Wrack Burial Limits Germination and Establishment of Yellow Flag Iris (Iris pseudacorus L.)
Abstract
:1. Introduction
2. Results
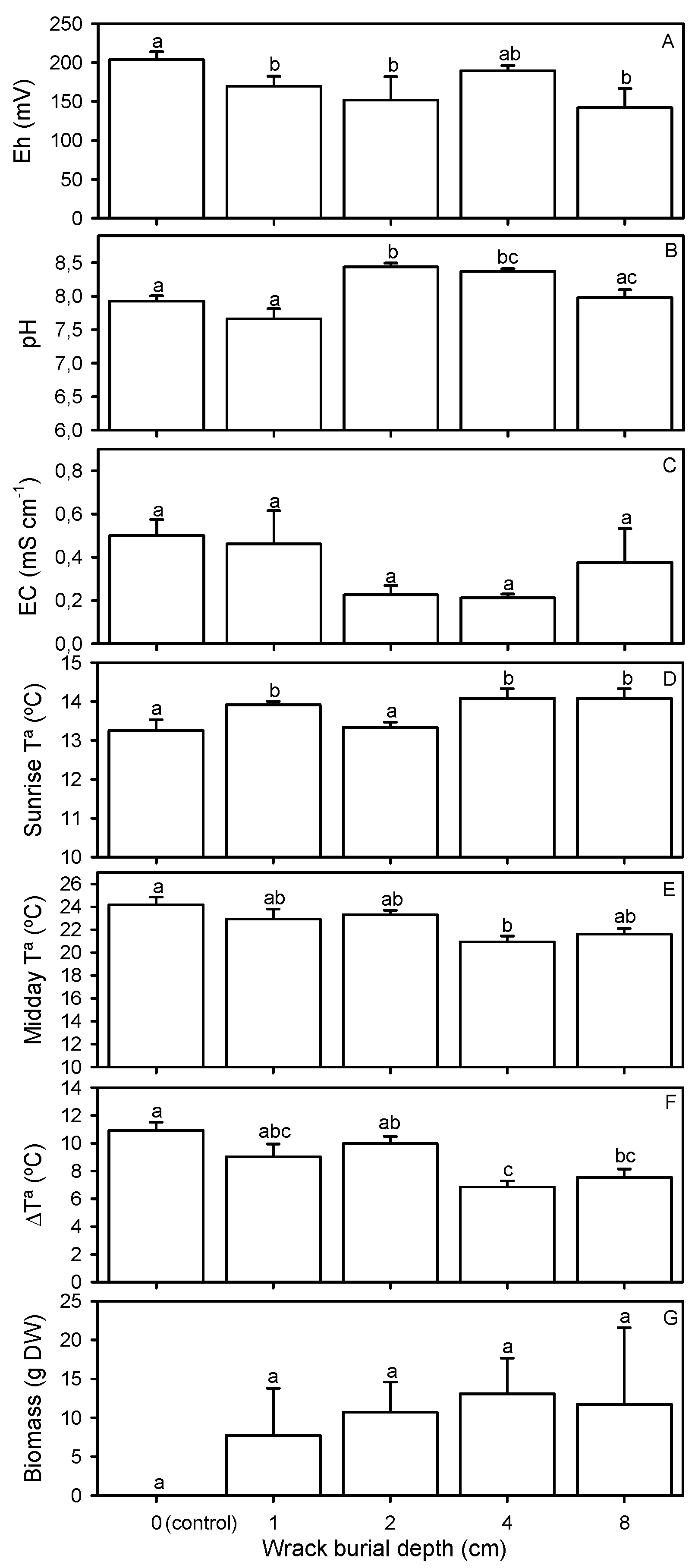
2.1. Environmental Conditions
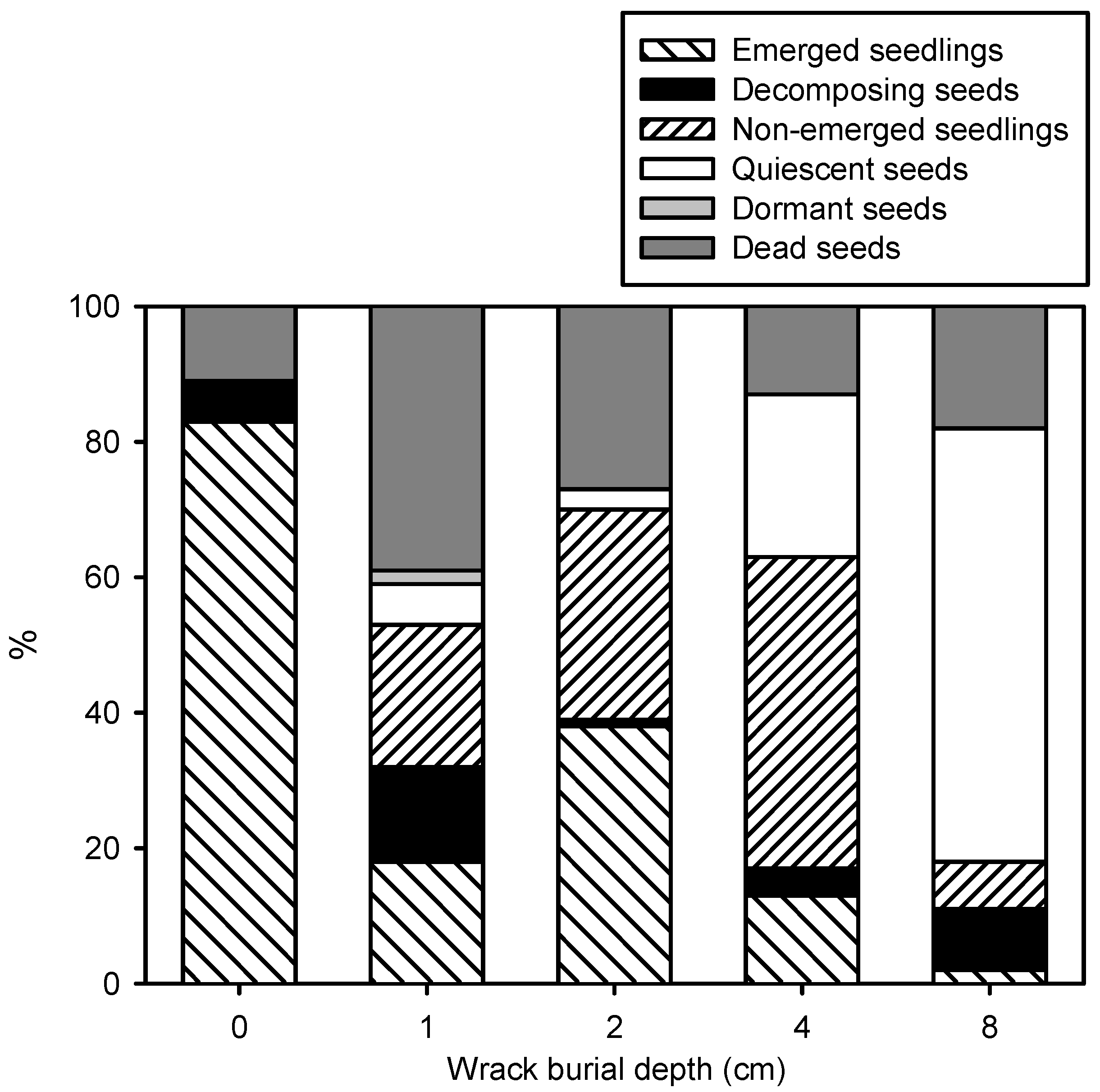
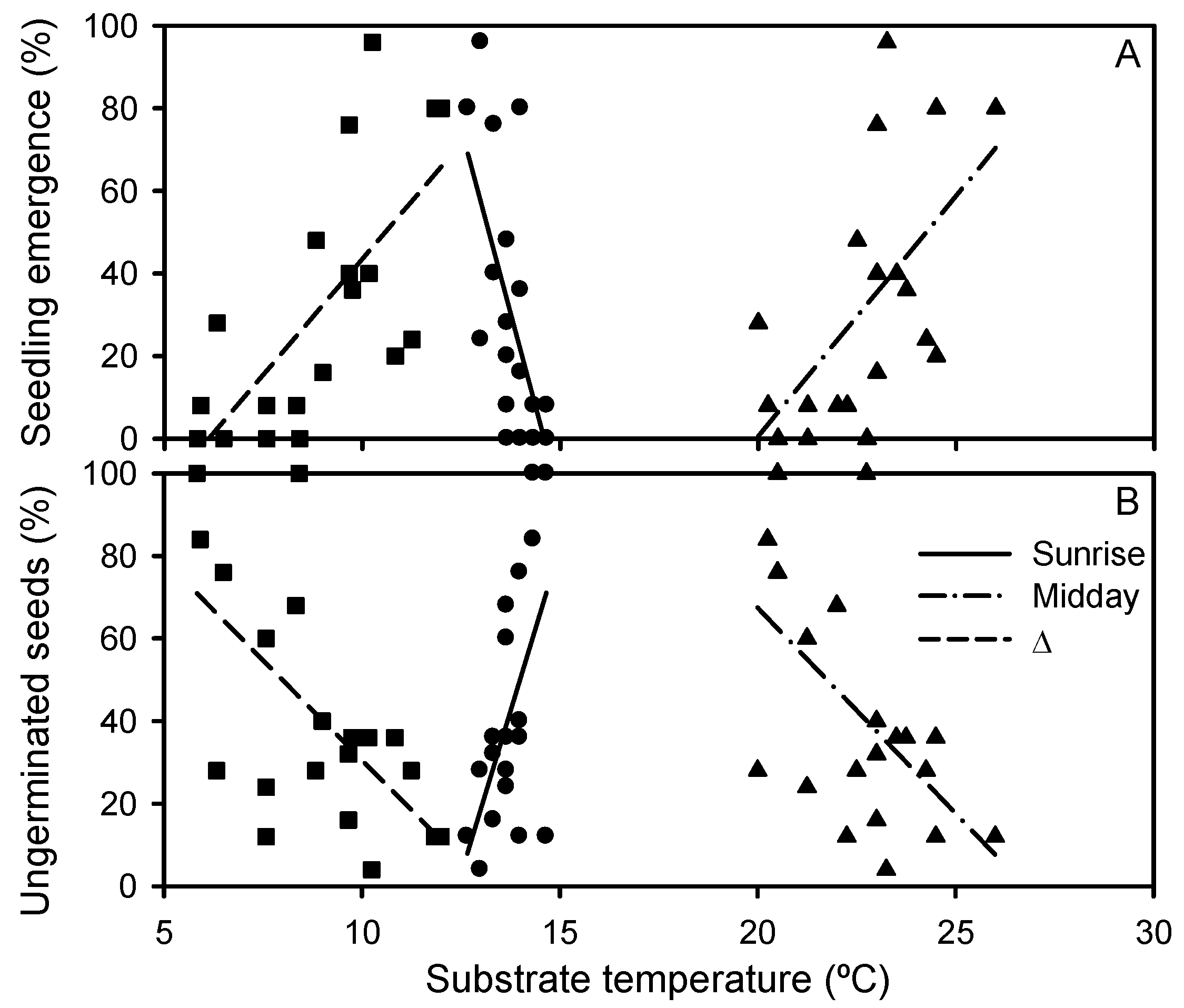
2.2. Plant Traits
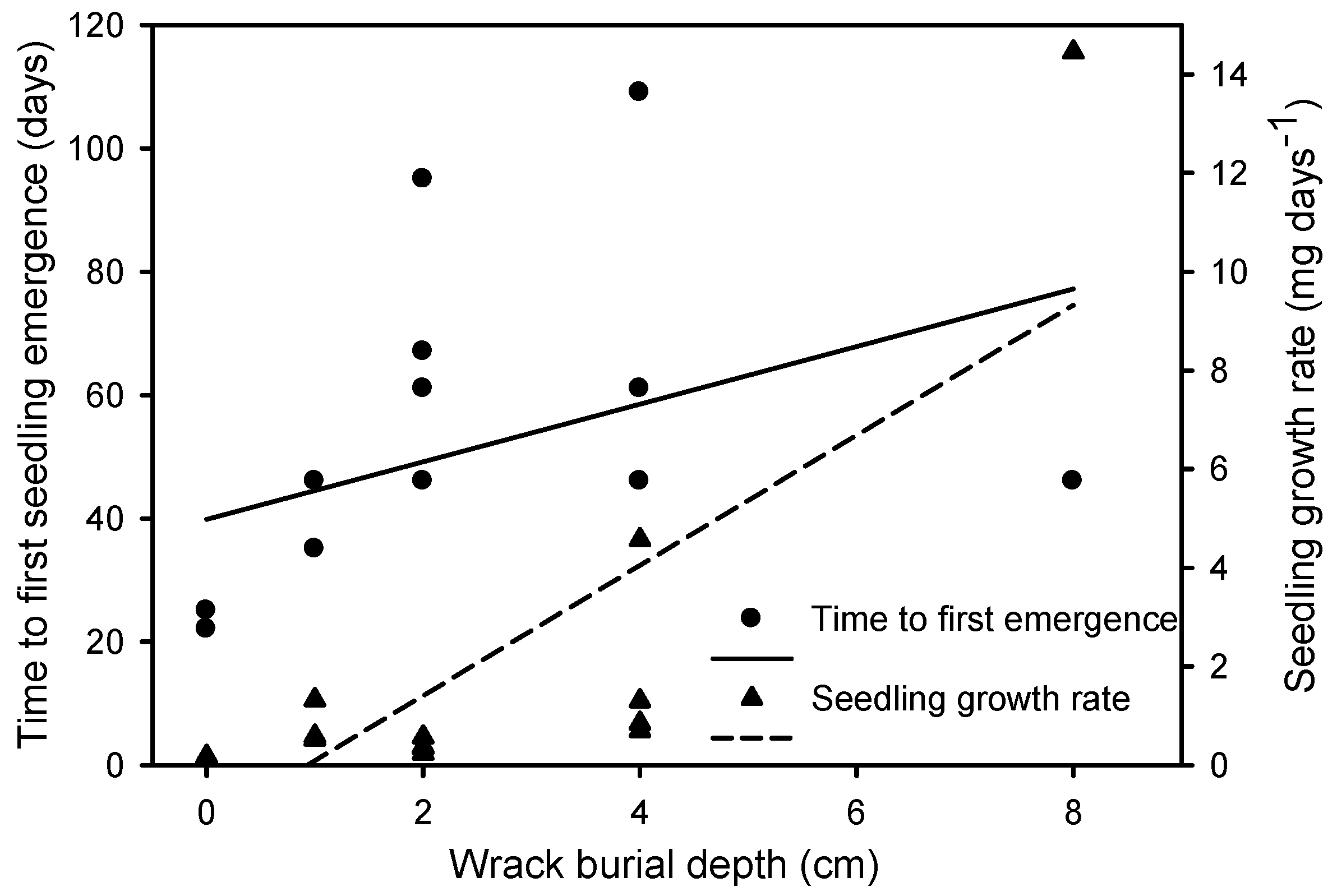
2.3. Recovery Experiment
3. Materials and Methods
3.1. Seed and Wrack Material
3.2. Burial Experiment
3.3. Environmental Conditions
3.4. Plant Traits
3.5. Recovery Experiment
3.6. Statistical Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutterman, Y. Strategies of seed dispersal and germination in plants inhabiting deserts. Bot. Rev. 1994, 60, 373–425. [Google Scholar] [CrossRef]
- Rees, M.; Condit, R.; Crawley, M.; Pacala, S.; Tilman, D. Long-term studies of vegetation dynamics. Science 2001, 293, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.F.; Deng, Z.W.; An, S.Q.; Wang, Z.S.; Liu, Y.H.; Yan, O.Y.; Zhou, C.F.; Zhi, Y.B.; Li, H.L. Habitat choice and seed-seedling conflict of Spartina alterniflora on the coast of China. Hydrobiologia 2009, 630, 287–297. [Google Scholar] [CrossRef]
