Review on Rice Husk Biochar as an Adsorbent for Soil and Water Remediation
Abstract
:1. Introduction
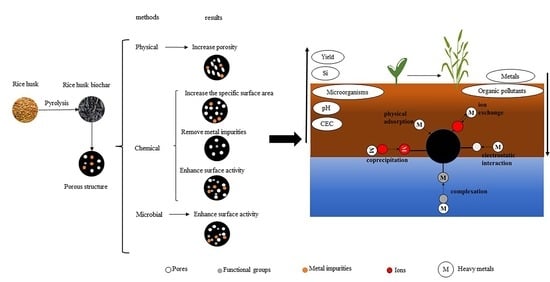
2. Preparation of Rice Husk Biochar
3. Properties of Rice Husk Biochar
4. Modification of Rice Husk Biochar
4.1. Physical Modification
4.2. Chemical Modification
- (1)
- Acid/alkali modification
- (2)
- Metal (metal oxide) modification
- (3)
- Functional group modification
4.3. Microbial Modification
5. Rice Husk Biochar as an Adsorbent
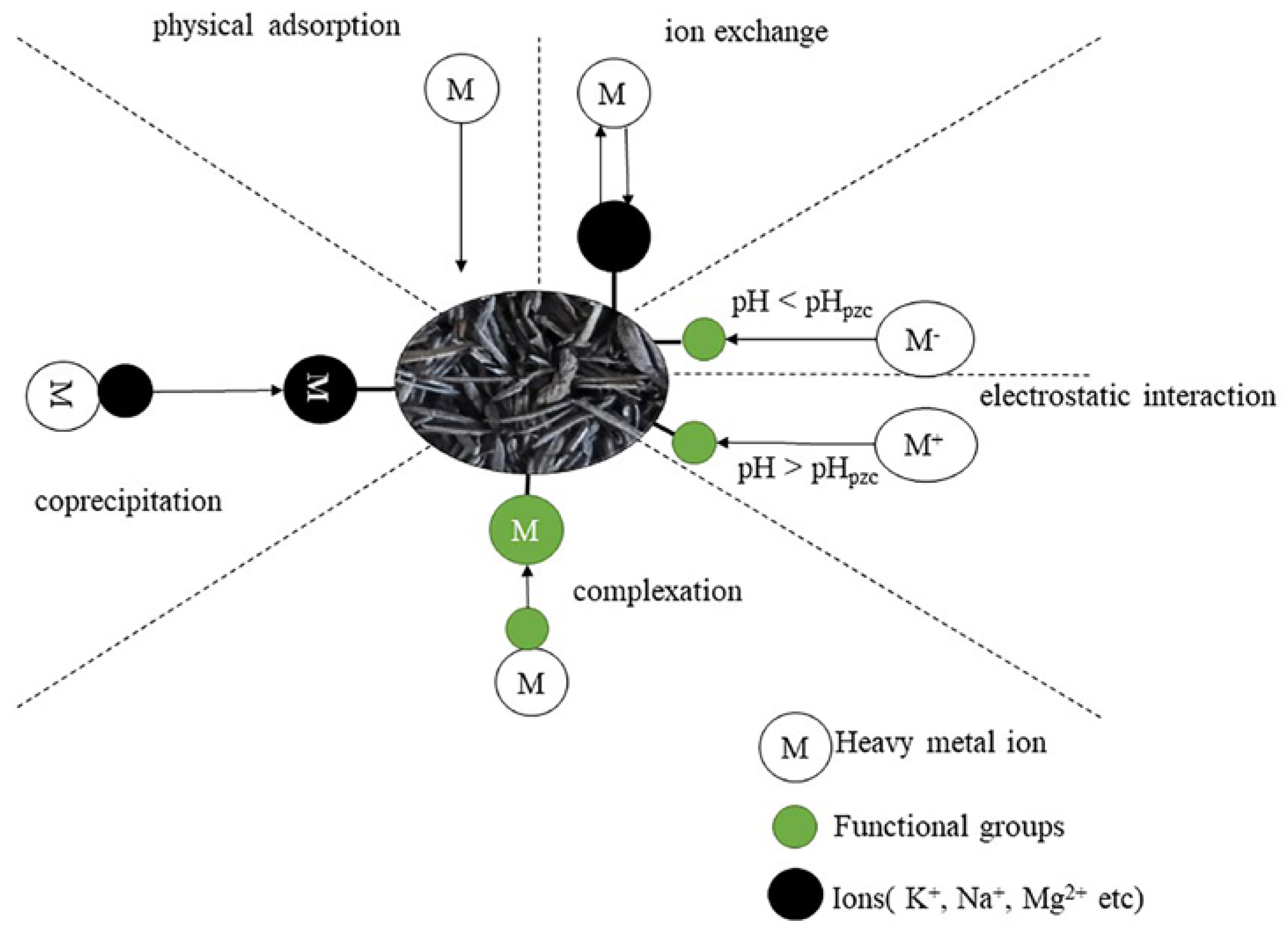
5.1. Adsorption of Heavy Metals in Water
5.2. Adsorption of Organic Matter in Water
6. Rice Husk Biochar as a Soil Conditioner
6.1. Effect on Soil pH
6.2. Effect on Soil Cation Exchange
6.3. Effect on Soil Microorganisms
6.4. Effect on Heavy Metals in Soil
6.5. Effect on Organic Pollutants in Soil
6.6. Effect on Plant Growth
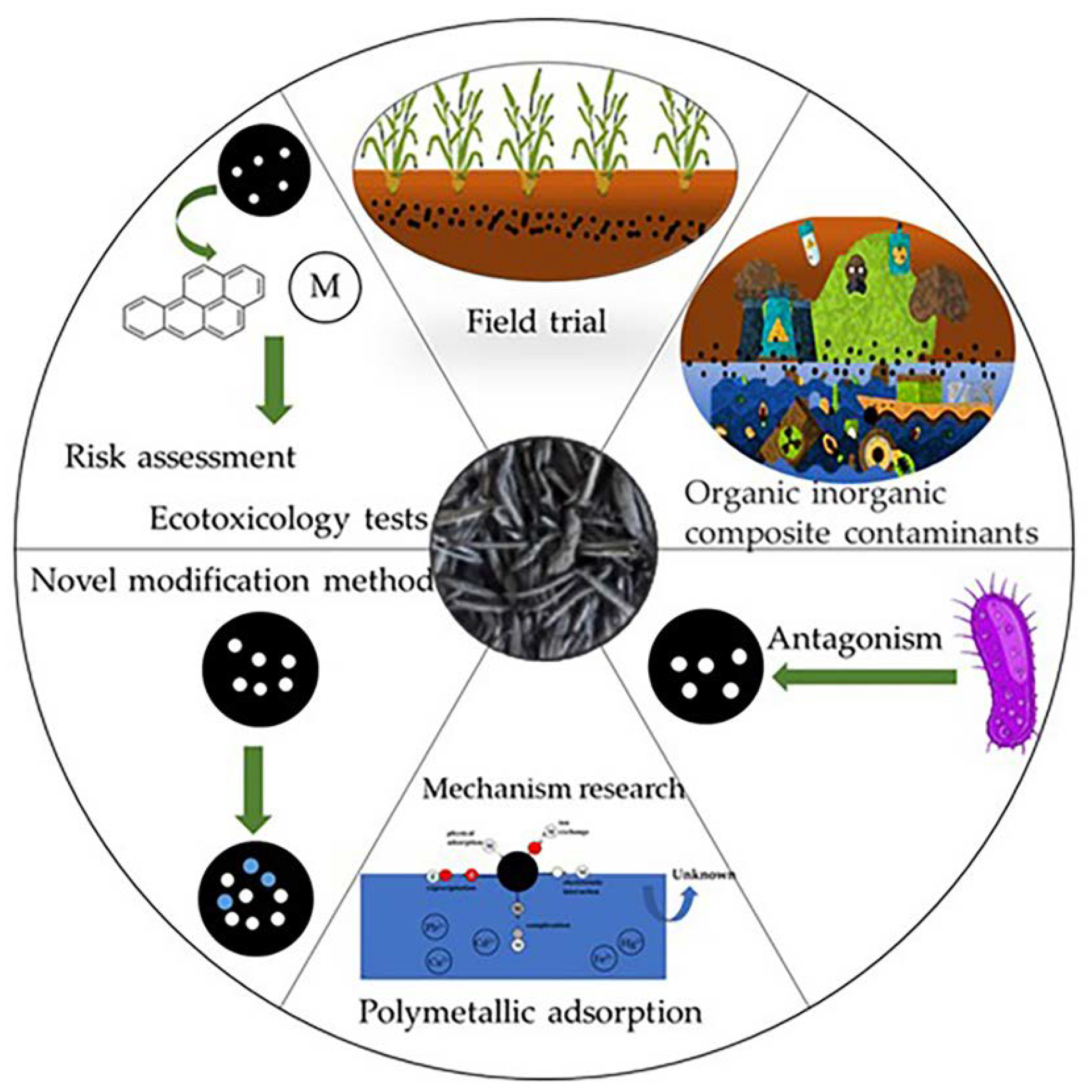
7. Conclusions and Future Perspectives
- (1)
- There is a necessity to strengthen the risk assessment and toxicology experiments on RHB as it is an adsorbent material with potential health hazards [161].
- (2)
- In the study of new RHB modification methods, the adsorption performance and secondary pollution characteristics of RHB must be comprehensively evaluated to design a nonpolluting modification method.
- (3)
- The natural environment often contains many different types of pollutants. Therefore, future research should focus on the comprehensive study of the sorption mechanism of RHB on one or several specific pollutants under coexistence of different types of pollutants (e.g., organic–inorganic composite pollutants and multiple heavy metal solutions).
- (4)
- To better utilize RHB in soil improvement, it is necessary to conduct pot trial studies to evaluate interactions between soil microorganisms and RHB, such as antagonistic and synergistic effects [162].
- (5)
- Long-term and regional field trials are required to study the value of RHB in agricultural applications to provide an accurate assessment of the use of RHB in this field.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Xu, L.; Jiang, W.; Xuan, Y.; Lu, W.; Li, Z.; Yang, S.; Gu, Z. Adsorption mechanism of rhein-coated Fe3O4 as magnetic adsorbent based on low-field NMR. Environ. Sci. Pollut. Res. 2021, 28, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Zeng, J.; Zhu, F.; Luo, X.; Feng, J.; He, J.; Xue, S. Geochemical partitioning and spatial distribution of heavy metals in soils contaminated by lead smelting. Environ. Pollut. 2022, 307, 119486. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, M.; Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Patra, J.; Panda, S.; Dhal, N. Biochar as a low-cost adsorbent for heavy metal removal: A review. Int. J. Res. Biosci. 2017, 6, 1–7. [Google Scholar]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Yi, Z. After-Effects of Hydrochar Amendment on Water Spinach Production, N Leaching, and N2O Emission from a Vegetable Soil under Varying N-Inputs. Plants 2022, 11, 3444. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Wang, L.; Rinklebe, J.; Tack, F.M.; Hou, D. A review of green remediation strategies for heavy metal contaminated soil. Soil Use Manag. 2021, 37, 936–963. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T. Biochar: A sustainable solution. Environ. Dev. Sustain. 2021, 23, 6642–6680. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Ju, M.; Liu, L. Preparation and modification of biochar materials and their application in soil remediation. Appl. Sci. 2019, 9, 1365. [Google Scholar] [CrossRef] [Green Version]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Z.; Liu, C.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 2022, 305, 135457. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Fleig, O.P.; Raymundo, L.M.; Trierweiler, L.F.; Trierweiler, J.O. Study of Rice Husk Continuous Torrefaction as a Pretreatment for Fast Pyrolysis. J. Anal. Appl. Pyrolysis 2020, 154, 104994. [Google Scholar] [CrossRef]
- Vieira, F.R.; Luna, C.M.R.; Arce, G.L.; Ávila, I. Optimization of slow pyrolysis process parameters using a fixed bed reactor for biochar yield from rice husk. Biomass Bioenergy 2020, 132, 105412. [Google Scholar] [CrossRef]
- Satbaev, B.; Yefremova, S.; Zharmenov, A.; Kablanbekov, A.; Yermishin, S.; Shalabaev, N.; Satbaev, A.; Khen, V. Rice husk research: From environmental pollutant to a promising source of organo-mineral raw materials. Materials 2021, 14, 4119. [Google Scholar] [CrossRef]
- Mohammadi, A.; Cowie, A.; Mai, T.L.A.; de la Rosa, R.A.; Kristiansen, P.; Brandao, M.; Joseph, S. Biochar use for climate-change mitigation in rice cropping systems. J. Clean. Prod. 2016, 116, 61–70. [Google Scholar] [CrossRef]
