Triterpenoids in Jujube: A Review of Composition, Content Diversity, Pharmacological Effects, Synthetic Pathway, and Variation during Domestication
Abstract
1. Introduction
2. Results
2.1. Types of Triterpenoids in Jujube
2.1.1. Triterpenes
2.1.2. Saponins
2.2. Dynamic Changes in Triterpenoid Content in Jujube and Sour Jujube
2.3. Biological Activity of Triterpenes in Jujube and Sour Jujube
2.3.1. Anti-Cancer Activity
2.3.2. Antioxidant and Hepatoprotective Activities
2.3.3. Anti-Viral Activity
2.3.4. Immune and Anti-Inflammatory Activities
2.3.5. Other Biological Activity
2.4. Pharmacokinetic Study of Jujube and Sour Jujube Triterpenoids
2.5. Synthetic Pathways and Regulation of Jujube and Sour Jujube Terpenoids
2.6. Effects of Domestication on Jujube and Sour Jujube Triterpene Metabolism
3. Conclusions and Perspectives
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Luo, Z.; Liu, Z.; Zhao, J.; Deng, W.; Wei, H.; Liu, P.; Liu, M. Genome Size Variation within Species of Chinese Jujube (Ziziphus jujuba Mill.) and Its Wild Ancestor Sour Jujube (Z. acidojujuba Cheng et Liu). Forests 2019, 10, 460. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Z.; Li, S.; Lian, Q.; Fu, P.; He, Y.; Qiao, J.; Xu, K.; Liu, L.; Wu, M.; et al. Genomic analyses of diverse wild and cultivated accessions provide insights into the evolutionary history of jujube. Plant Biotechnol. J. 2021, 19, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Bao, T.; Mo, J.; Ni, J.; Chen, W. Research advances in bioactive components and health benefits of jujube (Ziziphus jujuba Mill.) fruit. J. Zhejiang Univ. Sci. B 2021, 22, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, X.S.; Liu, M.J. Research progress on chemical constituents and utilization of sour jujube (Z. acidojujuba Cheng et Liu). North. Hortic. 2017, 5, 184–188. [Google Scholar]
- Liu, M.; Wang, J.; Wang, L.; Liu, P.; Zhao, J.; Zhao, Z.; Yao, S.; Stănică, F.; Liu, Z.; Wang, L.; et al. The historical and current research progress on jujube-a superfruit for the future. Hortic. Res. 2020, 7, 119. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Maiwulanjiang, M.; Zhang, W.L.; Zhan, J.Y.; Lam, C.T.; Zhu, K.Y.; Yao, P.; Choi, R.C.; Lau, D.T.; et al. Chemical and biological assessment of Ziziphus jujuba fruits from China: Different geographical sources and developmental stages. J. Agric. Food Chem. 2013, 61, 7315–7324. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Duan, J.X.; Tang, Y.P.; Qian, D.W. Progress on resource chemistry of the medicinal plants ingenus Ziziphus. Mod. Chin. Med. 2012, 14, 1–5. [Google Scholar] [CrossRef]
- Liu, S.; Lv, Y.; Tang, Z.; Zhang, Y.; Xu, H.; Zhang, D.; Cui, C.; Liu, H.; Sun, H.; Song, Z.; et al. Ziziphus jujuba Mill., a plant used as medicinal food: A review of its phytochemistry, pharmacology, quality control and future research. Phytochem. Rev. 2021, 20, 507–541. [Google Scholar] [CrossRef]
- El Maaiden, E.; El Kharrassi, Y.; Qarah, N.A.S.; Essamadi, A.K.; Moustaid, K.; Nasser, B. Genus Ziziphus: A comprehensive review on ethnopharmacological, phytochemical and pharmacological properties. J. Ethnopharmacol. 2020, 15, 112950. [Google Scholar] [CrossRef]
- Sakna, S.T.; Maghraby, Y.R.; Abdelfattah, M.S.; Farag, M.A. Phytochemical diversity and pharmacological effects of triterpenes from genus Ziziphus: A comprehensive review. Phytochem. Rev. 2022, 1–26. [Google Scholar] [CrossRef]
- Prakash, O.; Usmani, S.; Singh, R.; Singh, N.; Gupta, A.; Ved, A. A panoramic view on phytochemical, nutritional, and therapeutic attributes of Ziziphus mauritiana Lam.: A comprehensive review. Phytother. Res. 2021, 35, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2013, 30, 1028–1065. [Google Scholar] [CrossRef] [PubMed]
- Mallavadhani, U.V.; Chandrashekhar, M.; Shailaja, K.; Ramakrishna, S. Design, synthesis, anti-inflammatory, cytotoxic and cell based studies of some novel side chain analogues of myrrhanones A & B isolated from the gum resin of Commiphora mukul. Bioorg. Chem. 2019, 82, 306–323. [Google Scholar] [CrossRef]
