Antibacterial Potential of Microwave-Assisted Extraction Prepared Hydrolates from Different Salvia Species
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition
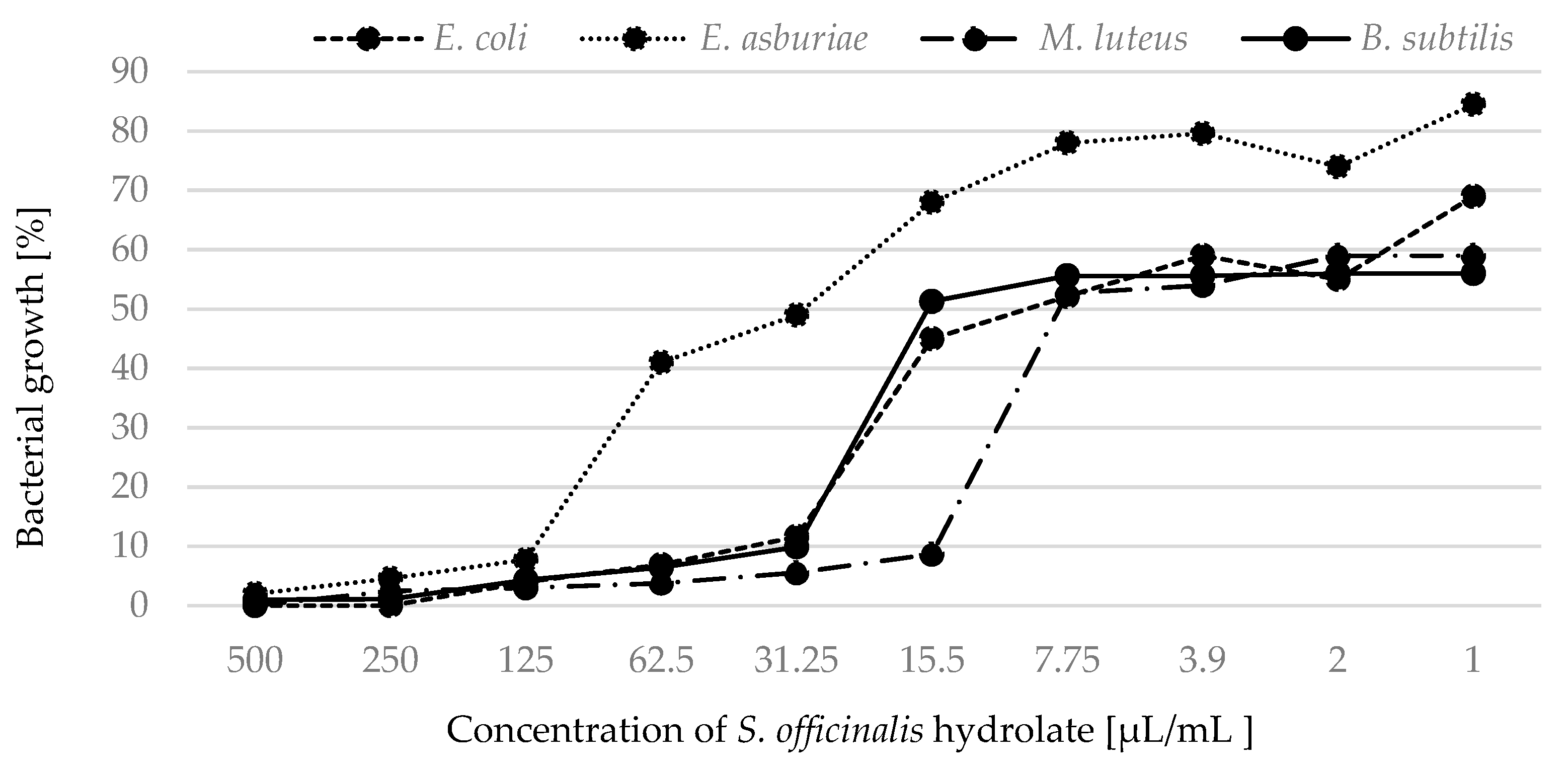
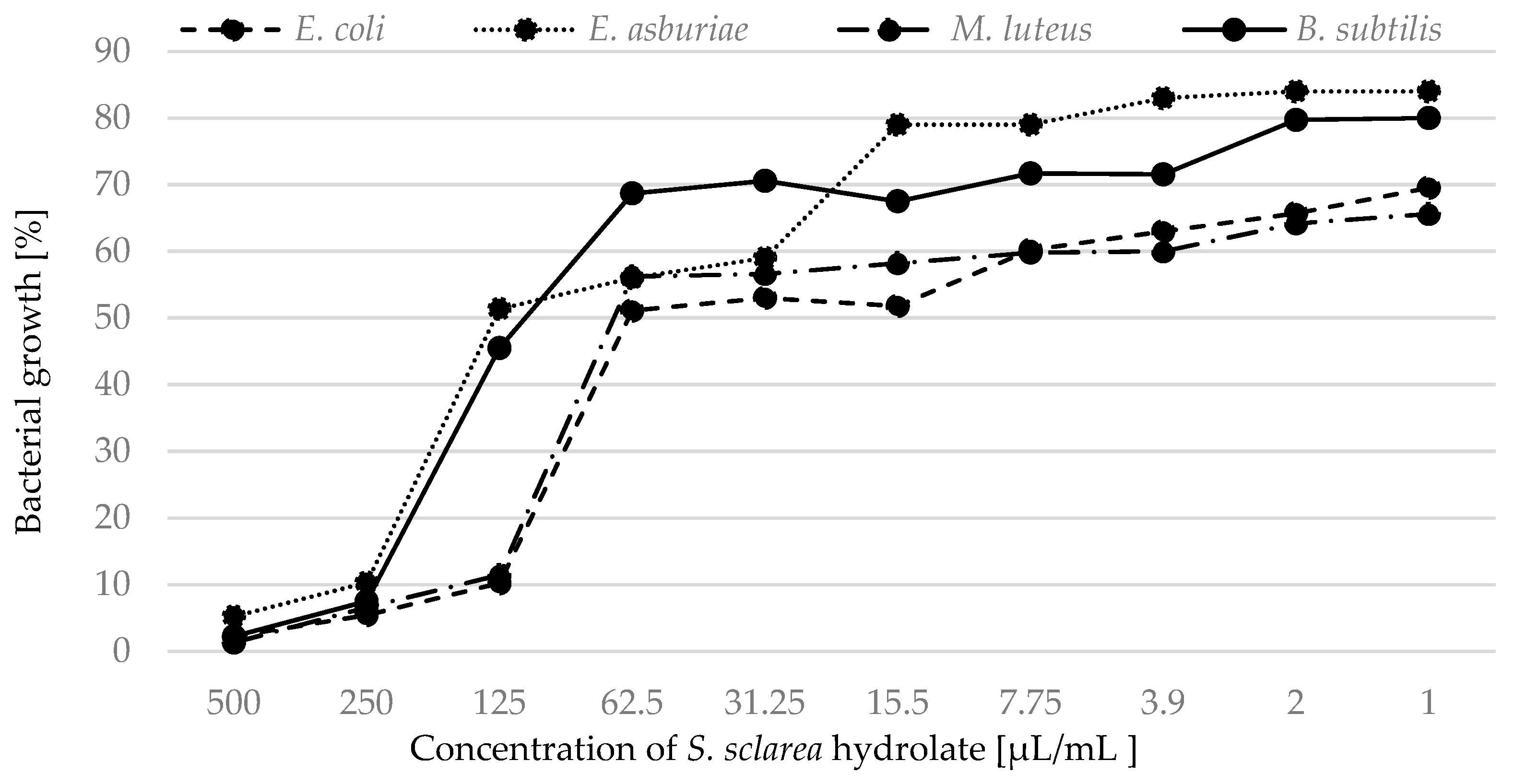
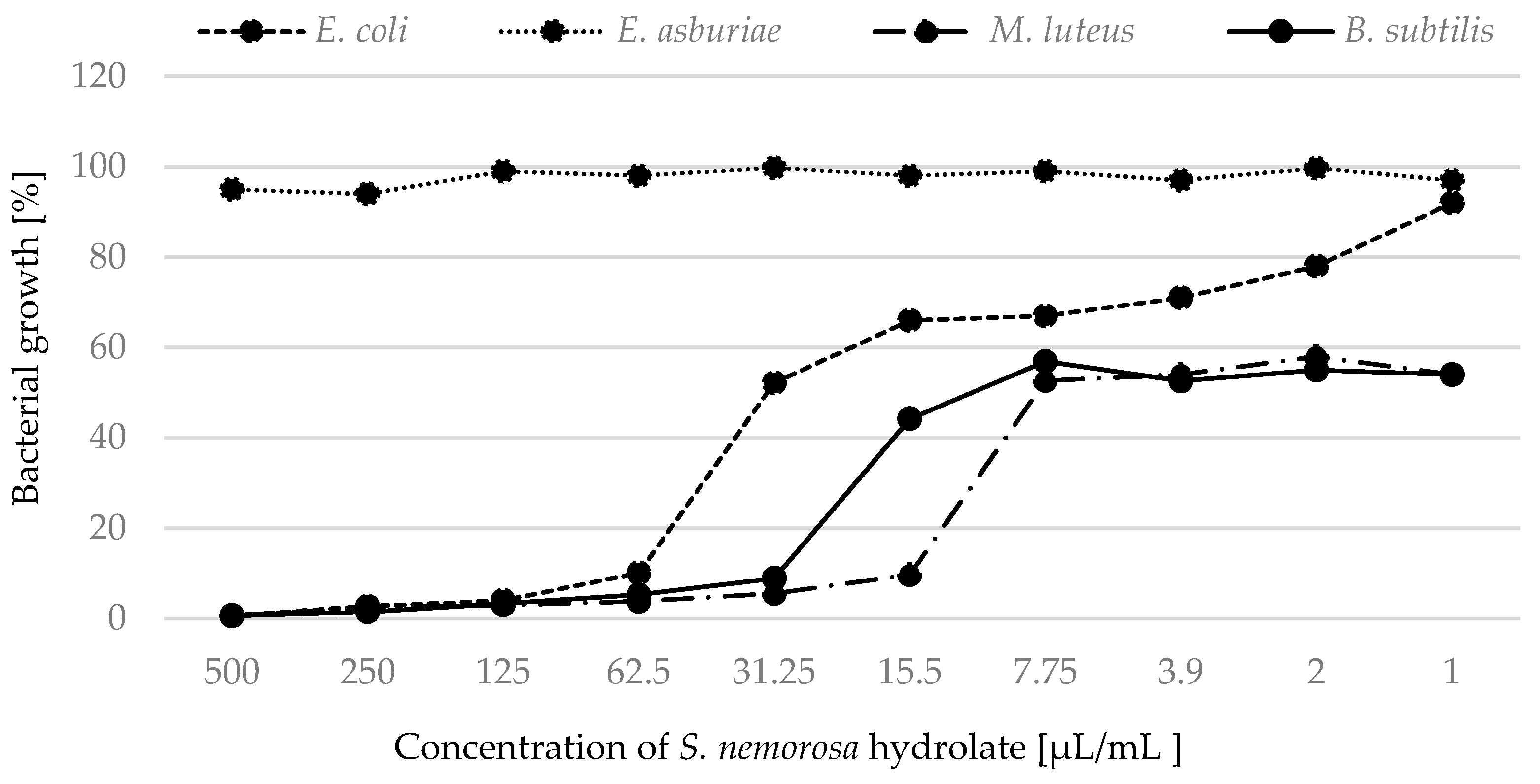
2.2. Antimicrobial Activity
2.3. Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Isolation of Hydrolates
4.2. GC- MS Analysis of Salvia Hydrolates
4.3. Free Radical Scavenging Assays
4.4. Bacterial Strains
4.5. Preparation of the Inoculum
4.6. Determination of Minimum Inhibitory Concentration
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Živković, J.; Ristić, M.; Kschonsek, J.; Westphal, A.; Mihailović, M.; Filipović, V.; Böhm, V. Comparison of chemical profile and antioxidant capacity of seeds and oils from Salvia sclarea and Salvia officinalis. Chem. Biodivers. 2017, 14, e1700344. [Google Scholar] [CrossRef]
- Delamare, L.A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the esential oil of Salvia officinalis L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Cabral, C.; Fereirra, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-Bdour, T.H.; Salgueiro, L. Essential Oil of Common Sage (Salvia officinalis L.) from Jordan: Assessment of Safety in Mammalian Cells and Its Antifungal and Anti-Inflammatory Potential. BioMed Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef] [Green Version]
- Ovidi, E.; Laghezza Masci, V.; Zambelli, M.; Tiezzi, A.; Vitalini, S.; Garzoli, S. Laurus nobilis, Salvia sclarea and Salvia officinalis Essential Oils and Hydrolates: Evaluation of Liquid and Vapor Phase Chemical Composition and Biological Activities. Plants 2021, 10, 707. [Google Scholar] [CrossRef]
- Sefidkon, F.; Khajavi, M.S. Chemical composition of the essential oils of two Salvia species from Iran: Salvia verticillata L. and Salvia santolinifolia Boiss. Flavour Frag. J. 1999, 14, 77–78. [Google Scholar] [CrossRef]
