Photosynthetic Pigment and Carbohydrate Profiling of Fucus vesiculosus from an Iberian Coastal Lagoon
Abstract
1. Introduction
2. Materials and Methods
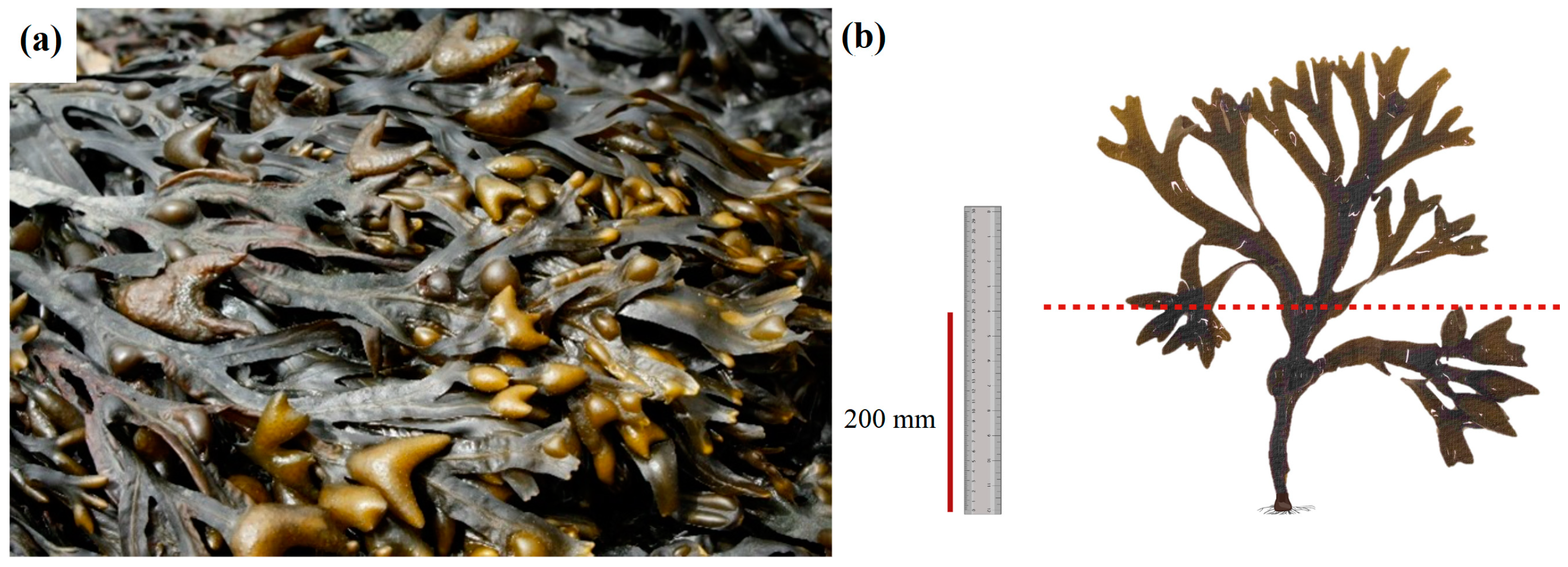
2.1. Macroalgae Sampling
2.2. Chlorophyll a Fluorescence
2.3. Carbohydrate Composition
2.4. Photosynthetic Pigment Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Blue Economy—Towards a Strong and Sustainable EU Algae Sector. Commission Communication COM(2022)592. 2022. Available online: https://oceans-and-fisheries.ec.europa.eu/system/files/2022-11/COM-2022-592_en.pdf (accessed on 10 January 2023).