- Madasu, C.; Karri, S.; Sangaraju, R.; Sistla, R.; Uppuluri, M.V. Synthesis and biological evaluation of some novel 1,2,3-triazole hybrids of myrrhanone B isolated from Commiphora mukul gum resin: Identification of potent antiproliferative leads active against prostate cancer cells (PC-3). Eur. J. Med. Chem. 2020, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Z.; Wimmer, Z. Selected plant triterpenoids and their amide derivatives in cancer treatment: A review. Phytochemistry 2022, 203, 113340. [Google Scholar] [CrossRef] [PubMed]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, M.L.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Zou, Q.P.; Lu, C.Z. Spectral characteristics of lupane-type triterpenoids form natural products. J. Hunan Univ. Chin. Med. 2013, 33, 10–32. [Google Scholar]
- Guo, S.; Duan, J.A.; Tang, Y.P.; Su, S.L.; Qian, D.W. Triterpenoid acids from Ziziphus jujuba. Chem. Nat. Compd. 2011, 47, 138–139. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Tang, Y.P.; Qian, Y.F.; Zhao, J.L.; Qian, D.W. Triterpenoids from the fruits of Ziziphus jujuba var. spinosa. Biochem. Syst. Ecol. 2011, 39, 4–6. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Hayashida, A.; Tsurushima, K.; Nagai, R.; Yoshitomi, M.; Daiguji, N.; Sakashita, N.; Takeya, M.; Tsukamoto, S.; Ikeda, T. Triterpenoids isolated from Zizyphus jujuba inhibit foam cell formation in macrophages. J. Agric. Food Chem. 2011, 59, 4544–4552. [Google Scholar] [CrossRef]
- Lee, S.M.; Min, B.S.; Lee, C.G.; Kim, K.S.; Kho, Y.H. Cytotoxic triterpenoids from the fruits of Zizyphus jujuba. Planta Med. 2003, 69, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Pan, Q.; Min, Z.D. A novel lupine triterpene from seeds of Ziziphus jujuba var. spinosa. Chin. Tradit. Herb. Drugs 2006, 2, 168–171. [Google Scholar]
- Wang, J.R.; Zhang, J.; Yin, Z.Q.; Liang, J.Y.; Ye, W.C. Chemical constituents from the seeds of Ziziphus jujuba var. spinosa. Chin. J. Nat. Med. 2008, 4, 268–270. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Tang, Y.P.; Yang, N.Y.; Qian, D.W.; Su, S.L.; Shang, E.X. Characterization of triterpenic acids in fruits of ziziphus species by HPLC-ELSD-MS. J. Agric. Food Chem. 2010, 58, 6285–6289. [Google Scholar] [CrossRef]
- Qiao, A.; Wang, Y.; Xiang, L.; Zhang, Z.; He, X. Triterpenoids of sour jujube show pronounced inhibitory effect on human tumor cells and antioxidant activity. Fitoterapia 2014, 98, 137–142. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Tang, Y.; Qian, Y.; Zhao, J.; Qian, D.; Su, S.; Shang, E. Simultaneous qualitative and quantitative analysis of triterpenic acids.; saponins and flavonoids in the leaves of two Ziziphus species by HPLC-PDA-MS/ELSD. J. Pharm. Biomed Anal. 2011, 56, 264–270. [Google Scholar] [CrossRef]
- Kang, K.B.; Kim, J.W.; Oh, W.K.; Kim, J.; Sung, S.H. Cytotoxic Ceanothane- and Lupane-Type Triterpenoids from the Roots of Ziziphus jujuba. J. Nat. Prod. 2016, 79, 2364–2375. [Google Scholar] [CrossRef] [PubMed]
- Grishko, V.V.; Tolmacheva, I.A.; Pereslavtseva, A.V. Triterpenoids with a Five-Membered a-Ring: Distribution in Nature, Transformations, Synthesis, and Biological Activity. Chem. Nat. Compd. 2015, 51, 1–21. [Google Scholar] [CrossRef]
- Che, Y.; Li, S.T.; Zhang, Y.Q. Chemical constituents of Ziziphus jujuba var. spinosa root. Chem. Ind. For. Prod. 2012, 32, 83–86. [Google Scholar]
- Wu, Y.; Chen, M.; Du, M.B.; Yue, C.H.; Li, Y.Y.; Zhu, M.; Liu, C.; Wang, D.Y.; Liu, J.G.; Hu, Y.L. Chemical constituents from the fruit of Zizyphus jujuba Mill. var. spinose. Biochem. Syst. Ecol. 2014, 57, 182–186. [Google Scholar] [CrossRef]
- Su, B.N.; Cuendet, M.; Farnsworth, N.R.; Fong, H.H.; Pezzuto, J.M.; Kinghorn, A.D. Activity-guided fractionation of the seeds of Ziziphus jujuba using a cyclooxygenase-2 inhibitory assay. Planta Med. 2002, 12, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Lin, B.F.; Liu, K.C. Three triterpene esters from Zizyphus jujuba. Phytochemistry 1996, 43, 847–851. [Google Scholar] [CrossRef]
- Ruan, J.; Sun, F.; Hao, M.; Han, L.; Yu, H.; Lin, F.; Wang, L.; Cao, G.; Zhang, Y.; Wang, T. Structurally diverse triterpenes obtained from the fruits of Ziziphus jujuba Mill. as inflammation inhibitors by NF-κB signaling pathway. Food Funct. 2021, 12, 4496–4503. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, J.G.; Lee, Y.H.; Lee, C.G.; Min, B.S.; Kim, J.H.; Lee, H.K. Anti-complementary activity of triterpenoides from fruits of Zizyphus jujuba. Biol. Pharm. Bull. 2004, 27, 1883–1886. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Tang, Y.P.; Duan, J.A.; Su, S.L.; Ding, A.W. Two new terpenoids from fruits of Ziziphus jujuba. Chin. Chem. Lett. 2009, 20, 197–200. [Google Scholar] [CrossRef]