- Craft, J.D.; Satyal, P.; Setzer, W.N. The Chemotaxonomy of Common Sage (Salvia officinalis) Based on the Volatile Constituents. Medicines 2017, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Boutebouhart, H.; Didaoui, L.; Tata, S.; Sabaou, N. Effect of Extraction and Drying Method on Chemical Composition, and Evaluation of Antioxidant and Antimicrobial Activities of Essential Oils from Salvia officinalis L. J. Essent. Oil Bear. Plants 2019, 3, 717–727. [Google Scholar] [CrossRef]
- Chizzola, R. Composition and Variability of the Essential Oil of Salvia nemorosa (Lamiaceae) from the Vienna Area of Austria. Nat. Prod. Commun. 2012, 17, 1671–1672. [Google Scholar] [CrossRef] [Green Version]
- Güllüce, M.; Özer, H.; Bar, I.Ş.Ö.; Daferera, D.; Şahín, F.; Polissiou, M. Chemical Composition of the Essential Oil of Salvia aethiopis L. Turk. J. Biol. 2006, 30, 231–233. [Google Scholar]
- Carrubba, A.; La Torre, R.; Picaglia, R.; Marotti, M. Characterization of an Italian biotype of clary sage (Salvia sclarea L.) grown in a semi-arid Mediterranean environment. Flavour Frag. J. 2002, 17, 91–194. [Google Scholar] [CrossRef]
- Siebert, D.J. Salvia divinorum and salvinorin A: New pharmacologic findings. J. Ethnopharmacol. 1994, 43, 53–56. [Google Scholar] [CrossRef] [PubMed]
- El Euch, S.K.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Farimani, M.M.; Bahadori, M.B.; Koulaei, S.A.; Salehi, P.; Ebrahimi, S.N.; Khavasi, H.R.; Hamburger, M. New ursane triterpenoids from Salvia urmiensis Bunge: Absolute configuration and anti-proliferative activity. Fitoterapia 2015, 106, 1–6. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Farah, A.; Mattazi, N.; Fadil, M.; Chraibi, M.; Benbrahim, K.F. Esential oils analysis and antibacterial activity of the leaves of Rosmarinus officinalis, Salvia officinalis and Menta piperita cultivated in Agadir (Morocco). Int. J. Pharm. Pharm. Sci. 2015, 7, 73–79. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ouedrhiri, W.; Farah, A.; El Abed, E.; Koraichi, S.I. Antibacterial activity of extractfrom Salvia officinalis and Rosmarinus officinalis obtained by sonication and maceration methods. Int. J. Pharm. Sci. 2014, 6, 167–170. [Google Scholar]
