Unleashing the Potential of Medicinal Plants in Benin: Assessing the Status of Research and the Need for Enhanced Practices
Abstract
1. Introduction
- -
- Aloe vera: Aloe vera is a succulent plant that is commonly used for medicinal purposes. In Benin, it is used to treat various ailments, including skin conditions, digestive problems, and infections.
- -
- Neem: Neem is a tree that is widely used in Benin for its medicinal properties. It is used to treat a variety of conditions, including fever, malaria, and skin diseases.
- -
- Hibiscus: Hibiscus is a plant that is used in Benin to make a popular drink known as bissap. The drink is made by steeping the leaves of the hibiscus plant in hot water and adding sugar or honey. It is believed to have a range of health benefits, including reducing high blood pressure and boosting the immune system.
- -
- Moringa: Moringa is a tree that is used in Benin for its nutritional and medicinal properties. It is rich in vitamins and minerals and is used to treat a variety of conditions, including anemia, diabetes, and high blood pressure.
- -
- Baobab: Baobab is a tree that is native to Africa and is used in Benin for its medicinal and nutritional properties. The fruit of the baobab tree is rich in vitamin C, fiber, and antioxidants, and is used to treat a variety of conditions, including diarrhea, constipation, and respiratory infections.
- -
- Ginger: Ginger is a root that is widely used in Benin for its medicinal and culinary properties. It is used to treat a variety of conditions, including nausea, indigestion, and inflammation.
- -
- Turmeric: Turmeric is a spice that is widely used in Benin for its medicinal and culinary properties. It is used to treat a variety of conditions, including arthritis, digestive problems, and skin conditions.
- -
- African pepper: African pepper, also known as grains of paradise, is a spice that is used in Benin for its culinary and medicinal properties. It is used to treat a variety of conditions, including digestive problems, fever, and respiratory infections.
2. Results
- -
- University of Abomey-Calavi: The University of Abomey-Calavi is the largest and oldest university in Benin, located in the city of Abomey-Calavi. It has several faculties, including the Faculty of Agricultural Sciences, which offers programs in agronomy, plant breeding, soil science, and agroforestry. It has also the Polytechnic School of Abomey-Calavi, which offers programs in a variety of engineering and technology disciplines.
- -
- University of Parakou: The University of Parakou is located in the city of Parakou and offers programs in a variety of disciplines, including agriculture and environmental sciences. The Faculty of Agronomic Sciences offers programs in agronomy, agribusiness, and animal production.
- -
- National University of Science, Technology, Engineering and Mathematics of Abomey (UNSTIM): The National University of Science, Technology, Engineering and Mathematics of Abomey (UNSTIM) is located in the city of Abomey and offers programs in a variety of disciplines, including agriculture and natural sciences.
- -
- National School of Agriculture: The National School of Agriculture is located in the city of Porto-Novo and offers programs in agricultural sciences, including crop production, livestock production, and agribusiness.
2.1. Biological Activities Studies
2.2. Toxicological Studies
2.3. Phytochemical Study
3. Discussion
- -
- Health improvement: The study can provide valuable insights into the biological, toxicological, and phytochemical aspects of the medicinal plants commonly used in the country, which can inform and improve healthcare practices and policies.
- -
- Economic benefits: By identifying the most promising medicinal plants and the most important areas of research, the study can help support the development of the medicinal plant industry in the country, potentially leading to economic benefits.
- -
- Scientific advancement: The study can also contribute to the advancement of scientific knowledge in the field of medicinal plants, providing a better understanding of the plants used in traditional medicine and their potential health benefits.
- -
- Capacity building: By highlighting the current state of research at national universities, the study can help identify areas where capacity building is needed and inform future investment in research and development.
4. Materials and Methods
- -
- The first step was to search the following search engines (Google Scholar, Pubmed, Sciencedirect, and FreeFullPDF) for scientific information on the medicinal plants studied in Benin, using the following expressions or word groups: “Biological activities”; “Pharmacological activities”; “Toxicity”; “Phytochemistry” “Phytochemical screening”, and “Quantitative screening”. This search was performed in both French and English. The information was collected according to each of the four major universities of Benin (the University of Abomey-Calavi, University of Parakou, National University of Agriculture of Kétou, and the National University of Science, Technology, Engineering and Mathematics of Abomey).
- -
- The second step consisted of a critical analysis of the methodological approach of each of the scientific studies identified in order to identify the main limitations of the methodologies used and to propose possible actions to improve research practices. For the scientific articles whose authors were in collaboration via several research institutions, the authorship of the article was defined by considering the corresponding author from one of the national universities of Benin.
- -
- The last step consisted of making a synthesis of the information collected about the different pharmacological, toxicological, and phytochemical activities by university.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akoegninou, A.; Van Der burg, W.; Van der Maesen, L. Flore Analytique du Bénin [Analytical Flora of Benin]; Backhuys: Kerkwerve, The Netherlands, 2006; 195p. [Google Scholar]
- OMS. Stratégie de l’ OMS Pour la Médecine Traditionnelle Pour 2002–2005; Organisation Mondiale de la Santé: Geneva, Switzerland, 2002. [Google Scholar]
- Dougnon, T.V.; Hounsa, E.; Agbodjento, E.; Koudokpon, H.; Legba, B.; Fabiyi, K.; Afaton, A.; Sintondji, K.; Akpode, B.; Klotoé, J.R.; et al. Toxicological Characterization of Ten Medicinal Plants of the Beninese Flora Used in the Traditional Treatment of Diarrheal Diseases. Evid. Based Complement. Altern. Med. ECAM 2021, 2021, 6676904. [Google Scholar] [CrossRef] [PubMed]
- INSAE. Enquête Démographique Générale; Ministère de la Prospective du Développement et de l’Evaluation de l’Action Publique, Institut National de la Statistique et de l’Analyse Economique, Republique du Bénin: Cotonou, Bénin, 2013. [Google Scholar]
- Adjanohoun, E.; Adjakidje, V.; Ahyi, M.; Ake Assi, L.; Akoegninou, A.; D’Almeida, J. Contribution Aux Etudes Ethnobotaniques et Floristiques En Republique Populaire Du Bénin, 2nd ed.; Agence de Coopération Culturelle et Technique: Paris, France, 1989; 100p. [Google Scholar]
- WHO. Traditional Medicine Strategy; WHO Library Cataloguing-in-Publication Data; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Adjalian, E.; Bothon, F.T.D.; Yehouenou, B.; Noudogbessi, J.-P.; Figueredo, G.; Avlessi, F.; Sohounhloue, D.C.K. GC/MS and GC/FID Analysis and Evaluation of Antimicrobial Performance of Aframomum sceptrum essential Oils of Benin. World J. Pharm. Sci. 2014, 2, 787–792. [Google Scholar]
- Adounkpe, F.; Ayena, A.; Aholoukpe, V.; Dougnon, V.; Klotoe, J.-R.; Medehouenou, M.; Baba-Moussa, L. Use of the Leaves of Alchornea cordifolia (Schumach. & Thonn.) Müll (Euphorbiaceae). Bull. Natl. Res. Cent. 2022, 46, 132–145. [Google Scholar]
- Ahouandjinou, J.-M.; Adjou, E.S.; Kpatinvo, B.; Allavo, U.; Dahouenon-Ahoussi, E.; Sohounhloue, D.C. Biological Properties of Essential Oils from Eucalyptus camaldulensis and Ocimum gratissimum against Sitophilus spp. Isolated from Stored Traditional Yams Chips. J. Pharmacogn. Phytochem. 2021, 10, 24–27. [Google Scholar]
- Ahouansou, C.; Houngbeme, A.; Fagla Medegan, S.; Catteau, L.; Fagbohoun, L.; Kotchoni, S.; Gbaguidi, F. Isolation and Idendification of Two Triterpenes with Larvicidal Potential in Launaea taraxacifolia (Asteracae) on Anopheles gambiae by HPLC. Int. J. Curr. Res. Biosci. Plant Biol. 2018, 5, 17–24. [Google Scholar] [CrossRef]
- Alitonou, G.A.; Sessou, P.; Tchobo, P.F.; Noudogbessi, J.-P.; Avlessi, F.; Yehouenou, B.; Menut, C.; Villeneuve, P.; Sohounhloue, D.C.K. Chemical Composition and Biological Activities of Essential Oils of Chenopodium ambrosioides L. Collected in Two Areas of Benin. Int. J. Biosci. 2012, 7, 16–24. [Google Scholar]
- Amoussa, A.M.; Lagnika, L.; Sanni, A. Acacia ataxacantha (Bark): Chemical Composition and Antibacterial Activity of the Extracts. Int. J. Pharm. Pharm. Sci. 2014, 6, 138–141. [Google Scholar]
- Anago, E.; Lagnika, L.; Gbenou, J.; Loko, F.; Moudachirou, M.; Sanni, A. Antibacterial Activity and Phytochemical Study of Six Medicinal Plants Used in Benin. Pak. J. Biol. Sci. PJBS 2011, 14, 449–455. [Google Scholar] [CrossRef][Green Version]
- Avlessi, F.; Alitonou, G.; Djenontin, S.; Tchobo, F.P.; Boniface, Y.; Chantal, M.; Sohounhloue, D. Chemical Composition and Biological Activities of the Essential Oil Extracted from Fresh Leaves of Chromoleana odorata (L. Robinson) Growing in Benin. ISCA J. Biol. Sci. 2012, 1, 7–13. [Google Scholar]
- Ayéna, A.C.; Anani, K.; Dosseh, K.; Agbonon, A.; Gbeassor, M. Comparative Study of Antimicrobial, Anti-Inflammatory, and Antioxidant Activities of Different Parts from Pterocarpus santalinoides l’Her. Ex. DC (Fabaceae). Evid. Based Complement. Altern. Med. ECAM 2021, 2021, 8938534. [Google Scholar] [CrossRef]
- Badé, F.T.; Dah-Nouvlessounon, D.; Sina, H.; Nanoukon, C.; Moussè, W.; Aklesso, N.; Sylvestre, A.; Majoie, T.; Martial, N.; Lehmane, H.; et al. Phytochemical Screening and Antimicrobial Activity of Desmodium ramosissimum. Am. J. Plant Sci. 2019, 11, 51–63. [Google Scholar]
- Bero, J.; Ganfon, H.; Jonville, M.-C.; Frédérich, M.; Gbaguidi, F.; DeMol, P.; Moudachirou, M.; Quetin-Leclercq, J. In Vitro Antiplasmodial Activity of Plants Used in Benin in Traditional Medicine to Treat Malaria. J. Ethnopharmacol. 2009, 122, 439–444. [Google Scholar] [CrossRef]
- Boya, B.; Ahoyo, T.; Sina, H.; Dougnon, V.; Christine, N.; Dah-Nouvlessounon, D.; Amousou, D.; Sossa, C.; Houssa, F.; Baba-Moussa, L. Antibiotics Resistance Profile and Antibacterial Activities of Combretum micranthum and Combretum adenogonium Extracts on Clinical Isolated Vibrio cholerae. Am. J. Infect. Dis. Microbiol. 2019, 7, 57–64. [Google Scholar]
- Dah-Nouvlessounon, D.; Adoukonou-Sagbadja, H.; Nafan, D.; SINA, H.; Noumavo, A.D.P.; Baba-Moussa, F.; Adjanohoun, A.; Joachim Djimon, G.; Baba-Moussa, L. Antimicrobial, Antioxidant, Cytotoxic Activities and Phytochemical Assessment of Cola acuminata Used in Benin. Int. J. Pharm. Pharm. Sci. 2015, 7, 102–109. [Google Scholar]
- Dah-Nouvlessounon, D.; Baba-Moussa, F.; Adjanohoun, A.; Sina, H.; Noumavo, A.D.P.; Adoukonou-Sagbadja, H.; Nafan, D.; Christine, N.; Anago, F.; Baba-Moussa, L. Phytochemical Screening and Biological Activities of Garcinia kola (Bark, Leaves and Seeds) Collected in Benin. Afr. J. Microbiol. Res. 2015, 9, 1716–1727. [Google Scholar]
- Dèdéhou, V.F.G.N.; Alowanou, G.G.; Nonviho, G.; Zinsou, F.; Houngbèmè, G.A.; Béhingan, B.M.; Agbassou, F.; Aboudou, H.K.; Hounzangbé-Adoté, S.M. Nutritional and Anticoccidial Properties of Papaya (Carica papaya) Leaf Meal on Growing Rabbits. Sci. J. Vet. Adv. 2022, 10, 316–324. [Google Scholar]
- Dègnon, R.G.; Allagbé, A.C.; Adjou, E.S.; Dahouenon-Ahoussi, E. Antifungal Activities of Cymbopogon Citratus Essential Oil against Aspergillus Species Isolated from Fermented Fish Products of Southern Benin. J. Food Qual. Hazards Control 2019, 1, 1–12. [Google Scholar] [CrossRef]
- Dougnon, V.; Klotoé, J.; Senou, M.; Roko, G.; Dougnon, G.; Fabiyi, K.; Afoussatou, A.; Aniambossou, V.; Assogba, P.; Bankolé, H.; et al. Chemical Composition, Cytotoxicity and Antibacterial Activity of Selected Extracts of Euphorbia hirta, Citrus aurantifolia and Heterotis rotundifolia on Enteropathogenic Bacteria. EC Microbiol. 2017, 12, 180–195. [Google Scholar]
- Dougnon, V.; Hounsa, E.; Agbodjento, E.; Lunga, P.; Legba, B.B.; Sintondji, K.M.; Afaton, A.; Klotoé, J.; Baba-Moussa, L.; Bankolé, H. Percentage Destabilization Effect of Some West African Medicinal Plants on the Outer Membrane of Various Bacteria Involved in Infectious Diarrhea. BioMed Res. Int. 2021, 2021, 4134713. [Google Scholar] [CrossRef]
