Effects of Flavonoids and Phenols from Moringa oleifera Leaf Extracts on Biofilm Processes in Xanthomonas campestris pv. campestris
Abstract
:1. Introduction
2. Results
2.1. Effects of Single Phenols on Membrane Permeability
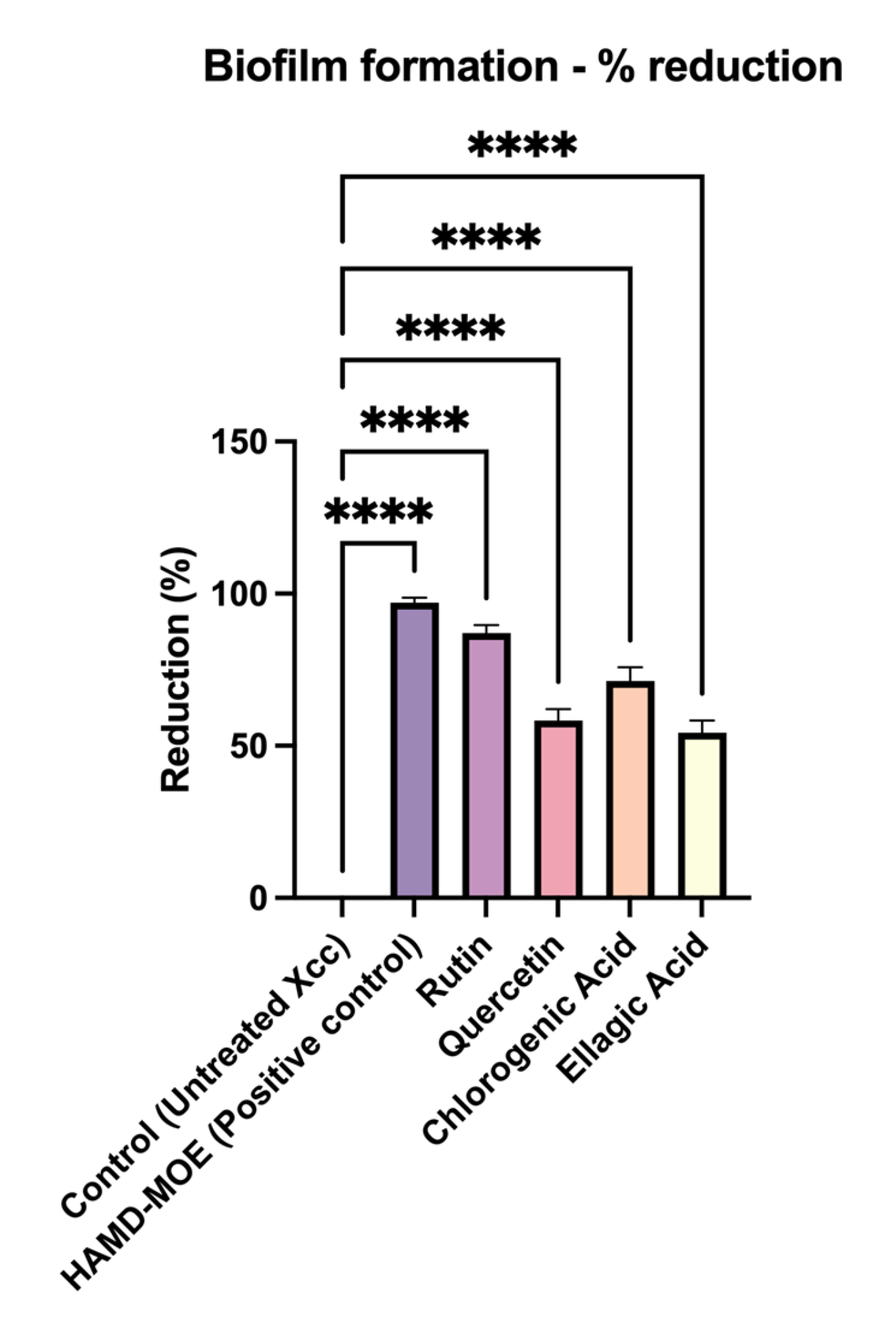
2.2. Effects of Single Phenols on Biofilm Formation