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Wernberg, T.; Krumhansl, K.; Filbee-Dexter, K.; Pedersen, M.F. Status and trends for the world’s kelp forests. In World Seas: An Environmental Evaluation; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 57–78. [Google Scholar]
- Leal, M.C.; Munro, M.H.G.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef]
- Moreira, A.; Cruz, S.; Marques, R.; Cartaxana, P. The underexplored potential of green macroalgae in aquaculture. Rev. Aquac. 2022, 14, 5–26. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical constituents and biological activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef]
- Morya, V.K.; Kim, J.; Kim, E.K. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 71–82. [Google Scholar] [CrossRef]
- Haggag, Y.; Abd Elrahman, A.; Ulber, R.; Zayed, A. Fucoidan in pharmaceutical formulations: A comprehensive review for smart drug delivery systems. Mar. Drugs 2023, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Nunes, C.; Coimbra, M.A. The potential of fucose-containing sulfated polysaccharides as scaffolds for biomedical applications. Curr. Med. Chem. 2019, 26, 6399–6411. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D. Brown algae carbohydrates: Structures, pharmaceutical properties, and research challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef]
- Oliveira, C.; Ferreira, A.S.; Novoa-Carballal, R.; Nunes, C.; Pashkuleva, I.; Neves, N.M.; Coimbra, M.A.; Reis, R.L.; Martins, A.; Silva, T.H. The key role of sulfation and branching on fucoidan antitumor activity. Macromol. Biosci. 2017, 17, 1600340. [Google Scholar] [CrossRef]
- De Souza, M.C.R.; Marques, C.T.; Dore, C.M.G.; da Silva, F.R.F.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Cavalier-Smith, T.; Chao, E.E.Y. Phylogeny and megasystematics of phagotrophic Heterokonts (kingdom Chromista). J. Mol. Evol. 2006, 62, 388–420. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Bishayee, A.; Fimognari, C. On a beam of light: Photoprotective activities of the marine carotenoids astaxanthin and fucoxanthin in suppression of inflammation and cancer. Mar. Drugs 2020, 18, 544. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-ΚB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef]
- Woo, M.N.; Jeon, S.M.; Kim, H.J.; Lee, M.K.; Shin, S.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef]
- Dias, M.; Lopes, F.; Dekeyser, I. Hydrological characterisation of Ria de Aveiro, Portugal, in early summer. Oceanol. Acta 1999, 22, 473–485. [Google Scholar] [CrossRef]
- Vargas, C.I.C.; Vaz, N.; Dias, J.M. An evaluation of climate change effects in estuarine salinity patterns: Application to Ria de Aveiro shallow water system. Estuar. Coast. Shelf Sci. 2017, 189, 33–45. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.A.; Delgadillo, I.; Waldron, K.W.; Selvendran, R.R. Isolation and analysis of cell wall polymers from olive pulp. In Plant Cell Wall Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 17, pp. 19–44. [Google Scholar] [CrossRef]
- Selvendran, R.R.; March, J.F.; Stephen, R.G. Determination of aldoses and uronic acid content of vegetable fiber. Anal. Biochem. 1979, 96, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Ponce, N.M.A.; Stortz, C.A. A comprehensive and comparative analysis of the fucoidan compositional data across the Phaeophyceae. Front. Plant Sci. 2020, 11, 556312. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.R.; Cartaxana, P.; Brotas, V. HPLC Determination of phytoplankton and microphytobenthos pigments: Comparing resolution and sensitivity of a C18 and a C8 method. Liminology Oceanogr. Methods 2007, 5, 363–370. [Google Scholar] [CrossRef]
- Kraay, G.W.; Zapata, M.; Veldhuis, M.J.W. Separation of chlorophylls c1, c2, and c3 of marine phytoplankton by reversed-phase-C18-High performance liquid chromatography. J. Phycol. 1992, 28, 708–712. [Google Scholar] [CrossRef]
- Elvidge, E.C.L.; Phang, S.M.; Sturges, W.T.; Malin, G. The effect of desiccation on the emission of volatile bromocarbons from two common temperate macroalgae. Biogeosciences 2015, 12, 387–398. [Google Scholar] [CrossRef]
