, C.; Tiwari, M.; Wang, K.; Marcos, S.A.; Savatin, D.V.; Urs, M.J.; Zhiponova, M.K.; Gudesblat, G.E.; Vanhoutte, I. POLAR-guided signalling complex assembly and localization drive asymmetric cell division. Nature 2018, 563, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhao, Y.; Shi, H.; Li, J.; Wang, Y.; Tang, D. BRASSINOSTEROID-SIGNALING KINASE1 Phosphorylates MAPKKK5 to Regulate Immunity in Arabidopsis. Plant Physiol. 2018, 176, 2991–3002. [Google Scholar] [CrossRef] [Green Version]
- Hua, W.P.; Zhang, Y.; Song, J.; Zhao, L.J.; Wang, Z.Z. De novo transcriptome sequencing in Salvia miltiorrhiza to identify genes involved in the biosynthesis of active ingredients. Genomics 2011, 98, 272–279. [Google Scholar]
- Singh, A.; Breja, P.; Khurana, J.P.; Khurana, P. Wheat Brassinosteroid-Insensitive1 (TaBRI1) Interacts with Members of TaSERK Gene Family and Cause Early Flowering and Seed Yield Enhancement in Arabidopsis. PLoS ONE 2016, 11, e0153273. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Chen, M.; Xu, C.J.; Bai, L.; Yin, X.R.; Li, X.; Allan, A.C.; Ferguson, I.B.; Chen, K.S. Transcriptomic analysis of Chinese bayberry (Myrica rubra) fruit development and ripening using RNA-Seq. BMC Genom. 2012, 13, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.; Xu, Q.; Da, X.; Guo, F.; Ding, Y.; Deng, X. Transcriptome changes during fruit development and ripening of sweet orange (Citrus sinensis). BMC Genom. 2012, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Xie, S.; Xiao, Q.; Wei, B.; Zheng, L.; Wang, Y.; Cao, Y.; Zhang, X.; Long, T.; Li, Y. Sucrose and ABA regulate starch biosynthesis in maize through a novel transcription factor, ZmEREB156. Sci. Rep. 2016, 10, 27590. [Google Scholar] [CrossRef] [Green Version]
- Plotkin, J.B. Transcriptional regulation is only half the story. Mol. Syst. Biol. 2010, 24, 406. [Google Scholar] [CrossRef]
- Chen, J.; Nolan, T.M.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yin, Y. Arabidopsis WRKY46, WRKY54, and WRKY70 Transcription Factors Are Involved in Brassinosteroid-Regulated Plant Growth and Drought Responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Rozhon, W.; Bigeard, J.; Pflieger, D.; Husar, S.; Pitzschke, A.; Teige, M.; Jonak, C.; Hirt, H.; Poppenberger, B. Brassinosteroid-regulated GSK3/Shaggy-like kinases phosphorylate mitogen-activated protein (MAP) kinase kinases, which control stomata development in Arabidopsis thaliana. J. Biol. Chem. 2013, 288, 7519–7527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Schuck, S.; Wu, J.; Yang, P.; Döring, A.C.; Zeier, J.; Tsuda, K. A MPK3/6-WRKY33-ALD1-Pipecolic Acid Regulatory Loop Contributes to Systemic Acquired Resistance. Plant Cell 2018, 30, 2480–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkenbihl, R.P.; Diezel, C.; Somssich, I.E. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol. 2012, 159, 266–285. [Google Scholar] [CrossRef] [Green Version]
- Divi, U.K.; Rahman, T.; Krishna, P. Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. Plant Biotechnol. J. 2016, 14, 419–432. [Google Scholar] [CrossRef]
- Nykiel, M.; Gietler, M.; Fidler, J.; Prabucka, B.; Rybarczyk, P.A.; Graska, J.; Boguszewska, M.D.; Muszyńska, E.; Morkunas, I.; Labudda, M. Signal Transduction in Cereal Plants Struggling with Environmental Stresses: From Perception to Response. Plants 2022, 11, 1009. [Google Scholar] [CrossRef]
- Woo, N.S.; Badger, M.R.; Pogson, B.J. A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Methods 2008, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Anuradha, S.; Rao, S. Effect of 24-epibrassinolide on the photosynthetic activity of radish plants under cadmium stress. Photosynthetica 2009, 47, 317–320. [Google Scholar] [CrossRef]
- Saglam, C.S. The Effect of Epibrassinolide on Senescence in Wheat Leaves. Acta Physiol. Plant. 2004, 26, 18–19. [Google Scholar]
- Fedina, E.O.; Karimova, F.G.; Tarchevsky, I.A.; Toropygin, I.Y.; Khripach, V.A. Effect of epibrassinolide on tyrosine phosphorylation of the calvin cycle enzymes. Russ. J. Plant Physiol. 2008, 55, 193–200. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ashraf, M.; Athar, H.U.R. Athar, Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? Plant Growth Regul. 2008, 55, 51–64. [Google Scholar] [CrossRef]