- Sonkoly, J.; Valkó, O.; Balogh, N.; Godó, L.; Kelemen, A.; Kiss, R.; Miglécz, T.; Tóth, E.; Tóth, K.; Tóthmérész, B.; et al. Germination response of invasive plants to soil burial depth and litter accumulation is species-specific. J. Veg. Sci. 2020, 31, 1079–1087. [Google Scholar] [CrossRef]
- Panetta, F.D.; Timmins, S. Evaluating the feasibility of eradication for terrestrial weed incursions. Plant Prot. Q. 2004, 19, 5–11. [Google Scholar]
- Grewell, B.J.; Gillard, M.B.; Futrell, C.J.; Castillo, J.M. Seedling Emergence from Seed Banks in Ludwigia hexapetala-Invaded Wetlands: Implications for Restoration. Plants 2019, 8, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.M.; Rubio-Casal, A.E.; De Cires, A.; Figueroa, E.M.; Pickart, A.J.; Castillo, J.M. Burial effects on seed germination and seedling emergence of two halophytes of contrasting seed size. Plant Ecol. Divers. 2020, 13, 339–349. [Google Scholar] [CrossRef]
- Huss, J.C.; Gierlinger, N. Functional packaging of seeds. New Phytol. 2021, 230, 2154–2163. [Google Scholar] [CrossRef]
- Facelli, J.M.; Pickett, S.T. Plant litter: Its dynamics and effects on plant community structure. Bot. Rev. 1991, 57, 1–32. [Google Scholar] [CrossRef]