- Che, Y.; Zheng, B.Q.; Teng, Y.R.; Zhang, Y.R. Chemical constituents from the leaves of Ziziphus jujuba var. spinosa. Chin. Tradit. Pat. Med. 2012, 34, 686–688. [Google Scholar]
- Guo, S.; Duan, J.H.; Qian, D.W.; Tang, Y.P. Chemical constituents of Ziziphus plants:research advances. J. Int. Pharm. Res. 2013, 40, 702–710. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Shimono, N.; Arihara, S. Antisweet substances, jujubasaponins I–III from zizyphus jujuba revised structure of ziziphin. Tetrahedron Lett. 1991, 32, 7059–7062. [Google Scholar] [CrossRef]
- Huang, X.; Mao, Y.; Li, H.; Wang, Y.; Liu, Y. Difference in chemical constituents of Ziziphi Spinosae Semen from different producing areas by UHPLC-LTQ-Orbitrap MS based metabolomics. Mod. Chin. Med. 2021, 23, 2077–2087. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Shimono, N.; Arihara, S. Antisweet Natural Products. VI. Jujubasaponins IV.; V and Vi from Zizyphus jujuba MILL. Chem. Pharm. Bull. 1992, 40, 2275–2278. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.X.; Zhao, J.L.; Qian, Y.F.; Tang, Y.P.; Qian, D.W. Resource chemical constituents from sarcocarp of Ziziphus jujuba var. spinosa. Chin. Tradit. Herb. Drugs 2012, 43, 1905–1909. [Google Scholar]
- Yoshikawa, M.; Murakami, T.; Ikebata, A.; Wakao, S.; Murakami, N.; Matsuda, H.; Yamahara, J. Bioactive saponins and glycosides. X. On the constituents of zizyphi spinosi semen, the seeds of Zizyphus jujuba Mill. var. spinosa Hu (1): Structures and histamine release-inhibitory effect of jujubosides A1 and C and acetyljujuboside B. Chem. Pharm. Bull. 1997, 45, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, B.; Yao, S. Simultaneous analysis and identification of main bioactive constituents in extract of Zizyphus jujuba var. sapinosa (Zizyphi spinosi semen) by high-performance liquid chromatography-photodiode array detection-electrospray mass spectrometry. Talanta 2007, 71, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.S.; Lee, J.; Kim, Y.; Kang, S.S. A New Saponin from the Seeds of Zizyphus jujuba var. spinosa. Bull. Korean Chem. Soc. 2013, 34, 657–660. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Wang, D.; Liu, J.; Hu, Y. Triterpenoid saponins from Ziziphus jujuba var. spinosa. Chem. Nat. Compd. 2013, 49, 677–681. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, B.; Luo, D.; Chen, L.Y.; Hou, Y.L.; Dai, Y.; Yao, X.S. New triterpene glycosides from Ziziphi Spinosae Semen. Fitoterapia 2013, 90, 185–191. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, X.S.; Hu, Z.H. Dammarane-type saponins from Ziziphus jujube. J. Asian Nat. Prod. Res. 2014, 16, 200–205. [Google Scholar] [CrossRef]
- Fu, Q.; Yuan, H.; Chen, J.; Shi, J. Dammarane-type saponins from Ziziphus jujube and their inhibitory effects against TNF-α release in LPS-induced RAW 246.7 macrophages. Phytochem. Lett. 2016, 16, 169–173. [Google Scholar] [CrossRef]
- Masullo, M.; Cerulli, A.; Montoro, P.; Pizza, C.; Piacente, S. In depth LC-ESIMSn-guided phytochemical analysis of Ziziphus jujuba Mill. leaves. Phytochemistry 2019, 159, 148–158. [Google Scholar] [CrossRef]
- Matsuda, H.; Murakami, T.; Ikebata, A.; Yamahara, J.; Yoshikawa, M. Bioactive saponins and glycosides. XIV. Structure elucidation and immunological adjuvant activity of novel protojujubogenin type triterpene bisdesmosides, protojujubosides A, B, and B1, from the seeds of Zizyphus jujuba var. spinosa (Zizyphi Spinosi Semen). Chem. Pharm. Bull. 1999, 47, 1744–1748. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Ye, L. Chemical constituents in seeds of Zizyphus jujuba var. spinosa. Chin. Tradit. Herb. Drugs 2009, 40, 1534–1536. [Google Scholar]
- Wang, J.Z.; Yang, J.S. Structural Elucidation of Triterpene Saponins from the seed of Zizyphus jujuba Mill. Chin. J. Org. Chem. 2008, 1, 69–72. [Google Scholar]
- Bai, Y.J.; Cheng, G.; Tao, J.; Wang, B.; Zhao, Y.J.; Liu, Y.; Ma, L.B.; Tu, G.Z. Structure identification of jujuboside E. Acta Pharm. Sin. 2003, 12, 934–937. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Tang, Y.; Su, S.; Shang, E.; Ni, S.; Qian, D. High-performance liquid chromatography--two wavelength detection of triterpenoid acids from the fruits of Ziziphus jujuba containing various cultivars in different regions and classification using chemometric analysis. J. Pharm. Biomed Anal. 2009, 49, 1296–1302. [Google Scholar] [CrossRef]