- Tepe, B.; Donmez, F.; Unlu, M.; Candan, F.; Daferera, D.; Unlu, V.G.; Polisiou, M.; Sokmen, A. Antimicrobial and antioxidative activities of the essential oil and methanol extracts of Salvia crypthanta (Montbret et Aucher ex Bent) and Salvia multicaulis. Food Chem. 2004, 84, 519–525. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polisiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Ćulafić, D.M.; Vuković-Gačić, B.; Knežević-Vukčević, J.; Stanković, S.; Simić, D. Comparative study on the antibacterial activity of volatiles from sage (Salvia officinalis L.). Arch. Biol. Sci. 2005, 57, 173–178. [Google Scholar] [CrossRef]
- Smigielski, K.; Prusinowska, R.; Stobiecka, A.; Kunicka-Styczynska, A.; Gruska, R. Biological properties and chemical composition of essential oils from flowers and aerial parts of lavender (Lavandula angustifolia). J. Essent. Oil Bear. Plants 2018, 21, 1303–1314. [Google Scholar] [CrossRef]
- Hay, Y.O.; Abril-Sierra, M.A.; Sequeda-Castaneda, L.G.; Bonnafous, C.; Raynaud, C. Evaluation of combinations of essential oils and essential oils with hydrosols on antimicrobial and antioxidant activities. J. Pharm. Pharmacogn. Res. 2018, 6, 216–230. [Google Scholar]
- Lira, P.D.; Reeta, D.; Tkacik, E.; Ringuelet, J.; Coussio, J.D.; van Baren, C.; Bandoni, A.L. Essential oil and by-products of distillation of bay leaves (Laurus nobilis L.) from Argentina. Ind. Crops Prod. 2009, 30, 259–264. [Google Scholar] [CrossRef]
- D’Amato, S.; Serio, A.; Lopez, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Hamedi, A.; Moheimani, S.M.; Sakhteman, A.; Etemadfard, H.; Moein, M. An overview on indications and chemical composition of aromatic waters (hydrosols) as functional beverages in Persian nutrition culture and folk medicine for hyperlipidemia and cardiovascular conditions. J. Evid.-Based Integr. Med. 2017, 22, 544–561. [Google Scholar] [CrossRef]