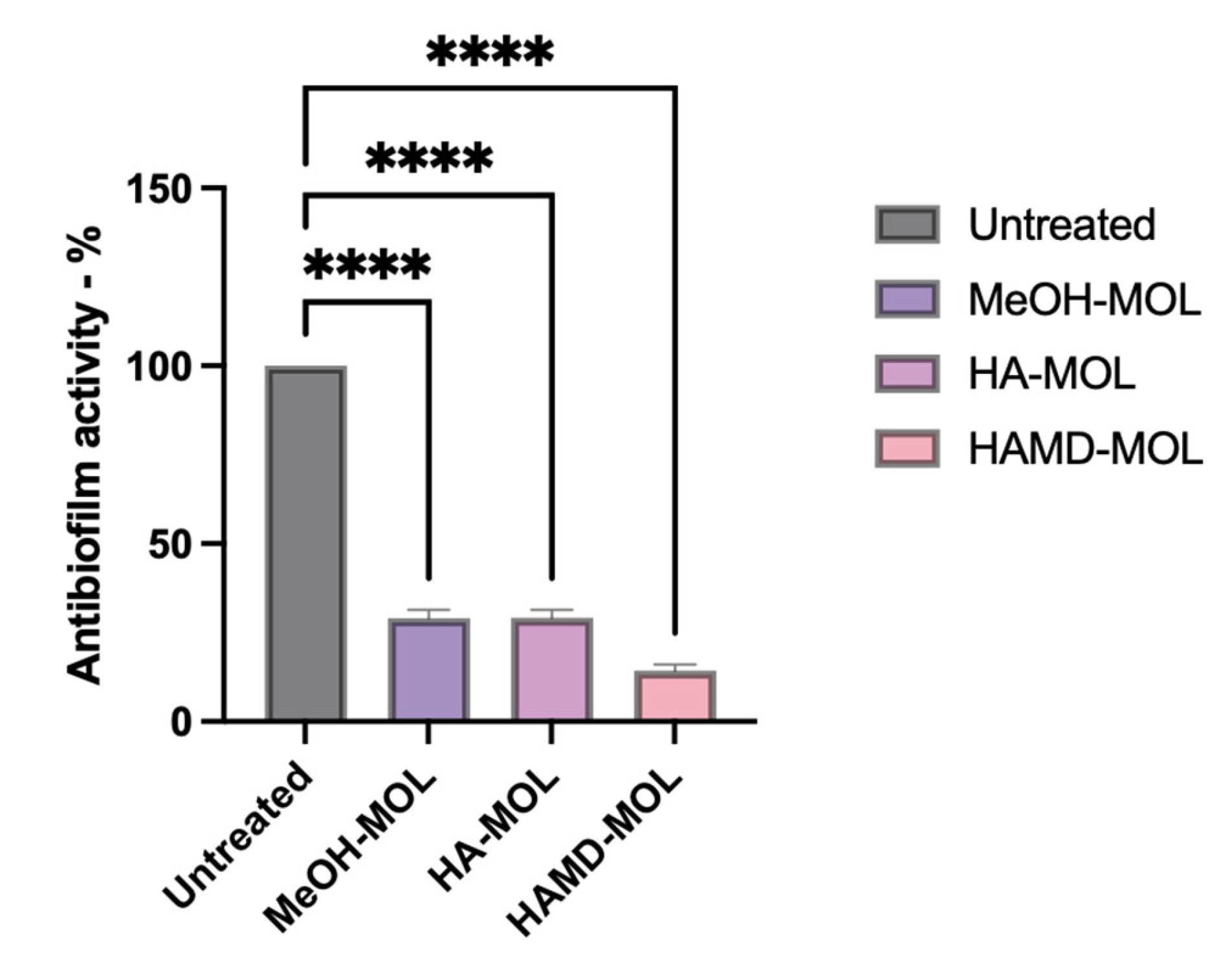
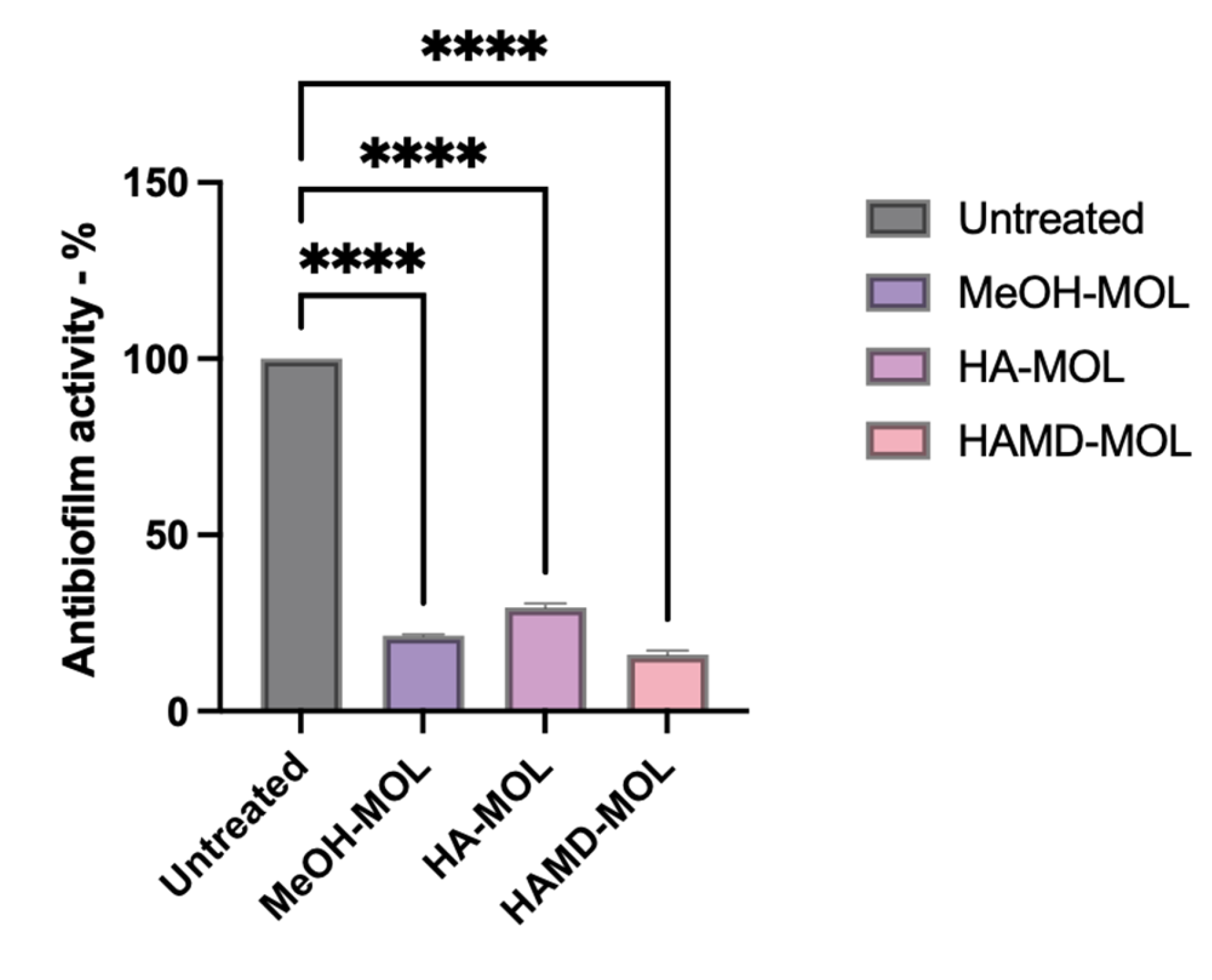
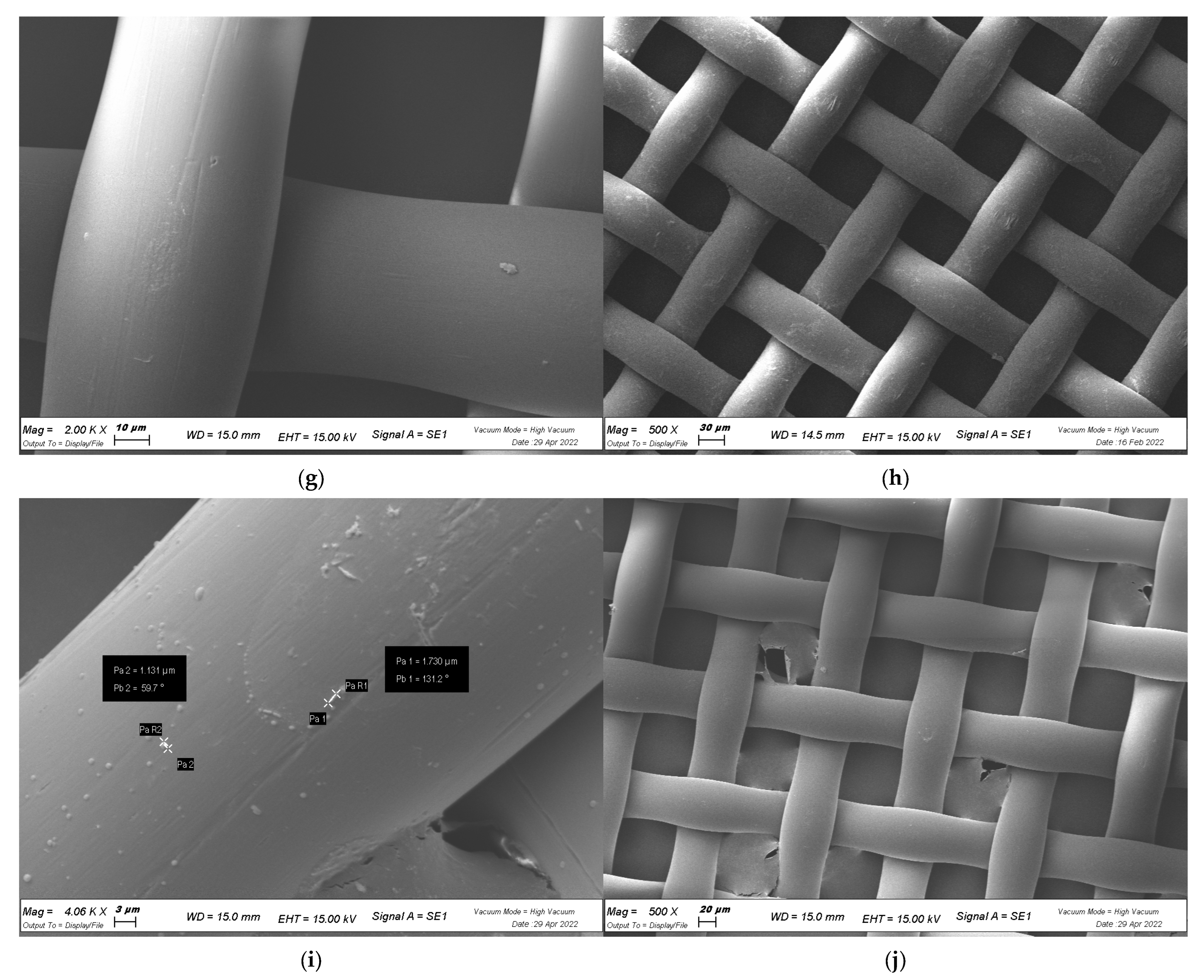
2.3. Assay of Biofilm Formation and Removal on Abiotic Surfaces
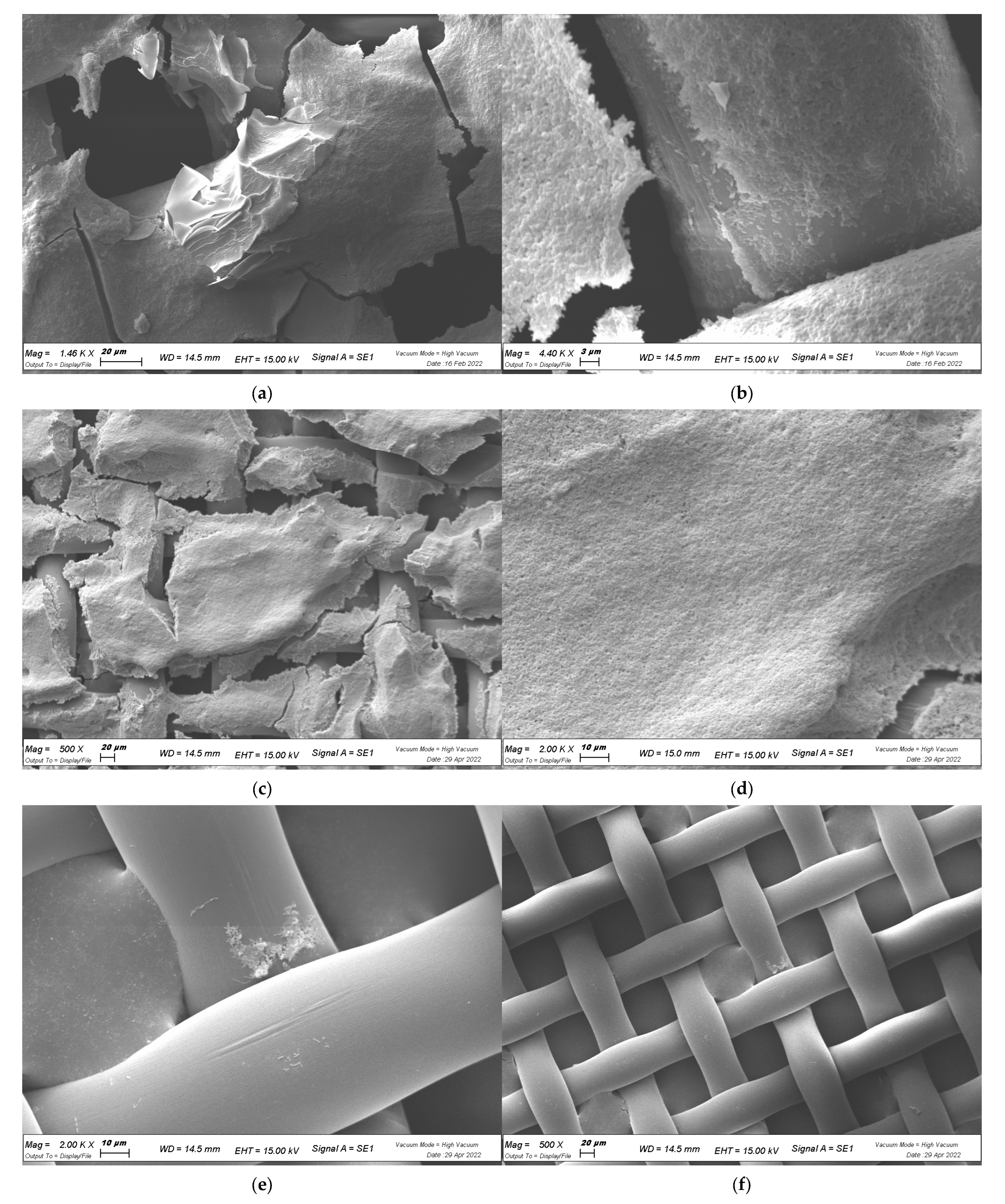
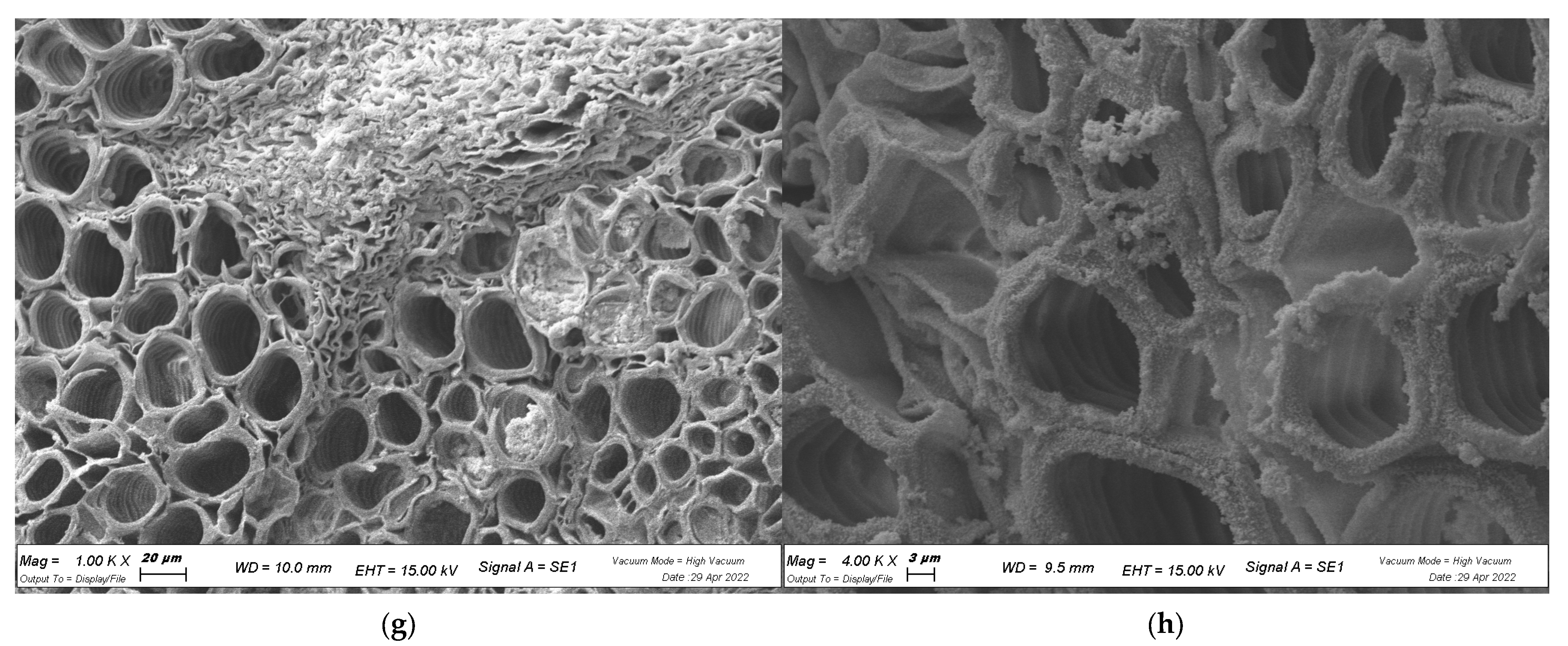
2.4. In Planta Biofilm Formation
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Media
4.2. Plant Material and Extraction Methods
- -
- Hydroalcoholic extract (HA-MOL): 10 g of powder was mixed with 200 mL of hydroalcoholic solution (ethanol:water, 70:30) at room temperature for 1 h under magnetic stirring, then filtered and concentrated in vacuum to provide the desired dry hydroalcoholic extract.
- -
- Methanolic extract (MeOH-MOL): 5 g of powder was mixed with 100 mL of methanol and subjected to two sonication cycles (40 °C, 60 min, 80%) with subsequent centrifugation. The supernatant was concentrated under vacuum to obtain the desired product.
- -
- Hydroalcoholic extract with maltodextrin (HAMD-MOL): dried M. oleifera Lam. leaves were extracted with 50% ethanol (raw material:solvent, 1:10) for 45 min at 45 °C. After filtration, concentration, and pasteurization, the extract was spray-dried using maltodextrin, obtaining a fine powder.
