Antibiotic-Potentiating Activity of the Schinus terebinthifolius Raddi Essential Oil against MDR Bacterial Strains
Abstract
:1. Introduction
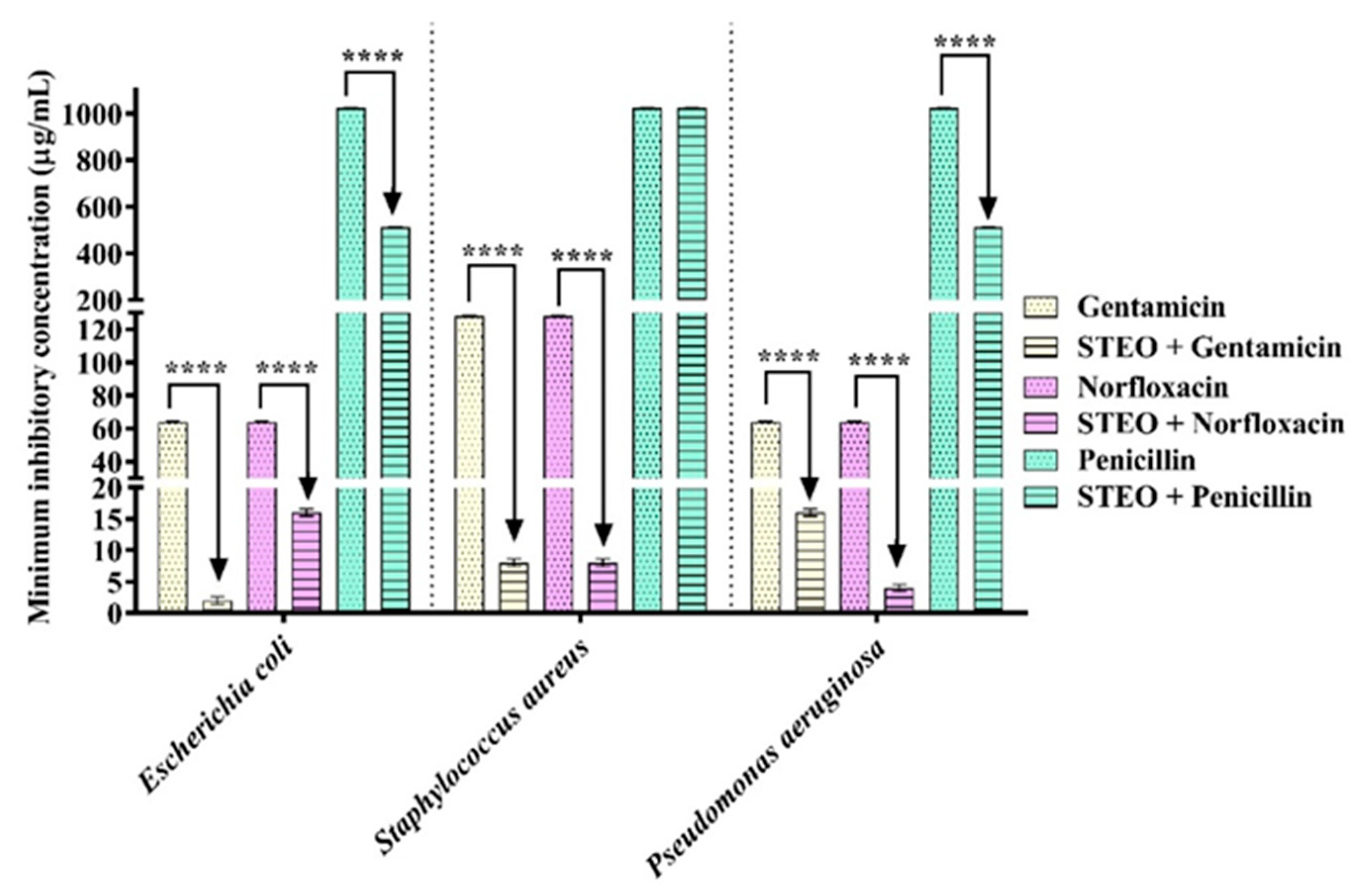
2. Results
3. Discussion
4. Materials and Methods
4.1. Botanical Material
4.2. Essential Oil Extraction and Characterization
4.3. Bacterial Strains
4.4. Bacterial Inocula
4.5. Drugs and Reagents
4.6. Minimum Inhibitory Concentration (MIC) Determination
4.7. Analysis of Antibiotic Activity Modulation
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conceição, A.K.C.; dos Santos Lira, Á.G.; Moreira, O.J.M.; de Sousa, L.M.R.; Pereira, H.J.M.; de Abreu, V.H.R.; Vieira, T.A. Plantas medicinais: Um saber tradicional como alternativa no processo de cura. Rev. Agroecossistemas 2018, 10, 238–254. [Google Scholar] [CrossRef] [Green Version]
- Castro, M.R.; Figueiredo, F.F. Saberes tradicionais, biodiversidade, práticas integrativas e complementares: O uso de plantas medicinais no SUS. Hygeia-Rev. Bras. De Geogr. Médica E Da Saúde 2019, 15, 56–70. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.L.S.; Rosal, L.F.; Oliveira, M.F.S.; Batista, R.F.; Montão, D.P. Conhecimentos sobre plantas medicinais de comunidades tradicionais em Viseu/Pará: Valorização e conservação. Rev. Bras. De Agroecol. 2019, 14, 72–83. [Google Scholar]
- Loureiro, R.J.; Roque, F.; Rodrigues, A.T.; Herdeiro, M.T.; Ramalheira, E. O uso de antibióticos e as resistências bacterianas: Breves notas sobre a sua evolução. Rev. Port. De Saúde Pública 2016, 34, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Lima, W.G.; Ramos-Alves, M.C.; Soares, A.C. From psychiatric disorders to antibiotic therapy: Repositioning of chlorprom-azine as an antibacterial agent. Rev. Colomb. De Cienc. Químico-Farm. 2019, 48, 5–28. [Google Scholar] [CrossRef]
- Garcia, R.; Garcia, F.A.D.O.; Pereira, P.S.; Coutinho, H.D.M.; Siyadatpanah, A.; Norouzi, R.; Wilairatana, P.; Pereira, M.D.L.; Nissapatorn, V.; Tintino, S.R.; et al. Microbial resistance: The role of efflux pump superfamilies and their respective substrates. Life Sci. 2022, 295, 120391. [Google Scholar] [CrossRef]
- Vikesland, P.J.; Pruden, A.; Alvarez, P.J.J.; Aga, D.; Bürgmann, H.; Li, X.; Manaia, C.M.; Nambi, I.; Wigginton, K.; Zhang, T.; et al. Toward a Comprehensive Strategy to Mitigate Dissemination of Environmental Sources of Antibiotic Resistance. Environ. Sci. Technol. 2017, 51, 13061–13069. [Google Scholar] [CrossRef] [Green Version]
- Brunder, W.; Schmidt, H.; Karch, H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 1997, 24, 767–778. [Google Scholar] [CrossRef]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit: A critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef]