- Šilha, D.; Švarcová, K.; Bajer, T.; Královec, K.; Tesarová, E.; Moucková, K.; Pejchalová, M.; Bajerová, P. Chemical composition of natural hydrolates and their antimicrobial activity. Molecules 2020, 25, 5654. [Google Scholar] [CrossRef]
- Rossi, C.; Maggio, F.; Chaves-López, C.; Valbonetti, L.; Berrettoni, M.; Paparella, A.; Serio, A. Effectiveness of selected essential oils and one hydrolate to prevent and remove Listeria monocytogenes biofilms on polystyrene and stainless steel food-contact surfaces. J. Appl. Microbiol. 2021, 132, 1866–1876. [Google Scholar] [CrossRef]
- Vosoughi, N.; Gomarian, M.; Pirbalouti, A.G.; Khaghani, S.; Malekpoor, F. Essential oil composition and total phenolic, flavonoid contents, and antioxidant activity of sage (Salvia officinalis L.) extract under chitosan application and irrigation frequencies. Ind. Crops Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Mandras, N.; Nostro, A.; Roana, J.; Scalas, D.; Banche, G.; Ghisetti, V.; Del Re, S.; Fucale, G.; Cuffini, A.M.; Tullio, V. Liquid and vapourphase antifungal activities of essential oils against Candida albicans and non-albicans Candida. BMC Complement. Altern. Med. 2016, 16, 330. [Google Scholar] [CrossRef] [Green Version]
- Selim, S.; Almuhayawi, M.S.; Alqhtani, H.; Al Jaouni, S.K.; Saleh, F.M.; Warrad, M.; Hagagy, N. Anti-Salmonella and antibiofilm potency of Salvia officinalis L. essential oil against antibiotic-resistant Salmonella enterica. Antibiotics 2022, 11, 489. [Google Scholar] [CrossRef]
- Politi, M.; Ferrante, C.; Menghini, L.; Angelini, P.; Flores, G.A.; Muscatello, B.; Braca, A.; De Leo, M. Hydrosols from Rosmarinus officinalis, Salvia officinalis, and Cupressus sempervirens: Phytochemical analysis and bioactivity evaluation. Plants 2022, 11, 349. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.E.; Shirtliff, M.E. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1463–1469. [Google Scholar] [CrossRef] [Green Version]