- Sadeghi Afjeh, M.; Bagheri Marandi, G.; Zohuriaan-Mehr, M.J. Nitrate removal from aqueous solutions by adsorption onto hydrogel-rice husk biochar composite. Water Environ. Res. 2020, 92, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Upgrading the rice husk char obtained by flash pyrolysis for the production of amorphous silica and high quality activated carbon. Bioresour. Technol. 2014, 170, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Liou, T.-H.; Wang, P.-Y. Utilization of rice husk wastes in synthesis of graphene oxide-based carbonaceous nanocomposites. Waste Manag. 2020, 108, 51–61. [Google Scholar] [CrossRef]
- Qu, J.; Li, B.; Wei, T.; Li, C.; Liu, B. Effects of rice-husk ash on soil consistency and compactibility. Catena 2014, 122, 54–60. [Google Scholar] [CrossRef]
- Khalid, Z.B.; Siddique, M.N.I.; Nayeem, A.; Adyel, T.M.; Ismail, S.B.; Ibrahim, M.Z. Biochar application as sustainable precursors for enhanced anaerobic digestion: A systematic review. J. Environ. Chem. Eng. 2021, 9, 105489. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Teng, F.; Zhang, Y.; Wang, D.; Shen, M.; Hu, D. Iron-modified rice husk hydrochar and its immobilization effect for Pb and Sb in contaminated soil. J. Hazard. Mater. 2020, 398, 122977. [Google Scholar] [CrossRef]
- Selvaranjan, K.; Navaratnam, S.; Gamage, J.; Thamboo, J.; Siddique, R.; Zhang, J.; Zhang, G. Thermal and environmental impact analysis of rice husk ash-based mortar as insulating wall plaster. Constr. Build. Mater. 2021, 283, 122744. [Google Scholar] [CrossRef]
- Venkatanarayanan, H.K.; Rangaraju, P.R. Evaluation of sulfate resistance of Portland cement mortars containing low-carbon rice husk ash. J. Mater. Civ. Eng. 2014, 26, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.; Tang, L.; Dong, H.; Huang, B. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Ndirangu, S.M.; Liu, Y.; Xu, K.; Song, S. Risk evaluation of pyrolyzed biochar from multiple wastes. J. Chem. 2019, 2019, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Chatzimichailidou, S.; Xanthopoulou, M.; Tolkou, A.K.; Katsoyiannis, I.A. Biochar Derived from Rice by-Products for Arsenic and Chromium Removal by Adsorption: A Review. J. Compos. Sci. 2023, 7, 59. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Characterization of biochars produced from oil palm and rice husks and their adsorption capacities for heavy metals. Int. J. Environ. Sci. Technol. 2014, 11, 967–976. [Google Scholar] [CrossRef]
- Severo, F.F.; da Silva, L.S.; Moscôso, J.S.C.; Sarfaraz, Q.; Rodrigues Júnior, L.F.; Lopes, A.F.; Marzari, L.B.; Molin, G.D. Chemical and physical characterization of rice husk biochar and ashes and their iron adsorption capacity. SN Appl. Sci. 2020, 2, 1286. [Google Scholar] [CrossRef]
- Singh, C.; Tiwari, S.; Gupta, V.K.; Singh, J.S. The effect of rice husk biochar on soil nutrient status, microbial biomass and paddy productivity of nutrient poor agriculture soils. Catena 2018, 171, 485–493. [Google Scholar] [CrossRef]
- Shackley, S.; Carter, S.; Knowles, T.; Middelink, E.; Haefele, S.; Sohi, S.; Cross, A.; Haszeldine, S. Sustainable gasification–biochar systems? A case-study of rice-husk gasification in Cambodia, Part I: Context, chemical properties, environmental and health and safety issues. Energy Policy 2012, 42, 49–58. [Google Scholar] [CrossRef]
- Adebajo, S.O.; Oluwatobi, F.; Akintokun, P.O.; Ojo, A.E.; Akintokun, A.K.; Gbodope, I.S. Impacts of rice-husk biochar on soil microbial biomass and agronomic performances of tomato (Solanum lycopersicum L.). Sci. Rep. 2022, 12, 1787. [Google Scholar] [CrossRef]
- V Varela Milla, O.; Rivera, E.B.; Huang, W.-J.; Chien, C.; Wang, Y.-M. Agronomic properties and characterization of rice husk and wood biochars and their effect on the growth of water spinach in a field test. J. Soil Sci. Plant Nutr. 2013, 13, 251–266. [Google Scholar] [CrossRef] [Green Version]
- Prakongkep, N.; Gilkes, R.J.; Wiriyakitnateekul, W.; Duangchan, A.; Darunsontaya, T. The effects of pyrolysis conditions on the chemical and physical properties of rice husk biochar. Int. J. Mater. Sci. 2013, 3, 97–103. [Google Scholar]
- Asadi, H.; Ghorbani, M.; Rezaei-Rashti, M.; Abrishamkesh, S.; Amirahmadi, E.; Chengrong, C.; Gorji, M. Application of rice husk biochar for achieving sustainable agriculture and environment. Rice Sci. 2021, 28, 325–343. [Google Scholar] [CrossRef]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for environmental sustainability in the energy-water-agroecosystem nexus. Renew. Sustain. Energy Rev. 2021, 149, 111379. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Ryms, M.; Kosakowski, W. Thermal biomass conversion: A review. Processes 2020, 8, 516. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Calheiro, D.; Kieling, A.G.; Moraes, C.A.M.; Rocha, T.L.A.C.; Brehm, F.A.; Modolo, R.C.E. Characterization of rice husk ash produced using different biomass combustion techniques for energy. Fuel 2016, 165, 351–359. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Khalil, U.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Alyemeni, M.N.; Wijaya, L. Adsorption-reduction performance of tea waste and rice husk biochars for Cr (VI) elimination from wastewater. J. Saudi Chem. Soc. 2020, 24, 799–810. [Google Scholar] [CrossRef]