- Zhang, J.; Maun, M.A. Potential for seed bank formation in seven Great Lakes sand dune species. Am. J. Bot. 1994, 81, 387–394. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Dong, M.; Huang, Z.Y. Effects of sand burial and seed size on seed germination and seedling emergence of Psammochloa villosa. Chin. J. Plant Ecol. 2005, 5, 730–739. [Google Scholar]
- Zhang, J.; Maun, M.A. Sand burial effects on seed germination, seedling emergence and establishment of Panicum virgatum. Holaractic Ecol. 1990, 13, 56–61. [Google Scholar] [CrossRef]
- Tobe, K.; Zhang, L.; Omasa, K. Seed germination and seedling emergence of three annuals growing on desert sand dunes in China. Ann. Bot. 2005, 95, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Egawa, C.; Tsuyuzaki, S. The effects of litter accumulation through succession on seed bank formation for small- and large-seeded species. J. Veg. Sci. 2013, 24, 1062–1073. [Google Scholar] [CrossRef]
- van Egmond, E.M.; van Bodegon, P.M.; van Hal, J.R.; van Logtestijn, R.S.P.; Broekman, R.A.; Berg, M.P.; Aerts, R. Growth of pioneer beach plants is strongly driven by buried macroalgal wrack, whereas macroinvertebrates affect plant nutrient dynamics. J. Exp. Mar. Biol. Ecol. 2019, 514, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Brewer, J.S.; Levine, J.M.; Bertness, M.D. Interactive effects of elevation and burial with wrack on plant community structure in some Rhode Island salt marshes. J. Ecol. 1998, 86, 125–136. [Google Scholar] [CrossRef]
- Tate, A.S.; Battaglia, L.L. Community disassembly and reassembly following experimental storm surge and wrack application. J. Veg. Sci. 2013, 24, 46–57. [Google Scholar] [CrossRef]
- Platt, W.J.; Joseph, D.; Ellair, D.P. Hurricane wrack generates landscape-level heterogeneity in coastal pine savanna. Ecography 2015, 38, 63–73. [Google Scholar] [CrossRef]
- Windham, L. Comparison of biomass production and decomposition between Phragmites australis (common reed) and Spartina patens (salt hay grass) in brackish tidal marshes of New Jersey, USA. Wetlands 2001, 21, 179–188. [Google Scholar] [CrossRef]
- Adam, P. Saltmarsh Ecology; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Huiskes, A.H.L.; Koutstaal, B.P.; Herman, P.M.J.; Beeftink, W.G.; Markusse, M.M.; de Munck, W. Seed dispersal of halophytes in tidal salt