- Baydar, H.; Sangun, M.K.; Erbas, S.; Kara, N. Comparison of arome compounds indistilled end extracted products of sage (Salvia officinalis L.). J. Essent. Oil-Bear. Plants 2013, 16, 39–44. [Google Scholar] [CrossRef]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef]
- Tavares, C.S.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Hydrolates: A review on their volatiles composition, biological properties and potential uses. Phytochem. Rev. 2022, 21, 1661–1737. [Google Scholar] [CrossRef]
- Stan (Tudora), C.; Muscalu, A.; Burnichi, F.; Popescu, C.; Gatea, F.; Sicuia, O.-A.; Vladut, N.V.; Israel-Roming, F. Evaluation of essential oils and hydrolate from a new hyssops variety (Hyssopus officinalis L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12639. [Google Scholar] [CrossRef]
- Wedakoon, C.N.; Sakaguchi, M. Combined effect of sodium cholride and clove on growth and biogenic amine formation of Enterobacter aerogenes in mackerel muscle extract. J. Food Prot. 1993, 56, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Kuzma, L.; Kalemba, D.; Rozalski, M.; Rozalska, B.; Więckowska-Szakiel, M.; Krajewska, U.; Wysokinska, H. Chemical composition and biological activities of essential oil from Salvia sclarea plants regenerated in vitro. Molecules 2009, 14, 1438–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saǧdıç, O.; Özcan, M. Antibacterial activity of Turkish spice hydrosols. Food Control 2003, 14, 141–143. [Google Scholar] [CrossRef]
- Tornuk, F.; Cankurt, H.; Ozturk, I.; Sagdiç, Ö.; Bayram, O.; Yetim, H. Efficacy of various plant hydrosols as natural food sanitizers in reducing Escherichia coli O157:H7 and Salmonella typhimurium on fresh cut carrots and apples. Int. J. Food Microbiol. 2011, 148, 30–35. [Google Scholar] [CrossRef]
- Ozturk, I.; Tornuk, F.; Saǧdıç, O.; Kisi, O. Application of non-linear models to predict inhibition effects of various plant hydrosols on Listeria monocytogenes inoculated on fresh-cut apples. Foodborne Pathog. Dis. 2012, 9, 607–616. [Google Scholar] [CrossRef]