- Dunnigan, L.; Ashman, P.J.; Zhang, X.; Kwong, C.W. Production of biochar from rice husk: Particulate emissions from the combustion of raw pyrolysis volatiles. J. Clean. Prod. 2018, 172, 1639–1645. [Google Scholar] [CrossRef]
- Cai, W.; Dai, L.; Liu, R. Catalytic fast pyrolysis of rice husk for bio-oil production. Energy 2018, 154, 477–487. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, H.; Shao, J.; Chen, Y.; Feng, Y.; Wang, X.; Chen, H. Effects of hydrofluoric acid pre-deashing of rice husk on physicochemical properties and CO2 adsorption performance of nitrogen-enriched biochar. Energy 2015, 91, 903–910. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Ahmad, M.; Seo, D.-C.; Cho, J.-S.; Lee, S.-E.; Lee, S.S.; Ok, Y.S. Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J. Hazard. Mater. 2015, 290, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Paethanom, A.; Yoshikawa, K. Influence of pyrolysis temperature on rice husk char characteristics and its tar adsorption capability. Energies 2012, 5, 4941–4951. [Google Scholar] [CrossRef] [Green Version]
- Abrishamkesh, S.; Gorji, M.; Asadi, H.; Bagheri-Marandi, G.; Pourbabaee, A. Effects of rice husk biochar application on the properties of alkaline soil and lentil growth. Plant Soil Environ. 2015, 61, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.R.; Uemura, Y.; Osman, N.; Yusup, S. Production and evaluation of physicochemical characteristics of paddy husk bio-char for its C sequestration applications. BioEnergy Res. 2015, 8, 1800–1809. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Z.; Zhang, M.; Rinklebe, J.; Ma, Y.; Hou, D. Effect of production temperature and particle size of rice husk biochar on mercury immobilization and erosion prevention of a mercury contaminated soil. J. Hazard. Mater. 2021, 420, 126646. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Liu, G.; Yousaf, B.; Ali, M.U.; Ullah, H.; Munir, M.A.M.; Liu, R. Contrasting effects of operating conditions and biomass particle size on bulk characteristics and surface chemistry of rice husk derived-biochars. J. Anal. Appl. Pyrolysis 2018, 134, 281–292. [Google Scholar] [CrossRef]
- Wei, L.; Huang, Y.; Li, Y.; Huang, L.; Mar, N.N.; Huang, Q.; Liu, Z. Biochar characteristics produced from rice husks and their sorption properties for the acetanilide herbicide metolachlor. Environ. Sci. Pollut. Res. 2017, 24, 4552–4561. [Google Scholar] [CrossRef]
- Shi, J.; Fan, X.; Tsang, D.C.W.; Wang, F.; Shen, Z.; Hou, D.; Alessi, D.S. Removal of lead by rice husk biochars produced at different temperatures and implications for their environmental utilizations. Chemosphere 2019, 235, 825–831. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, X.; Ke, S.; Shao, J.; Yang, H.; Zhang, S.; Chen, H. Effect of different biomass species and pyrolysis temperatures on heavy metal adsorption, stability and economy of biochar. Ind. Crops Prod. 2022, 186, 115238. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total Environ. 2020, 713, 136433. [Google Scholar] [CrossRef]
- Pratiwi, E.P.A.; Hillary, A.K.; Fukuda, T.; Shinogi, Y. The effects of rice husk char on ammonium, nitrate and phosphate retention and leaching in loamy soil. Geoderma 2016, 277, 61–68. [Google Scholar] [CrossRef]
- Yi, S.; Gao, B.; Sun, Y.; Wu, J.; Shi, X.; Wu, B.; Hu, X. Removal of levofloxacin from aqueous solution using rice-husk and wood-chip biochars. Chemosphere 2016, 150, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Mayakaduwa, S.; Herath, I.; Ok, Y.S.; Mohan, D.; Vithanage, M. Insights into aqueous carbofuran removal by modified and non-modified rice husk biochars. Environ. Sci. Pollut. Res. 2017, 24, 22755–22763. [Google Scholar] [CrossRef]
- Huang, F.; Gao, L.-Y.; Wu, R.-R.; Wang, H.; Xiao, R.-B. Qualitative and quantitative characterization of adsorption mechanisms for Cd2+ by silicon-rich biochar. Sci. Total Environ. 2020, 731, 139163. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Cowie, A.L.; Mai, T.L.A.; Brandao, M.; de la Rosa, R.A.; Kristiansen, P.; Joseph, S. Climate-change and health effects of using rice husk for biochar-compost: Comparing three pyrolysis systems. J. Clean. Prod. 2017, 162, 260–272. [Google Scholar] [CrossRef]