4.3. Effects of Single Phenols on Membrane Permeability
4.4. Effects of Single Phenols on Biofilm Formation
4.5. Assay of Biofilm Formation and Removal on Abiotic Surface
4.6. Biofilm Formation in Planta and Total Viable Count
4.7. Visualization of Biofilm with SEM
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vicente, J.G.; Holub, E.B. Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol. Plant Pathol. 2012, 14, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Crossman, L.; Dow, J. Biofilm formation and dispersal in Xanthomonas campestris. Microbes Infect. 2004, 6, 623–629. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-W.; Cao, X.-Q.; Poplawsky, A.R. Chemical Structure, Biological Roles, Biosynthesis and Regulation of the Yellow Xanthomonadin Pigments in the Phytopathogenic Genus Xanthomonas. Mol. Plant-Microbe Interact. 2020, 33, 705–714. [Google Scholar] [CrossRef] [Green Version]
- He, Y.-W.; Zhang, L.-H. Quorum sensing and virulence regulation in Xanthomonas campestris. FEMS Microbiol. Rev. 2008, 32, 842–857. [Google Scholar] [CrossRef] [Green Version]
- Gudesblat, G.E.; Torres, P.S.; Vojnov, A.A. Xanthomonas campestris Overcomes Arabidopsis Stomatal Innate Immunity through a DSF Cell-to-Cell Signal-Regulated Virulence Factor. Plant Physiol. 2008, 149, 1017–1027. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Dey, S.S.; Bhatia, R.; Batley, J.; Kumar, R. Molecular breeding for resistance to black rot [Xanthomonas campestris pv. campestris (Pammel) Dowson] in Brassicas: Recent advances. Euphytica 2018, 214, 196. [Google Scholar] [CrossRef]
- Ryan, R.P.; Ryan, D.J.; Sun, Y.-C.; Li, F.-M.; Wang, Y.; Dowling, D.N. An acquired efflux system is responsible for copper resistance in Xanthomonas strain IG-8 isolated from China. FEMS Microbiol. Lett. 2007, 268, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Thapa, S.; Babadoost, M. Effectiveness of Chemical Compounds and Biocontrol Agents for Management of Bacterial Spot of Pumpkin Caused by Xanthomonas cucurbitae. Plant Health Prog. 2016, 17, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Kotchoni, O.S.; Torimiro, N.; Gachomo, E.W. Control of Xanthomonas campestris pv. Vignicoia in cowpea following seed and seedling treatment with hydrogen peroxide and N-heterocyclic pyridinium chlorochromate. J. Plant Pathol. 2007, 89, 361–367. Available online: https://www.jstor.org/stable/41998414 (accessed on 9 January 2023).
- Liu, Z.; Wang, H.; Wang, J.; Lv, J.; Xie, B.; Luo, S.; Wang, S.; Zhang, B.; Li, Z.; Yue, Z.; et al. Physical, chemical, and biological control of black rot of brassicaceae vegetables: A review. Front. Microbiol. 2022, 13, 1023826. [Google Scholar] [CrossRef]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef] [PubMed]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Suteu, D.; Rusu, L.; Zaharia, C.; Badeanu, M.; Daraban, G.M. Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products. Appl. Sci. 2020, 10, 8913. [Google Scholar] [CrossRef]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, R.; Macchi, G.; Caproni, A.; Sicurella, M.; Buratto, M.; Salvatori, F.; Pappadà, M.; Manfredini, S.; Baldisserotto, A.; Marconi, P. Control of Erwinia amylovora Growth by Moringa oleifera Leaf Extracts: In Vitro and in Planta Effects. Plants 2022, 11, 957. [Google Scholar] [CrossRef]
- Fontana, R.; Caproni, A.; Buzzi, R.; Sicurella, M.; Buratto, M.; Salvatori, F.; Pappadà, M.; Manfredini, S.; Baldisserotto, A.; Marconi, P. Effects of Moringa oleifera Leaf Extracts on Xanthomonas campestris pv. campestris. Microorganisms 2021, 9, 2244. [Google Scholar] [CrossRef]
- Chhikara, N.; Kaur, A.; Mann, S.; Garg, M.; Sofi, S.A.; Panghal, A. Bioactive compounds, associated health benefits and safety considerations of Moringa oleifera L.: An updated review. Nutr. Food Sci. 2020, 51, 255–277. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pamplona Pagnossa, J.; Blasi, F.; Cossignani, L.; Hilsdorf Piccoli, R.; Zengin, G.; Montesano, D.; Cocconcelli, P.S.; Lucini, L. Phenolic profiling and in vitro bioactivity of Moringa oleifera leaves as affected by different extraction solvents. Food Res. Int. 2020, 127, 108712. [Google Scholar] [CrossRef]
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Bhattacharya, A.; Tiwari, P.; Sahu, P. A review of the phytochemical and pharmacological characteristics of Moringa oleifera. J. Pharm. Bioallied Sci. 2018, 10, 181–191. [Google Scholar] [CrossRef]