- Dougnon, V.; Hounsa, E.; Koudokpon, H.; Agbodjento, E.; Afaton, A.; Sintondji, K.; Klotoe, J.R.; Segbo, J.; Baba-Moussa, L. Assessment of the Antibacterial Effect of Khaya senegalensis on Some Gram-Negative Bacteria. Bull. Natl. Res. Cent. 2021, 45, 98–107. [Google Scholar] [CrossRef]
- Fandohan, P.; Gbenou, J.D.; Gnonlonfin, B.; Hell, K.; Marasas, W.F.O.; Wingfield, M.J. Effect of Essential Oils on the Growth of Fusarium verticillioides and Fumonisin Contamination in Corn. J. Agric. Food Chem. 2004, 52, 6824–6829. [Google Scholar] [CrossRef] [PubMed]
- Fanou, B.A.; Klotoe, J.R.; Dougnon, V.; Assogba, P.; Agbodjento, E.; Koudokpon, C.H.; Fah, L.; Sintondji, K.; Kpoze, R.; Loko, F. Efficacy of Extracts of Cyanthillium cinereum, Khaya senegalensis and Lippia multiflora on Candida Strains Isolated From Urine Samples in Benin (West Africa). Front. Trop. Dis. 2022, 3, 49. [Google Scholar] [CrossRef]
- Houmènou, V.; Adjatin, A.; Assogba, F.M.; Gbenou, J.D.; Akoègninou, A. Etude Phytochimique Et De Cytotoxicité De Quelques Plantes Utilisées Dans Le Traitement De La Stérilité Féminine Au Sud-Bénin. Eur. Sci. J. 2018, 14, 156–172. [Google Scholar] [CrossRef]
- Houngbeme, A.G.; Gandonou, C.; Yehouenou, B.; Kpoviessi, S.; Sohounhloue, D.; Moudachirou, M.; Gbaguidi, F. Phytochemical Analysis, Toxicity and Antibacterial Activity of Benin Medicinal Plants Extracts Used in the Treatment of Sexually Transmitted Infections Associated with Hiv/Aids. Int. J. Pharm. Sci. Res. 2014, 5, 1739–1745. [Google Scholar]
- Hounzangbe-Adote, M.S.; Zinsou, F.E.; Affognon, K.J.; Koutinhouin, B.; N’Diaye, M.A.; Moutairou, K. Efficacité antiparasitaire de la poudre de graines de papaye (Carica papaya) sur les strongles gastro-intestinaux des moutons Djallonké au sud du Bénin. Rev. D’élevage Méd. Vét. Pays Trop. 2001, 54, 225–229. [Google Scholar] [CrossRef][Green Version]
- Kakpo, A.B.; Yayi, E.; Lenta, B.N.; Assogba, F.M.; Toklo, P.M.; Boyom, F.F.; Baba-Moussa, L.; Gbenou, J. Phytochemistry and Anti-Bacterial Activity of Thirteen Plants Used in Traditional Medicine to Treat Typhoid Fever in Benin. Int. J. Innov. Appl. Stud. 2019, 25, 1034–1047. [Google Scholar]
- Klotoé, J.; Fanou, B.; Agbodjento, E.; Houehou, A.; Fah, L.; Dougnon, V.; Assogba, P.; Loko, F. Antifungal Activity of Ocimum gratissimum L., Lantana camara L. & Pteleopsis suberosa Engl. & Dies Used in the Treatment of Vulvovaginal Candidiasis in Benin. Future J. Pharm. Sci. 2021, 7, 237. [Google Scholar] [CrossRef]
- Koudokpon, H.; Armstrong, N.; Dougnon, T.V.; Fah, L.; Hounsa, E.; Bankolé, H.S.; Loko, F.; Chabrière, E.; Rolain, J.M. Antibacterial Activity of Chalcone and Dihydrochalcone Compounds from Uvaria chamae Roots against Multidrug-Resistant Bacteria. BioMed Res. Int. 2018, 2018, 1453173. [Google Scholar] [CrossRef]
- Koudoro, Y.; Agbangnan, D.C.P.; Boniface, Y.; Tchobo, F.P.; Alitonou, G.; Avlessi, F.; Akpovi, A.; Dominique, S. Chemical Characterization and Biological Activities of Extracts from Two Plants (Cissus quadrangularis and Acacia polyacantha) Used in Veterinary Medicine in Benin. J. Pharmacogn. Phytochem. 2015, 3, 91–96. [Google Scholar]
- Koudoro, Y.; Wotto, V.; Christian, K.; Agbangnan, D.C.P.; Dominique, S. Phytochemical Screening, Antibacterial and Anti-Radical Activities of Daniellia oliveri Trunk Bark Extracts Used in Veterinary Medicine against Gastrointestinal Diseases in Benin. Int. J. Adv. Res. 2015, 3, 1190–1198. [Google Scholar]
- Lagnika, L.; Tchachedre, M.; Amoussa, A.M.; Amoussa, O.; Latoundji, K.; Sanni, A. Phytochemical Assessment, In Vitro Antimicrobial And Antioxidant Activities of Acacia hockii De Wild. Adv. Biol. Biomed. 2016, 3, 1–8. [Google Scholar]
- Lagnika, L.; Amoussa, A.M.O.; Adjileye, R.A.A.; Laleye, A.; Sanni, A. Antimicrobial, Antioxidant, Toxicity and Phytochemical Assessment of Extracts from Acmella uliginosa, a Leafy-Vegetable Consumed in Bénin, West Africa. BMC Complement. Altern. Med. 2016, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Lagnika, L.; Amoussa, A.M.; Adjovi, Y.; Sanni, A. Antifungal, Antibacterial and Antioxidant Properties of Adansonia digitata and Vitex doniana from Benin Pharmacopeia. J. Pharmacogn. Phytother. 2012, 4, 44–52. [Google Scholar] [CrossRef]
- Lagnika, L.; Anago, E.; Sanni, A. Screening for Antibacterial, Antioxidant Activity and Toxicity of Some Medicinal Plants Used in Benin Folkloric Medicine. J. Med. Plants 2011, 5, 773–777. [Google Scholar]
- Lagnika, L.; Weniger, B.; Senecheau, C.; Sanni, A. Antiprotozoal Activities of Compounds Isolated from Croton lobatus l. Afr. J. Infect. Dis. 2009, 3, 1–10. [Google Scholar] [CrossRef]
- Legba, B.; Dougnon, V.; Chabi, Y.; Gbaguidi, C.; Aniambossou, A.; Deguenon, E.; Dougnon, J.; Kpodekon, M.; Baba-Moussa, L. Evaluation of In-Vivo Anti-Salmonella Activity of Uvaria chamae, Lantana camara and Phyllantus amarus Used in Benin, West Africa. BMC Vet. Res. 2020, 16, 49. [Google Scholar] [CrossRef]
- Legba, B.B.; Dougnon, V.; Ahoyo, A.; Agbankpe, A.; Hounmanou, Y.; Aniambossou, V.; Hounsa, E.; Fabiyi, K.; Afoussatou, A.; Assogba, P.; et al. Exploration of the Antibacterial and Chemical Potential of Some Beninese Pharmacopoiea Traditional Plants. Microbiol. Medica. 2018, 32, 990–998. [Google Scholar] [CrossRef][Green Version]
- Medoatinsa, E.; Agbangnan, D.C.P.; Viwami, F.; Bogninou-Agbidinoukoun, G.; Noudogbessi, J.; Lagnika, L.; Ahissou, H.; Sohounhloue, D. In Vitro Antiplasmodial And Antioxidant Activities of Ethanolic And Hydroethanolic Extracts of Hyptis suaveolens. World J. Pharm. Pharm. Sci. 2015, 4, 1–7. [Google Scholar]
- Ogougbé, E.; Cynthia, A.M.; Razack, O.; Rodrigue, H.; Anatole, L.; Julien, D. Phytochemical Screening, Antibacterial Activity And Acute Oral Toxicity of Aqueous And Ethanolic Extracts of Harrisonia abyssinica (Rutaceae) Leaf: Wild Plant Used In Benin Pharmacopeia. Eur. Sci. J. ESJ 2022, 18, 235. [Google Scholar]
- Olaye, T.; Tchobo, F.P.; Chabi, N.; Koudokpon, H.; Abou, A.; Olatoundé, M.; Latifou, L.; Guy, A.; Avlessi, F.; Dominique, S. Bioactive Compounds and Antimicrobial Potential of the Roots Extract of Anogeissus leiocarpa, a Chewing Stick Used for Oral Care in Benin Republic. J. Pharmacogn. Phytother. 2020, 12, 71–80. [Google Scholar] [CrossRef]
- Olounladé, P.A.; Azando, E.V.B.; Attakpa, E.Y.; Gbenou, J.D.; Alowanou, G.G.; Tchétan, E.; Dansou, C.C.; Hounzangbé-Adoté, M.S.; Gbaguidi, F.; Moudachirou, M.; et al. In Vitro Study on the Role of the Tannins of Newbouldia laevis and Zanthoxylum zanthoxyloides on Infective Larvae of Trichostrongylus colubriformis. Afr. J. Agric. Res. 2017, 12, 3513–3519. [Google Scholar]
- Olounladé, P.A.; Azando, E.V.B.; Hounzangbé-Adoté, M.S.; Ha, T.B.T.; Leroy, E.; Moulis, C.; Fabre, N.; Magnaval, J.F.; Hoste, H.; Valentin, A. In Vitro Anthelmintic Activity of the Essential Oils of Zanthoxylum zanthoxyloides and Newbouldia laevis against Strongyloides ratti. Parasitol. Res. 2012, 110, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, T.I.; Ategbo, J.-M.; Mensah, G.A.; Adote-Hounzangbe, S. Effet Antiparasitaire Des Graines de Papaye (Carica papaya) Chez l’aulacode (Thryonomys swinderianus Temminck, 1827) d’élevage: Cas Des Aulacodicultures Du Sud-Bénin. Int. J. Biol. Chem. Sci. 2010, 4, 1–8. [Google Scholar] [CrossRef]
- Salifou, S.; Houngnimassoun, A.; Dotche, I.; Sabbas, A.; Salifou, S. Larvicide Activity of Two Chemotypes of Hyptis suaveolens (Lamiaceae) Poit, 1806 and Alphacypermethrin on Larvae of Rhipicepalus (Boophilus) microplus (Can., 1887) (Acari: Ixodidae). J. Entomol. Zool. Stud. 2020, 8, 790–794. [Google Scholar]
- Sidi, I.Y.M.S.; Azando, E.V.B.; Olounlade, P.A.; Hounzangbe-Adote, M.S. Effets Combinés Des Feuilles de Newbouldia laevis et de Zanthoxylum zanthoxyloïdes Sur Les Nématodes Parasites Gastro-Intestinaux Des Ovins Djallonké. Int. J. Biol. Chem. Sci. 2015, 9, 2078–2090. [Google Scholar] [CrossRef][Green Version]
- Tante, C.O.; Djenontin, A.; Chitou, S.; Sewade, W.; Agbani, P.; Akogbeto, M.; Chougourou, D.C. Identification and Larvicidal Efficacy of Mosquito-Repelling Plants Used in Malaria Vector Control in South-East Benin. Tanzan. J. Sci. 2022, 48, 487–498. [Google Scholar] [CrossRef]
- Tiko, G.; Medjigbodo, A.; Rafiou, A.; Amoussa, A.M.; Amoussa, O.; Djogbenou, L.; Lagnika, L. Scientific Baseline Information for the Potential Use of Hibiscus surattensis L against Malaria: Phytochemistry and Biological Studies. J. Drug Deliv. Ther. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Tiko, G.H.; Amoussa, A.M.O.; Adamou, R.; Medjigbodo, A.A.; Djogbenou, L.S.; Lagnika, L. Assessment of Antiplasmodial and Antioxidant Activities, Total Phenolics and Flavonoids Content, and Toxicological Profile of Cola millenii K. Shum (Malvaceae). Int. J. Biochem. Res. Rev. 2020, 4, 47–60. [Google Scholar] [CrossRef]
- Tokoudagba, K.J.M.; Ahouansou, A.C.; Gbaguidi, F. Phytochemistry and Antibacterial Activity of Extracts of Parkia biglobosa (Mimosaceae) and Carissa edulis (Apocynaceae) | IJSAR. Int. J. Sci. Acad. Res. 2021, 2, 3241–3245. [Google Scholar]
- Yemoa, A.; Gbenou, J.; Affolabi, D.; Moudachirou, M.; Bigot, A.; Anagonou, S.; Portaels, F.; Martin, A.; Quetin-Leclercq, J. Beninese Medicinal Plants as a Source of Antimycobacterial Agents: Bioguided Fractionation and In Vitro Activity of Alkaloids Isolated from Holarrhena floribunda Used in Traditional Treatment of Buruli Ulcer. BioMed Res. Int. 2015, 2015, 835767. [Google Scholar] [CrossRef]
- Lagnika, L.; Amoussa, A.M. In Vitro Antibacterial Activity of Two Medicinal Plants Used in Bénin to Treat Microbial Infections. Indian J. Sci. 2014, 8, 10–15. [Google Scholar]
- Ganfon, H.; Houvohessou, J.-P.; Assanhou, A.G.; Bankole, H.S.; Gbenou, J. Activité Antibactérienne de l’extrait Éthanolique et Des Fractions de Anogeissus leiocarpa (DC) Guill. Et Perr. (Combretaceae). Int. J. Biol. Chem. Sci. 2019, 13, 643–651. [Google Scholar] [CrossRef]
- Toklo, P.M.; Yayi Ladekan, E.; Linden, A.; Hounzangbe-Adote, S.; Kouam, S.F.; Gbenou, J.D. Anthelmintic Flavonoids and Other Compounds from Combretum glutinosum Perr. Ex DC (Combretaceae) Leaves. Acta Crystallogr. Sect. C Struct. Chem. 2021, 77, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Ganfon, H.; Gbaguidi, F.; Frederich, M.; Moudachirou, M.; Quetin-Leclercq, J. In Vitro Evaluation of Antiplasmodial Activity of Plant Samples Used in Traditional Medicine in Benin. Planta Medica. 2008, 74, PF6. [Google Scholar] [CrossRef]
- Ladekan-Yayi, E.; Toklo, M.; Dah-Nouvlessounon, D.; Assogba, M.; Wouamba, S.C.; Wouamba, N.; Tchegnitegni, B.; Géorcelin, A.; Baba-Moussa, L.; Sylvie, A.; et al. Anthelmintic and Antimicrobial Activities of Tannin Extracts of Mitragyna inermis (Willd.) Kuntze (Rubiaceae) and Combretum glutinosum Perr. Ex DC (Combretaceae). Am. J. Appl. Chem. 2021, 9, 145–153. [Google Scholar] [CrossRef]
- Yovo, M.; Alitonou, G.; Philippe, S.; Dedome, L.; Tchobo, F.P.; Avlessi, F.; Menut, C.; Sohounhloue, D. Phytochemistry and Antibacterial Activity of Citrus sinensis Extracts against Three Pathogenic Bacteria in Benin. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1344–1352. [Google Scholar]
- Adjobimey, T.; Edayé, I.; Lagnika, L.; Gbenou, J.; Moudachirou, M.; Sanni, A. Activités antiplasmodiales in vitro de quelques plantes antipaludiques de la pharmacopée béninoise. Comptes Rendus Chim. 2004, 7, 1023–1027. [Google Scholar] [CrossRef]
- Amoussa, A.M.; Lagnika, L.; Tchachedre, M.; Laleye, A.; Sanni, A. Acute Toxicity and Antifungal Effects of Acacia ataxacantha (Bark). Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 661–668. [Google Scholar]
- Lagnika, L.; Gbaguidi, F.; Anago, E.; Adeoti, Z.; Moudachirou, M.; Sanni, A.; Quetin-Leclercq, J. Antibacterial and Antioxidant Activity of Three Compounds Isolated from Mitracarpus scaber. Int. J. Biol. Chem. Sci. 2010, 4, 30–41. [Google Scholar] [CrossRef][Green Version]
- Lagnika, L.; Anago, E.; Atindehou, M.; Adjahoutonon, B.; Dramane, K.; Sanni, A. Antimicrobial Activity of Crataeva religiosa Forst against Bacteria Isolated from Thryonomys swinderianus Temminck. Afr. J. Biotechnol. 2011, 10, 10034–10039. [Google Scholar]
- Baba-Moussa, F.; Adjanohoun, A.; Attakpa, E.; Kpavodé, L.; Joachim Djimon, G.; Kotchoni, S.; Sezan, A.; Fatiou, T.; Baba-Moussa, L. Antimicrobial Activity of Three Essential Oils from Benin on Five Oral Germs: Test of Mouthbaths. Ann. Biol. Res. 2012, 3, 5192–5199. [Google Scholar]
- Kougnimon, E.; Casimir, A.D.; Durand, D.-N.; Bawa, B.; Lamine, B.M.; Frédéric, L. Antioxidant and Antibacterial Activities of Terminalia superba Engl. and Diels (Combretaceae) Bark Extracts. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2836–2846. [Google Scholar]