marshes. J. Ecol. 1995, 83, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Morzaria-Luna, H.N.; Zedler, J.B. Does seed availability limit plant establishment during salt marsh restoration? Estuaries Coast 2007, 30, 12–25. [Google Scholar] [CrossRef]
- Rabinowitz, T.R.M.; Freene, L.; Glogowski, A.D.; Bowron, T.; van Proosdij, D.; Lundholm, J.T. Hitchhiking halophytes in wrack and sediment-laden ice blocks contribute to tidal marsh development in the Upper Bay of Fundy. Wetl. Ecol. Manag. 2022, 30, 375–388. [Google Scholar] [CrossRef]
- Pennings, S.C.; Richards, C.L. Effects of wrack burial in salt-stressed habitats: Batis maritima in a southwest Atlantic salt marsh. Ecography 1998, 21, 630–638. [Google Scholar] [CrossRef]
- Minchinton, T.E.; Bertness, M.D. Disturbance by wrack facilitates spread of Phragmites australis in a coastal marsh. J. Exp. Mar. Biol. Ecol. 2002, 281, 89–107. [Google Scholar] [CrossRef]
- Elmore, A.J.; Engelhardt, K.A.M.; Cadol, D.; Palinkas, C.M. Spatial patterns of plant litter in a tidal freshwater marsh and implications for marsh persistence. Ecol. Appl. 2016, 26, 846–860. [Google Scholar] [CrossRef]