- Tornuk, F.; Ozturk, I.; Sagdiç, Ö.; Yilmaz, A.; Erkmen, O. Application of predictive inactivation models to evaluate survival of Staphylococcus aureus in fresh-cut apples treated with different plant hydrosols. Int. J. Food Prop. 2014, 17, 587–598. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, L.; Tornuk, F.; Caliskan-Aydigan, O.; Zeki Durak, M.; Sagdiç, Ö. Decontamination of iceberg lettuce by some plant hydrosols. LWT-Food Sci. Technol. 2016, 74, 48–54. [Google Scholar] [CrossRef]
- Oral, N.; Vatansever, L.; Guven, A.; Gulmez, M. Antibacterial activity of some Turkish plant hydrosols. Kafkas Univ. Vet. Fak. Gerg. 2008, 14, 205–209. [Google Scholar]
- Jakubczyk, K.; Tuchowska, A.; Janda-Milczarek, K. Plant hydrolates—Antioxidant properties, chemical composition and potential applications. Biomed. Pharmacother. 2021, 142, 112033. [Google Scholar] [CrossRef] [PubMed]
- Senol, S.; Orhan, I.; Celep, F.; Kahraman, A.; Dogan, M.; Yilmaz, G.; Şeneraet, B. Survey of 55 Turkish Salvia taxa for their acetylcholinesterase inhibitory and antioxidant activities. Food Chem. 2010, 120, 34–43. [Google Scholar] [CrossRef]
- Jeshvaghani, Z.A.; Rahimmalek, M.; Talebi, M.; Hossein Goli, S.A. Comparison of total phenolic content and antioxidant activity in different Salvia species using three model systems. Ind. Crops Prod. 2015, 77, 409–414. [Google Scholar] [CrossRef]
- Ben Taari, M.; Msaada, K.; Hosni, K.; Marzouk, B. Fatty acids, phenolic changes and antioxidant activity of clary sage (Salvia sclarea L.) rosette leaves grown under saline conditions. Ind. Crops Prod. 2012, 38, 58–63. [Google Scholar] [CrossRef]
- Stagos, D.; Portesis, N.; Spanou, C.; Mossialos, D.; Aligiannis, N.; Chaita, E.; Panagoulis, C.; Reri, E.; Skaltsounis, L.; Tsatsakis, A.M.; et al. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem.Toxicol. 2012, 50, 4115–4124. [Google Scholar] [CrossRef]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 2006, 95, 200–204. [Google Scholar] [CrossRef]