- Xia, X.J.; Huang, L.F.; Zhou, Y.H.; Mao, W.H.; Shi, K.; Wu, J.X.; Asami, T.; Chen, Z.; Yu, J.Q. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 2009, 230, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhai, Z.; Tian, X.; Duan, L.; Li, Z. Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.). Plant Growth Regul. 2008, 56, 257–264. [Google Scholar] [CrossRef]
- Honnerová, J.; Rothová, O.; Holá, D.; KoOvá, M.; Kohout, L.; Kvasnica, M. The Exogenous Application of Brassinosteroids to Zea mays (L.) Stressed by Long-Term Chilling Does Not Affect the Activities of Photosystem 1 or 2. J. Plant Growth Regul. 2010, 29, 500–505. [Google Scholar] [CrossRef]
- Sam, O.; Núez, M.; Ruiz-Sánchez, M.C.; Dell, J.; Falcón, V.; Rosa, M.; Seoane, J. Effect of a Brassinosteroid Analogue and High Temperature Stress on Leaf Ultrastructure of Lycopersicon Esculentum. Biol. Plant. 2001, 44, 213–218. [Google Scholar] [CrossRef]
- Novoderezhkin, V.I.; Croce, R. The location of the low-energy states in Lhca1 favors excitation energy transfer to the core in the plant PSI-LHCI supercomplex. Photosynth. Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Li, H.; Yang, M.; Zhao, C.; Wang, Y.; Zhang, R. Physiological and proteomic analyses revealed the response mechanisms of two different drought-resistant maize varieties. BMC Plant Biol. 2021, 21, 513. [Google Scholar] [CrossRef]
- Peng, H.; Gao, J.; Song, X. Transcriptome analyses reveal photosynthesis-related genes involved in chloroplast development of the ems-induced maize mutant. Plant Biotechnol. Rep. 2022, 16, 565–578. [Google Scholar] [CrossRef]
- Liu, M.; Ma, W.; Su, X.; Zhang, X.; Lu, Y.; Zhang, S.; Yan, J.; Feng, D.; Ma, L.; Taylor, A. Mutation in a chlorophyll-binding motif of Brassica ferrochelatase enhances both heme and chlorophyll biosynthesis. Cell Rep. 2022, 41, 111758. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. A RNA-Seq Analysis of the Response of Photosynthetic System to Low Nitrogen Supply in Maize Leaf. Int. J. Mol. Sci. 2017, 18, 2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, A.; Wen, D.; Zhang, C. Maize Seed Germination Under Low-Temperature Stress Impacts Seedling Growth Under Normal Temperature by Modulating Photosynthesis and Antioxidant Metabolism. Front. Plant Sci. 2022, 13, 843033. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [Green Version]
- Beausoleil, S.A.; Villén, J.; Gerber, S.A.; Rush, J.; Gygi, S.P. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 2006, 24, 1285–1292. [Google Scholar] [CrossRef]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef] [Green Version]
| Sample | No. of Reads | HISAT2 | |
|---|---|---|---|
| No. of Mapped Reads | % of Mapped Reads | ||
| CK1 | 47,265,794 | 40,808,687 | 86.34 |
| CK2 | 36,858,382 | 32,171,708 | 87.28 |
| CK3 | 37,525,170 | 32,802,893 | 87.42 |
| EBR1 | 37,779,118 | 32,877,026 | 87.02 |
| EBR2 | 36,097,508 | 31,370,499 | 86.90 |
| EBR3 | 42,883,904 | 37,520,436 | 87.49 |
| Brz1 | 43,591,944 | 38,366,049 | 88.01 |
| Brz2 | 45,891,392 | 40,287,334 | 87.79 |
| Brz3 | 39,977,534 | 35,050,577 | 87.68 |
| Control | Treat | Up-Regulated | Down-Regulated | Total |
|---|---|---|---|---|
| Ck | EBR | 128 | 169 | 297 |
| Ck | Brz | 99 | 152 | 251 |
| EBR | Brz | 202 | 161 | 363 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; He, X.; Gao, Y.; Liu, W.; Song, J.; Zhang, J. Integrative Analysis of Transcriptome, Proteome, and Phosphoproteome Reveals Potential Roles of Photosynthesis Antenna Proteins in Response to Brassinosteroids Signaling in Maize. Plants 2023, 12, 1290. https://doi.org/10.3390/plants12061290
Li H, He X, Gao Y, Liu W, Song J, Zhang J. Integrative Analysis of Transcriptome, Proteome, and Phosphoproteome Reveals Potential Roles of Photosynthesis Antenna Proteins in Response to Brassinosteroids Signaling in Maize. Plants. 2023; 12(6):1290. https://doi.org/10.3390/plants12061290
Chicago/Turabian StyleLi, Hui, Xuewu He, Yuanfen Gao, Wenjuan Liu, Jun Song, and Junjie Zhang. 2023. "Integrative Analysis of Transcriptome, Proteome, and Phosphoproteome Reveals Potential Roles of Photosynthesis Antenna Proteins in Response to Brassinosteroids Signaling in Maize" Plants 12, no. 6: 1290. https://doi.org/10.3390/plants12061290