- Reidenbaugh, T.G.; Banta, W.C. Origin and effects of tidal wrack in a Virginia salt marsh. Gulf Res. Rep. 1980, 6, 393–401. [Google Scholar]
- Bertness, M.D.; Ellison, A.M. Determinants of pattern in a New England salt marsh plant community. Ecol. Monogr. 1987, 57, 129–147. [Google Scholar] [CrossRef]
- Valiela, I.; Rietsma, C.S. Disturbance of salt marsh vegetation by wrack mats in Great Sippewissett marsh. Oecologia 1995, 102, 106–112. [Google Scholar] [CrossRef]
- Tolley, P.M.; Christian, R.R. Effects of increased inundation and wrack deposition on a high salt marsh plant community. Estuaries 1999, 22, 944–954. [Google Scholar] [CrossRef]
- Ungar, I.A. Seed germination and seed-bank ecology in halophytes. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 529–544. [Google Scholar]
- Hartman, J.; Caswell, H.; Valiela, I. Effects of wrack accumulation on salt marsh vegetation. In Proceedings of the 17th European Marine Biology Symposium; Cabioch, C., Glemaree, M., Samain, J., Eds.; Olsen and Olsen: Fredensborg, Denmark, 1983; pp. 99–102. [Google Scholar]
- Encyclopedia of Life. Yellow Flag, Iris psuedacorus L. Available online: http://eol.org (accessed on 17 March 2023).