- Song, L.J.; Zhang, L.; Ma, Y.J.; Lian, W.S.; Liu, Y.G.; Wang, Y.H. Optimized extraction of total triterpenoids from Jujube (Ziziphus jujuba Mill.) and comprehensive analysis of triterpenic acids in different cultivars. Plants 2020, 9, 412. [Google Scholar] [CrossRef]
- Xue, X.F.; Zhao, A.L.; Wang, Y.K.; Ren, H.Y.; Li, D.K.; Li, Y. Analysis of triterpenoid acid contents in fruits 219 jujube germplasm resources. Acta Bot. Boreali-Occident. Sin. 2021, 41, 480–492. [Google Scholar]
- Zhao, A.L.; LI, D.K.; Wang, Y.K.; Sui, K.L.; Cao, Y.Q. Evaluation on nutritional characteristics and germplasm screening of different chinese jujube cultivars. J. Plant Genet. Resour. 2010, 11, 811–816. [Google Scholar]
- Ruan, K.H.; Tang, Z.S.; Liu, H.B.; Wang, M.; Song, Z.S. Quality evaluation of sour jujube pulp from different origins. Cent. South Pharm. 2021, 5, 896–901. [Google Scholar]
- Peng, Y.F. Analysis of Chemical Bioactive Components and Extraction Technique of Wax in Jujube. Ph.D. Thesis, Hebei Agricultural University, Baoding, China, 2008. [Google Scholar]
- Wen, C.; Zhang, Z.; Shi, Q.; Yue, R.; Li, X. Metabolite and Gene Expression Analysis Underlying Temporal and Spatial Accumulation of Pentacyclic Triterpenoids in Jujube. Genes 2022, 13, 823. [Google Scholar] [CrossRef]
- Ding, S.H.; Wang, R.R.; Zhang, J.H.; Xie, Q.T.; Li, G.Y.; Shan, Y. Changes of bioactive compounds and antioxidant capacities of jujube(Zizyphus jujuba)fruit cv ‘Jinsixiaozao’ during different growth/ripening stages of growth. Sci. Technol. Food Ind. 2017, 38, 74–79+86. [Google Scholar] [CrossRef]
- Chang, X.J. Study on contents of phenolics.; organic acids, triterpenoid acids, vitamin C of red jujube with different maturity and antioxidant activities. Storage Process 2021, 21, 28–32. [Google Scholar]
- Tian, J.; Li, C.M.; Li, Q.L. Optimal harvest period of jujube fruit based on cyclic nucleotides and triterpene acids content. China Food Addit. 2018, 10, 62–68. [Google Scholar]
- Yang, X.J.; Liu, Y.R.; Tang, Z.S.; Song, Z.X.; Chang, B.J.; Zhao, Y.T.; Zhao, M.L. Study on the differential changes of primary and secondary metabolites between gree and ripe Zizyphus jujuba var. spinosa fruits based on principal component analysis. Nat. Prod. Res. Dev. 2022, 34, 824–835. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Qian, D.; Tang, Y.; Wu, D.; Su, S.; Wang, H.; Zhao, Y. Content variations of triterpenic acid.; nucleoside.; nucleobase.; and sugar in jujube (Ziziphus jujuba) fruit during ripening. Food Chem. 2015, 167, 468–474. [Google Scholar] [CrossRef]
- Miao, L.J. The Analysis of Triterpenoids of the Fruit of Chinese Jujube. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2006. [Google Scholar]
- Shin, M.; Lee, B.M.; Kim, O.; Tran, H.N.K.; Lee, S.; Hwangbo, C.; Min, B.S.; Lee, J.H. Triterpenoids from Ziziphus jujuba induce apoptotic cell death in human cancer cells through mitochondrial reactive oxygen species production. Food Funct. 2018, 17, 3895–3905. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J. Extraction, Identification and Anti-Prostate Cancer Activity of Triterpenoids from Ziziphus Jujube mill. cv. ‘Jinsixiaozao’. Master’s Thesis, Shandong University, Jinan, China, 2019. [Google Scholar]
- Cui, X.Q. Study on Chemical Component Analysis and Biological Activity of Z.jujuba Fruits and Leaves. Master’s Thesis, Northwestern University, Evanston, IL, USA, 2017. [Google Scholar]
- Sun, Y.F.; Song, C.K.; Viernstein, H.; Unger, F.; Liang, Z.S. Apoptosis of human breast cancer cells induced by microencapsulated betulinic acid from sour jujube fruits through the mitochondria transduction pathway. Food Chem. 2013, 138, 1998–2007. [Google Scholar] [CrossRef]
- Wang, X.Y.; Du, G.R.; Li, H. Progress of analytical methods for antioxidant capacity in vitro. J. Food Sci. Biotechnol. 2012, 31, 247–252. [Google Scholar]
- Liang, R.; Wu, J.H.; Zhou, X.S.; Wang, D.L.; Lao, F.; Liao, F.; Meng, L.F.; Guo, X.F. A review of anioxidant components of jujube fruit. Food Res. Dev. 2019, 40, 211–218. [Google Scholar]
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y.; Ren, F. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem. Toxicol. 2010, 48, 1461–1465. [Google Scholar] [CrossRef]
- Fang, C.Q.; Zhang, W.J.; Zhang, J.Z.; Qu, N.; Cao, Z.H.; Pan, J.H.; Lei, Y.T.; Luo, H.M.; Han, D. Research status of the construction and application of common liver injury animal models. Chin. J. Clin. Pharmacol. 2022, 38, 276–280. [Google Scholar] [CrossRef]