- Císarová, M.; Hleba, L.; Tančinová, D.; Florková, M.; Foltinová, D.; Charousová, I.; Vrbová, K.; Božik, M.; Klouček, P. Inhibitory effect of essential oils from some lamiaceae species on growth of Eurotium spp. isolated from bread. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 857–862. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical And Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, 8th ed.; CLSI Document M7-A8; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
- Hleba, L.; Vuković, N.; Horská, E.; Petrová, J.; Sukdolak, S.; Kačániová, M. Phenolic profile and antimicrobial activities to selected microorganisms of some wild medical plant from Slovakia. Asian Pac. J. Trop. Dis. 2014, 4, 269–274. [Google Scholar] [CrossRef]
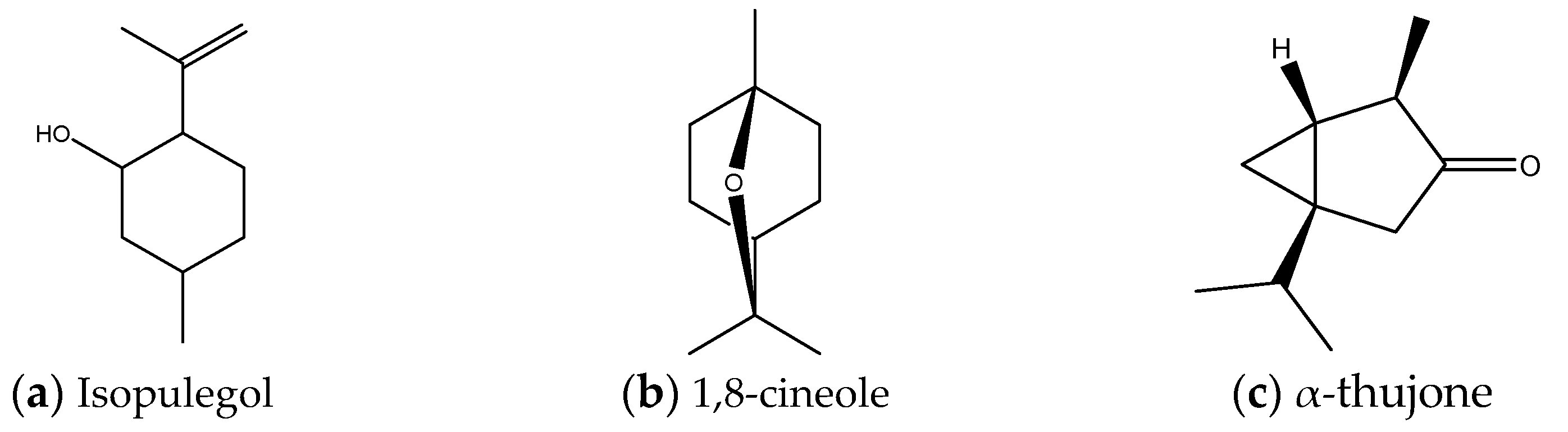
| RI b | Compound | S. officinalisc | S. nemorosac | S. aethiopisc | S. sclareac | S. divinorumc |
|---|---|---|---|---|---|---|
| 982 | (-)-β-Pinene a | - | 1.31% | - | 1.20% | 3.15% |
| 1034 | 1,8-Cineole a | 19.57% | 15.67% | 11.75% | 4.71% | 6.29% |
| 1100 | (-)-Linalool a | 4.01% | 2.68% | 3.70% | 3.44% | 3.40% |
| 1117 | α- and β-Thujone | 5.57% | 14.10% | 13.78% | 11.50% | 10.95% |
| 1137 | Unidentified | - | - | 1.03% | 1.13% | 1.21% |
| 1146 | (-)-Isopulegol | 57.11% | 47.20% | 56.54% | 40.26% | 38.22% |
| 1167 | (-)-Borneol a | 5.41% | 4.74% | 5.06% | 5.28% | 6.55% |
| 1178 | Menthol (+/−) a | - | 2.02% | - | - | 2.71% |
| 1197 | Carvomenthenol a | 1.12% | - | - | - | - |
| 1403 | Unidentified | - | - | - | - | 1.38% |
| 1413 | Unidentified | - | 1.50% | 1.15% | - | - |
| 1475 | α-Cubebene | - | - | - | 3.90% | 4.10% |
| 1517 | Caryophyllene | - | - | - | 3.28% | 2.71% |
| 1552 | α-Caryophyllene | - | - | - | 3.34% | 3.04% |
| 1579 | Germacrene D | - | - | - | 4.59% | 4.21% |
| 1585 | Unidentified | - | - | - | - | 1.29% |
| 1623 | Unidentified | - | - | - | - | 1.10% |
| 1625 | Unidentified | - | - | - | - | 1.20% |