- Garoy, E.Y.; Gebreab, Y.B.; Achila, O.O.; Tekeste, D.G.; Kesete, R.; Ghirmay, R.; Kiflay, R.; Tesfu, T. Methicillin-resistant Staphylococcus aureus (MRSA): Prevalence and antimicrobial sensitivity pattern among patients—A multicenter study in Asmara, Eritrea. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 8321834. [Google Scholar] [CrossRef] [Green Version]
- Boucher, H.W.; Corey, G.R. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holanda, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Yin, J.; Xiao, H.; Chen, S.; Liu, G.; Tan, B.; Li, N.; Peng, Y.; Li, T.; Zeng, B.; et al. Intestinal Microbiota-Derived GABA Mediates Interleukin-17 Expression during Enterotoxigenic Escherichia coli Infection. Front. Immunol. 2017, 7, 685. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.B.; Xiao, Y.H.; Zhang, Q.Y.; Zhou, M.; Liao, S.G. Hepatoprotective natural triterpenoids. Eur. J. Med. Chem. 2018, 145, 691–716. [Google Scholar] [CrossRef] [PubMed]
- Amison, R.T.; O’Shaughnessy, B.G.; Arnold, S.; Cleary, S.J.; Nandi, M.; Pitchford, S.C.; Bragonzi, A.; Page, C.P. Platelet Depletion Impairs Host Defense to Pulmonary Infection with Pseudomonas aeruginosa in Mice. Am. J. Respir. Cell Mol. Biol. 2018, 58, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrêa, R.C.G.; Heleno, S.A.; Alves, M.J.; Ferreira, I.C. Bacterial Resistance: Antibiotics of last generation used in clinical practice and the rise of natural products as new therapeutic alternatives. Curr. Pharm. Des. 2020, 26, 815–837. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.; Chan, A.N.; Wright, G.D. The Antibiotic Resistome: A Guide for the Discovery of Natural Products as Antimicrobial Agents. Chem. Rev. 2021, 121, 3464–3494. [Google Scholar] [CrossRef]
- Ferreira, I.V.; Mendes-Costa, M.C. Atividade antimicrobiana de extratos etanólicos de Banisteriopsis anisandra (Malpighiaceae) e interações medicamentosas. RSBO Rev. Sul-Bras. De Odontologia 2019, 16, 94. [Google Scholar]
- Miranda, C.C.S.; Salazar, V.A.C.; Lima, H.R.S.; Oliveira, K.D.; de Araújo, F.S.; Maria, B.; Brito, A.O.; Ester, P.; Letícia, S.; Bárbara, R.; et al. Atividade antibiótica de extratos e óleos essenciais frente à cepa Propionibacterium acnes. Res. Soc. Dev. 2020, 9, e2569108070. [Google Scholar] [CrossRef]
- Freitas, P.R.; de Araújo, A.C.J.; Barbosa, C.R.D.S.; Muniz, D.F.; Rocha, J.E.; Neto, J.B.D.A.; da Silva, M.M.C.; Pereira, R.L.S.; da Silva, L.E.; Amaral, W.D.; et al. Characterization and antibacterial activity of the essential oil obtained from the leaves of Baccharis coridifolia DC against multiresistant strains. Microb. Pathog. 2020, 145, 104223. [Google Scholar] [CrossRef]