- Magnusson, G. Diurnal measurements of Fv/Fm used to improve productivity estimates in macroalgae. Mar. Biol. 1997, 130, 203–208. [Google Scholar] [CrossRef]
- Pearson, G.; Kautsky, L.; Serrão, E. Recent evolution in Baltic Fucus vesiculosus: Reduced tolerance to emersion stresses compared to intertidal (North Sea) populations. Mar. Ecol. Prog. Ser. 2000, 202, 67–79. [Google Scholar] [CrossRef]
- Huovinen, P.; Gõmez, I. UV Sensitivity of vegetative and reproductive tissues of two Antarctic brown algae is related to differential allocation of phenolic substances. Photochem. Photobiol. 2015, 91, 1382–1388. [Google Scholar] [CrossRef]
- Klindukh, M.; Ryzhik, I.; Makarov, M. Changes in physiological and biochemical parameters of Barents Sea Fucus vesiculosus Linnaeus 1753 in response to low salinity. Aquat. Bot. 2022, 176, 103469. [Google Scholar] [CrossRef]
- Nygård, C.A.; Ekelund, N.G.A. Photosynthesis and UV-B tolerance of the marine alga Fucus vesiculosus at different sea water salinities. J. Appl. Phycol. 2006, 18, 461–467. [Google Scholar] [CrossRef]
- Takolander, A.; Leskinen, E.; Cabeza, M. Synergistic effects of extreme temperature and low salinity on foundational macroalga Fucus vesiculosus in the Northern Baltic Sea. J. Exp. Mar. Biol. Ecol. 2017, 495, 110–118. [Google Scholar] [CrossRef]
- Meichssner, R.; Stegmann, N.; Cosin, A.S.; Sachs, D.; Bressan, M.; Marx, H.; Krost, P.; Schulz, R. Control of fouling in the aquaculture of Fucus vesiculosus and Fucus serratus by regular desiccation. J. Appl. Phycol. 2020, 32, 4145–4158. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Shevchenko, N.M.; Tarbeeva, D.V.; Zvyagintseva, T.N. Comparative study of polysaccharides from reproductive and sterile tissues of five brown seaweeds. Mar. Biotechnol. 2012, 14, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Nishide, E.; Anzai, H.; Uchida, N.; Nisizawa, K. Sugar constituents of fucose-containing polysaccharides from various japanese brown algae. Hydrobiologia 1990, 204, 573–576. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The biochemical composition and antioxidant properties of Fucus vesiculosus from the Arctic Region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Optimisation of fucoxanthin extraction from Irish seaweeds by response surface methodology. J. Appl. Phycol. 2017, 29, 1027–1036. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Aðalbjörnsson, B.V. Sugaring-out: A novel sample preparation method for determination of fucoxanthin in Icelandic edible seaweeds. J. Appl. Phycol. 2021, 33, 515–521. [Google Scholar] [CrossRef]
- Ramus, J.; Lemons, F.; Zlmmerman, C. Adaptation of light-harvesting pigments to downwelling light and the consequent photosynthetic performance of the eulittoral rockweeds Ascophyllum nodosum and Fucus vesiculosus. Mar. Biol. 1977, 42, 293–303. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resende, A.C.R.; Pereira, R.; Nunes, C.; Cruz, S.; Calado, R.; Cartaxana, P. Photosynthetic Pigment and Carbohydrate Profiling of Fucus vesiculosus from an Iberian Coastal Lagoon. Plants 2023, 12, 1324. https://doi.org/10.3390/plants12061324
Resende ACR, Pereira R, Nunes C, Cruz S, Calado R, Cartaxana P. Photosynthetic Pigment and Carbohydrate Profiling of Fucus vesiculosus from an Iberian Coastal Lagoon. Plants. 2023; 12(6):1324. https://doi.org/10.3390/plants12061324
Chicago/Turabian StyleResende, Ana C. R., Rui Pereira, Cláudia Nunes, Sónia Cruz, Ricardo Calado, and Paulo Cartaxana. 2023. "Photosynthetic Pigment and Carbohydrate Profiling of Fucus vesiculosus from an Iberian Coastal Lagoon" Plants 12, no. 6: 1324. https://doi.org/10.3390/plants12061324
APA StyleResende, A. C. R., Pereira, R., Nunes, C., Cruz, S., Calado, R., & Cartaxana, P. (2023). Photosynthetic Pigment and Carbohydrate Profiling of Fucus vesiculosus from an Iberian Coastal Lagoon. Plants, 12(6), 1324. https://doi.org/10.3390/plants12061324