| 1680 | Unidentified | - | - | - | 1.28% | - |
| 1689 | Naphthalene | - | 1.75% | 1.26% | 6.41% | - |
| - | Total | 92.79% | 90.95% | 94.27% | 90.31% | 91.52% |
| Tested Hydrolates | E. coli | E. asburiae | M. luteus | B. subtilis | ||||
|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | |
| S. officinalis | 27.50 | 42.12 | 56.65 | 123.16 | 5.69 | 7.81 | 18.43 | 31.25 |
| S. nemorosa | 38.82 | 62.58 | >500 | >500 | 8.51 | 15.63 | 11.26 | 28.14 |
| S. aethiopis | >500 | >500 | 216.59 | 407.13 | >500 | >500 | >500 | >500 |
| S. sclarea | 74.99 | 125.41 | 141.41 | 253.01 | 81.68 | 136.38 | 106.58 | 201.11 |
| S. divinorum | >500 | >500 | 325.61 | 472.61 | >500 | >500 | >500 | >500 |
| Tested Hydrolates | Antioxidant Activity (%) | TEAC (mg/mL) |
|---|---|---|
| S. officinalis | 23.25 ± 0.26 | 52.16 ± 2.85 |
| S. nemorosa | 14.61 ± 0.09 | 31.08 ± 1.09 |
| S. aethiopis | 10.62 ± 0.96 | 24.12 ± 0.96 |
| S. sclarea | 18.99 ± 0.58 | 42.12 ± 1.58 |
| S. divinorum | 6.42 ± 0.57 | 14.41 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ürgeová, E.; Uváčková, Ľ.; Vaneková, M.; Maliar, T. Antibacterial Potential of Microwave-Assisted Extraction Prepared Hydrolates from Different Salvia Species. Plants 2023, 12, 1325. https://doi.org/10.3390/plants12061325
Ürgeová E, Uváčková Ľ, Vaneková M, Maliar T. Antibacterial Potential of Microwave-Assisted Extraction Prepared Hydrolates from Different Salvia Species. Plants. 2023; 12(6):1325. https://doi.org/10.3390/plants12061325
Chicago/Turabian StyleÜrgeová, Eva, Ľubica Uváčková, Miroslava Vaneková, and Tibor Maliar. 2023. "Antibacterial Potential of Microwave-Assisted Extraction Prepared Hydrolates from Different Salvia Species" Plants 12, no. 6: 1325. https://doi.org/10.3390/plants12061325
APA StyleÜrgeová, E., Uváčková, Ľ., Vaneková, M., & Maliar, T. (2023). Antibacterial Potential of Microwave-Assisted Extraction Prepared Hydrolates from Different Salvia Species. Plants, 12(6), 1325. https://doi.org/10.3390/plants12061325