- Harsha, P.S.S.; Lavelli, V. Effects of Maltodextrins on the Kinetics of Lycopene and Chlorogenic Acid Degradation in Dried Tomato. Molecules 2019, 24, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, M.L.; Malafaia, C.B.; Macedo, A.J.; Silva, M.V.; Mariano, R.L.R.; Souza, E.B. Biofilm formation by Xanthomonas campestris pv. viticola affected by abiotic surfaces and culture media. Trop. Plant Pathol. 2017, 43, 146–151. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Nero, T.; Mukherjee, S.; Olson, R.; Yan, J. Searching for the Secret of Stickiness: How Biofilms Adhere to Surfaces. Front. Microbiol. 2021, 12, 686793. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaaslan, M.; Şengün, F.; Cansu, Ü.; Başyiğit, B.; Sağlam, H.; Karaaslan, A. Gum arabic/maltodextrin microencapsulation confers peroxidation stability and antimicrobial ability to pepper seed oil. Food Chem. 2020, 337, 127748. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.T.; Call, D.R.; Beyenal, H. Maltodextrin enhances biofilm elimination by electrochemical scaffold. Sci. Rep. 2016, 6, 36003. [Google Scholar] [CrossRef] [Green Version]
- Papoutsis, K.; Golding, J.B.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M. Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neupane, P.; Bhuju, S.; Thapa, N.; Bhattarai, H.K. ATP Synthase: Structure, Function and Inhibition. Biomol. Concepts 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P.R. Antioxidant Activity and Total Phenolic Content of Moringa oleifera Leaves in Two Stages of Maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial Activity of a New Class of Phosphorylated and Modified Flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef] [Green Version]
- Hooda, H.; Singh, P.; Bajpai, S. Effect of quercitin impregnated silver nanoparticle on growth of some clinical pathogens. Mater. Today Proc. 2020, 31, 625–630. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Alnour, T.M.; Ahmed-Abakur, E.H.; Elssaig, E.H.; Abuduhier, F.M.; Ullah, M.F. Antimicrobial synergistic effects of dietary flavonoids rutin and quercetin in combination with antibiotics gentamicin and ceftriaxone against E. coli (MDR) and P. mirabilis (XDR) strains isolated from human infections: Implications for food–medicine interactions. Ital. J. Food Sci. 2022, 34, 34–42. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Šolmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Liu, H.N.; Liu, Y.; Hu, L.L.; Suo, Y.L.; Zhang, L.; Jin, F.; Feng, X.A.; Teng, N. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult. Sci. 2014, 93, 347–353. [Google Scholar] [CrossRef]
- Lin, E.-S.; Luo, R.-H.; Huang, C.-Y. A Complexed Crystal Structure of a Single-Stranded DNA-Binding Protein with Quercetin and the Structural Basis of Flavonol Inhibition Specificity. Int. J. Mol. Sci. 2022, 23, 588. [Google Scholar] [CrossRef]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as Inhibitors of Antibiotic Resistant Bacteria—Mechanisms Underlying Rutin Interference with Bacterial Virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.-J.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three faces of biofilms: A microbial lifestyle, a nascent multicellular organism, and an incubator for diversity. Npj Biofilms Microbiomes 2021, 7, 80. [Google Scholar] [CrossRef]
- Esertaş, Ü.Z.; Kara, Y.; Kiliç, A.O.; Kolayli, S. A comparative study of antimicrobial, anti-quorum sensing, anti-biofilm, anti-swarming, and antioxidant activities in flower extracts of pecan (Carya illinoinensis) and chestnut (Castanea sativa). Arch. Microbiol. 2022, 204, 589. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Felício, M.R.; Boas, E.V.; Gonçalves, S.; Costa, F.F.; Samy, R.P.; Santos, N.C.; Franco, O.L. New frontiers for anti-biofilm drug development. Pharmacol. Ther. 2016, 160, 133–144. [Google Scholar] [CrossRef]
- Manner, S.; Fallarero, A. Screening of Natural Product Derivatives Identifies Two Structurally Related Flavonoids as Potent Quorum Sensing Inhibitors against Gram-Negative Bacteria. Int. J. Mol. Sci. 2018, 19, 1346. [Google Scholar] [CrossRef] [Green Version]
- Hamrita, B.; Noumi, E.; Hafi, F.; Nazzaro, F.; Snoussi, M. Phytochemical composition and antimicrobial, and anti-quorum sensing activities of Punica granatum L. methanolic extract. Iran. J. Microbiol. 2022, 14, 373–382. [Google Scholar] [CrossRef]
- Gianni, D.; Galli, E.; Bernardini, M.L. Biologia dei Microrganismi. 2019. Available online: https://online.universita.zanichelli.it/deho-3ed/- (accessed on 2 August 2022).