- Phuong, H.T.; Uddin, M.; Kato, Y. Characterization of biochar from pyrolysis of rice husk and rice straw. J. Biobased Mater. Bioenergy 2015, 9, 439–446. [Google Scholar] [CrossRef]
- Angn, D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, G.; He, S.; Deng, F.; Pang, Q.; Xu, Y.; Yao, H. Efficient removal of elemental mercury by magnetic chlorinated biochars derived from co-pyrolysis of Fe(NO3)3 -laden wood and polyvinyl chloride waste. Fuel 2019, 239, 982–990. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Bushra, B.; Remya, N. Biochar from pyrolysis of rice husk biomass—Characteristics, modification and environmental application. Biomass Convers. Biorefinery 2020, 1–12. [Google Scholar] [CrossRef]
- Han, X.; He, Y.; Zhao, H.; Wang, D. Optimization of preparation conditions of activated carbon from the residue of desilicated rice husk using response surface methodology. Korean J. Chem. Eng. 2014, 31, 1810–1817. [Google Scholar] [CrossRef]
- Menya, E.; Olupot, P.; Storz, H.; Lubwama, M.; Kiros, Y. Production and performance of activated carbon from rice husks for removal of natural organic matter from water: A review. Chem. Eng. Res. Des. 2018, 129, 271–296. [Google Scholar] [CrossRef]
- Akhil, D.; Lakshmi, D.; Kartik, A.; Vo, D.-V.N.; Arun, J.; Gopinath, K.P. Production, characterization, activation and environmental applications of engineered biochar: A review. Environ. Chem. Lett. 2021, 19, 2261–2297. [Google Scholar] [CrossRef]
- Xiao, Y.; Lyu, H.; Tang, J.; Wang, K.; Sun, H. Effects of ball milling on the photochemistry of biochar: Enrofloxacin degradation and possible mechanisms. Chem. Eng. J. 2020, 384, 123311. [Google Scholar] [CrossRef]
- Lee, J.-H.; Heo, Y.-J.; Park, S.-J. Effect of silica removal and steam activation on extra-porous activated carbons from rice husks for methane storage. Int. J. Hydrogen Energy 2018, 43, 22377–22384. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Panwar, N.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Convers. Biorefinery 2020, 1–23. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, W.; Mašek, O.; Chen, X.; Gu, B.; Sharma, B.K.; Cao, X. Roles of phosphoric acid in biochar formation: Synchronously improving carbon retention and sorption capacity. J. Environ. Qual. 2017, 46, 393–401. [Google Scholar] [CrossRef]
- Amen, R.; Bashir, H.; Bibi, I.; Shaheen, S.M.; Niazi, N.K.; Shahid, M.; Hussain, M.M.; Antoniadis, V.; Shakoor, M.B.; Al-Solaimani, S.G. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chem. Eng. J. 2020, 396, 125195. [Google Scholar] [CrossRef]
- He, R.; Yuan, X.; Huang, Z.; Wang, H.; Jiang, L.; Huang, J.; Tan, M.; Li, H. Activated biochar with iron-loading and its application in removing Cr (VI) from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123642. [Google Scholar] [CrossRef]
- Goswami, R.; Shim, J.; Deka, S.; Kumari, D.; Kataki, R.; Kumar, M. Characterization of cadmium removal from aqueous solution by biochar produced from Ipomoea fistulosa at different pyrolytic temperatures. Ecol. Eng. 2016, 97, 444–451. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Lin, P.-Y.; Hsieh, S.-L.; Kirankumar, R.; Patel, A.K.; Singhania, R.-R.; Dong, C.-D.; Chen, C.-W.; Hsieh, S. Engineered mesoporous biochar derived from rice husk for efficient removal of malachite green from wastewaters. Bioresour. Technol. 2022, 347, 126749. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Lin, Q.; Cheng, S.; Guo, J.; Yao, X.; Liu, Q.; Yin, G.; Liu, D. Enhanced adsorption of Cd(II) from aqueous solution by a magnesium oxide–rice husk biochar composite. Environ. Sci. Pollut. Res. 2018, 25, 14032–14042. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lawluvy, Y.; Shi, Y.; Ighalo, J.O.; He, Y.; Zhang, Y.; Yap, P.-S. Adsorption of cadmium and lead from aqueous solution using modified biochar: A review. J. Environ. Chem. Eng. 2022, 10, 106502. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of biochar and modified biochar in heavy metal contaminated soil: A descriptive review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Cai, L.; Guo, J.; Wang, Y.; Ji, L.; Song, W. Preparation and characterization of macroalgae biochar nanomaterials with highly efficient adsorption and photodegradation ability. Materials 2018, 11, 1709. [Google Scholar] [CrossRef] [Green Version]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Zhang, A.; Li, X.; Xing, J.; Xu, G. Adsorption of potentially toxic elements in water by modified biochar: A review. J. Environ. Chem. Eng. 2020, 8, 104196. [Google Scholar] [CrossRef]
- O′Connor, D.; Peng, T.; Li, G.; Wang, S.; Duan, L.; Mulder, J.; Cornelissen, G.; Cheng, Z.; Yang, S.; Hou, D. Sulfur-modified rice husk biochar: A green method for the remediation of mercury contaminated soil. Sci. Total Environ. 2018, 621, 819–826. [Google Scholar] [CrossRef]