- Ganfon, H.; Bero, J.; Tchinda, A.T.; Gbaguidi, F.; Gbenou, J.; Moudachirou, M.; Frédérich, M.; Quetin-Leclercq, J. Antiparasitic Activities of Two Sesquiterpenic Lactones Isolated from Acanthospermum hispidum D.C. J. Ethnopharmacol. 2012, 141, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Salifou, S.; Daga, D.F.; Attindehou, S.; Deguenon, R.; Biaou, C.F. Antiparasitic Effects of the Water Extract from Chenopodium ambrosioides L. (Chenopodiaceae) against Some Gastrointestinal Nematodes in West African Long Legged Goats. J. Parasitol. Vector Biol. 2013, 5, 13–16. [Google Scholar]
- Tchatchedre, M.; Amoussa, A.M.O.; Atindehou, M.; Nacoulma, A.P.; Sanni, A.; Lagnika, L. Anti-Quorum Sensing, Antibacterial, Antioxidant Activities, and Phytoconstituents Analysis of Medicinal Plants Used in Benin: Acacia macrostachya (Rchb. Ex DC.). J. Appl. Biol. Biotechnol. 2020, 8, 84–93. [Google Scholar]
- Lagnika, L.; Prodjinonto, U.; Attioua, B.; Sanni, A. Chemical Analysis, Antimicrobial and Antioxidant Activities of Eight Extracts from Schrankia leptocarpa L. Afr. J. Biotechnol. 2012, 11, 13739–13745. [Google Scholar] [CrossRef]
- Gandonou Dossa, C.; Bambola, B.; Toukourou, H.; Gbaguidi Ahokannou, F.; Dansou, C.; Awede, B.; Laleye, A.; Ahissou, H. Chemical Composition And Antimicrobial Activity of The Essential Oils of Four Varieties of Lippia multiflora In Benin. Univers. J. Pharm. Res. 2019, 3, 11–19. [Google Scholar]
- Kpadonou, D.; Kpoviessi, S.; Bero, J.; Agbani, P.; Gbaguidi, F.; Kpadonou-Kpoviessi, B.; Sinsin, B.; Frédérich, M.; Quetin-Leclercq, J. Chemical Composition, in Vitro Antioxidant and Antiparasitic Properties of the Essential Oils of Three Plants Used in Traditional Medicine in Benin. J. Med. Plant Res. 2019, 13, 17–23. [Google Scholar]
- Koudoro, Y.; Yovo, M.; Boniface, Y.; Agbangnan, D.C.P.; Tchobo, F.P.; Alitonou, G.; Sohounhloue, D. Chemical Study, Antiradical and Antibacterial Potential of the Extracts of Ximenia americana and Cussonia arborea of Benin. World J. Pharm. Sci. 2012, 2, 1626–1635. [Google Scholar]
- Sacramento, I.T.; Mensah, G.A.; Ategbo, J.-M. Comparative Effect of Lemon Seeds and Albendazole a Veterinary Anthelmintics on Gastrointestinal Parasites of Farmed Grasscutter: Case of Breeding Grasscutter in Southern Benin. Pharmacopée Médecine Tradit. Afr. 2022, 21, 36–45. [Google Scholar]
- Sakirigui, A.; Yayi Ladékan, E.; Fagbohoun, L.; Chabi Sika, K.; Assogba, F.; Gbenou, J. Comparative Phytochemical Analysis and Antimicrobial Activity of Extracts of Seed and Leaf of Persea americana Mill. Acad. J. Med. Plants 2020, 8, 79–89. [Google Scholar]
- Kpadonou, D.; Sina, H.; Kpadonou-Kpoviessi, B.; Agbani, P.; Allanto, F.; Atchade, B.; Gbaguidi, F.; Joachim Djimon, G.; Baba-Moussa, L.; Salomé, K. Comparative Study of Antimicrobial and Toxic Activities of Seven Medicinal Plants of the Beninese Flora. Chem. Res. J. 2022, 7, 69–78. [Google Scholar]
- Guezodje, T.; Agbankpe, A.J.; Dassou, G.; Dougnon, T.; Dicko, A.; Yedomonhan, H. Cytotoxicity, Antimicrobial Activities And Chemical Properties of Ten Plants From The Benin Pharmacopoeia Used For Oral Care. Int. J. Pharm. Sci. Res. 2021, 12, 6642–6652. [Google Scholar]
- Sidi, I.Y.M.S.; Alowanou, G.G.; Tchétan, E.; Aminou, M.Y.; Hounzangbé-Adoté, S.M.; Babatoundé, S. Effets De La Digestion Gastrique Sur Les Propriétés Anthelminthiques De Zanthoxylum zanthoxyloides (Lam.) Zepernick & Timlerto Et De Newbouldia laevis (P.Beauv.) Sur Haemonchus contortus. Eur. Sci. J. ESJ 2017, 13, 204. [Google Scholar]
- Weniger, B.; Lagnika, L.; Vonthron-Sénécheau, C.; Adjobimey, T.; Gbenou, J.; Moudachirou, M.; Brun, R.; Anton, R.; Sanni, A. Evaluation of Ethnobotanically Selected Benin Medicinal Plants for Their in Vitro Antiplasmodial Activity. J. Ethnopharmacol. 2004, 90, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Alowanou, G.G.; Azando, E.V.B.; Adenilé, A.D.; Koudandé, D.O.; Chrysostome, C.A.M.; Hounzangbé-Adoté, S.M. Evaluation of the in Vivo Anthelmintic Properties of Mitragyna inermis (Willd.) as a Livestock Dewormer against Parasitic Hematophagous Worm Haemonchus contortus Infections in Different Breeds of Lambs. Trop. Anim. Health Prod. 2020, 52, 309–319. [Google Scholar] [CrossRef]
- Yovo, M.; Alitonou, G.A.; Yedomonhan, H.; Tchobo, F.; Dedome, O.; Sessou, P.; Avlessi, F.; Menu, C.; Sohounhloué, D. First Report on Chemical Composition and Antimicrobial Activity of Artabotrys velutinus Scott-Elliot Extracts against Some Clinical Strains in Benin. Am. J. Appl. Chem. 2016, 4, 71–76. [Google Scholar] [CrossRef]
- Dèdéhou, V.F.G.N.; Babatoundé, S.; Hounzangbé-Adoté, M.S. In Vitro Effect of Degradation in Sacco in the Rumen on the Anthelmintic Properties of Parkia biglobosa and Pterocarpus erinaceus on Haemonchus contortus. Eur. Sci. J. ESJ 2018, 14, 87. [Google Scholar] [CrossRef]
- Alowanou, G.G.; Olounladé, P.A.; Akouèdegni, G.C.; Faihun, A.M.L.; Koudandé, D.O.; Hounzangbé-Adoté, S. In Vitro Anthelmintic Effects of Bridelia ferruginea, Combretum glutinosum, and Mitragyna inermis Leaf Extracts on Haemonchus contortus, an Abomasal Nematode of Small Ruminants. Parasitol. Res. 2019, 118, 1215–1223. [Google Scholar] [CrossRef]
- Agbankpe, A.; Dougnon, V.; Bankole, S.; Houngbegnon, O.; Dah-Nouvlessounon, D.; Baba-Moussa, L. In Vitro Antibacterial Effects of Crateva adansonii, Vernonia amygdalina and Sesamum radiatum Used for the Treatment of Infectious Diarrhoeas in Benin. J. Infect. Dis. Ther. 2016, 4, 2332-0877. [Google Scholar]
- Sidi, I.; Alowanou, G.; Olounlade, P.; Dedehou, N.; Hounzangbe Adote, M.S. In Vitro Combined Effects of Zanthoxylum zanthoxyloides and Newbouldia laevis Methanolic Extracts on Three Life-Cycle Stages of the Parasitic Nematode, Haemonchus contortus. J. Anim. Health Prod. 2016, 4, 128–133. [Google Scholar] [CrossRef]
- Lagnika, L.; Attioua, B.; Vonthron-Sénécheau, C.; Kaiser, M.; Lobstein, A.; Sanni, A.; Weniger, B. In Vitro Preliminary Study of Antiprotozoal Effect of Four Medicinal Plants from Benin. J. Med. Plant Res. 2013, 7, 556–560. [Google Scholar]
- Medoatinsa, S.E.; Agbangnan, C.P.D.; Bogninou, G.S.R.; Ahissou, H.; Bothon, F.T.D.; Wottoalentin, V.; Félicien, A.; Sohounhloue, D. Phytochemical Profile and Antioxidant Potential of 12 Antimalarial Recipes Used in Lacustrine Sreas in Benin. Int. J. Pharm. Sci. Invent. 2016, 5, 16–21. [Google Scholar]
- Chokki, M.; Zongo, C.; Dah-Nouvlessounon, D.; Cudălbeanu, M.; Noumavo, P.; Ghinea, I.O.; Furdui, B.; Savadogo, A.; Dinica, R.M.; Baba-Moussa, L. Phytochemical Screening and Antimicrobial Activity of Momordica charantia L. and Morinda lucida Benth Extracts from Benin. Afr. J. Microbiol. Res. 2020, 14, 426–435. [Google Scholar]
- Chabi-Sika, K.; Sina, H.; Boya, B.; Mama-Sirou, I.; Kpangon, L.; Salami, H.A.; Kelomey, A.; Roko, G.; Assogba, S.A.; Adoko, M.Y. Phytochemical Screening and Antimicrobial Activity of Sarcocephalus latifolius Smith Roots Extracts. Biotechnol. J. Int. 2022, 26, 54–62. [Google Scholar] [CrossRef]
- Lagnika, L.; Fantodji, M.; Sanni, A. Phytochemical Study And Antibacterial, Antifungal And Antioxidant Properties of Bridelia ferruginea And Pteleopsis suberosa. Int. J. Pharm. Sci. Res. 2012, 3, 2130–2136. [Google Scholar]
- Sessou, P.; Yaovi, B.; Yovo, M.; Gamedjo, J.; Dossa, F.; Aguidissou, O.; Boko, K.; Alitonou, G.; Farougou, S.; Sohounhloue, D. Phytochemistry and Antibacterial Activity of Plants Extracts Compared with Two Commercial Antibiotics against E. coli Responsible for Avian Colibacillosis in Benin. Int. J. Phytomed. 2018, 10, 168–174. [Google Scholar] [CrossRef]
- Yovo, M.; Dedome, S.-L.O.; Sessou, P.; Alitonou, G.A.; Tchobo, F.P.; Avlessi, F.; Sohounhloue, D.C.K. Phytochmical Studies and Biological Activities of Extracts from Two Medicinal Plants Used in Benin to Treat Skin Infections and Septicemies. Int. J. Innov. Appl. Stud. 2020, 28, 507–514. [Google Scholar]
- Sounouvou, H.; Toukourou, H.; Catteau, L.; Fatiou, T.; Evrard, B.; Van Bambeke, F.; Gbaguidi, F.; Quetin-Leclercq, J. Antimicrobial Potentials of Essential Oils Extracted from West African Aromatic Plants on Common Skin Infections. Sci. Afr. 2021, 11, e00706. [Google Scholar] [CrossRef]
- Yemoa, A.L.; Martin, A.; Affolabi, D.; Gbenou, J.D. Antimycobacterial Screening of Plants from Benin on Mycobacterium ulcerans, the Causal Agent of Buruli Ulcer. West Afr. J. Res. Health 2014, 3, 15–18. [Google Scholar]
- Kpoviessi, B.; Yayi, E.; Salomé, K.; Gbaguidi, F.; Yehouenou, B.; Quetin-Leclercq, J.; Figueredo, G.; Moudachirou, M.; Accrombessi, G. Chemical Variation of Essential Oil Constituents of Ocimum gratissimum L. from Benin, and Impact on Antimicrobial Properties and Toxicity against Artemia salina Leach. Chem. Biodivers. 2012, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Dougnon, V.; Toussaint, S.; Agbankpe, J.; Aniambossou, A.; Amadou, A.; Dougnon, J.; Baba-Moussa, L. Evaluation of the Antibacterial Activity of Jatropha multifida Sap and Artemisia annua Extract on Some Clinical Strains Responsible of Urinary Tract Infections. Indian J. Sci. Technol. 2019, 12, 1–10. [Google Scholar]
- Nounagon, M.S.; Dah-Nouvlessounon, D.; N’tcha, C.; Akorede, S.M.; Sina, H.; Noumavo, P.A.; Baba-Moussa, F.; Bade, F.; Adjadohoun, A.; Baba-Moussa, L. Phytochemical Screening and Biological Activities of Combretum Adenogonium Leaves Extracts. Eur. Sci. J. 2017, 1313, 1857–78818. [Google Scholar] [CrossRef]
- Bonou, J.; Baba-Moussa, F.; Adeoti, Z.; Ahouandjinou, H.; Dougnon, V.; Dossa, D.; Gbenou, J.D.; Toukourou, F.; Baba-Moussa, L. Antimicrobial Activity of Essential Oils of Lippia multiflora, Eugenia caryophyllata, Mentha piperita and Zingiber officinale on Five Oral-Dental Microorganisms. J. Pharmacogn. Phytochem. 2016, 5, 271–276. [Google Scholar]
- Nounagnon, S.M.; N’tcha, C.; Sina, H.; Noumavo, P.; Dah-Nouvlessounon, D.; Assogba, F.; Gbénou, J.; Baba-Moussa, L. Antimicrobial Activities of Combretum micranthum Extracts on Staphylococcus aureus Strains Isolated from Skin Infections and Some Reference Strains. Asian J. Plant Sci. Res. 2016, 6, 40–47. [Google Scholar]
- Onzo, C.F.; Azokpota, P.; Dah-Nouvlessounon, D.; Lehmane, T.H.; Adjatin, A.; Baba-Moussa, L. Évaluation de l’activité Antimicrobienne de Quatre Feuilles Utilisées Comme Emballages Dans l’artisanat Agroalimentaire Au Bénin. J. Appl. Biosci. 2015, 95, 9015–9027. [Google Scholar] [CrossRef]
- Chabi Sika, K.; Sina, H.; Adoukonou-Sagbadja, H.E.A.; Roko, G.; Aliou, S.; Kifouli, A.; Ahanchede, A.; Baba-Moussa, L. Antimicrobial Activity of Anacardium occidentale L. Leaves and Barks Extracts on Pathogenic Bacteria. Afr. J. Microbiol. Res. 2014, 8, 2458–2467. [Google Scholar] [CrossRef]
- Bossou, A.F.A.D.; Dokpè, S.; Atindehou, M.; Sophie, G.; Bogninou, R.; Koudoro, Y.; Bothon, F.; Tchachedre, M.; Agbangnan, D.C.P.; Avlessi, F.; et al. Evaluation of Antiradical and Antibacterial Activities of Hydroethanolic Extract of Spondias mombin Leaves from Benin. IOSR J. Pharm. 2020, 10, 11–16. [Google Scholar]
- Sakirigui, A.; Ladekan, E.; Fagbonou, L.; Chabi Sika, K.; Assogba, M.; Joachim Djimon, G. Comparative Antimicrobial Activity of Volatile And Non-Volatile Extracts of Cymbopogon citratus Leaves. Int. J. Pharmacogn. 2019, 7, 234–239. [Google Scholar]
- Bothon, F.T.D.; Abou, L.; Atindehou, M.; Yarou, K.A.; Ewedje, E.-E.B.; Agbangnan, P.C.; Lagnika, L.; Wafo, E.; Avlessi, F. Evaluation of Antibacterial, Free Radical Scavenging Activities and Phytochemical Composition of Boswellia dalzielii Hutch Extracts. J. Pharmacogn. Phytochem. 2019, 8, 414–418. [Google Scholar]
- Koudoro, Y.; Raphaël, D.E.; Dassou, G.H.; Atindehou, M.; Agbangnan, D.C.P.; Pascal, C.; Guy, A.; Avlessi, F.; Dinica, R.-M.; Dominique, S.C. Phytochemical Screening, Antioxidant Capacity, Antibacterial and Anti-Inflammatory Activities of Ethanolic Extract of Cordia senegalensis Leaves, a Plant Used in Benin to Treat Skin Diseases. Chem. Res. J. 2021, 6, 137–146. [Google Scholar]
- Koudoro, Y.; Agbangnan, D.C.P.; Bothon, F.; Reine, S.; Guy, A.; Avlessi, F.; Codjo, S.; Dominique, K. Métabolites Secondaires et Activités Biologiques Des Extraits de l’écorce de Tronc de Khaya senegalensis, Une Plante à Usage Vétérinaire Récoltée Au Bénin. Int. J. Innov. Appl. Stud. 2018, 23, 441–450. [Google Scholar]
- Gbenou, J.D.; Sakirigui, A.; Yayi, E.; Assogba, M.; Gbénou, J. Phytochemical Composition and Potential Antimicrobial Activity of Extracts of Two Neglected Seeds (Mangifera indica L. and Persea Americana L.) in Benin. IOSR J. Appl. Chem. 2020, 13, 21–26. [Google Scholar]