- Cai, T.J.; Wang, R.Z.; Wei, J.H.; Xue, Y.; Lei, H.J.; Xu, H.D. Protective effect of betulinic acid and total triterpenoid acids of red jujubes on alcoholic liver injury in mice. Food Sci. 2018, 39, 191–195. [Google Scholar]
- Kim, C.; Jeong, Y.H.; Kim, N.; Ryu, S.H.; Bae, J.S. Hepatoprotective functions of jujuboside B. J. Nat. Med. 2023, 77, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Wang, G.F.; Wang, M.M.; Wang, X.D.; Zhang, S.S.; Tian, Y.; He, X.L. The analysis of Antioxidaant capacities of triterpenoids of Chinese dates from North of Shaanxi. Mol. Plant Breed. 2017, 15, 3267–3271. [Google Scholar] [CrossRef]
- Cai, T.J.; Lei, H.J.; Wang, R.Z.; Xu, Y.F.; Xu, H.D. Study on triterpenic acids purification from jujube by microporous resins and its antioxidant ativity. Sci. Technol. Food Ind. 2017, 38, 159–165. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liang, Z.S.; Liu, Z.; Huang, J.; Yan, H. Study on antibacterial and antioxidant activities of triterpenoid saponins in sour jujube fruits. Sci. Technol. Food Ind. 2012, 6, 139–142. [Google Scholar] [CrossRef]
- Hong, E.H.; Song, J.H.; Kang, K.B.; Sung, S.H.; Ko, H.J.; Yang, H. Anti-Influenza Activity of Betulinic Acid from Zizyphus jujuba on Influenza A/PR/8 Virus. Biomol. Ther. 2015, 23, 345–349. [Google Scholar] [CrossRef]
- Xiao, S.L.; Wang, H.; Wang, Q.; Han, X.; Xu, R.K.; Meng, K.; Tian, Z.Y.; Zhang, L.H.; Zhou, D.M. Recent progresses on pentacyclic triterpene-based antiviral agents. Sci. Sin. 2015, 45, 865–883. [Google Scholar]
- Yu, M.; Si, L.; Wang, Y.; Wu, Y.; Yu, F.; Jiao, P.; Shi, Y.; Wang, H.; Xiao, S.; Fu, G.; et al. Discovery of pentacyclic triterpenoids as potential entry inhibitors of influenza viruses. J. Med. Chem. 2014, 57, 10058–10071. [Google Scholar] [CrossRef]
- Zhang, Y.H. Immune Factor and Immune Activity of Zizyphus Jujuba cv.huizao from Aksu.; Xinjiang. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2016. [Google Scholar]
- Zou, M.; Zhang, Y.H.; Chen, Y.L.; Ma, X.F.; Wang, G.X.; Ma, C.; Zhu, F.T. Immune interaction of bioactive components in Ziziphus jujuba cv.huizao fruits from Aksu. Food Sci. 2018, 39, 201–206. [Google Scholar]
- Masullo, M.; Montoro, P.; Autore, G.; Marzocco, S.; Pizza, C.; Piacente, S. Quali-quantitative determination of triterpenic acids of Ziziphus jujuba fruits and evaluation of their capability to interfere in macrophages activation inhibiting NO release and iNOS expression. Food Res. Int. 2015, 77, 109–117. [Google Scholar] [CrossRef]
- Yu, L.; Jiang, B.P.; Luo, D.; Shen, X.C.; Guo, S.; Duan, J.A.; Tang, Y.P. Bioactive components in the fruits of Ziziphus jujuba Mill. against the inflammatory irritant action of Euphorbia plants. Phytomedicine 2012, 19, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Kitamura, K.; Irie, K.; Naruse, S.; Matsuura, T.; Uemae, T.; Taira, S.; Ohigashi, H.; Murakami, S.; Takahashi, M.; et al. Triterpenoids Isolated from Ziziphus jujuba Enhance Glucose Uptake Activity in Skeletal Muscle Cells. J. Nutr. Sci. Vitaminol. 2017, 63, 193–199. [Google Scholar] [CrossRef]
- Lee, D.; Kang, K.B.; Kim, H.W.; Park, J.S.; Hwang, G.S.; Kang, K.S.; Choi, S.; Yamabe, N.; Kim, K.H. Unique Triterpenoid of Jujube Root Protects Cisplatin-induced Damage in Kidney Epithelial LLC-PK1 Cells via Autophagy Regulation. Nutrients 2020, 12, 677. [Google Scholar] [CrossRef] [PubMed]
- Ninave, P.B.; Patil, S.D. Antiasthmatic potential of Zizyphus jujuba Mill and Jujuboside B.—Possible role in the treatment of asthma. Respir. Physiol. Neurobiol. 2019, 260, 28–36. [Google Scholar] [CrossRef]
- Cao, L.J.; Zhan, S.Y.; Ji, X.Y.; Zheng, B.H.; Ye, C.Y.; Chen, Z.Y.; Liu, G.Q.; Ding, B.Y. Research advance in multi-component pharmacokinetics of Chinese herbal extracts in the recent five years. China J. Chin. Mater. Med. 2021, 46, 3270–3287. [Google Scholar] [CrossRef]
- Gong, Z.P.; Chen, Y.; Zhang, R.J.; Yang, Q.; Zhu, X.X. Advances on pharmacokinetics of traditional Chinese medicine under disease state. China J. Chin. Mater. Med. 2015, 40, 169–173. [Google Scholar]
- Li, Y.; Guo, S.; Hua, T.; Wang, Y.; Wei, D.; Zhao, M.; Su, S.; Duan, J.A. Comparative pharmacokinetics of triterpenic acids in normal and immunosuppressed rats after oral administration of Jujubae Fructus extract by UPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2018, 1077, 13–21. [Google Scholar] [CrossRef]