- Ejim, L.; Farha, M.A.; Falconer, S.B.; Wildenhain, J.; Coombes, B.K.; Tyers, M.; Brown, E.D.; Wright, G. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat. Chem. Biol. 2011, 7, 348–350. [Google Scholar] [CrossRef]
- Freires, I.A.; Denny, C.; Benso, B.; De Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Silva, J.P.L.; Duarte-Almeida, J.M.; Perez, D.V.; Franco, B.D.G.D.M. Óleo essencial de orégano: Interferência da composição química na atividade frente a Salmonella enteritidis. Food Sci. Technol. 2010, 30, 136–141. [Google Scholar] [CrossRef] [Green Version]
- de Medeiros, V.M.; Nascimento, Y.M.D.; Souto, A.L.; Madeiro, S.A.L.; Costa, V.C.D.O.; Silva, S.M.P.; Silva, V.D.S.F.; Agra, M.D.F.; de Siqueira-Júnior, J.P.; Tavares, J.F. Chemical composition and modulation of bacterial drug resistance of the essential oil from leaves of Croton grewioides. Microb. Pathog. 2017, 111, 468–471. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Owolabi, M.S.; Ogundajo, A.L.; Dosoky, N.S.; Setzer, W.N. Chemical Composition and Antimicrobial Potential of Essential Oils of Leaf and Stem Bark of Haematostaphis barteri Hook. f. (Anacardiaceae). J. Essent. Oil Bear. Plants 2020, 23, 583–593. [Google Scholar] [CrossRef]
- Lamboro, T.; Mengistu, M.; Hordofa, T.G. Phytochemical screening, characterization of essential oil and antimicrobial activity of Schinus molle (Anacardiaceae) collected from Eastern Hararghe, Ethiopia. Braz. J. Nat. Sci. 2020, 3, 305. [Google Scholar] [CrossRef]
- Linden, M.; Brinckmann, C.; Feuereisen, M.M.; Schieber, A. Effects of structural differences on the antibacterial activity of biflavonoids from fruits of the Brazilian peppertree (Schinus terebinthifolius Raddi). Food Res. Int. 2020, 133, 109134. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.; Augusta, I.M.; Braz, M.V.D.C.; Riger, C.J.; Prudêncio, E.R.; Sawaya, A.C.H.F.; Sampaio, G.R.; Torres, E.A.F.D.S.; Saldanha, T. Aroeira fruit (Schinus terebinthifolius Raddi) as a natural antioxidant: Chemical constituents, bioactive compounds and in vitro and in vivo antioxidant capacity. Food Chem. 2020, 315, 126274. [Google Scholar] [CrossRef]
- Macedo, N.B.; Gutierrez, D.D.; Santos, A.S.; Pereira, R.O.; Gandhi, G.R.; Silva, M.D.G.D.O.E.; Vidal, A.; Júnior, L.J.Q.; Quintans, J.D.S.S.; Silva, A.M. Bioactive Compounds from Schinus terebinthifolius Raddi and Their Potential Health Benefits. In Phytopharmaceuticals: Potential Therapeutic Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021; p. 363. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Composition and Antimicrobial Activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. Essential Oils and Their Major Monoterpene Alcohols Alone and in Combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef]