- Hayasaka, D.; Fujiwara, S.; Uchida, T. Impacts of invasive Iris pseudacorus L. (yellow flag) establishing in an abandoned urban pond on native semi-wetland vegetation. J. Integr. Agric. 2018, 17, 1881–1887. [Google Scholar] [CrossRef] [Green Version]
- Mopper, S.; Wiens, K.C.; Goranova, G.A. Competition, salinity, and clonal growth in native and introduced irises. Am. J. Bot. 2016, 103, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Gervazoni, P.; Sosa, A.; Franceschini, C.; Coetzee, J.; Faltlhauser, A.; Fuentes-Rodriguez, D.; Martínez, A.; Hill, M. The alien invasive yellow flag (Iris pseudacorus L.) in Argentinian wetlands: Assessing geographical distribution through different data sources. Biol. Inv. 2020, 22, 3183–3193. [Google Scholar] [CrossRef]
- Gaskin, J.F.; Pokorny, M.L.; Mangold, J.M. An unusual case of seed dispersal in an invasive aquatic; yellow flag iris (Iris pseudacorus). Biol. Inv. 2016, 18, 2067–2075. [Google Scholar] [CrossRef]
- Gillard, M.B.; Castillo, J.M.; Mesgaran, M.B.; Futrell, C.J.; Grewell, B.J. High aqueous salinity does not preclude germination of invasive Iris pseudacorus from estuarine populations. Ecosphere 2021, 12, e03486. [Google Scholar] [CrossRef]
- Gillard, M.B.; Castillo, J.M.; Mesgaran, M.B.; Futrell, C.J.; Grewell, B.J. Germination niche breadth of invasive Iris pseudacorus (L.) suggests continued recruitment from seeds with global warming. Am. J. Bot. 2022, 109, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Alexeeva, N.B.; Boltenkov, E.V.; Mironova, L.N. Some peculiarities of seed morphology of the Iris (Iridaceae) species from the Russian Far East. Bot. Zhurn. 2011, 96, 851–857. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Forcella, F.; Benech Arnold, R.L.; Sanchez, R.; Ghersa, C.M. Modeling seedling emergence. Field Crops Res. 2000, 67, 123–139. [Google Scholar] [CrossRef]
- Maun, M.A.; Riach, S. Morphology of caryopses, seedlings and seedling emergence of the grass Calamovilfa longifolia from various depths in sand. Oecologia 1981, 49, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Baskin, J.M.; Baskin, C.C.; Bu, H.Y.; Du, G.Z.; Ma, M. Effect of diurnal fluctuating versus constant temperatures on germination of 445 species from the Eastern Tibet Plateau. PLoS ONE 2013, 8, e69364. [Google Scholar] [CrossRef] [Green Version]
- AEMET. Available online: https://opendata.aemet.es/centrodedescargas/inicio (accessed on 17 March 2023).
- Baskin, J.M.; Baskin, C.C. The annual dormancy cycle in buried weed seeds: A continuum. Bioscience 1985, 35, 492–498. [Google Scholar] [CrossRef]
- Harper, J.L.; Benton, R.A. The behaviour of seeds in soil. The germination of seeds on the surface of a water supplying substrate. J. Ecol. 1966, 54, 151–156. [Google Scholar] [CrossRef]
- Wijte, A.H.; Gallagher, J.L. Effect of oxygen availability and salinity on early life history stages of salt marsh plants. II. Early seedling development advantage of Spartina alterniflora over Phragmites australis (Poaceae). Am. J. Bot. 1996, 83, 1343–1350. [Google Scholar] [CrossRef]
- Sutherland, W.J. Iris pseudacorus L. J. Ecol. 1990, 78, 833–848. [Google Scholar] [CrossRef]
- Castillo, J.M.; Abbas, A.; Rubio-Casal, A.E.; de Cires, A.; Figueroa, E.; Nieva, F.J.J. Wrack burial reduces germination and establishment of the invasive cordgrass Spartina densiflora. NeoBiota 2014, 21, 65–79. [Google Scholar] [CrossRef]
- Guan, B.; Zhang, L.; Li, M.; Zhang, H.; Zhang, X.; Han, G.; Yu, J. The sediment burial depth and salinity control the early developments of Suaeda salsa in the Yellow River Delta. Nord. J. Bot. 2021, 39, e02956. [Google Scholar] [CrossRef]