- Chen, M.; Wang, F.; Zhang, D.-L.; Yi, W.-M.; Liu, Y. Effects of acid modification on the structure and adsorption NH4+-N properties of biochar. Renew. Energy 2021, 169, 1343–1350. [Google Scholar] [CrossRef]
- Yang, G.-X.; Jiang, H. Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res. 2014, 48, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Gai, C.; Guo, Y.; Peng, N.; Liu, T.; Liu, Z. N-Doped biochar derived from co-hydrothermal carbonization of rice husk and Chlorella pyrenoidosa for enhancing copper ion adsorption. RSC Adv. 2016, 6, 53713–53722. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Cheng, H.; Sun, Y.; Wang, X.; Zou, S.; Ye, G.; Huang, H.; Ye, D. Hierarchical porous carbon fabricated from cellulose-degrading fungus modified rice husks: Ultrahigh surface area and impressive improvement in toluene adsorption. J. Hazard. Mater. 2020, 392, 122298. [Google Scholar] [CrossRef] [PubMed]
- Narzari, R.; Bordoloi, N.; Chutia, R.S.; Borkotoki, B.; Gogoi, N.; Bora, A.; Kataki, R. Biochar: An overview on its production, properties and potential benefits. Biol. Biotechnol. Sustain. Dev. 2015, 1, 13–40. [Google Scholar]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Wang, J.; Lu, S.; Yan, J. Enhanced adsorption for Pb (II) and Cd (II) of magnetic rice husk biochar by KMnO 4 modification. Environ. Sci. Pollut. Res. 2019, 26, 8902–8913. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, B.; Wang, R.; Zhao, Z. Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: A review. Environ. Sci. Pollut. Res. 2017, 24, 26297–26309. [Google Scholar] [CrossRef]
- Yrjälä, K.; Ramakrishnan, M.; Salo, E. Agricultural waste streams as resource in circular economy for biochar production towards carbon neutrality. Curr. Opin. Environ. Sci. Health 2022, 26, 100339. [Google Scholar] [CrossRef]
- Sanka, P.M.; Rwiza, M.J.; Mtei, K.M. Removal of Selected Heavy Metal Ions from Industrial Wastewater Using Rice and Corn Husk Biochar. Water Air Soil Pollut. 2020, 231, 244. [Google Scholar] [CrossRef]
- Amen, R.; Yaseen, M.; Mukhtar, A.; Klemeš, J.J.; Saqib, S.; Ullah, S.; Al-Sehemi, A.G.; Rafiq, S.; Babar, M.; Fatt, C.L.; et al. Lead and cadmium removal from wastewater using eco-friendly biochar adsorbent derived from rice husk, wheat straw, and corncob. Clean. Eng. Technol. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Higashikawa, F.S.; Conz, R.F.; Colzato, M.; Cerri, C.E.P.; Alleoni, L.R.F. Effects of feedstock type and slow pyrolysis temperature in the production of biochars on the removal of cadmium and nickel from water. J. Clean. Prod. 2016, 137, 965–972. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, S.; Liu, J.; Su, S.; Liang, Q.; Zeng, X.; Li, T. Study of the effect of pyrolysis temperature on the Cd2+ adsorption characteristics of biochar. Appl. Sci. 2018, 8, 1019. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Bolan, N.S.; Tsang, D.C.W.; Hou, D. Green immobilization of toxic metals using alkaline enhanced rice husk biochar: Effects of pyrolysis temperature and KOH concentration. Sci. Total Environ. 2020, 720, 137584. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, Z.; Feng, R.; Zhang, Y. Characterization of corncob-derived biochar and pyrolysis kinetics in comparison with corn stalk and sawdust. Bioresour. Technol. 2014, 170, 76–82. [Google Scholar] [CrossRef]
- Suwunwong, T.; Hussain, N.; Chantrapromma, S.; Phoungthong, K. Facile synthesis of corncob biochar via in-house modified pyrolysis for removal of methylene blue in wastewater. Mater. Res. Express 2020, 7, 015518. [Google Scholar] [CrossRef]
- Jing, F.; Sohi, S.P.; Liu, Y.; Chen, J. Insight into mechanism of aged biochar for adsorption of PAEs: Reciprocal effects of ageing and coexisting Cd2+. Environ. Pollut. 2018, 242, 1098–1107. [Google Scholar] [CrossRef]
- Pipíška, M.; Krajčíková, E.K.; Hvostik, M.; Frišták, V.; Ďuriška, L.; Černičková, I.; Kaňuchová, M.; Conte, P.; Soja, G. Biochar from Wood Chips and Corn Cobs for Adsorption of Thioflavin T and Erythrosine B. Materials 2022, 15, 1492. [Google Scholar] [CrossRef]
- Wan, S.; Hua, Z.; Sun, L.; Bai, X.; Liang, L. Biosorption of nitroimidazole antibiotics onto chemically modified porous biochar prepared by experimental design: Kinetics, thermodynamics, and equilibrium analysis. Process Saf. Environ. Prot. 2016, 104, 422–435. [Google Scholar] [CrossRef]