- Deguenon, E.L.M.; Dougnon, V.; Senou, M.; Hounmanou, Y.; Bankole, H.S.; Dokpomiwa, H.; Baba-Moussa, L.; Agbankpe, A. Biological And Chemical Activities of Some Beninese Plant’s Extracts. Int. J. Biol. Pharm. Allied Sci. 2017, 6, 2333–2358. [Google Scholar]
- Kabré, W.; Dah-Nouvlessounon, D.; Hama-Ba, F.; Abiola, A.; Guinnin, F.; Sina, H.; Kohonou, N.A.; Atchadé Pascal, T.; Senou, M.; Savadogo, A.; et al. Mung Bean (Vigna radiata (L.) R. Wilczek) from Burkina Faso Used as Antidiabetic, Antioxidant and Antimicrobial Agent. Plant 2022, 2022, 3556. [Google Scholar] [CrossRef]
- Senou, M.; Atchadé Pascal, T.; Dougnon, V.; Agossadou, A.; Assogba, M.; Kinsiclounon, E.; Koudokpon, H.; Fah, L.; Fanou, B.; Akpovi, C.D.; et al. Efficiency of Sorghum Bicolor Extract in the Treatment of Induced Anemia on Wistar Rats International Journal of Biosciences. Int. J. Biosci. 2016, 2, 4–13. [Google Scholar]
- Mignanwandé, Z.F.; Johnson, R.C.; Hounkpatin, A.S.Y.; Boni, G.; Houndeton, A.G.; Houéto, E.E.; Kpètèhoto, W.H.; Amoussa, M.O. Antibacterial Activities of the Ethanolic Extract of Crateva adansonii DC. (Capparidaceae) Harvested in Dassa-Zoumè in Central Bénin. Open J. Med. Microbiol. 2020, 10, 46–57. [Google Scholar] [CrossRef]
- Senou, M.; Lokonon, J.E.; Abissi, E.O.-T.R.; Agbogba, F.; Dehou, R.J.; Medoatinsa, E.; Tchogou, P.; Cachon, B.F.; Houngbeme, A.; Attakpa, E.; et al. Antibacterial Activity and Toxicity of the Sap and Aqueous Extract of the Leaves of Jatropha multifida Linn. J. Biosci. Med. 2022, 10, 171–182. [Google Scholar]
- Dehou, R.J.; Abissi, Y.G.; Kpossou, G.; Tchogou, P.; Lokonon, E.; Agbogba, F.; Anago, E.; Lamine, B.-M. Bactericidal Effect of the Aqueous Extract of the Leaves of Lantana camara L. (Verbenaceae), a Plant Used in Benin in the Treatment of Skin Infections. J. Appl. Biosci. 2021, 4, 18–26. [Google Scholar]
- Olounlade, P.; Konmy, B.S.B.; Azando, E.; Allou, S.; Baba-Moussa, L. Moringa oleifera, Ocimum gratissimum and Vernonia amygdalina as a Natural Antiparasitic Alternative in Growing Rabbits. Livest. Res. Rural Dev. 2021, 33, 1–7. [Google Scholar]
- China, T.; Sabbas, A.; Gbangboche, A.; Salifou, S. In vitro anthelmintic activity of acetonic and methanolic extracts of khaya senegalensis stem bark on Haemonchus contortus. World J. Pharm. Pharm. Sci. 2016, 5, 138–147. [Google Scholar]
- Dakpogan, H.B.; Houndonougbo, P.V.; Pomalegni, S.C.B.; Ahounou, J.; Chrysostome, C. Evaluation of the Effect of Phyllanthus amarus, jatropha Curcas and Piliostigma thonningii on Experimental Chicken Coccidiosis. J. Anim. Plant Sci. 2019, 42, 7269–7278. [Google Scholar] [CrossRef]
- Tchetan, E.; Olounladé, P.A.; Azando, E.V.B.; Khaliq, H.A.; Ortiz, S.; Houngbeme, A.; Alowanou, G.G.; Koura, B.I.; Akouedegni, G.C.; Houinato, M.R.B.; et al. Anthelmintic Activity, Cytotoxicity, and Phytochemical Screening of Plants Used to Treat Digestive Parasitosis of Small Ruminants in Benin (West Africa). Animals 2022, 12, 2718. [Google Scholar] [CrossRef] [PubMed]
- Dakpogan, H.B.; Houndonougbo, V.P.; Sègbédji, J.; Mensah, G.A.; Salifou, S. Antiparasitic Activity of Papaya Seed Extract (Carica papaya) in Free-Range Local Breed Chicken (Gallus gallus) Production System in Ketou District. J. Anim. Plant Sci. 2019, 41, 6896–6902. [Google Scholar] [CrossRef]
- Houngnimassou, H.M.A.; Attindehou, S.; Salifou, S.; Koumodji, K.D.; Salifou, S. Effets Strongylicides in Vitro de l’extrait Aqueux de Feuilles de Ficus exasperata Valh. 1805 (Moraceae). Int. J. Biol. Chem. Sci. 2017, 11, 1012–1020. [Google Scholar] [CrossRef][Green Version]
- Honvou, S.H.S.; Aboh, B.A.; Mensah, S.E.P.; Akakpo, R.P.A.; Atchade, T.G.S.; Dougnon, J.T.; Mensah, G.A. Effet Des Granules de Feuilles de Moringa oleifera Sur Les Oocystes et La Croissance Ponderale Des Lapereaux Au Benin. J. Rech. Sci. L’Uni. Lomé 2017, 19, 63–72. [Google Scholar]
- Olounladé, P.A.; Eloi, A.Y.; Bertrand, A.E.V.; Sylvie, H.-A.M.; Hervé, H. Effet In Vivo De Newbouldia laevis (Bignoniaceae) Sur Des Strongles Gastro-Intestinaux Des Moutons. Eur. Sci. J. ESJ 2017, 13, 335–345. [Google Scholar]
- Tchetan, E.; Azando, E.V.; Olounladé, P.A.; Alowanou, G.G.; Hounzangbé-Adoté, S.M. In Vitro Effects of Tannin and Extracts of Bridelia ferruginea and Mitragyna inermis on the Exsheathment of Infective Larvae of Haemonchus contortus. Int. J. Vet. Sci. Med. 2020, 8, 93–99. [Google Scholar] [CrossRef]
- Afouda, L.; Baimey, H.; Bachabi, F.X.; Sero-Kpera, D.H.; Balogoun, R. Effet de L’hyptis (Hyptis Suaveolens), Du Neem (Azadirachta indica), Du Vernonia (Vernonia amygdalina), et de L’amarante (Amaranthus spp.) Sur Les Nematodes A Galles (Meloidogyne Spp.) En Cultures Maraîcheres. Agron. Afr. 2012, 24, 1–10. [Google Scholar]
- Adehan, S.; Biguezoton, A.; Adakal, H.; Dossa, F.; Dougnon, T.; Youssao, E.; Philippe, S.; Aboh, A.; Youssao Abdou Karim, I.; Assogba, N.; et al. Acaricidal Activity of Ethanolic and Volatile Extracts of The Leaves of Selected Plants Used in Veterinary Pharmacopeia on The Larvae of Rhipicephalus microplus in Benin. Alex. J. Vet. Sci. 2016, 49, 1–11. [Google Scholar]
- Adinci, K.J.; Akpo, Y.; Tonouhewa, A.; Yessinou, R.; Philippe, S.; Yovo, M.; Adehan, S.; Adoligbe, C.; Mensah, G.; Assogba, M.; et al. In Vitro Evaluation of the Acaricidal Effect of Vegetal Oils Extracted from the Kernel of Thevetia peruviana and Annona muricata on the Rhipicephalus (Boophilus) microplus Larvae. Sci. J. Vet. Adv. 2017, 6, 162–169. [Google Scholar]
- Ahoton, D.; Assogba, M.; Chodaton Zinsou, M.; Ladekan, E.; Moudachirou, M.; Joachim Djimon, G. Phenolic Compounds’ Dosage and Antioxidant Activities of Ethanolic and Aqueous Extracts of Cassytha filiformis’ Linne (Lauraceae) Lianas Saprophyte of Melaleuca quinquenervia (Cav.) ST Blake Myrtaceae. Chem. Res. J. 2021, 6, 94–104. [Google Scholar]
- Alitonou, G.; Tchobo, F.P.; Philippe, S.; Avlessi, F.; Menut, C.; Sohounhloue, D. Chemical Composition, Antiradical and Anti-Inflammatory Activities of Four Annonaceae from Benin. Int. J. Pharm. Chem. Biol. Sci. 2013, 2013, 914–923. [Google Scholar]
- Allanto, F.; Kpadonou-Kpoviessi, B.; Agnimonhan, F.H.; Dah-Nouvlessounon, D.; Agbani, P.; Atchade, B.; Gbaguidi, F.; Baba-Moussa, L.; Joachim Djimon, G.; Salomé, K. Influence of Chemical Composition on the Antioxidant Activity and Toxicity of Essential Oils of Cymbopogon nardus (l.) Rendle and Eucalyptus camaldulensis Dehnh Acclimatized in Benin. Pharm. Chem. J. 2022, 9, 58–66. [Google Scholar]
- Amoussa, A.M.O.; Bourjot, M.; Lagnika, L.; Vonthron-Sénécheau, C.; Sanni, A. Acthaside: A New Chromone Derivative from Acacia ataxacantha and Its Biological Activities. BMC Complement. Altern. Med. 2016, 16, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Bogninou, G.S.; Houndékon, B.A.P.; Marcelline, G.; Pascal, A.D.C.; Worou, C.N.; Hounankpon, Y.; Félicien, A. Phytochemical Composition and Antioxydant Capacity of Abelmoschus esculentus l. Fresh Immature Fruits. Am. J. Food Sci. Technol. 2018, 6, 223–227. [Google Scholar]
- Bothon, F.; Debiton, E.; Yedomonhan, H.; Avlessi, F.; Teulade, J.-C.; Sohounhloue, D. α-Glucosidase Inhibition, Antioxidant and Cytotoxicity Activities of Semi- Ethanolic Extracts of Bridellia ferruginea Benth. and Ceiba pentandra L. Gaerth from Benin. Res. J. Chem. Sci. 2012, 2, 2231–2606. [Google Scholar]
- Chodaton-Zinsou, M.; Assogba, M.; Yayi, E.; Gbaguidi, F.; Moudachirou, M.; Joachim Djimon, G. Phytochemical Composition, Biological Activities of Croton lobatus L. Leaves, Hydrolysis Effect on Activities and Chemical Composition. Int. J. Appl. Chem. 2020, 8, 13–22. [Google Scholar]
- Fanou, B.; Klotoé, J.; Dougnon, V.; Soha, A.; Mahudro, Y.; Frédéric, L. Etude Comparative de La Composition Chimique, de l’activité Antiradicalaire et de La Toxicité de Quatre Plantes Utilisées Dans Le Traitement Traditionnel Des Candidoses Au Bénin. Pharm. Et Médecine Tradit. Afr. 2021, 4, 54–64. [Google Scholar]
- Gbonsou, A.I.; Assogba, F.M.; Kinsou, L.D.; Ahoton, D.; Yovo, M.; Gbenou, J.; Atrevi, N.; Edorh, P. A Comparative Analysis of the Secondary Metabolites, Polyphenol Contents and Antioxidant Potential of Ethanolic, Hydro-Ethanolic and Aqueous Extracts of Annona muricata Linn Leaves in Abomey-Calavi, Benin. J. Bio. Env. Sci. 2021, 18, 1–9. [Google Scholar]
- Kanfon, R.E.; Gnawe, M.; Dossa, C.P.A.; Yedomonhan, H.; Wotto, D.V.; Sohounhloue, C.K.D. Caractérisation Physico-Chimique et Évaluation de l’activité Antiradicalaire Des Extraits de Sept Morphotypes de Gombo (Abelmoschus spp.) Cultivés Au Bénin. Int. J. Biol. Chem. Sci. 2018, 12, 1447–1458. [Google Scholar] [CrossRef]
- Klotoé, J.R.; Agbodjento, E.; Dougnon, V.T.; Yovo, M.; Sacramento, T.I.; Déguénon, E.; Dougnon, J.T.; Atègbo, J.M. Exploration of the Chemical Potential and Antioxidant Activity of Some Plants Used in the Treatment of Male Infertility in Southern Benin. J. Pharm. Res. Int. 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Kohonou, A.; Chabi, N.; Dah-Nouvlessounon, D.; Nounagnon, M.; Sognigbe, B.; Haziz, S.; Christine, N.; Christian, K.A.; Halfane, L.; Victorien, D.; et al. Antioxidant, Anti-Inflammatory Efficacy and HPLC Analysis of Annona muricata Leaves Extracts from Republic of Benin. Am. J. Plant Sci. 2020, 11, 803–818. [Google Scholar]
- Osseni, M.; Agbangnan, D.C.P.; Bossou, A.F.A.D.; Yedomonhan, H.; Avlessi, F.; Sohounhloue, D. Radical Scavenging Activities and Study of Volatile Compounds of Three Plants Used in Traditional Medicine in Benin: Anchomanes difformis, Parkia biglobosa and Polyalthia longifolia. Int. J. Innov. Appl. Stud. 2014, 9, 1609–1619. [Google Scholar]
- Winsou, I.; Anago, E.; Dah-Nouvlessounon, D.; Eustache, A.; Martial, N.; Roko, G.; Baba-Moussa, L. Phenolic Profile and Antioxidant, Anti-Inflammatory Activity of Annona senegalensis, Ipomoea batatas, Terminalia superba and Psidium guajava Linn Extracts Used in Benin. Am. J. Plant Sci. 2022, 13, 1296–1310. [Google Scholar]
- Guinnin, F.F.D.; Sacramento, I.T.; Ategbo, J.-M.; Agbangnan, C.D.P. Physico-Chemical Composition and Radicalscavenging Activity Evaluation of the Extracts of Aristolochia albida Duch. (Aristolochiaceae) of Benin. J. Appl. Biosci. 2016, 107, 10460–10470. [Google Scholar] [CrossRef][Green Version]
- Amoussou, B.F.; Badoussi, E.; Houndji, S.; Odjegnide, S.; Gbenou, J.; Kayode, P. Essai de Production d’extrait Bioactif et Bio-Aromatisant à Partir Des Racines de Mondia whitei (Hook f.) Skeels (Apocynaceae) d’écologie Béninoise. Eur. Sci. J. 2019, 15, 339–360. [Google Scholar]
- Dossou-Agoin, G.; Ganfon, H.; Assogba, M.F.; Gbankoto, A.; Gbenou, J.; Laleye, A. Antioxidant Activities of the Aqueous Extracts of Pedalium murex D. Royen EX L. Fruit and Leafy Stem. Eur. J. Med. Plants 2021, 32, 1–9. [Google Scholar] [CrossRef]
- Amoussa, A.M.O.; Sanni, A.; Lagnika, L. Antioxidant Activity and Total Phenolic, Flavonoid and Flavonol Contents of the Bark Extracts of Acacia ataxacantha. J. Pharmacogn. Phytochem. 2015, 4, 172–178. [Google Scholar]
- Ahodegnon, D.K.; Gnansounou, M.; Bogninou, R.G.; Kanfon, E.R.; Chabi, B.; Dossa, P.C.A.; Anago, E.A.; Ahoussi, E.; Wotto, V.; Sohounhloue, D.C. Biochemical Profile and Antioxidant Activity of Parkia biglobosa and Tamarindus indica Fruits Acclimated in Benin. Int. J. Adv. Res. 2018, 6, 702–711. [Google Scholar]
- Laleye, A.F.; Azando, B.E.V.; Pascal Abiodoun, O.; Tohouegnon, T.; Laleye, A.; Ahissou, H. Evaluation of Antihyperglycemic, Antiradical and Acute Oral Toxicity Activities of Aqueous Leaves Extract of Moringa oleifera Lam (Moringaceae) from Benin in Normal Rats. Afr. J. Biotechnol. 2017, 16, 1513–1519. [Google Scholar] [CrossRef][Green Version]
- Chokki, M.; Cudălbeanu, M.; Zongo, C.; Dah-Nouvlessounon, D.; Ghinea, I.O.; Furdui, B.; Raclea, R.; Savadogo, A.; Baba-Moussa, L.; Avamescu, S.M.; et al. Exploring Antioxidant and Enzymes (A-Amylase and B-Glucosidase) Inhibitory Activity of Morinda lucida and Momordica charantia Leaves from Benin. Foods 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Kpètèhoto, H.W.; Amoussa, A.M.O.; Johnson, R.C.; Houéto, E.E.M.; Mignanwandé, F.M.Z.; Yédomonhan, H.; Loko, F.; Bankolé, H.; Lagnika, L. Phytochemical Analysis and Antioxidant Potential of Ocimum gratissimum Linn (Lamiaceae) Commonly Consumed in the Republic of Benin. J. Appl. Biol. Biotechnol. 2019, 7, 75–83. [Google Scholar]
- Koudoro Yaya, A.; Morand, A.J.; Andreea, B.D.; Théophile, O.; Pascal, A.D.C.; Alain, A.G.; Félicien, A.; Mihaela, D.R.; Dominique, S.C.K. Phytochemical Analysis, Antioxidant and Anti-Inflammatory Activities of Chassalia kolly Leaves Extract, a Plant Used in Benin to Treat Skin Illness. GSC Biol. Pharm. Sci. 2021, 15, 63–72. [Google Scholar] [CrossRef]