- Li, Y.; Guo, S.; Ren, Q.; Wei, D.; Zhao, M.; Su, S.; Tang, Z.; Duan, J.A. Pharmacokinetic Comparisons of Multiple Triterpenic Acids from Jujubae Fructus Extract Following Oral Delivery in Normal and Acute Liver Injury Rats. Int. J. Mol. Sci. 2018, 19, 2047. [Google Scholar] [CrossRef]
- Du, C.; Yan, Y.; Shen, C.; Cui, X.; Pei, X.; Qin, X. Comparative pharmacokinetics of six major compounds in normal and insomnia rats after oral administration of Ziziphi Spinosae Semen aqueous extract. J. Pharm. Anal. 2020, 10, 385–395. [Google Scholar] [CrossRef]
- Song, P.; Zhang, Y.; Ma, G.; Zhang, Y.; Zhou, A.; Xie, J. Gastrointestinal Absorption and Metabolic Dynamics of Jujuboside A.; A Saponin Derived from the Seed of Ziziphus jujuba. J. Agric. Food Chem. 2017, 65, 8331–8339. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Li, C. Biosynthesis of Plant Triterpenoid Saponins in Microbial Cell Factories. J. Agric. Food Chem. 2018, 21, 12155–12165. [Google Scholar] [CrossRef] [PubMed]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Y.; Yin, X.; Wang, X.; Qi, X.; Xue, Z. Diverse triterpene skeletons are derived from the expansion and divergent evolution of 2,3-oxidosqualene cyclases in plants. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 113–132. [Google Scholar] [CrossRef]
- Seki, H.; Tamura, K.; Muranaka, T. P450s and UGTs: Key Players in the Structural Diversity of Triterpenoid Saponins. Plant Cell Physiol. 2015, 56, 1463–1471. [Google Scholar] [CrossRef]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Nguyen, K.V.; Pongkitwitoon, B.; Pathomwichaiwat, T.; Viboonjun, U.; Prathanturarug, S. Effects of methyl jasmonate on the growth and triterpenoid production of diploid and tetraploid Centella asiatica (L.) Urb. hairy root cultures. Sci. Rep. 2019, 10, 18665. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2019, 46, 197–212. [Google Scholar] [CrossRef]
- Chen, X.; Chen, R.; Wang, Y.; Wu, C.; Huang, J. Genome-Wide Identification of WRKY Transcription Factors in Chinese jujube (Ziziphus jujuba Mill.) and Their Involvement in Fruit Developing, Ripening, and Abiotic Stress. Genes 2019, 10, 360. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, Z.; Shi, Q.; Duan, X.; Du, J.; Wu, C.; Li, X. Methyl Jasmonate- and Salicylic Acid-Induced Transcription Factor ZjWRKY18 Regulates Triterpenoid Accumulation and Salt Stress Tolerance in Jujube. Int. J. Mol. Sci. 2023, 15, 3899. [Google Scholar] [CrossRef]
- Huang, J.; Chen, X.; He, A.; Ma, Z.; Gong, T.; Xu, K.; Chen, R. Integrative Morphological.; Physiological.; Proteomics Analyses of Jujube Fruit Development Provide Insights Into Fruit Quality Domestication From Wild Jujube to Cultivated Jujube. Front. Plant Sci. 2021, 12, 773825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shi, Q.; Wang, B.; Ma, A.; Wang, Y.; Xue, Q.; Shen, B.; Hamaila, H.; Tang, T.; Qi, X.; et al. Jujube metabolome selection determined the edible properties acquired during domestication. Plant J. 2022, 109, 1116–1133. [Google Scholar] [CrossRef] [PubMed]
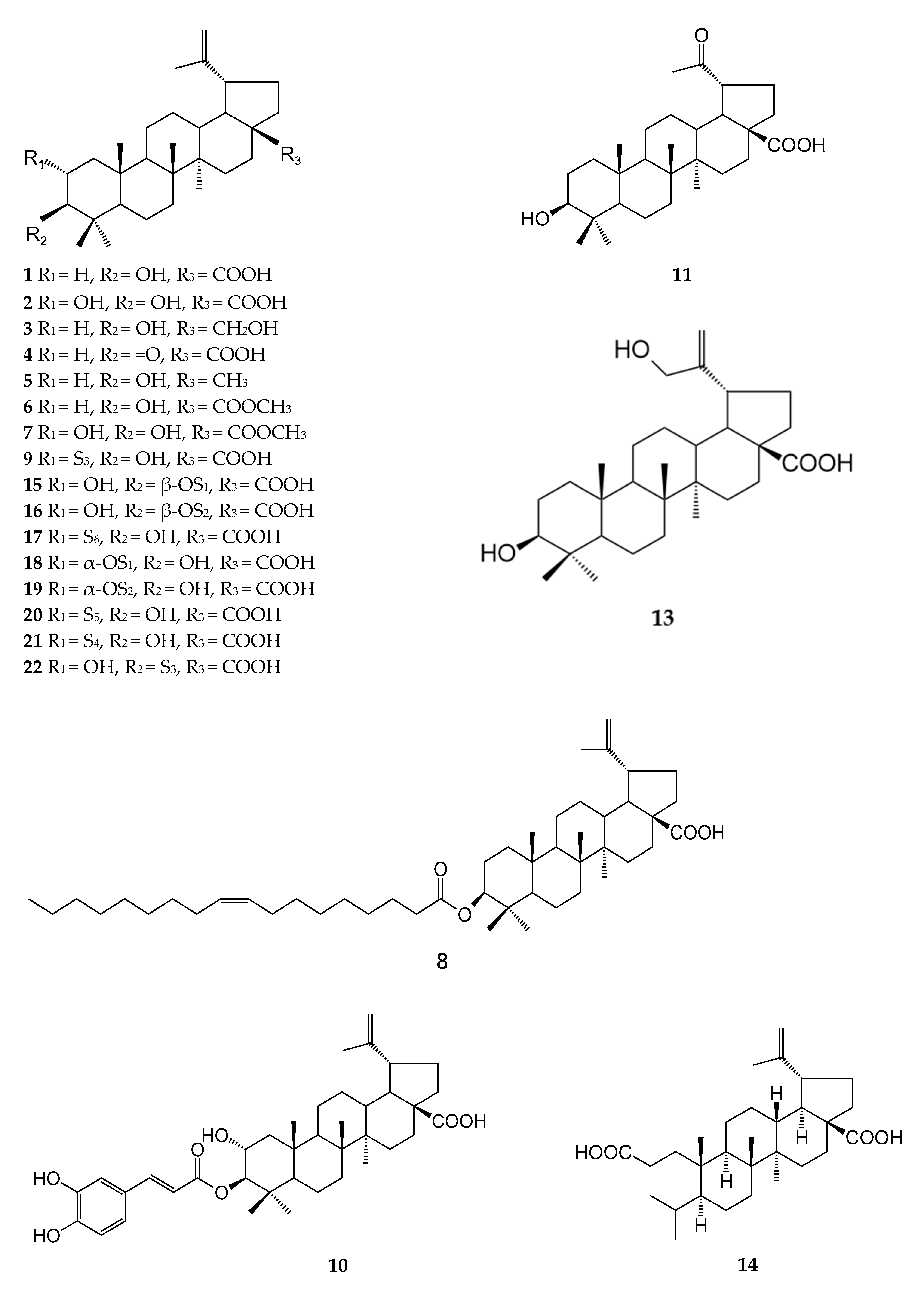









| Number | Type | Compound Name | Source | Reference |
|---|---|---|---|---|
| 1 | Lupane | Betulinic acid | ZJ and ZA | [18,19,20] |
| 2 | Lupane | Alphitolic acid | ZJ and ZA | [18,19,20] |
| 3 | Lupane | Betulin | ZJ and ZA | [19,20] |
| 4 | Lupane | Betulonic acid | ZJ and ZA | [19,20,21] |
| 5 | Lupane | Lupeol | ZA | [23,29] |
| 6 | Lupane | Methyl betulinate | ZA | [23] |
| 7 | Lupane | Alphitolic acid methyl ester | ZA | [22,30] |
| 8 | Lupane | 3-O-[9(Z)-octadecenoyl] betulinic acid | ZJ | [31] |
| 9 | Lupane | 2-O-protocatechuoylalphitolic acid | ZJ and ZA | [27,32] |
| 10 | Lupane | 2α-Hydroxypyracrenic acid | ZA | [32] |
| 11 | Lupane | Platanic acid | ZA | [30] |
| 12 | Lupane | 2α,3β,20-Trihydroxylupane-28-oic acid | ZJ | [33] |
| 13 | Lupane | 3β,30-Dihydroxylup-20(29)-en-28-oic acid | ZJ | [33] |
| 14 | Lupane | (3α,4β,5β,8α,9β,10α,13α,14β,15β)-13-Carboxy-4,9-dimethyl-15-(1-methylethenyl)-3-(1-methylethyl)-18-norandrostane-4-propanoic acid | ZJ | [33] |
| 15 | Lupane | 3-O-Trans-p-coumaroyl alphitolic acid | ZJ | [34] |
| 16 | Lupane | 3-O-Cis-p-coumaroyl alphitolic acid | ZJ | [34] |
| 17 | Lupane | 2-O-Benzoylalphitolic acid | ZJ | [27] |
| 18 | Lupane | 2-O-Trans-p-coumaroylalphitolic acid | ZJ | [27] |
| 19 | Lupane | 2-O-Cis-p-coumaroylalphitolic acid | ZJ | [27] |
| 20 | Lupane | 2-O-Vanilloylalphitolic acid | ZJ | [27] |