- Xu, D.; Cao, J.; Li, Y.; Howard, A.; Yu, K. Effect of pyrolysis temperature on characteristics of biochars derived from different feedstocks: A case study on ammonium adsorption capacity. Waste Manag. 2019, 87, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, Y.; Xing, B.; Qin, X.; Zhang, C.; Xia, H. Lead and cadmium clean removal from wastewater by sustainable biochar derived from poplar saw dust. J. Clean. Prod. 2021, 314, 128074. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Wolny-Koładka, K.; Gondek, K.; Gałązka, A.; Gawryjołek, K. Effect of Coapplication of Biochar and Nutrients on Microbiocenotic Composition, Dehydrogenase Activity Index and Chemical Properties of Sandy Soil. Waste Biomass Valorization 2020, 11, 3911–3923. [Google Scholar] [CrossRef] [Green Version]
- Manna, S.; Singh, N.; Purakayastha, T.J.; Berns, A.E. Effect of deashing on physico-chemical properties of wheat and rice straw biochars and potential sorption of pyrazosulfuron-ethyl. Arab. J. Chem. 2020, 13, 1247–1258. [Google Scholar] [CrossRef]
- Rajabi, H.; Hadi Mosleh, M.; Prakoso, T.; Ghaemi, N.; Mandal, P.; Lea-Langton, A.; Sedighi, M. Competitive adsorption of multicomponent volatile organic compounds on biochar. Chemosphere 2021, 283, 131288. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Wang, X.; Feng, K.; Su, J.; Dong, J. The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef]
- Tofan, L. Polymeric Biomass Derived Adsorbents for Co (II) Remediation, Recycling and Analysis. Polymers 2022, 14, 1647. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R. Comparison of characteristics of twenty-one types of biochar and their ability to remove multi-heavy metals and methylene blue in solution. Fuel Process. Technol. 2017, 160, 55–63. [Google Scholar] [CrossRef]
- Bansal, M.; Garg, R.; Garg, V.K.; Garg, R.; Singh, D. Sequestration of heavy metal ions from multi-metal simulated wastewater systems using processed agricultural biomass. Chemosphere 2022, 296, 133966. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk-and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Z.; Fan, X.; Tan, L.; Jiang, Y.; Zheng, W.; Han, F.X.; Liang, Y. A comparative study on adsorption of cadmium and lead by hydrochars and biochars derived from rice husk and Zizania latifolia straw. Environ. Sci. Pollut. Res. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Min, Y.; Feng, Q.; Mcgrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Román, S.; Ledesma, B.; González, J.F.; Al-Kassir, A.; Engo, G.; Álvarez-Murillo, A. Two stage thermal regeneration of exhausted activated carbons. Steam gasification of effluents. J. Anal. Appl. Pyrolysis 2013, 103, 201–206. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Roh, H.; Choi, Y.-L.; Koduru, J.R.; Yang, J.-K.; Chang, Y.-Y. Influencing factors on sorption of TNT and RDX using rice husk biochar. J. Ind. Eng. Chem. 2015, 32, 178–186. [Google Scholar] [CrossRef]
- Xu, G.; Wei, L.; Sun, J.; Shao, H.; Chang, S. What is more important for enhancing nutrient bioavailability with biochar application into a sandy soil: Direct or indirect mechanism? Ecol. Eng. 2013, 52, 119–124. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification-a critical review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef]
- Oladele, S.O. Changes in physicochemical properties and quality index of an Alfisol after three years of rice husk biochar amendment in rainfed rice–Maize cropping sequence. Geoderma 2019, 353, 359–371. [Google Scholar] [CrossRef]
- Kartika, K.; Lakitan, B.; Wijaya, A.; Kadir, S.; Widuri, L.I.; Siaga, E.; Meihana, M. Effects of particle size and application rate of rice-husk biochar on chemical properties of tropical wetland soil, rice growth and yield. Aust. J. Crop Sci. 2018, 12, 817–826. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Niu, C.; Jiang, J.; Sun, H. Positive Effects of Biochar on the Degraded Forest Soil and Tree Growth in China: A Systematic Review. Phyton-Int. J. Exp. Bot. 2022, 91, 1601–1616. [Google Scholar] [CrossRef]
- Ghorbani, M.; Asadi, H.; Abrishamkesh, S. Effects of rice husk biochar on selected soil properties and nitrate leaching in loamy sand and clay soil. Int. Soil Water Conserv. Res. 2019, 7, 258–265. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Gao, X.; Cheng, H.Y.; Valle, I.D.; Liu, S.; Masiello, C.A.; Silberg, J.J. Charcoal Disrupts Soil Microbial Communication through a Combination of Signal Sorption and Hydrolysis. ACS Omega 2016, 1, 226–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefaniuk, M.; Oleszczuk, P. Addition of biochar to sewage sludge decreases freely dissolved PAHs content and toxicity of sewage sludge-amended soil. Environ. Pollut. 2016, 218, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Bian, R.; Shi, W.; Luo, J.; Li, W.; Wang, Y.; Joseph, S.; Gould, H.; Zheng, J.; Zhang, X.; Liu, X.; et al. Copyrolysis of food waste and rice husk to biochar to create a sustainable resource for soil amendment: A pilot-scale case study in Jinhua, China. J. Clean. Prod. 2022, 347, 131269. [Google Scholar] [CrossRef]