- Orth, R.J.; Harwell, M.C.; Bailey, E.M.; Bartholomew, A.; Jawad, J.T.; Lombana, A.V.; Moore, K.A.; Rhode, J.M.; Woods, H.E. A review of issues in seagrass seed dormancy and germination: Implications for conservation and restoration. Mar. Ecol. Prog. Ser. 2000, 200, 227–288. [Google Scholar] [CrossRef] [Green Version]
- Benvenuti, S.; Macchia, M.; Miele, S. Light, temperature and burial depth effects on Rumex obtusifolius seed germination and emergence. Weed Res. 2000, 41, 177–188. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B. Some ecophysiological aspects of seed germination in halophytes. In Halophytes Utilization and Regional Sustainable Development of Agriculture; Liu, X., Liu, M., Eds.; Meteorological Press of China: Beijing, China, 2002; pp. 59–68. [Google Scholar]
- Reza, E.; Hendrickx, F.; Maelfait, J.P.; Hoffman, M. The effect of successional stage and salinity on the vertical distribution of seeds in salt marsh soils. Flora 2010, 205, 442–448. [Google Scholar]
- Polo-Ávila, A.; Infante-Izquierdo, M.D.; Soto, J.M.; Hermoso-López, V.; Nieva, F.J.J.; Castillo, J.M.; Muñoz-Rodríguez, A.F. Contrasting propagule dispersal and halophyte seed banks along the intertidal gradient. Mar. Ecol. Prog. Ser. 2019, 616, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Maun, M.A. Adaptations of plants to burial in coastal sand dunes. Canad. J. Bot. 1998, 76, 713–738. [Google Scholar] [CrossRef]
- Parker, V.T.; Simpson, R.L.; Leck, M.A. Pattern and process in the dynamics of seed banks. In Ecology of Soil Seed Banks; Leck, M.A., Parker., V.T., Simpson, R.L., Eds.; Academic Press Inc.: San Diego, CA, USA, 1989; pp. 367–384. [Google Scholar]
- Gallego-Tévar, B.; Grewell, B.J.; Whitcraft, C.R.; Futrell, J.C.; Bárcenas-Moreno, G.; Castillo, J.M. Contrasted impacts of Yellow Flag Iris (Iris pseudacorus) on plant diversity in tidal wetlands within Its native and invaded distribution ranges. Diversity 2022, 14, 326. [Google Scholar] [CrossRef]
| LM or GLM (d.f. = 4) | |
|---|---|
| Environmental variables | |
| Substrate redox potential (mV) | χ2 = 10387.300, p < 0.0001 |
| Substrate pH | F = 11.427, p < 0.0001 |
| Substrate electrical conductivity (mS cm−1) | F = 1.323, p = 0.306 |
| Substrate temperature at sunrise (°C) | F = 3.943, p = 0.022 |
| Substrate temperature at midday (°C) | F = 4.473, p = 0.014 |
| Daily temperature variation in the substrate (°C) | F = 7.236, p = 0.002 |
| Biomass of plant species (g) (transformed by √x) | F = 2.114, p = 0.130 |
| Plant traits | |
| Seedling emergence (%) | F = 39.703, p < 0.0001 |
| Time to first seedling emergence (days) | F = 3.558, p = 0.031 |
| Seedling growth rate (mg day−1) | χ2 = 277.010, p < 0.0001 |
| Decomposing seeds (%) | F = 1.137, p = 0.377 |
| Germinated and non-emerged seeds (%) | F = 5.522, p = 0.006 |
| Non-decomposing and ungerminated seeds (%) | F = 7.233, p = 0.002 |
| Quiescent seeds (%) | χ2 = 39.187, p < 0.0001 |
| Dormant seeds (%) | χ2 = 16.000, p < 0.003 |
| Dead seeds (%) | F = 2.839, p = 0.062 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, J.M.; Gallego-Tévar, B.; Grewell, B.J. Wrack Burial Limits Germination and Establishment of Yellow Flag Iris (Iris pseudacorus L.). Plants 2023, 12, 1510. https://doi.org/10.3390/plants12071510
Castillo JM, Gallego-Tévar B, Grewell BJ. Wrack Burial Limits Germination and Establishment of Yellow Flag Iris (Iris pseudacorus L.). Plants. 2023; 12(7):1510. https://doi.org/10.3390/plants12071510
Chicago/Turabian StyleCastillo, Jesús M., Blanca Gallego-Tévar, and Brenda J. Grewell. 2023. "Wrack Burial Limits Germination and Establishment of Yellow Flag Iris (Iris pseudacorus L.)" Plants 12, no. 7: 1510. https://doi.org/10.3390/plants12071510
APA StyleCastillo, J. M., Gallego-Tévar, B., & Grewell, B. J. (2023). Wrack Burial Limits Germination and Establishment of Yellow Flag Iris (Iris pseudacorus L.). Plants, 12(7), 1510. https://doi.org/10.3390/plants12071510