- Djehoue, R.; Amoussa, A.M.O.; Sanni, A.; Lagnika, L. Phytochemical Composition and Antioxidant Property of Dissotis rotundifolia Used for Malaria Management in South Benin. J. Med. Plants 2020, 8, 23–29. [Google Scholar]
- Tokoudagba, K.J.M.D.; Gbaguidi, F.A. Phytochemical Study And Evaluation of The Antioxidant Activity of Two Medicinal Plants In Benin. World J. Pharm. Res. 2021, 7, 94–103. [Google Scholar]
- Dassou, E.M.; Glinma, B.; Assogba, M.F.; Gbenou, J. Phytochemical Study, Antioxidant and Anticonvulsant Activities of Aqueous Extract of Leaves of Opilia celtidifolia (Guill. Et Perr.) Endl. Ex Walp. Opiliaceae, from Benin. IOSR J. Pharm. Biol. Sci. 2022, 17, 43–55. [Google Scholar]
- Tokoudagba, J.-M.; Gandonou, C.D.; Houngue, U.; Auge, C.; Schini-Kerth, V.B. Phytochemistry and Vasorelaxant Activity of Some Plants Used Traditionally against High Blood Pressure in Benin. World J. Pharm. Sci. 2018, 6, 40–48. [Google Scholar]
- Adjileye, R.A.; Amoussa, A.M.O.; Lagnika, L. Trema orientalis L. and Dialium guineense Wild. Used to Manage Hypertension in Benin: Phytochemical Study and Antioxidant Activity. J. Med. Plants Stud. 2019, 7, 43–48. [Google Scholar]
- Adjileye, R.; Amoussa Abdou Madjid, O.; Rafiou, A.; Awede, B.; Laleye, A.; Lagnika, L. UHPLC-DAD Characterization of Bioactive Secondary Metabolites from Ocimum americanum and Pupalia lappacea Extracts: Antioxidant Activity and Antihypertensive Effects on L-NAME-Induced Hypertensive Rats. J. Pharmacogn. Phytother. 2019, 11, 17–27. [Google Scholar] [CrossRef]
- Yomakou, R.; Glinma, B.; Assogba, M.; Akpako, H.; Ahoton, D.; Aikpe, F.; Yayi, E.; Joachim Djimon, G. Phytochemistry, Metabolites Quantification and Antioxidant Activity of Calotropis procera (Ait.) and Ficus umbellata (Vahl.), Plants Traditionally Used against Hemorrhoids in Benin. Int. J. Curr. Res. Chem. Pharm. 2022, 2, 8–15. [Google Scholar]
- Fadeyi, O.G.; Assogba, F.M.; Chabi, D.; Yorou, N.S.; Gbenou, J.D. Ethnomycology, Myco-Chemical Analyzes and Antioxidant Activity of Eleven Species of the Genus amanita (Basidiomycota, Fungi) from Benin (West Africa). J. Pharmacogn. Phytochem. 2019, 8, 335–341. [Google Scholar]
- Koukoui, O.; Agbangnan, D.C.P.; Medoatinsa, E.; Prigent, S.; Nusse, O.; Combettes, L.; Sohounhloue, D. Chemical Profile, Cytotoxicity Anti-Radical and Hypolipidemic Activities of Tridax procumbens of Benin. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1441–1452. [Google Scholar]
- Médine, K.; Pascal, A.; Sylviane, B.; Mahudro, Y.; Oliver, N.; Laurent, C.; Dominique, S. Phytochemical Study and Evaluation of Cytotoxicity, Antioxidant and Hypolipidemic Properties of Launaea taraxacifolia Leaves Extracts on Cell Lines HepG2 and PLB985. Am. J. Plant Sci. 2015, 6, 1768. [Google Scholar]
- Seton, S.; Koukoui, O.; Koudoro, Y.; Amagbegnon, J.B.; Betira, M.; Sonounameto, F.; Agbangnan, C.P. Comparative Phytochemical Analysis and Antiradical Activity of Five Plants Used for the Treatment of Type 2 Diabetes in Benin. J. Pharm. Res. Int. 2022, 34, 34–43. [Google Scholar] [CrossRef]
- Mama, I.; Attakpa, E.; Béhanzin, J.; Amoussa, A.M.; Machioud, S.; Rodrigue, A.; Guinnin, F.; Lagnika, L.; Sezan, A.; Baba-Moussa, L.; et al. The Effect of Methanolic Leaf Extract of Boerhavia diffusa Linn. (Nictaginaceae) on the Activities of Antidiabetic, Anti-Inflammatory and Antioxidant Enzymes in Experimental Diabetes. J. Pharm. Res. Int. 2018, 24, 1–25. [Google Scholar]
- Roko, G.; Dougnon, V.; Hounkpatin, A.; Klotoé, J.; Baba-Moussa, L. Anti-Inflammatory, Analgesic and Antipyretic Properties of Ethanolic Extracts of Three Plants of Beninese’s Pharmacopoeia: Euphorbia hirta, Citrus aurantifolia and Heterotis rotundifolia. Asian J. Biol. 2020, 8, 1–89. [Google Scholar] [CrossRef][Green Version]
- Gbenou, J.D.; Ahounou, J.F.; Akakpo, H.B.; Laleye, A.; Yayi, E.; Gbaguidi, F.; Baba-Moussa, L.; Darboux, R.; Dansou, P.; Moudachirou, M. Phytochemical Composition of Cymbopogon citratus and Eucalyptus citriodora Essential Oils and Their Anti-Inflammatory and Analgesic Properties on Wistar Rats. Mol. Biol. Rep. 2013, 40, 1127–1134. [Google Scholar] [CrossRef]
- Attakpa, E.; Sezan, A.; Baba-Moussa, L.; Seri, B.; Gbéassor, M.; Khan, N. Sclerocarya birrea Oil Modulates Human T-Lymphocyte Differentiation. Am. J. Pharmatech Res. 2015, 5, 190–206. [Google Scholar]
- Senou, M.; Atchadé Pascal, T.; Agbogba, F.; Lokonon, J.; Medoatinsa, E.; Kanfon, R.; Dougnon, V.; Agbangnan, D.C.P.; Boko, C.; Loko, F.; et al. The ethyl acetate fraction of Psorospermum febrifugum spachroot barks aqueous extract is a good stimulator of hematopoiesis. Int. J. Adv. Res. 2020, 8, 1018–1026. [Google Scholar] [CrossRef]
- Lokonon, J.E.; Tchogou, P.; Agbogba, F.; Abissi, G.; Medoatinsa, E.; Koudoro, Y.; Agbangnan, P.; Anago, E.; Baba-Moussa, L.; Senou, M. Anti-Sickling Activity of Daniellia oliveri (Rolfe) Hutch. & Dalziel. Bark Aqueous Extracts in the Management of Sickle Cell Disease in Benin. J. Appl. Biosci. 2021, 168, 17446–17455. [Google Scholar]
- Adjou, E.S.; Cabral, D.L.V.; Soumanou, M.M. Insecticidal and Repellent Effects of Essential Oils from Leaves of Hyptis suaveolens and Ocimum canum against Tenebroides mauritanicus (L.) Isolated from Peanut in Post-Harvest. J. Für Verbrauch. Leb. 2019, 14, 25–30. [Google Scholar] [CrossRef]
- Abagli, Z.; Alavo, T.; Avlessi, F.; Moudachirou, M. Potential of the Bush Mint, Hyptis suaveolens Essential Oil for Personal Protection Against Mosquito Biting. J. Am. Mosq. Control Assoc. 2012, 28, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kindomihou, V.; Tosso, F.; Sikirou, R.; Assogbadjo, A.; Mensah, G. Efficiency of Botanical Extracts on Bee Pests in the North-Western of Benin. Acad. J. Agric. Res. 2018, 2, 331–339. [Google Scholar]
- Tepa-Yotto, G.T.; Adandonon, A.; Adikpeto, M.B.S. Potentiel des extraits de plantes insecticides pour la gestion de la chenille légionnaire d’automne Spodoptera frugiperda au Bénin. Sci. Technol. Substain. Agric. 2021, 1, 1–8. [Google Scholar]
- Tepa-Yotto, G.; Dahoueto, B.; Winsou, J.; Hounmakou, E.; Billa, A.-A.; Nonfodji, A.; Honfo, F.F.M.; Akadiri, M.A.; Atta, P.; Dassi, R.; et al. Efficacité En Laboratoire et Sur Le Terrain Des Cendres et de Divers Insecticides Biorationnels Sur La Chenille Légionnaire d’automne, Spodoptera frugiperda J. E. Smith (Lepidoptera, Noctuidae) Au Bénin. Rev. RAMReS 2022, 10, 43–52. [Google Scholar]
- Adjagba, M.; Razack, O.; Adounkpe, F.; Agbanlinsou, A.; Lagnika, L.; Djego, G.; Darboux, R.; Gbaguidi, F.; Laleye, A. Effect of Fractions from Carissa edulis (Forssk.) Vahl (Apocynaceae) Leaves on Hypertension Induced in Wistars Rats. World J. Adv. Res. Rev. 2013, 15, 424–431. [Google Scholar] [CrossRef]
- Adjagba, M.; Awede, B.; Osseni, R.; Hountondji, C.; Dougnon, G.; Lagnika, L.; Darboux, R.; Laleye, A. Antihypertensive Effect of Extracts from Crateva adansonii DC.Ssp. adansonii in the Wistar Rats. Int. J. Biol. Chem. Sci. 2017, 11, 2604–2615. [Google Scholar] [CrossRef][Green Version]
- Quenum, C.T.; Ahissou, H.; Gouthon, P.; Laleye, A. Etude de l’activité Antihypertensive d’une Association de Plantes (Schrankia leptocarpa, Garcinia kola et Ocimum americanum) Chez Le Rat Wistar. Int. J. Biol. Chem. Sci. 2014, 8, 2685–2695. [Google Scholar] [CrossRef]
- Koutchiko, S.H.; Attakpa Sèlidji, E.; Guinnin, F.; Sènou, M.; Amoussa Abdou, M.; Lagnika, L.; Sina, H.; Yédomonhan, H.; Baba-Moussa, L. Antioxidative Effects and Mechanisms of Antihypertensive Potential of Croton gratissimus Burch and Schrankia leptocarpa DC in Rats. Ann. Hypertens. 2022, 2, 1011. [Google Scholar]
- Adjagba, M.; Awede, B.; Lagnika, L.; Nondichao, K.; Razack, O.; Darboux, R.; Laleye, A. Antihypertensive Activity of Different Fractions of Tridax procumbens Crude Aqueous Extract in Wistar Rats. J. Physiol. Pharmacol. Adv. 2015, 5, 1255–1267. [Google Scholar]
- Gbankoto, A.; Sindete, M.; Adjagba, M.; Sangare, M.; Selidji, A.; Awede, B. Antihypertensive Effects of Moringa oleifera Leaf Extract Lam. (Moringaceae) in NG-Nitro-L-Arginine-Methyl Ester-Induced Hypertensive Rats. Natl. J. Physiol. Pharm. Pharmacol. 2019, 9, 1257–1266. [Google Scholar] [CrossRef]
- Attakpa, E.; Chabi, N.; Bertin, G.; Atègbo, J.-M.; Seri, B.; Khan, N. Moringa oleifera-Rich Diet and T-Cell Calcium Signaling in Hypertensive Rats. Physiol. Res. 2017, 66, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Koukoui, E.; Michodjehoun, L.; Hoteyi, I.; Sezan, A. Hypotensive Activity of Tridax procubens Hydro Ethanolic Extract: Roles of Transport of Sodium and Potassium in Rat Wistar. Am. J. Pharm.Tech. Res. 2018, 1, 8–17. [Google Scholar]
- Avaligbe, C.; Joachim Djimon, G.; Salomé, K.; Accrombessi, G.; Moudachirou, M.; Gbeassor, M. Antihemolytic Properties of Extracts of Six Plants Used in the Traditional Treatment of Sickle Cell Disease in Benin. J. Appl. Pharm. Sci. 2012, 2, 8–13. [Google Scholar]
- Lokonon, E.; Senou, M.; Abissi, Y.G.; Dehou, R. Evaluation of the Anti-Sickle Cell Activity of Uvaria chamea P. Beauv. Roots Aqueous Extract. Int. J. Biol. 2022, 14, 1–8. [Google Scholar]
- Fah, L.; Dougnon, V.; Avocefohoun, A.; Koudokpon, H.; Aniambossou, V.; Assogba, P.; Casimir, D.A.; Loko, F. Évaluation Des Propriétés Biologiques de Launaea taraxacifolia, Un Légume Feuille Utilisé Dans Le Traitement Du Diabète Au Bénin. Afr. Sci. 2018, 14, 202–216. [Google Scholar]
- Fah, L.; Koudokpon, H.; Dougnon, J.; Klotoe, J.R.; Fanou, B.; Dougnon, T.V.; Loko, F. Evaluation des propriétés antihyperglycémiantes de Schwenckia americana L.: Une plante utilisée dans le traitement du diabète au Bénin. Ethnopharmacologia 2015, 54, 1–9. [Google Scholar]
- Agossou, E.; Ahokpe, M.; Behanzin, J.; Takin, M.; Yessoufou, G.; Zohoun, L.; Baba-Moussa, L.; Sezan, A. Effect of the Ethanolic Extract of Khaya Senegalensis on Some Biochemical Parameters on Rabbit’s in Glucose Overload Condition. Am. J. Plant Sci. 2015, 6, 240–248. [Google Scholar] [CrossRef]
- Takin, M.; Ahokpe, M.; Zohoun, L.; Assou, E.; Aivodji, N.; Agossou, E.; Sezan, A. Effect of Total Khaya senegalensis (Meliaceae) Barks Extracts on Hepatic Liberation of Glucose. Natl. J. Physiol. Pharm. Pharmacol. 2014, 4, 112–117. [Google Scholar]
- Tollo, B.; Chougourou, D.C.; Todohoue, C.M. Anti-Hyperglycaemic and Lipid Profile Regulatory Properties of Moringa oleifera in Subjects At Early Stages of Type 2 Diabetes Mellitus. Emj Eur. Med. J. 2016, 4, 99–105. [Google Scholar]
- Yessoufou, A.; Gbenou, J.; Grissa, O.; Hichami, A.; Simonin, A.-M.; Tabka, Z.; Moudachirou, M.; Moutairou, K.; Khan, N.A. Anti-Hyperglycemic Effects of Three Medicinal Plants in Diabetic Pregnancy: Modulation of T Cell Proliferation. BMC Complement. Altern. Med. 2013, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Houndjo, C.; Wilfrid, A.; Justin, K.; Marc Abel, A.; Pierre, H.D.; Moudachirou, M.; Joachim, D.G. Comparative Study of Antihyperglycemic Activity of Aqueous Extracts from the Leaves of Bridelia ferruginea, Lophira lanceolata and Oxytenanthera abyssinica, with Their Mixture. Int. J. Curr. Res. Chem. Pharm. Sci. 2017, 4, 22–33. [Google Scholar]
- Lalèyè, O.A.F.; Ahissou, H.; Assogba, M.F.; Azando, V.B.E.; Olounladé, A.P.; Agbo’Saga, K.F.; Lalèyè, A. In Vivo Hypoglycemic Activity and Acute Oral Toxicity of Ethanolic and Aqueous Leaves Extract of Momordica charantia Linn (Cucurbitaceae) from Benin. J. Chem. Pharm. Res. 2015, 7, 376–385. [Google Scholar]
- Kotin, L.M.; Assogba, F.M.; Akakpo, H.B.; Aikpe, J.F.A.; Godonou, J.-B.; Dansou, P.H.; Gbenou, J.D. Phytochemical, Toxicity and Antihyperglycaemic Effects of Zea mays Linn Leaves’ Extracts. J. Drug Deliv. Ther. 2022, 12, 86–92. [Google Scholar] [CrossRef]
- Seton, S.; Koukoui, O.; Amagbegbon, J.B.; Betira, M.; Sonounameto, F.; Assou, E.; Sezan, A. Toxicological and Anti-Hyperglycemic Studies of Five Plants Used for the Treatment of Type 2 Diabetes in Benin. J. Drug Deliv. Ther. 2022, 12, 47–53. [Google Scholar] [CrossRef]
- Assou, C.; Anago, E.; Senou, M.; Agbogba, F.; Agniwo, P.; Lokonon, J.E.; Abissi, Y.; Hlouedje, W.; Tchogou, P.; Houngbeme, A. Anti-Hyperglycemic Effect of Momordica charantia Green Fruit Extract. Int. J. Pharm. Sci. Invent. 2022, 12, 47–53. [Google Scholar]
- Amagbegnon, J.-B.; Koukoui, O.; Agbangnan, P.C.; Seton, S.; Betira, M.; Medegan, S.; Loko, L.; Dougnon, V.; Djogbenou, L.; Sezan, A. Evaluation of the Preventive and Therapeutic Activities of Tridax procumbens against Hyperglycemia and Hyperlipidemia Induced in Wistar Rats. Pharmacol. Pharm. 2021, 12, 127–140. [Google Scholar] [CrossRef]
- Gomina, M.; Salifou, T.; Djidonou, G.; Zinsou, S. Effect of the Garcinia kola Seed on Glycemia, Creatininemia and Aminotransferases in Adult Subjects. Inf. Conhecimento Para A Saúde 2020, 5, 24–28. [Google Scholar] [CrossRef]