- Derakhshan Nejad, Z.; Jung, M.C. The effects of biochar and inorganic amendments on soil remediation in the presence of hyperaccumulator plant. Int. J. Energy Environ. Eng. 2017, 8, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Singh Karam, D.; Nagabovanalli, P.; Sundara Rajoo, K.; Fauziah Ishak, C.; Abdu, A.; Rosli, Z.; Melissa Muharam, F.; Zulperi, D. An overview on the preparation of rice husk biochar, factors affecting its properties, and its agriculture application. J. Saudi Soc. Agric. Sci. 2022, 21, 149–159. [Google Scholar] [CrossRef]
- Duwiejuah, A.B.; Abubakari, A.H.; Quainoo, A.K.; Amadu, Y. Review of biochar properties and remediation of metal pollution of water and soil. J. Health Pollut. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.-L.; Cai, C.; Liang, J.-H.; Huang, Q.; Chen, Z.; Huang, Y.-Z.; Arp, H.P.H.; Sun, G.-X. The effects of biochars from rice residue on the formation of iron plaque and the accumulation of Cd, Zn, Pb, As in rice (Oryza sativa L.) seedlings. Chemosphere 2012, 89, 856–862. [Google Scholar] [CrossRef]
- Ibrahim, M.; Khan, S.; Hao, X.; Li, G. Biochar effects on metal bioaccumulation and arsenic speciation in alfalfa (Medicago sativa L.) grown in contaminated soil. Int. J. Environ. Sci. Technol. 2016, 13, 2467–2474. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.; Chen, Z.; Cai, C.; Wang, X.; Huang, Y.; Xiao, B.; Sun, G. Effect of biochars from rice husk, bran, and straw on heavy metal uptake by pot-grown wheat seedling in a historically contaminated soil. BioResources 2013, 8, 5965–5982. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Zhao, L.; Gao, B.; Xu, X.; Cao, X. The interfacial behavior between biochar and soil minerals and its effect on biochar stability. Environ. Sci. Technol. 2016, 50, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Zama, E.F.; Reid, B.J.; Arp, H.P.H.; Sun, G.-X.; Yuan, H.-Y.; Zhu, Y.-G. Advances in research on the use of biochar in soil for remediation: A review. J. Soils Sediments 2018, 18, 2433–2450. [Google Scholar] [CrossRef] [Green Version]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Singh, C.; Tiwari, S.; Singh, J. Impact of rice husk biochar on nitrogen mineralization and methanotrophs community dynamics in paddy soil. Int. J. Pure Appl. Biosci. 2017, 5, 428–435. [Google Scholar] [CrossRef]
- Dong, D.; Feng, Q.; Mcgrouther, K.; Yang, M.; Wang, H.; Wu, W. Effects of biochar amendment on rice growth and nitrogen retention in a waterlogged paddy field. J. Soils Sediments 2015, 15, 153–162. [Google Scholar] [CrossRef]
- Tubaña, B.S.; Heckman, J.R. Silicon in soils and plants. Silicon Plant Dis. 2015, 6, 7–51. [Google Scholar]
- Wang, Y.; Xiao, X.; Zhang, K.; Chen, B. Effects of biochar amendment on the soil silicon cycle in a soil-rice ecosystem. Environ. Pollut. 2019, 248, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Dietz, K.-J. Silicon as versatile player in plant and human biology: Overlooked and poorly understood. Front. Plant Sci. 2015, 6, 994. [Google Scholar] [CrossRef] [Green Version]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and plants: Current knowledge and future prospects. J. Plant Growth Regul. 2021, 40, 906–925. [Google Scholar] [CrossRef]
- Rizwan, M.; ur Rehman, M.Z.; Ali, S.; Abbas, T.; Maqbool, A.; Bashir, A. Biochar is a potential source of silicon fertilizer: An overview. Biochar Biomass Waste 2019, 225–238. [Google Scholar]
- Natasha, N.; Shahid, M.; Khalid, S.; Bibi, I.; Naeem, M.A.; Niazi, N.K.; Tack, F.M.; Ippolito, J.A.; Rinklebe, J. Influence of biochar on trace element uptake, toxicity and detoxification in plants and associated health risks: A critical review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2803–2843. [Google Scholar] [CrossRef]
- Azhar, M.; ur Rehman, M.Z.; Ali, S.; Qayyum, M.F.; Naeem, A.; Ayub, M.A.; ul Haq, M.A.; Iqbal, A.; Rizwan, M. Comparative effectiveness of different biochars and conventional organic materials on growth, photosynthesis and cadmium accumulation in cereals. Chemosphere 2019, 227, 72–81. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Chen, B. Biochar impacts on soil silicon dissolution kinetics and their interaction mechanisms. Sci. Rep. 2018, 8, 8040. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xiao, X.; Xu, Y.; Chen, B. Environmental effects of silicon within biochar (sichar) and carbon–silicon coupling mechanisms: A critical review. Environ. Sci. Technol. 2019, 53, 13570–13582. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Rangecroft, S.; Emmett, B.A.; Deluca, T.H.; Jones, D.L. Is biochar a source or sink for polycyclic aromatic hydrocarbon (PAH) compounds in agricultural soils? Gcb Bioenergy 2013, 5, 96–103. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sarkar, B.; Aralappanavar, V.K.; Mukhopadhyay, R.; Basak, B.B.; Srivastava, P.; Marchut-Mikołajczyk, O.; Bhatnagar, A.; Semple, K.T.; Bolan, N. Biochar-microorganism interactions for organic pollutant remediation: Challenges and perspectives. Environ. Pollut. 2022, 308, 119609. [Google Scholar] [CrossRef] [PubMed]