| 21 | Lupane | 2-O-p-Hydroxybenzoylalphitolic acid | ZJ | [27] |
| 22 | Lupane | 3-O-Protocatechuoylalphitolic acid | ZJ | [27] |
| 23 | Oleanane | Oleanolic acid | ZJ and ZA | [18,19,21] |
| 24 | Oleanane | Oleanonic acid | ZJ and ZA | [19,20,21] |
| 25 | Oleanane | Maslinic acid | ZJ and ZA | [19,24] |
| 26 | Oleanane | 3-O-Trans-p-coumaroyl maslinic acid | ZJ | [21,33] |
| 27 | Oleanane | 3-O-Cis-p-coumaroyl maslinic acid | ZJ | [21,34] |
| 28 | Oleanane | Hydroxyoleanonic acid lactone | ZA | [19] |
| 29 | Oleanane | 11-Oxo-maslinic acid | ZJ | [33] |
| 30 | Oleanane | 2-O-Trans-p-coumaroyl maslinic acid | ZJ | [33] |
| 31 | Oleanane | 3,4-Seco-olean-12-ene-3,28-dioic acid | ZJ | [33] |
| 32 | Oleanane | 2α-Cis-p-coumaroyloxy-2α,3β,23α-trihydroxy-olean-12-en-28-oic acid | ZJ | [33] |
| 33 | Ursane | Pomolic acid | ZJ and ZA | [19,20] |
| 34 | Ursane | Pomonic acid | ZJ and ZA | [19,20,24] |
| 35 | Ursane | Pomolic acid 28-methyl ester | ZJ | [20] |
| 36 | Ursane | Ursolic acid | ZJ and ZA | [19,24,35] |
| 37 | Ursane | Ursonic acid | ZJ and ZA | [18,19] |
| 38 | Ursane | Corosolic acid | ZA | [19] |
| 39 | Ursane | Cecropiacic acid | ZJ and ZA | [19,33] |
| 40 | Ursane | 3β,13β-dihydroxy-urs-11-en-28-oic acid | ZA | [25] |
| 41 | Ursane | 3β-hydroxy-urs-20(30)-en-28-oic acid | ZA | [25] |
| 42 | Ursane | 2α,3β-dihydroxy-urs-20(30)-en-28-oic acid | ZA | [25] |
| 43 | Ursane | 3β,12β,13β-trihydroxy-ursan-28-oic acid | ZA | [25] |
| 44 | Ursane | 2α,3β,13β-trihydroxy-urs-11-en-28-oic acid | ZA | [25] |
| 45 | Ursane | 2α,3β,13β,23-tetrahydroxy-urs-11-en-28-oic acid | ZA | [25] |
| 46 | Ursane | 2α,3β,28-trihydroxy-urs-20(30)-ene | ZA | [25] |
| 47 | Ursane | 2α,3β,12β,13β-tetrahydroxy-ursan-28-oic acid | ZA | [25] |
| 48 | Ursane | Euscaphic acid | ZJ | [33] |
| 49 | Ursane | 2β,19α-Hydroxyursolic acid | ZJ | [33] |
| 50 | Ursane | Jacoumaric acid | ZJ | [33] |
| 51 | Ursane | 2-Oxopomolic acid | ZJ | [33] |
| 52 | Ursane | (1S,2S,4aR,4bS,6aS,9R,10S,10aS,12aR)-6a-carboxy-1,2,3,4,4a,4b,5,6,6a,7,8,9,10,10a,12,12a-hexadecahydro-1,4a,4b,9,10-pentamethyl-2-(1-methylethyl)-1-chrysenepropanoic acid | ZJ | [33] |
| 53 | Ceanothane | Epiceanothic acid | ZJ | [18,19,30] |
| 54 | Ceanothane | Ceanothic acid | ZJ | [18,19,30] |
| 55 | Ceanothane | Ceanothic acid 2-methyl ester | ZJ | [20] |
| 56 | Ceanothane | Ceanothic acid 28-methyl ester | ZJ | [20] |
| 57 | Ceanothane | Zizyberanal acid | ZJ | [35] |
| 58 | Ceanothane | Zizyberanalic acid/colubrinic acid | ZJ and ZA | [34,36] |
| 59 | Ceanothane | Isoceanothic acid | ZJ | [29] |
| 60 | Ceanothane | 3-O-Protocatechuoylceanothic acid | ZJ | [32] |
| 61 | Ceanothane | Ceanothenic acid | ZJ and ZA | [18,26] |
| 62 | Ceanothane | Zizyberenalic acid | ZJ | [21,34] |
| 63 | Ceanothane | 3-O-Vanilloylceanothic acid | ZJ | [27] |
| 64 | Ceanothane | 24-Hydroxyceanothic acid | ZJ | [27] |
| 65 | Ceanothane | 3-Dehydroxyceanothetric acid | ZJ | [27] |
| 66 | Ceanothane | 3-Dehydroxyceanothetric acid 2-methyl ester | ZJ | [27] |
| 67 | Ceanothane | Ceanothetric acid 2-methyl ester | ZJ | [27] |
| 68 | Ceanothane | Epiceanothic acid 2-methyl ester | ZJ | [27] |
| 69 | Ceanothane | 3-O-Methylzizyberanalic acid | ZJ | [27] |
| 70 | Ceanothane | 3-O-Protocatechuoylceanothic acid 2-methyl ester | ZJ | [27] |
| 71 | Ceanothane | 3-O-Vanilloylepiceanothic acid | ZJ | [27] |
| 72 | Ceanothane | 3-O-Vanilloylceanothic acid 2-methyl este | ZJ | [27] |
| 73 | Ceanothane | 3-O-p-Hydroxybenzoylceanothic acid | ZJ | [27] |
| 74 | Ceanothane | 3-O-p-Hydroxybenzoylepiceanothic acid | ZJ | [27] |
| 75 | Ceanothane | 2-O-Protocatechuoylisoceanothanolic acid | ZJ | [27] |
| 76 | Ceanothane | 3-Dehydroxyceanothan-27α-carboxy-28β,19β-olide | ZJ | [27] |
| 77 | Ceanothane | 3-O-Protocatechuoylceanothan-28β,19β-olide | ZJ | [27] |
| 78 | Ceanothane | 2,28-Dinor-24-hydroxylup-1,17(22)-dien-27-oic acid | ZJ | [27] |
| 79 | Ceanothane | 7β-O-Vanilloyl-3-dehydroxyceanothetric acid 2-methyl ester | ZJ | [27] |
| Number | Type | Compound Name | Source | Reference |
|---|---|---|---|---|
| 1 | I | Jujuba saponin I | ZJ and ZA | [38,39] |
| 2 | I | Jujuba saponin II | ZJ | [38] |
| 3 | I | Jujuba saponin III | ZJ | [38] |
| 4 | I | Ziziphin | ZJ | [38] |
| 5 | I | Ziziphus saponin I | ZJ and ZA | [40,41] |
| 6 | I | Ziziphus saponin II | ZJ and ZA | [40,41] |
| 7 | I | Ziziphus saponin III | ZJ | [40] |
| 8 | I | Acetyljujuboside B | ZA | [42] |