- Aivodji, N.; Chabi, C.; Ahokpe, M.; Sezan, A. Annona muricata Reduced Hepatocytes Differentiation Caused by Cycas revoluta in Wistar Rats. OSR J. Pharm. Biol. Sci. 2018, 14, 6–12. [Google Scholar]
- Hoteyi, S.; Koukoui, E.; Michodjehoun, L. Annona muricata Reduced Proliferation Caused By Cycas revoluta in Wistar Rats. Glob. J. Res. Anal. 2018, 3, 25–32. [Google Scholar]
- Michodjehoun, L.; Koukoui, E.; Houndefo, T.; Hounsinou, S.; Aivodji, N.; Ahokpe, M.; Sezan, A. Action of Ethyl Extracts of Annona muricata in the Traditional Treatment of Cervical Cancer in the Wistar Rat. Int. J. Curr. Res. Multidiscip. 2018, 2, 1–9. [Google Scholar]
- Houndefo, T.; Gbaguidi, B.N.; Hoteyi, I.; Gbenou, J.D.; Sezan, A. Apoptotic Action of Ethanolic Extracts of The Leaves of Hexalobus Monopetalus (Annonaceae) On Cervical Cancer in Wistar Rats. Int. J. Med. Res. Pharm. Sci. 2018, 4, 1–12. [Google Scholar]
- Senou, M.; Tchogou, A.; Agbangnan, D.; Dougnon, T.; Ogué, P.; Agossadou, A.; Lalèyè, A.; Loko, F.; Agbonon, A.; Sèzan, A. Efficacy of Ethyl Acetate Fraction of Cocos nucifera Aqueous Extract on the Treatment of Anemia. Int. J. Pharm. Sci. Invent. 2017, 6, 44–51. [Google Scholar]
- Medoatinsa, S.E.; Dossa, C.P.A.; Atchade, S.P.; Bogninou, G.S.R.; Dosseh, K.; Kpatcha, T.; Agbonon, A.; Ahissou, H.; Sohounhloue, D. Antipyretic and Antianemic Activities of Three Anti-Malaria Recipes from South Benin on Wistar Rats. Am. J. Pharmacol. Sci. 2017, 5, 57–62. [Google Scholar]
- Tchogou, A.P.; Senou, M.; Dougnon, T.V.; Agossadou, A.; Assogba, F.; Kinsiclounon, E.G.; Ewedje, E.; Agbangnan, D.C.P.; Gbenou, J.; Laleye, A.; et al. The Aqueous Extract of Cocos nucifera L. (Arecaceae) Effectively Treat Induced Anemia. Experimental Study on Wistar Rats. Int. J. Biol. 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Tchogou, A.P.; Sènou, M.; Agbogba, F.; Lokonon, J.E.; Medoatinsa, S.E.; Agbangnan Dossa, C.P.; Loko, F.; Agbonon, A.; Sèzan, A.; Cotonou, R.; et al. Efficacy of Butanolic Fraction of Cocos nucifera’s Root Aqueous Extract on Induced Anemia Treatment. Int. J. Biol. 2021, 13, 49–57. [Google Scholar] [CrossRef]
- Agbogba, F.; Isabelle, S.T.; Pascal, T.A.; Espérance, M.; Rose, K.E.; Eugène, A.; Pascal, A.D.C.; Frédéric, L.; Anatole, L.; Jean-Marc, A. The Aqueous Extract of the Root Bark of Psorospermum febrifugum Spach Effectively Corrects Anaemia. Experimental Study on Wistar Rats. J. Appl. Biosci. 2019, 139, 14137–14146. [Google Scholar]
- Adoligbe, C.; Gangbe, R.; Adinci, J.; Mantip, S.; Farougou, S. Treatment of Girolando Cattle Dermatophilosis Using a Combination of Different Plant Extracts in the Municipality of Abomey-Calavi, Republic of Benin. Vet. World 2021, 14, 2750–2756. [Google Scholar] [CrossRef]
- Sirou, M.; Ibrahima, A.; Attakpa, E.; Rodrigue, A.; Amoussa, A.M.; Guinnin, F.; Lagnika, L.; Baba-Moussa, L.; Seri, B.; Khan, N. Protective Effects of Boerhavia diffusa Linn. (Nictaginaceae) on Liver of Streptozotocin-Induced Diabetic Rats. Eur. J. Biomed. 2019, 6, 19–25. [Google Scholar]
- Attakpa, E.; Amina, M.; Machioud, S.; Guinnin, F.; Rodrigue, A.; Amoussa, A.M.; Lagnika, L.; Baba-Moussa, L.; Seri, B.; Khan, N. Molecular Mechanisms of Hypoglycemic and Antioxidative Effects of Phyllanthus amarus on Streptozotocin-Induced Diabetic Rats. J. Endocrinol. Diabetes 2018, 5, 1–16. [Google Scholar]
- Fachinan, R.; Fagninou, A.; Nekoua, M.P.; Amoussa, A.M.; Adjagba, M.; Lagnika, L.; Lalèyè, A.; Moutairou, K.; Yessoufou, A. Evidence of Immunosuppressive and Th2 Immune Polarizing Effects of Antidiabetic Momordica Charantia Fruit Juice. BioMed Res. Int. 2017, 2017, 9478048. [Google Scholar] [CrossRef] [PubMed]
- Attakpa, E.S.; Sangaré, M.M.; Béhanzin, G.J.; Ategbo, J.-M.; Seri, B.; Khan, N.A. Moringa olifeira Lam. Stimulates Activation of the Insulin-Dependent Akt Pathway. Antidiabetic Effect in a Diet-Induced Obesity (DIO) Mouse Model. Folia Biol. 2017, 63, 42–51. [Google Scholar]
- Senou, M.; Lokonon, J.E.; Ayitchehou, G.; Agbogba, F.; Dehou, R.J.; Medoatinsa, E.; Tchogou, P.; Cachon, B.F.; Houngbeme, A.; Attakpa, E. Antidiabetic Activity of Aqueous Extracts of Laurus nobilis, a Spice Used by Beninese Traditional Therapists. Am. J. Med. Sci. 2021, 9, 115–119. [Google Scholar] [CrossRef]
- Koukoui, O.; Agbangnan, P. Antiradical Activity of Five Plants Used for the Treatment of Type 2 Diabetes in Benin: A Comparative and Analytical Overview. Curr. Overv. Pharm. Sci. 2022, 1, 27–43. [Google Scholar]
- Loko, L.; Fagla Medegan, S.; Parfait, K.; Ahouansou, C.; Joelle, T.; Glinma, B.; Dougnon, V.; Koukoui, O.; Djogbenou, L.; Tamo, M.; et al. Bioactivity of Essential Oils of Cymbopogon citratus (DC) Stapf and Cymbopogon nardus (L.) W. Watson from Benin against Dinoderus porcellus Lesne (Coleoptera: Bostrichidae) Infesting Yam Chips. Int. J. Trop. Insect Sci. 2020, 41, 511–524. [Google Scholar] [CrossRef]
- Aïzoun, N.; Koura, K.; Adjatin, A.; Aïzoun, N.; Koura, K.; Adjatin, A. Effect of Aqueous Extract of Lemon (Citrus Limon) on Anopheles gambiae Sensu Lato (Diptera: Culicidae) Larvae Tolerance in Malaria Vector Control in Dogbo District in South-Western Republic of Benin, West Africa. GSC Biol. Pharm. Sci. 2021, 17, 1–7. [Google Scholar] [CrossRef]
- Aïzoun, N.; Adjatin, A.; Alowanou, G. Effect of Coconut Oil on Anopheles gambiae Sensu Lato (Diptera: Culicidae) Larvae Tolerance in Malaria Vector Control in Dogbo District in South-Western Benin, West Africa. GSC Adv. Res. Rev. 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Aïzoun, N.; Adjatin, A.; Koukoui, O.; Chougourou, D. Larvicidal Activities of Ethanolic Extracts of Hyptis suaveolens Linn (Lamiaceae) and Azadirachta indica (Meliaceae) Leaves and Their Phytochemical Properties in Malaria Vector Control in Dogbo District in South-Western Benin, West Africa. Glob. J. Eng. Technol. Adv. 2022, 12, 113–120. [Google Scholar] [CrossRef]
- Aïkpe, J.; Akakpo, B.; Assogba, M.; Godonou, J.-B.; Sondjo, G.; Dansou, P.; Joachim Djimon, G. Antitussive Properties of Aqueous Extracts of Sterculia setigera Delile (Sterculiaceae) and Mixture of Aframomum melegueta K. Schum (Zingiberaceae)—Citrus aurantifolia (Christm. and Panzer) Swingle (Rutaceae). J. Pharmacogn. Phytochem. 2020, 9, 1624–1627. [Google Scholar] [CrossRef]
- Sindete, M.; Rharass, T.; Gbankoto, A.; Yemoa, A.; Ganfon, H.; Adjagba, M.; Ribou, A.-C. A Comparative Study of Caesalpinia bonduc (L.) Roxb. Root Extracts on Sexual Behaviour in Male Wistar Rats. Andrologia 2021, 53, e14072. [Google Scholar] [CrossRef] [PubMed]
- Sindete, M.; Gbankoto, A.; Ganfon, H.; Yemoa, A.; Dramane, K.; Laleye, A. Ethanol Extract of Caesalpinia bonduc (L.) Roxb Root Improves the Sexual Performance of Male Wistar Rats. Int. J. Med. Sci. Public Health 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Gbankoto, A.; Anago, E.; Houndjo, P.A.; Adjahouinou, D.C.; Gbaguidi, F. Effect of Aqueous and Ethanolic Extracts of Caesalpinia bonduc Root on Sexual Behaviour of Male Wistar Rats. Int. J. Multidiscip. Curr. Res. 2015, 3, 55–62. [Google Scholar]
- Assogba, F.M.; Aderomou, C.; Agbodjogbe, W.; Moudachirou, M.; Gbenou, J.D. Evaluation of Diuretic Properties from Elaeis guineensis Jacq. (Arecaceae) Leaves Aqueous Extractin Wistar Rat. J. Chem. Pharm. Res. 2015, 7, 12–22. [Google Scholar]
- Agbodjogbe, W.K.D.D.; Aïkpe, J.; Ayedoun, M.A.; Assogba, M.; Dansou, P.; Joachim Djimon, G. Diuretic and Natriuretic Activities from Ten Medicinal Plants Used in South Benin. J. Chem. Pharm. Res. 2015, 7, 1145–1152. [Google Scholar]
- Dandjesso, C.; Klotoé, J.; Dougnon, V.; Segbo, J.; Atègbo, J.-M.; Gbaguidi, F.; Fah, L.; Fanou, B.; Loko, F.; Dramane, K. Phytochemistry and Hemostatic Properties of Some Medicinal Plants Sold as Anti-Hemorrhagic in Cotonou Markets (Benin). Indian J. Sci. Technol. 2012, 5, 1822–1835. [Google Scholar] [CrossRef]
- Dougnon, V.; Klotoe, J.R.; Sègbo, J.; Atègbo, J.M.; Edorh, A.P.; Gbaguidi, F.; Hounkpatin, A.; Dandjesso, C.; Fah, L.; Fanou, B.; et al. Evaluation of the Phytochemical and Hemostatic Potential of Jatropha multifida Sap. Afr. J. Pharm. Pharmacol. 2012, 6, 1943–1948. [Google Scholar]
- Klotoé, J.; Dougnon, V.; Atègbo, J.-M.; Adounkpè, F.; Segbo, J.; Fanou, B.; Fah, L.; Edorh, P.; Dandjesso, C.; Koudokpon, H.; et al. In Vitro Evaluation of Hemostatic Properties of the Sap of Jatropha multifida L. (Euphorbiaceae). J. Med. Plants Res. 2012, 6, 4567–4572. [Google Scholar]
- Klotoe, J.R.; Ategbo, J.M.; Dougnon, V.; Loko, F.; Dramane, K. Hemostatic Effect of Jatropha multifida L. (Euphorbiaceae) in Rats Having Coagulation Disorders. J. Appl. Biol. Biotechnol. 2017, 5, 26–29. [Google Scholar]
- Sangare, M.; Klotoe, J.R.; Dougnon, V.; Ategbo, J.M.; Laleye, A.; Edorh, P.; Fah, L.; Senou, M.; Loko, F.; Dramane, L.K. Evaluation of the Hepatoprotective Activity of Gomphrena celosioides (Amaranthaceae) on Wistar Rats Intoxicated with Tetrachloride Carbon. Int. J. Curr. Res. 2012, 4, 67–72. [Google Scholar]
- Sangare, M.M.; Sina, H.; Bayala, B.; Baba-Moussa, L.S.; Ategbo, J.M.; Senou, M.; Dramane, K.L. Évaluation de la dose efficace de l’extrait aqueux de Gomphrena celosioides face à une hépatopathie induite par le tétrachlorure de carbone. Phytothérapie 2014, 12, 393–398. [Google Scholar] [CrossRef]
- Dougnon, T.V.; Bankolé, H.S.; Klotoé, J.R.; Sènou, M.; Fah, L.; Koudokpon, H.; Akpovi, C.; Dougnon, T.J.; Addo, P.; Loko, F.; et al. Treatment of Hypercholesterolemia: Screening of Solanum macrocarpon Linn (Solanaceae) as a Medicinal Plant in Benin. Avicenna J. Phytomed. 2014, 4, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ahounou, J.F.; Ouedraogo, G.G.; Gbenou, J.D.; Ouedraogo, S.; Agbodjogbe, W.K.; Dansou, P.H.; Moudachirou, M. Spasmolytic Effects of Aqueous Extract of Mixture from Aframomumum melegueta (K Schum)—Citrus aurantifolia (Christm and Panzer) on Isolated Trachea from Rat. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2012, 9, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Agbodjento, E.; Klotoé, J.R.; Dougnon, T.V.; Sacramento, T.I.; Dougnon, T.J.; Atègbo, J.-M. Effect of Rourea coccinea on Ethanol-Induced Male Infertility in Wistar Albino Rats. Andrologia 2021, 53, e14047. [Google Scholar] [CrossRef]
- Dossou-Agoin, G.B.; Gbankoto, A.; Azonbakin, S.; Osseni, R.; Yemoa, A.; Lalèyè, A. Aqueous Extract of Pedalium murex D. Royen Ex L. Leafy Stem Protects against Lead Induced Testicular Toxicity in Wistar Rats. J. Complement. Integr. Med. 2022, 19, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Gomina, M.; Dabrigui, B.; Salifou, T.; Alassani, A.; Cisse, I.; Djidonou, G.; Baba-Moussa, L.; Simon, A.; Zinsou, S. Effect of Daily Consumption of Nigella sativa Seed on Serum Lipids in Adult Subjects. Int. J. Adv. Biochem. Res. 2021, 5, 24–28. [Google Scholar] [CrossRef]
- Ahomadegbe, M.; Toklo, M.; Glinma, B.; Dah-Nouvlessounon, D.; Assogba, M.; Baba-Moussa, L.; Ladekan, E.; Joachim Djimon, G. Anti-Diarrheal Activity of Mangifera indica L. (Anacardiaceae) Leaf Extracts, an Antimicrobial Plant of the Beninese Flora. Int. J. Pharm. Tech. Res. 2022, 15, 32–40. [Google Scholar]
- Adoho, A.C.; Konmy, B.B.S.; Olounladé, P.A.; Azando, E.V.; Hounzangbé-Adoté, M.S.; Gbangboché, B.A. Phytochemistry and Larval Toxicity of Ipomea asarifolia, Commelina diffusa, Acalypha ciliata and Eleusine indica against Artemia salina. Int. J. Vet. Sci. 2022, 11, 121–128. [Google Scholar]
- Agbankpe, A.; Bankolé, S.; Assogba, M.; Dougnon, V.; Yèhouénou, B.; Joachim Djimon, G.; Baba-Moussa, L. Phytochemical Screening and Cytotoxic Analysis of Three Local Vegetables Used in the Treatment of Bacterial Diarrhoea in Southern Benin (West Africa): A Comparative Study. Br. Biotechnol. J. 2015, 9, 1–13. [Google Scholar] [CrossRef]
- Agbodjento, E.; Klotoé, J.R.; Sacramento, T.I.; Dougnon, T.V.; Déguenon, E.; Agbankpé, J.; Fabiyi, K.; Assogba, P.; Hounkanrin, M.-P.; Akotegnon, R.; et al. Larval Cytotoxic and Subacute Toxicity of Gardenia ternifolia, Rourea coccinea, and Cassytha filiformis Used in Traditional Medicine of Benin (West Africa). J. Toxicol. 2020, 2020, 8843575. [Google Scholar] [CrossRef]
- Degla, L.; Olounlade, P.; Sabbas, A.; Hounzangbe-Adote, M.; Lagnika, L. Comparative Study of Acute Oral Toxicity of Essential Oils of Ocimum gratissimum, Hyptis suaveolens and Psidium guajava in Wistar Rats. Int. J. Pharmacogn. Life Sci. 2021, 2, 1–5. [Google Scholar] [CrossRef]
- Dougnon, V.; Bankole, H.S.; Edorh, P.; Dougnon, J.T.; Klotoe, J.R.; Loko, F.; Boko, M. Cytotoxicity of Leaves and Fruits of Solanum macrocarpon Linn (Solanaceae) against Shrimp Larvae (Artemia salina Leach). Res. J. Recent Sci. 2013, 2, 6–9. [Google Scholar]