| Al (mg/kg) | Ca (mg/kg) | Si (mg/kg) | Mg (mg/kg) | As (mg/kg) | Reference |
|---|---|---|---|---|---|
| 0.02% | 0.08% | 0.55 | Chatzimichailidou et al., 2023 [33] | ||
| 189.00 | 691.00 | 11% | 357.00 | Samsuri et al., 2014 [34] | |
| 0.81 | 35% | 0.50 | Severo et al., 2020 [35] | ||
| 225 | 168 | 184 | Singh et al., 2018 [36] | ||
| 92–543 | 66–199 | 162–658 | 1.79–2.50 | Shackley et al., 2012 [37] | |
| 8.24 cmol/kg | 36.23 | 6.29 cmol/kg | Adebajo et al., 2022 [38] | ||
| 220 | 171 | 182 | Varela et al., 2013 [39] | ||
| 793 | 2245 | 166,338 | 900 | 3 | Prakongkep et al., 2013 [40] |
| 500 | 7804 | 149,449 | 1840 | ||
| 212 | 1340 | 193,748 | 1683 | 3 |
| Temperature (°C) | pH | H/C | O/C | SSA (m2/kg) | Reference |
|---|---|---|---|---|---|
| 250–300 | 7.4 | 0.79 | 0.22 | Abrishamkesh et al., 2015 [53] | |
| 300 | 5.7 | 0.09 | 0.42 | 8.0 | Shen et al., 2021 [55] |
| 500 | 9.8 | 0.04 | 0.14 | 25.0 | |
| 700 | 10.8 | 0.02 | 0.06 | 195 | |
| 300 | 8.65 | 1.192 | 0.264 | 6.54 | Abbas et al., 2018 [56] |
| 400 | 10.28 | 0.717 | 0.134 | 12.50 | |
| 500 | 11.36 | 0.578 | 0.067 | 20.11 | |
| 600 | 12.19 | 0.416 | 0.034 | 22.47 | |
| 300 | 7.47 | 0.89 | 0.61 | 2.57 | Wei et al., 2017 [57] |
| 500 | 10.47 | 0.42 | 0.53 | 18.4 | |
| 750 | 10.51 | 0.0199 | 0.679 | 53.08 | |
| 300 | 7.1 | 0.0695 | 0.3145 | 0.632 | Shi et al., 2019 [58] |
| 500 | 9.5 | 0.0459 | 0.1334 | 45.274 | |
| 700 | 9.8 | 0.0236 | 0.0848 | 193.149 | |
| 400 | 0.91 | 0.12 | 4.589 | Liao et al., 2022 [59] | |
| 600 | 0.54 | 0.06 | 34.782 | ||
| 350 | 6.41 | 1.10 | 0.52 | 11.61 | Pariyar et al., 2020 [60] |
| 450 | 6.92 | 0.91 | 0.30 | 18.58 | |
| 550 | 7.89 | 0.65 | 0.17 | 248.99 | |
| 650 | 7.97 | 0.58 | 0.11 | 280.97 | |
| 600 | 9.7 | 0.11 | 0.15 | 179.0 | Pratiwi et al., 2016 [61] |
| 300 | 0.0745 | 0.462 | 1.39 | Yi et al., 2016 [62] | |
| 600 | 0.0396 | 0.201 | 168.0 | ||
| 300 | 6.24 | 0.75 | 0.38 | 68.77 | Mayakaduwa et al., 2017 [63] |
| 500 | 7.17 | 0.51 | 0.37 | 169.81 | |
| 700 | 9.87 | 0.32 | 0.12 | 236.74 | |
| 700 | 10.72 | 0.47 | 0.24 | 242.53 | Huang et al., 2020 [64] |
| Biomass | SSA (m2/g) | H/C | O/C | Ash (%) | Reference |
|---|---|---|---|---|---|
| Rice husk | 292.595 | 0.05 | 0.35 | 66.56 | Jia et al., 2018 [104] |
| Rice husk | 118.2 | 0.422 | 35.4 | Severo et al., 2020 [35] | |
| Rice husk | 193.14 | 0.023 | 0.084 | 54.0 | Shi et al., 2019 [58] |
| Rice husk | 280.97 | 0.58 | 0.11 | Pariyar et al., 2020 [60] | |
| Rice husk | 181.9 | 0.021 | 0.052 | 38.26 | Wang et al., 2020 [105] |
| Corn cob | 180.1 | 0.15 | 0.60 | Liu et al., 2014 [106] | |
| Corn cob | 655.80 | 0.133 | 1.042 | 1.25 | Suwunwong et al., 2020 [107] |
| Corn cob | 14.589 | 0.136 | 0.772 | 8.30 | Liao et al., 2022 [59] |
| Corn cob | 0.37 | 0.14 | 4.0 | Jing et al., 2018 [108] | |
| Corn cob | 10.38 | 0.025 | 0.112 | 5.25 | Pipíška et al., 2022 [109] |
| sawn wood | 243.1 | 0.08 | 0.29 | Liu et al., 2014 [106] | |
| sawn wood | 32.8 | 0.35 | 0.11 | Wan et al., 2016 [110] | |
| sawn wood | 2.946 | 0.08 | 0.71 | 1.2 | Xu et al., 2019 [111] |
| sawn wood | 86.59 | 0.147 | 1.205 | 1.42 | Cheng et al., 2021 [112] |
| Wheat straw | 0.67 | 0.072 | 0.286 | 118 g/kg | Mierzwa-Hersztek et al., 2020 [113] |
| Wheat straw | 20.38 | 0.430 | 0.156 | 22.5 | Manna et al., 2020 [114] |
| Wheat straw | 58.38 | 0.040 | 0.443 | Rajabi et al., 2021 [115] | |
| Wheat straw | 2.94 | 0.73 | 0.19 | 16.12 | Chen et al., 2020 [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zheng, Z.; Li, H.; Xu, D.; Li, X.; Xiang, L.; Tu, S. Review on Rice Husk Biochar as an Adsorbent for Soil and Water Remediation. Plants 2023, 12, 1524. https://doi.org/10.3390/plants12071524
Li Z, Zheng Z, Li H, Xu D, Li X, Xiang L, Tu S. Review on Rice Husk Biochar as an Adsorbent for Soil and Water Remediation. Plants. 2023; 12(7):1524. https://doi.org/10.3390/plants12071524
Chicago/Turabian StyleLi, Zheyong, Zhiwei Zheng, Hongcheng Li, Dong Xu, Xing Li, Luojing Xiang, and Shuxin Tu. 2023. "Review on Rice Husk Biochar as an Adsorbent for Soil and Water Remediation" Plants 12, no. 7: 1524. https://doi.org/10.3390/plants12071524
APA StyleLi, Z., Zheng, Z., Li, H., Xu, D., Li, X., Xiang, L., & Tu, S. (2023). Review on Rice Husk Biochar as an Adsorbent for Soil and Water Remediation. Plants, 12(7), 1524. https://doi.org/10.3390/plants12071524