- He, Y.W.; Wu, J.; Cha, J.S.; Zhang, L.H. Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol. 2010, 10, 187. [Google Scholar] [CrossRef] [Green Version]
- Sabuquillo, P.; Cubero, J. Biofilm Formation in Xanthomonas arboricola pv. pruni: Structure and Development. Agronomy 2021, 11, 546. [Google Scholar] [CrossRef]
- Dunger, G.; Guzzo, C.R.; Andrade, M.O.; Jones, J.B.; Farah, C.S. Xanthomonas citri subsp. citri Type IV Pilus Is Required for Twitching Motility, Biofilm Development, and Adherence. Mol. Plant-Microbe Interact. 2014, 27, 1132–1147. [Google Scholar] [CrossRef] [Green Version]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and Their Derivatives from Plants as Antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Studies on lysogenesis I. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, L.C.; Scott, A.P.; Marfell, B.J.; Boughaba, J.A.; Chojnowski, G.; Waterhouse, N.J. Measuring Cell Death by Propidium Iodide Uptake and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087163. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, C.; Cypionka, H. Propidium ion enters viable cells with high membrane potential during live-dead staining. J. Microbiol. Methods 2017, 142, 79–82. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6. [Google Scholar]
- Sena-Vélez, M.; Redondo, C.; Gell, I.; Ferragud, E.; Johnson, E.; Graham, J.H.; Cubero, J. Biofilm formation and motility of Xanthomonas strains with different citrus host range. Plant Pathol. 2014, 64, 767–775. [Google Scholar] [CrossRef]
- Ozrenk, K.; Balta, F.; Çelik, F. Levels of fire blight (Erwinia amylovora) susceptibility of native apple, pear and quince germplasm from Lake Van Basin, Turkey. Eur. J. Plant Pathol. 2011, 132, 229–236. [Google Scholar] [CrossRef]
- Allan-Wojtas, P.; Hildebrand, P.; Braun, P.; Smith-King, H.; Carbyn, S.; Renderos, W. Low temperature and anhydrous electron microscopy techniques to observe the infection process of the bacterial pathogen Xanthomonas fragariae on strawberry leaves. J. Microsc. 2010, 239, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. Available online: http://www.ajevonline.org/content/16/3/144.abstract (accessed on 4 September 2022).
- Singh, V.; Guizani, N.; Musthafa, M.E.; Hakkim, L. Comparative Analysis of Total Phenolics, Flavonoid Content and Antioxidant Profile of Different Date Varieties (Phoenix dactylifera L.) From Sultanate of Oman. Available online: https://www.researchgate.net/publication/277309552 (accessed on 4 September 2022).
- Azieana, J.; Zainon, M.N.; Noriham, A.; Rohana, M.N. Total Phenolic and Flavonoid Content and Antioxidant Activities of Ten Malaysian Wild Mushrooms. OALib 2017, 4, e3987. [Google Scholar] [CrossRef]
- Nouman, W.; Anwar, F.; Gull, T.; Newton, A.; Rosa, E.; Domínguez-Perles, R. Profiling of polyphenolics, nutrients and antioxidant potential of germplasm’s leaves from seven cultivars of Moringa oleifera Lam. Ind. Crops Prod. 2016, 83, 166–176. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Manfredini, S.; Vertuani, S. Moringa oleifera leaf extracts as multifunctional ingredients for ‘natural and organic’ sunscreens and photoprotective preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef] [Green Version]
- Ahern, S.; Das, M.; Bhowmick, T.S.; Young, R.; Gonzalez, C.F. Characterization of Novel Virulent Broad-Host-Range Phages of Xylella fastidiosa and Xanthomonas. J. Bacteriol. 2013, 196, 459–471. [Google Scholar] [CrossRef]


| Control | MeOH-MOL | HA-MOL | HAMD-MOL | |
|---|---|---|---|---|
| Total Viable Count | 2.6 ± 1.44 × 108 CFU/mL | 3.03 ± 1.52 × 104 CFU/mL | 2.59 ± 2.1 × 104 CFU/mL | 6.02 ± 3.65 × 103 CFU/mL |
| Bacteria | Sample ID | Host | Province of Isolation | Year of Isolation |
|---|---|---|---|---|
| Xanthomonas campestris pv. campestris | 10863 | Brassica seeds | Bologna | 2011 |
| Xanthomonas campestris pv. campestris | 11043 | Brassica seeds | Bologna | 2011 |
| Xanthomonas campestris pv. campestris | 15616 | Brassica seeds | Forlì-Cesena | 2011 |
| Xanthomonas campestris pv. campestris | 15619 | Brassica seeds | Forlì-Cesena | 2012 |
| Xanthomonas campestris pv. campestris | 15622 | Brassica seeds | Forlì-Cesena | 2012 |
| Xanthomonas campestris pv. campestris | 30788 | Brassica seeds | Ravenna | 2014 |
| Xanthomonas campestris pv. campestris | 3586 | Brassica | DSMZ | 1995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, R.; Caproni, A.; Sicurella, M.; Manfredini, S.; Baldisserotto, A.; Marconi, P. Effects of Flavonoids and Phenols from Moringa oleifera Leaf Extracts on Biofilm Processes in Xanthomonas campestris pv. campestris. Plants 2023, 12, 1508. https://doi.org/10.3390/plants12071508
Fontana R, Caproni A, Sicurella M, Manfredini S, Baldisserotto A, Marconi P. Effects of Flavonoids and Phenols from Moringa oleifera Leaf Extracts on Biofilm Processes in Xanthomonas campestris pv. campestris. Plants. 2023; 12(7):1508. https://doi.org/10.3390/plants12071508
Chicago/Turabian StyleFontana, Riccardo, Anna Caproni, Mariaconcetta Sicurella, Stefano Manfredini, Anna Baldisserotto, and Peggy Marconi. 2023. "Effects of Flavonoids and Phenols from Moringa oleifera Leaf Extracts on Biofilm Processes in Xanthomonas campestris pv. campestris" Plants 12, no. 7: 1508. https://doi.org/10.3390/plants12071508