- Lagnika, L.; Amoussa, A.M.; Semiatou, A.; Adjovi, Y.; Sanni, A. In Vitro Antifungal and Antioxidant Activities of Two Benin Medicinal Plants. J. Med. Plants Res. 2014, 8, 513–519. [Google Scholar]
- Ohouko, O.F.H.; Koffi, K.; Novidzro, K.; Dougnon, V.; Agbonon, A.; Tozo, K.; Dougnon, T.; Messanvi, G. Phytochemical and Toxicological Studies of Acacia nilotica and Faidherbia albida Used in West African Traditional Medicine. Int. J. Recent Sci. Res. 2020, 3, 10–18. [Google Scholar]
- Osseni, R.; Akoha, S.; Adjagba, M.; Azonbakin, S.; Lagnika, L.; Awede, B.; Bigot, A.; Diouf, A.; Darboux, R.; Lalèyè, A. In Vivo Toxicological Assessment of the Aqueous Extracts of the Leaves of Carissa edulis (Apocynaceae) in Wistar Rats. Eur. J. Med. Plants 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Boko-Haya, Y.Y.; Ouinsavi, C.; Houngbeme, A.; Gbaguidi, F. Ethnic Differences in Use, Phytochemical Screening and Non-Poisonous Leaves of Phyllanthus amarus (Schum. & Thonn.) in North of Benin. J. Ethnobiol. Tradit. Med. Photon 2016, 126, 1185–1196. [Google Scholar]
- Sènou, M.; Tchogou, A.P.; Assogba, F.; Agossadou, A.; Dougnon, T.V.; Agbangnan, D.C.P.; Lalèyè, A.; Loko, F. Study of Biological Tolerance of Aqueous Extract of Sorghum bicolor. J. Appl. Biosci. 2017, 109, 10640–10648. [Google Scholar] [CrossRef][Green Version]
- Ahomadegbe, M.A.; Ladekan, E.Y.; Togbenou, N.; Assogba, F.; Agbonon, A.; Gbenou, J. Phytochemical and Toxicity Studies of the Leaves of Mangifera indica, Cajanus cajan and of Piliostigma thonningii, Acclimated in Benin, Used against Diarrheal Disease. J. Pharmacogn. Phytochem. 2018, 7, 2971–2978. [Google Scholar]
- Lagnika, L.; Tchachedre, M.; Laleye, A.; Sanni, A. Toxicological Effect of Aqueous Extracts of Croton lobatus L. And Schrankia leptocarpa L. In Rats Model. Pharmacol. Toxicol. Res. 2016, 2, 1–8. [Google Scholar]
- Legba, B.; Dougnon, V.; Deguenon, E.; Agbankpe, J.; Senou, M.; Aniambossou, A.; Gbaguidi, C.; Sintondji, K.; Baba-Moussa, L.; Dougnon, J. Toxicological Characterization of Six Plants of the Beninese Pharmacopoeia Used in the Treatment of Salmonellosis. J. Toxicol. 2019, 2019, 3530659. [Google Scholar] [CrossRef]
- Koutchiko, S.A.H.; Attakpa Selidji, E.; Akotegnon, R.; Guinnin, F.; Senou, M.; Amoussa Abdou, M.; Lagnika, L.; Sina, H.; Yedomonhan, H.; Baba-Moussa, F.; et al. Acute Toxicity Profile of Ethanolic Extracts of Croton gratissimus Burch and Schrankia leptocarpa DC in Rats: Medicinal Plants Used in the Treatment of Arterial Hypertension in Beninese Traditional Medicine. J. Physiol. Pathophysiol. 2022, 13, 17–26. [Google Scholar] [CrossRef]
- Guinnin, F.; Klotoé, J.; Ategbo, J. Acute Toxicity Evaluation of Ethanolic Extract of Aristolochia albida Duch. Leaves on Wistar Rats Liver and Kidney Functions. Int. J. Pharm. Pharm. Sci. 2017, 9, 35–45. [Google Scholar] [CrossRef][Green Version]
- Sessou, P.; Yovo, M.; Towanou, R.; Ayihou, Y.; Komagbe, G.S.; Adinci, J.; Ayaovi Yaovi, B.; Nestor Aguidissou, O.; Senou, M.; Alain Alitonou, G.; et al. Acute Toxicity of Aeollanthus pubescens Essential Oil with High Antimicrobial Potential against Multidrug Resistant Bacteria Isolated in Poultry Farms in Benin. Res. J. Pharmacogn. 2019, 6, 17–26. [Google Scholar]
- Aivodji, N.; Ahokpe, M.; Behanzin Sezan Alphons, J.; Sezan, A. Evaluation of the Toxicity of Annona muricata Leaf Extracts on Liver and Kidney Function and Investigation of Acute and Subacute Toxicity in Wistar Rats. Int. J. PharmTech Res. 2018, 8, 1–7. [Google Scholar]
- Alowanou, G.; Olounlade, P.A.; Azando, E.V.; Dossou, T.; Dedehou, V.F.; Daga, F.D.; Adenilé, A.D.; Hounzangbé-Adoté, S.M. Acute Oral Toxicity Activity of Aqueous Extract of Combretum Glutinosum Perr. Ex De Leaves in Wistar Rats. Inter. J. Pure App. Biosci. 2015, 3, 72–78. [Google Scholar]
- Chabi, N.W.; Konfo, C.T.; Adjagba, M.; Moussedikou, L.; Ahoussi-Dahouenon, E.; Laleye, A.; Gbaguidi, C.; Soumanou, M.M. Evaluation of the Toxicity of Hemizygia bracteosa (Benth) Plant Used in Traditional Medicine for the Treatment of Diabetes Mellitus in Benin. Am. J. Biomed. Res. 2015, 3, 40–44. [Google Scholar]
- Kangbeto Bidossessi, R.; Attakpa Sèlidji, E.; Guinnin, F.; Sènou, M.; Lagnika, L. Toxicological Assessment of Ethanolic Extracts of Annona senegalensis and Trichilia prieureana in the Treatment of Type 2 Diabetes in Benin. J. Physiol. Pathophysiol. 2022, 13, 27–35. [Google Scholar] [CrossRef]
- Senou, M.; Dehou, R.; Agbogba, F.; Tchogou, P.; Abissi, Y.; Houngbeme, A.; Kpossou, G.; Anago, E.; Attakpa, E. Safety of the Aqueous Extract of the Leaves of Senna alata (L.) Roxb. (Leguminosae-Caesalpinioideae), a Plant Used in Benin to Treat Infections. J. Biosci. Med. 2022, 10, 86–95. [Google Scholar] [CrossRef]
- Koukoui, O.; Seton, S.; Betira, M.; Amagbegnon, J.B.; Sonounameto, F.; Assou, E.; Sezan, A. Evaluation of the Safety and Efficacy of Extracts from the Leaves of Five Plants Used for the Treatment of Arterial Hypertension in Benin. Pharmacol. Amp Pharm. 2022, 13, 355–367. [Google Scholar] [CrossRef]
- Koukoui, O.; Senou, M.; Agbangnan, C.P.; Seton, S.; Koumayo, F.; Azonbakin, S.; Adjagba, M.; Laleye, A.; Sezan, A. Effective in vivo cholesterol and triglycerides lowering activities of hydroethanolic extract of Launaea taraxacifolia leaves. Int. J. Pharm. Sci. Res. 2017, 3, 2040. [Google Scholar]
- Koukoui, O.; Medegan, S.; Senou, M.; Guinra, A.; Koumayo, F.; Adjagba, M.; Agbagnan, P.; Laleye, A.; Sezan, A. Effect of Tridax procumbens hydroethanolic extract on wistar rats blood glucose, total cholesterol, and triglycerides. Int. J. Pharm. Biol. Sci. 2016, 6, 42–50. [Google Scholar]
- Lokonon, J.; Felicienne, A.; Medoatinsa, E.; Abissi, G.; Atchadé Pascal, T.; Koudoro, Y.; Agbangnan, D.C.P.; Eugenie, A.; Akpovi, C.D.; Senou, M. Safety of Uvaria chamae p. beauv roots aqueous extracts in wistar rats. Int. J. Adv. Res. 2022, 10, 782–787. [Google Scholar]
- Senou, M.; Atchadé, P.T.; Dougnon, V.; Agbangnan, D.C.P.; Ogue, P.; Agossadou, A.; Laleye, A.; Loko, F.; Agbonon, A.; Sezan, A.; et al. Safety of ethyl acetate fraction of Cocos nucifera root extract. Int. J. Adv. Res. 2017, 5, 1083–1090. [Google Scholar] [CrossRef]
- Senou, M.; Lokonon, J.E.; Tchogou, P.; Abissi, Y.G.; Medoatinsa, E. Safety of aqueous extracts from the bark of Daniellia oliveri (rolfe) hutch. and Dalziel in wistar rats. Am. J. Innov. Res. Appl. Sci. 2022, 10, 782–787. [Google Scholar]
- Assogba, P.; Dougnon, V.; Hounsa, E.; Badjabaissi, P.; Tari, R.M.; Klotoe, J.R.; Bankole, H.; Diallo, A. Assessment of Larval Toxicity and the Teratogenic Effect of Three Medicinal Plants Used in the Traditional Treatment of Urinary Tract Infections in Benin. BioMed Res. Int. 2021, 2021, 1401945. [Google Scholar] [CrossRef]
- Hounsa, E.; Dougnon, T.V.; Agbankpe, A.J.; Assogba, P.; Koudokpon, C.H.; Klotoe, J.-R.; Moussa, R.T.; Agbodjento, E.; Fabiyi, K.; Deguenon, E.; et al. Fetotoxicity and Subacute Toxicity of Some Plants Involved in the Treatment of Infectious Diarrhea in Benin. Front. Trop. Dis. 2022, 3, 56. [Google Scholar] [CrossRef]
- Agbogba, F.; Senou, M.; Atchadé, P.T.; Lokonon, J.; Sacramento, T.; Medoatinsa, E.; Kanfon, R.; Atakpa, E.; Dougnon, V.; Agbangnan, D.; et al. Ethyl Acetate Fraction of Psorospermum febrifugum Spach Aqueous Extract Did Not Exhibit Acute or Sub-Chronic Toxicity. Experimental Study on Wistar Rats. Int. J. Biol. 2019, 11, 123–131. [Google Scholar] [CrossRef]
- Fandohan, P.; Gnonlonfin, B.; Laleye, A.; Gbenou, J.D.; Darboux, R.; Moudachirou, M. Toxicity and Gastric Tolerance of Essential Oils from Cymbopogon citratus, Ocimum gratissimum and Ocimum basilicum in Wistar Rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 2493–2497. [Google Scholar] [CrossRef]
- Sindete, M.; Gbankoto, A.; Osseni, R.; Tossavi, N.D.; Azonbakin, S.; Baba-Moussa, L.; Laleye, A. A 90-Day Oral Toxicity Study of an Ethanolic Root Extract of Caesalpinia bonduc (L.) Roxb. in Wistar Rats. Evid. Based Complement. Altern. Med. ECAM 2021, 2021, 6620026. [Google Scholar] [CrossRef]
- Osseni, R.; Adanle, E.; Adjagba, M.; Bonaventure, A.; Hountohotegbe, T.; Bigot, A.; Raphael, D.; Laleye, A. Modification of Biochemical and Haematological Parameters during 90-Days Subchronic Toxicity Assessment of Carissa Edulis in Wistar Rats. J. Toxicol. Environ. Health Sci. 2017, 9, 7–13. [Google Scholar] [CrossRef][Green Version]
- Sangaré, M.M.; Atègbo, J.-M.; Klotoe, J.R.; Attakpa, E.; Guinnin, F.; Issotina, A.Z.; Dramane, K.L. Phytochemical Screening and Subacute Toxicity Evaluation of Stem Leaves of Monechma depauperatum (T. Anderson) on Wistar Rats Liver and Kidney Functions. J. Pharmacogn. Phytochem. 2017, 6, 320–324. [Google Scholar]
- Dougnon, V.; Klotoé, J.R.; Sègbo, J.; Aléodjrodo, E.P.; Sodipo, O.; Dougnon, F.; Dandjesso, C.; Loko, F.; Dramane, K. Hemostatic Activity Screening and Skin Toxicity of Sap of Jatropha multifida L. (Euphorbiaceae) Used in Traditional Medicine (Benin). Asian Pac. J. Trop. Dis. 2012, 2, 927–932. [Google Scholar]
- Kpadonou, D.; Kpadonou-Kpoviessi, B.; Glinma, B.; Orou, A.-A.S.; Agbani, P.; Gbaguidi, F.; Gbenou, J.; Baba-Moussa, L.; Kpoviessi, S. Effects of the Chemical Composition of Essential Oils from Seven Plants Used in Traditional Medicine in Benin on the Growth of Eleven Pathogenic Bacteria in Antimicrobial Control. J. Pharmacogn. Phytochem. 2022, 11, 23–31. [Google Scholar] [CrossRef]
- Nabede, A.; Sina, H.; Souho, T.; Melila, M.; Bade, F.T.; Adjanohoun, A.; Ouadja, B.; Baba-Moussa, L. Physicochemical Parameters of Blighia sapida (KD Koenig) Oil Extracted in Togo. Afr. J. Biochem. Res. 2022, 16, 63–70. [Google Scholar]
- Agbangnan, C.P.; Tachon, C.; Bonin, H.; Chrostowka, A.; Fouquet, E.; Sohounhloue, D. Phytochemical Study of a Tinctorial Plant of Benin Traditional Pharmacopoeia: The Red Sorghum (Sorghum caudatum) of Benin. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2012, 13, 121–135. [Google Scholar]
- Akotegnon, R.; Michodjehoun, L.; Behanzin, J.; Houeze, E.; Sezan, A. Impact Des Extraits Éthyliques Des Feuilles de Hexalobus monopétalus Sur Le Métabolisme Protéiques Des Rats Wistar. Int. J. Multidiscip. Curr. Res. 2018, 6, 1–8. [Google Scholar]
- Koukoui, O.; Médégan, S.; Houngbèmè, A.; Mansoura, B.; Gbaguidi, F. Propriétés nutritionnelles et phytochimiques de Launaea taraxacifolia et Tridax procumbens, deux alicaments sous utilisés au Bénin. Line 2017, 5, 1025–2355. [Google Scholar]
- Betira, M.; Koukoui, O.; Houngbeme, A.; Seton, S.; Amagbegnon, J.-B.; Sonounameto, F.; Sezan, A. Combination of the Leaves of Annona muricata, Launaea taraxacifolia and Tridax procumbens: An Herbal Medicine for the Prevention and Treatment of Hypertension, Atherosclerosis and Cardiovascular Diseases. Acta Sci. Pharm. Sci. 2021, 5, 2–8. [Google Scholar]
- Dossou, A.J.; Fandohan, A.B.; Omara, T.; Gbenou, J. Traditional Knowledge and Phytochemical Screening of Plants Used in Snakebite Prevention in Benin. Bull. Natl. Res. Cent. 2022, 46, 160–170. [Google Scholar] [CrossRef]
- Kossouoh, C.; Moudachirou, M.; Adjakidje, V.; Chalchat, J.-C.; Figuérédo, G. Essential Oil Chemical Composition of Annona muricata L. Leaves from Benin. J. Essent. Oil Res. 2007, 19, 307–309. [Google Scholar] [CrossRef]
- Bonou, J.; Ahouandjinou, H.; Baba-Moussa, F.; Adéoti, Z.; Dougnon, V.; Metongnon, I.; Gbenou, J.; Toukourou, F.; Baba-Moussa, L. Assessment of the Antimicrobial Activity of Essential Oils from Some Beninese Medicinal Plants: Influence of Different Tweens. Issues Biol. Sci. Pharm. Res. 2016, 4, 43–49. [Google Scholar]
- Lagnika, C.; Amoussa, A.M.O.; Sanni, A.; Lagnika, L. Effect of Blanching and Ultrasound on Drying Time, Physicochemical and Bioactive Compounds of Dried Cashew Apple. Am. J. Food Sci. Technol. 2019, 7, 227–233. [Google Scholar]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public. Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Gaillard, J. Etat Des Lieux Du Système National de Recherche Scientifique et Technique Du Bénin; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2010. [Google Scholar]
- World Health Organization. Regional Committee for Africa. Regional Strategy for Integrated Disease Surveillance and Response—2020–2030: Report of the Secretariat; Regional Office for Africa: Addis Ababa, Ethiopia, 2019. [Google Scholar]
- Mboussou, F.; Ndumbi, P.; Ngom, R.; Kamassali, Z.; Ogundiran, O.; Van Beek, J.; Williams, G.; Okot, C.; Hamblion, E.L.; Impouma, B. Infectious Disease Outbreaks in the African Region: Overview of Events Reported to the World Health Organization in 2018. Epidemiol. Infect. 2019, 147, e299. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications-detail-redirect/9789241509763 (accessed on 11 March 2023).