| 9 | I | Jujuboside A | ZA | [43] |
| 10 | I | Jujuboside B | ZA | [43] |
| 11 | I | Jujuboside A2 | ZA | [44] |
| 12 | I | Jujuboside C | ZA | [45] |
| 13 | I | Jujuboside A1 | ZA | [42,46] |
| 14 | I | Jujuboside B1 | ZA | [43,46] |
| 15 | I | Jujuboside I | ZA | [45,46] |
| 16 | I | Jujuboside D | ZJ | [47] |
| 17 | I | Jujuboside E | ZJ | [47] |
| 18 | I | Jujuboside H | ZJ | [48] |
| 19 | I | Jujuboside G | ZJ | [48] |
| 20 | I | Jujuboside F | ZJ | [48] |
| 21 | I | Jujuboside J | ZJ | [48] |
| 22 | I | Christinin A | ZJ | [49] |
| 23 | I | Christinin C | ZJ | [49] |
| 24 | II | Jujuboside III | ZA | [46] |
| 25 | II | Jujuboside IV | ZA | [46] |
| 26 | II | Jujubasaponin IV | ZJ | [40] |
| 27 | II | Jujubasaponin V | ZJ | [40] |
| 28 | III | Jujubasaponin VI | ZJ and ZA | [39,40] |
| 29 | IV | LotosideI | ZJ | [49] |
| 30 | IV | Lotoside II | ZJ | [49] |
| 31 | IV | Lotoside III | ZJ | [49] |
| 32 | V | Portojujuboside A | ZA | [50] |
| 33 | V | Portojujuboside B | ZA | [50] |
| 34 | V | Portojujuboside B1 | ZA | [50] |
| 35 | VI | Jujuboside H | ZA | [51] |
| 36 | VI | Jujuboside G | ZA | [52] |
| 37 | VII | Jujuboside E | ZA | [53] |
| 38 | VIII | Jujuboside II | ZA | [46] |
| 39 | IX | 3-O-[(β-D-glucopyranosyl (1→2)-β-D-glucopyr-anosyl-]-20-O-[(β-D-xylopyranosyl-(1→6)-β-D-glucopyranosyl]-2α,3β,12β-trihydroxy-dammar-25-en-24-one | ZJ | [49] |
| 40 | X | 3-O-[α-L-rhamnopyranosyl (1→2)-α-L-arabinopyranosyl-]-30 -[β-D-gluco-pyranosyl-(1→2)-β-D-glucopyranosyl]-3β,25,30-O-trihydroxy-16-one-20R,24R-epoxydammarane | ZJ | [49] |
| 41 | X | 3-O-[α-Lrhamnopyranosyl- (1→4)-α-L-arabinopyranosyl-]-30-O-[β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl]-3β,25,30-trihydroxy-16-one-20R,24R-epoxydammarane | ZJ | [49] |
| 42 | XI | 3-O-α-L-rhamnopyranosyl-(1→4)-α-L -rhamnopyr-anosyl-(1→2)-β-D-glucopyranosyl sidrigenin | ZJ | [49] |
| Biological Activity | Active Ingredient/Substance | Classification | Source | References |
|---|---|---|---|---|
| Anti-cancer activity | Pomlic acid, betulinic acid, 3-O-trans-p-coumaroyl alphitolic acid, oleanolic acid, 3-O-cis-p-Coumaroyl alphitolic acid, 2α,3β,19α-Trihydroxy-urs-12-en-28-oic acid, 2α,3β-Dihydroxy-urs-20(30)-en-28-oic acid, 2α,3β,28-Trihydroxy-urs-20(30)-ene | Triterpenes | ZJ and ZA | [68,69,70,71] |
| Antioxidant activity | Jujuboside B, 2α,3β-Dihydroxy-urs-20(30)-en-28-oic acid, 2α,3β,28-Trihydroxy-urs-20(30)-ene | Saponins and terpenoids | ZJ and ZA | [25,77] |
| Anti-inflammatory activity | Jujuboside F, Jujuboside G, Jujuboside H, Jujuboside J, Jujuboside I, Jujuboside A1, Jujuboside A, Jujuboside B, Jujuboside C, Jujubasaponin IV, Zizyberanalic acid, ceanothic acid, Alphitolic acid, oleanolic acid 3-O-trans-Coumaroyl alphitolic acid, oleanolic acid | Saponins and terpenoids | ZJ | [49,85,86] |
| Anti-viral activity | Betulinic acid | Triterpenes | ZJ | [80] |
| Antimicrobial activity | Total saponin compounds | Saponins | ZA | [87] |
| Renoprotective activity | 3-Dehydroxyceanothetric acid 2-methyl ester | Triterpenes | ZJ | [89] |
| Anti-asthmatic activity | Jujuboside B | Saponins | ZA | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, F.; Zhao, X.; Liu, F.; Luo, Z.; Chen, S.; Liu, Z.; Zhao, Z.; Liu, M.; Wang, L. Triterpenoids in Jujube: A Review of Composition, Content Diversity, Pharmacological Effects, Synthetic Pathway, and Variation during Domestication. Plants 2023, 12, 1501. https://doi.org/10.3390/plants12071501
Pan F, Zhao X, Liu F, Luo Z, Chen S, Liu Z, Zhao Z, Liu M, Wang L. Triterpenoids in Jujube: A Review of Composition, Content Diversity, Pharmacological Effects, Synthetic Pathway, and Variation during Domestication. Plants. 2023; 12(7):1501. https://doi.org/10.3390/plants12071501
Chicago/Turabian StylePan, Fuxu, Xuan Zhao, Fawei Liu, Zhi Luo, Shuangjiang Chen, Zhiguo Liu, Zhihui Zhao, Mengjun Liu, and Lili Wang. 2023. "Triterpenoids in Jujube: A Review of Composition, Content Diversity, Pharmacological Effects, Synthetic Pathway, and Variation during Domestication" Plants 12, no. 7: 1501. https://doi.org/10.3390/plants12071501
APA StylePan, F., Zhao, X., Liu, F., Luo, Z., Chen, S., Liu, Z., Zhao, Z., Liu, M., & Wang, L. (2023). Triterpenoids in Jujube: A Review of Composition, Content Diversity, Pharmacological Effects, Synthetic Pathway, and Variation during Domestication. Plants, 12(7), 1501. https://doi.org/10.3390/plants12071501