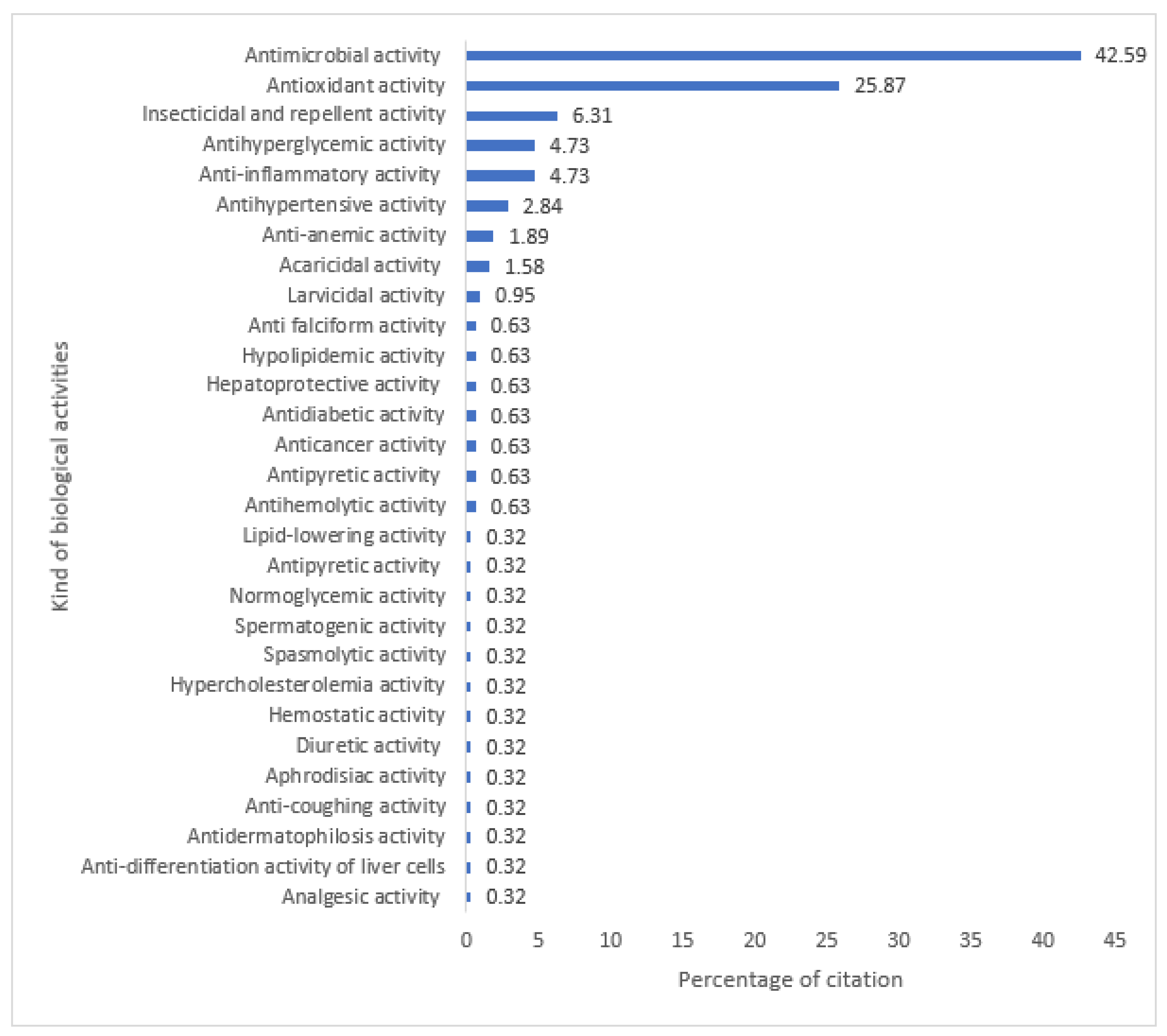


| Biological Activities | Methodology | Limitations–Prospects | Improvement of Research Practice | References |
|---|---|---|---|---|
| Antimicrobial activity |
Monitoring of the parasite load in lambs naturally and artificially infested with Haemonchus contortus and Trichostrongylus colubriformis larvae, then treated with dry leaf powders on one hand and consumption of fresh leaves of the plant on the other. | Research of the mechanism of the antibacterial effect, of the in vivo efficiency;
| Training on advanced tests to elucidate the mechanism of action of the antibacterial effect (in vitro and in vivo). Capacity building on standardised methods for elucidating the mechanism of pest control action. Capacity building on standardised methods for conducting in vivo anti-parasitic activity. Capacity building on the number of tests required to validate the anti-nematode activity of medicinal plant extracts. Capacity building on pharmacological evaluation methods. Dose selection and objective assessment of results. | [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124] |
| Acaricidal activity | In vitro test on the survival of ticks exposed to various concentrations of an extract. | [125,126] | ||
| Antioxidant activity | In vitro test, mostly using the DPPH method. | Research on the mechanism of the antioxidant effect, in vivo antioxidant effect. Failure to respect the number of tests required to validate the antioxidant activity. | Capacity building on the number of tests required to validate the antioxidant activity of medicinal plant extracts, their implementation, and the procedures for the in vivo exploration of the antioxidant effect with the key to the mode of action of this effect. | [11,13,14,15,19,20,34,35,37,39,43,53,64,70,71,73,74,88,88,91,103,105,106,110,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,156,157,158,159,160] |
| Anti-inflammatory activity | In vitro tests for the inhibition of enzymatic activity are the most widely used. In vivo test on rats by the administration of an extract, followed by the determination of the white blood cell count. | Research of the in vivo anti-inflammatory effect. No assay of inflammation markers. | Capacity building on the research of the in vivo mechanism of action of the anti-inflammatory effect of extracts. Capacity building on in vivo testing for inflammation markers. | [15,106,138,149,161,162,163,164,165,166] |
| Insecticidal and repellent activity | Multi-dose immersion test with increasing progression. Application of leaf powder directly on the larvae and spraying solutions with a hand sprayer in the boxes (in the laboratory). | Lack of justification for the infesting dose. No precision on the volume of extract sprayed. | Capacity building on the conduct of field and laboratory testing of insecticidal activity. | [167,168,169,170] |
| Spraying extracts on plant organs (field experimentation). | No details on the volume of extract sprayed. | Capacity building on the conduct of field and laboratory testing of insecticidal activity. | [170,171] | |
| Antihypertensive activity | In vivo test on Wistar rats with the exploration of few relevant parameters. | Advanced study with the consideration of several parameters to elucidate the mechanism of action. | Capacity building on adapted models to elucidate the mechanism of action. | [172,173,174,175,176,177,178,179] |
| Antihemolytic activities | In vitro assay on erythrocytes | . | [180,181] | |
| Antihyperglycemic activity | In vivo test on Wistar rats, followed by blood glucose determination. In-vivo testing on rats by the induction of metabolic disorder and the administration of extract concentrations and an evaluation of the effects. | Advanced study via more adapted models to elucidate the anti-diabetic effect of medicinal plants. Use of old model methods at the expense of more advanced methods. | Capacity building on advanced studies using more suitable models to elucidate the anti-diabetic effect of medicinal plants Capacity building on the design and execution of modern research protocols in pharmacology. | [146,182,183,184,185,186,187,188,189,190,191,192,193,194] |
| Analgesic activity | In vivo test on Wistar rats using the tail immersion method. | Advanced study via more adapted models. | Training on advanced tests to explore the analgesic effect. | [162] |
| Anti-differentiation or anticancer activity of liver cells | In vivo test on Wistar rats with methodological deficiencies. | Advanced study via more adapted models. | Training on advanced tests to explore the anticancer effect of medicinal plants. | [195,196,197,198] |
| Anti-anemic activity |
| Lack of precision on the justification of the doses used. | Capacity building on the design and execution of pharmacology research protocol. | [199,200,201,202,203] |
| Anti-dermatophilosis activity | Skin test on cattle with dermatophilosis and scabby lesions. | [204] | ||
| Anti-diabetic activity | Diabetes induction model with streptozotocin. | Advanced study on the research of the mechanism of action of the antidiabetic effect. | Capacity building on advanced studies to investigate the mechanism of action of the anti-diabetic effect. | [110,161,205,206,207,208] |
| In vivo test on normal rats without diabetes induction. | The experimental design is not consistent with the anti-diabetic effect being explored. | Capacity building on appropriate tests for diabetes induction and the exploration of the antidiabetic effects of plant extracts. | [209,210] | |
| Insecticidal and repellent activity | Test on the pest D. porcellus. | [211] | ||
| Larvicidal activity | In vitro test on larvae. | [212,213,214] | ||
| Anti-coughing activity | In vivo test on guinea pigs using the citric acid method. | [215] | ||
| Aphrodisiac activity | In vivo test with exploration of the parameters related to the aphrodisiac effect. | [216,217,218] | ||
| Diuretic activity |
| Advanced study on the most suitable models. | Training on advanced study via more suitable models. | [219,220] |
| Hemostatic activity | In vitro (anti-coagulation) and in vivo test to stop bleeding. | [221,222,223,224] | ||
| Hepatoprotective activity | In vivo test on Wistar rats after the induction of liver damage, followed by an evaluation of enzymatic and histological parameters. | Advanced study on adapted models allowing the elucidation of the mechanism of action. No assay of inflammation markers. | Training on advanced study via more suitable models. Capacity building on in-vivo tests for inflammation markers. | [194,225,226] |
| Hypercholesterolemia activity | Induction of hypercholesterolemia, followed by measurement of blood parameters. | Advanced study on the elucidation of the mechanism of action. | [227] | |
| Spasmolytic activity | Tested on isolated rat trachea in combination with an aqueous extract of Afromomum melegueta. | [228] | ||
| Spermatogenic activity | In vivo test on Wistar Rats, followed by assessment of reproductive parameters. | Advanced study on other models allowing the elucidation of the mechanism of action. | Training on advanced study via other models to elucidate the mechanism of action. | [229,230] |
| Normoglycemic activities | In vivo test on Wistar rats, followed by blood glucose determination. | Advanced study via more adapted models to elucidate the anti-diabetic effect of medicinal plants. | Capacity building on advanced studies using more suitable models to elucidate the anti-diabetic effect of medicinal plants. | [182] |
| Lipid-lowering activity | In-vivo test on rats by forced administration of 2 ml of pork fat and sugar water. | More suitable models should be considered. | Capacity building on the design and execution of the research protocol in pharmacology. | [231] |
| Antipyretic activity | In vivo test on Wistar Rats. | Advanced study on antipyretic properties. | Advanced study training via other models. | [162,200] |
| Antidiarrheal activity | In vivo test on Wistar Rats. | Advanced study on antidiarheal properties. | Advanced study training via other models. | [232] |
| Anticonvulsiant activity | In vivo test. | [152] |
| Toxicological Study | Methodology | Limitations—Prospects | Improvement of Research Practices | References |
|---|---|---|---|---|
| Larval cytotoxicity | In vitro test on the survival of Artemia salina larvae exposed to various concentrations of the extract. | Subjective assessment of the results. In vivo toxicological, cellular, and genotoxicity studies; research on heavy metals. | Capacity building on advanced in vivo, cellular and genotoxicity toxicology tests; heavy metals research. | [3,19,23,28,29,39,42,77,78,91,96,98,129,132,138,140,142,174,190,219,233,234,235,236,237,238,239,240,241,242] |
| Acute oral toxicity | In vivo single-dose test, followed by an exploration of clinical signs of toxicity after 14 days in accordance with OECD 413. | Advanced toxicity tests: cellular and genotoxicity; heavy metals research. The standard used does not correspond to the acute oral toxicity test. | Capacity building on advanced in vivo, cellular and genotoxicity toxicology tests; heavy metals research Training on the implementation of protocol 423 which is adapted to the acute oral toxicity test. | [3,44,53,63,113,146,182,189,200,236,240,243,244,245,246,247,248,249,250,251,252] |
| Cellular toxicity test | HepG2 cell test. | No limit noted. | [159] | |
| Acute toxicity test with 423 OECD | In vivo single-dose test followed by investigation of clinical signs of toxicity after 14 days in accordance with OECD 423. | No limit noted. | [191,192,209,253,254,255,256,257,258,259] | |
| Foetotoxicity | Test investigating the effect of plant extracts on the embryonic development of chicks. | Advanced toxicity tests: cellular and genotoxicity; heavy metals research. | Capacity building on advanced in vivo, cellular and genotoxicity toxicology tests; heavy metals research. | [260,261] |
| Sub-acute toxicity | In vivo test on rats, not according to the 407 OECD. | Non-compliance with the required standard in relation to chronic toxicity. Training on toxicity tests according to the required standards. | Capacity building on advanced in vivo, cellular and genotoxicity toxicology tests; heavy metals research. | [191,192,235,253,254,258,259,262,263,264,265,266] |
| Skin toxicity | Skin testing on Wistar rats. | Advanced toxicity tests: cellular and genotoxicity; heavy metals research. | Capacity building on advanced in vivo, cellular and genotoxicity toxicology tests; heavy metals research. | [267] |
| Cytotoxicity on human and animal cells | Test on human and animals cells. | [268] | ||
| Cytotoxicity in WI38 non-cancerous fibroblast cells using the MTT assay. | No limit. | Training on standards-based toxicity testing. | [118] |
| Phytochemical Study | Methodology | Limitations-Prospects | Improvement of Research Practices | References |
|---|---|---|---|---|
| Qualitative screening by staining and precipitation | Detection of the presence of major phytochemical groups, using the precipitation and staining method. | Subjective assessment of the results. Perspective of phytochemical study aiming at the identification and characterization of bioactive compounds of plant extracts. | Training on the use of chromatographic and spectroscopic methods for the characterization of bioactive compounds in plant extracts. | [13,19,20,23,28,29,38,43,44,53,61,67,70,72,74,76,77,78,82,89,90,91,92,93,103,104,105,106,107,108,109,110,111,120,125,132,133,141,142,145,146,148,149,150,151,152,153,157,160,163,172,176,182,187,188,190,192,193,200,220,221,233,234,239,241,243,252,266,269,270,271,272,273,274] |
| Screening for molecular identification of compounds | Identification of compounds by GC and GC/MS. | [73,96,129,156,163,268,275,276] | ||
| Qualitative screening by thin-layer chromatography | Detection of the presence of major phytochemical groups. | Lack of precision in the recognition of certain phytochemical groups. Prospects for phytochemical studies aimed at the identification and characterization of bioactive compounds in plant extracts. | Training on the use of chromatographic and spectroscopic methods for the characterization of bioactive compounds. | [71,156,173,270] |
| Screening for HPLC identification | Identification of molecules using HPLC. | [40,148,155] | ||
| Quantitative screening of polyphenols or other secondary metabolites | Spectrophotometric method. | Perspective of phytochemical study aiming at the identification and characterization of bioactive compounds of plant extracts. | Training on the use of chromatographic and spectroscopic methods for the characterization of bioactive compounds in plant extracts. | [42,78,92,93,103,105,106,108,109,118,132,137,144,145,146,148,151,154,158,277] |
| Total Number of Articles Included in This Study | 272 | |
|---|---|---|
| Year range | 2001–2022 | |
| Total number of plants | 269 | |
| Total number of articles per University | University of Abomey-Calavi | 218 |
| National University of Science, Technology, Engineering and Mathematics of Abomey | 35 | |
| University of Parakou | 7 | |
| National University of Agriculture | 12 | |
| Number of publications by language | English | 251 |
| French | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbodjento, E.; Lègba, B.; Dougnon, V.T.; Klotoé, J.R.; Déguénon, E.; Assogba, P.; Koudokpon, H.; Hanski, L.; Baba-Moussa, L.; Yayi Ladékan, E. Unleashing the Potential of Medicinal Plants in Benin: Assessing the Status of Research and the Need for Enhanced Practices. Plants 2023, 12, 1506. https://doi.org/10.3390/plants12071506
Agbodjento E, Lègba B, Dougnon VT, Klotoé JR, Déguénon E, Assogba P, Koudokpon H, Hanski L, Baba-Moussa L, Yayi Ladékan E. Unleashing the Potential of Medicinal Plants in Benin: Assessing the Status of Research and the Need for Enhanced Practices. Plants. 2023; 12(7):1506. https://doi.org/10.3390/plants12071506
Chicago/Turabian StyleAgbodjento, Eric, Boris Lègba, Victorien Tamègnon Dougnon, Jean Robert Klotoé, Esther Déguénon, Phénix Assogba, Hornel Koudokpon, Leena Hanski, Lamine Baba-Moussa, and Eléonore Yayi Ladékan. 2023. "Unleashing the Potential of Medicinal Plants in Benin: Assessing the Status of Research and the Need for Enhanced Practices" Plants 12, no. 7: 1506. https://doi.org/10.3390/plants12071506
APA StyleAgbodjento, E., Lègba, B., Dougnon, V. T., Klotoé, J. R., Déguénon, E., Assogba, P., Koudokpon, H., Hanski, L., Baba-Moussa, L., & Yayi Ladékan, E. (2023). Unleashing the Potential of Medicinal Plants in Benin: Assessing the Status of Research and the Need for Enhanced Practices. Plants, 12(7), 1506. https://doi.org/10.3390/plants12071506