- García-García, R.; López-Malo, A.; Palou, E. Bactericidal action of binary and ternary mixtures of carvacrol, thymol, and eugenol against Listeria innocua. J. Food Sci. 2011, 76, M95–M100. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef]
- Xu, J.-G.; Liu, T.; Hu, Q.-P.; Cao, X.-M. Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- Uliana, M.P.; Fronza, M.; da Silva, A.G.; Vargas, T.S.; Andrade, T.; Scherer, R. Composition and biological activity of Brazilian rose pepper (Schinus terebinthifolius Raddi) leaves. Ind. Crop. Prod. 2016, 83, 235–240. [Google Scholar] [CrossRef]
- Ennigrou, A.; Casabianca, H.; Vulliet, E.; Hanchi, B.; Hosni, K. Assessing the fatty acid, essential oil composition, their radical scavenging and antibacterial activities of Schinus terebinthifolius Raddi leaves and twigs. J. Food Sci. Technol. 2018, 55, 1582–1590. [Google Scholar] [CrossRef]
- Houghton, P.J.; Howes, M.-J.; Lee, C.C.; Steventon, G. Uses and abuses of in vitro tests in ethnopharmacology: Visualizing an elephant. J. Ethnopharmacol. 2007, 110, 391–400. [Google Scholar] [CrossRef]
- Cole, E.R.; Dos Santos, R.B.; Lacerda Júnior, V.; Martins, J.D.L.; Greco, S.J.; Cunha Neto, A. Chemical composition of essential oil from ripe fruit of Schinus terebinthifolius Raddi and evaluation of its activity against wild strains of hospital origin. Braz. J. Microbiol. 2014, 45, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.Z.M.; El-Hefny, M.; Ali, H.M.; Elansary, H.O.; Nasser, R.A.; El-Settawy, A.A.A.; Shanhorey, E.A.; Ashmawy, N.A.; Salem, A.Z.M. Antibacterial activity of extracted bioactive molecules of Schinus terebinthifolius ripened fruits against some pathogenic bacteria. Microb. Pathog. 2018, 120, 119–127. [Google Scholar] [CrossRef]
- Gundidza, M.; Gweru, N.; Magwa, M.L.; Mmbengwa, V.; Samie, A. The chemical composition and biological activities of essential oil from the fresh leaves of Schinus terebinthifolius from Zimbabwe. Afr. J. Biotechnol. 2009, 8, 7164–7169. [Google Scholar]
- EL-Nashar, H.A.S.; Mostafa, N.M.; El-Badry, M.A.; Eldahshan, O.A.; Singab, A.N.B. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) cabrera leaf and bark grown in Egypt. Nat. Prod. Res. 2021, 35, 5369–5372. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Freitas, P.R.; de Araújo, A.C.J.; Barbosa, C.R.D.S.; Muniz, D.F.; da Silva, A.C.A.; Rocha, J.E.; Oliveira-Tintino, C.D.D.M.; Ribeiro-Filho, J.; da Silva, L.E.; Confortin, C.; et al. GC-MS-FID and potentiation of the antibiotic activity of the essential oil of Baccharis reticulata (ruiz & pav.) pers. and α-pinene. Ind. Crop. Prod. 2020, 145, 112106. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Bezerra, C.F.; Camilo, C.J.; Do Nascimento Silva, M.K.; de Freitas, T.S.; Ribeiro-Filho, J.; Coutinho, H.D.M. Vanillin selectively modulates the action of antibiotics against resistant bacteria. Microb. Pathog. 2017, 113, 265–268. [Google Scholar] [CrossRef]
- Javadpour, M.M.; Juban, M.M.; Lo, W.C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De novo anti-microbial peptides with low mammalian cell toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef]
- Coutinho, H.D.; Costa, J.G.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of the Antibiotic Activity against a Multiresistant Escherichia coli by Mentha arvensis L. and Chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef]
| Constituent | % | RI * | Constituent | % | RI * |
|---|---|---|---|---|---|
| alpha-pinene | 24.3 | 937 | (E)-beta-farnesene + allo-aromadendrene | 0.9 | 1457 |
| alpha-fenchene | 0.3 | 951 | gama-muurolene | 16.6 | 1478 |
| sabinene | 1.0 | 975 | Bicyclogermacrene | 3.8 | 1492 |
| beta-pinene | 4.1 | 979 | alpha-muurolene | 0.5 | 1495 |
| myrcene | 13.7 | 991 | germacrene A | 1.1 | 1499 |
| para-cymene | 0.4 | 1025 | delta-cadinene | 1.8 | 1519 |
| limonene | 1.5 | 1030 | germacrene B | 0.6 | 1551 |
| (Z)-beta ocimene | 0.3 | 1039 | Spathulenol | 1.7 | 1573 |
| terpinen-4-ol | 0.9 | 1176 | caryophyllene oxide | 2.1 | 1577 |
| alpha-terpineol | 0.9 | 1189 | Viridiflorol | 0.6 | 1585 |
| alpha-copaene | 3.8 | 1373 | cubeban-11-ol | 0.4 | 1587 |
| beta-elemene | 1.6 | 1389 | epi-alpha-cadinol | 1.7 | 1637 |
| (E)-caryophyllene | 8.9 | 1416 | alpha-muurolol | 0.6 | 1641 |
| alpha-humulene | 1.1 | 1449 | alpha-cadinol | 2.3 | 1649 |
| Strain | Origin | Resistance Profile |
|---|---|---|
| E. coli 06 | Urine | Asb, Ca, Cef, Cfo, Cpm, Cro |
| P. aeruginosa 24 | Nasal Discharge | Ami, Cip, Cpm, Ctz, Imi, Lev, Mer, Ptz |
| S. aureus 10 | Rectal Swab | Oxa, Pen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa da Silva, M.M.; Bezerra de Araújo Neto, J.; Lucas dos Santos, A.T.; de Morais Oliveira-Tintino, C.D.; de Araújo, A.C.J.; Freitas, P.R.; da Silva, L.E.; do Amaral, W.; Deschamps, C.; de Azevedo, F.R.; et al. Antibiotic-Potentiating Activity of the Schinus terebinthifolius Raddi Essential Oil against MDR Bacterial Strains. Plants 2023, 12, 1587. https://doi.org/10.3390/plants12081587
Costa da Silva MM, Bezerra de Araújo Neto J, Lucas dos Santos AT, de Morais Oliveira-Tintino CD, de Araújo ACJ, Freitas PR, da Silva LE, do Amaral W, Deschamps C, de Azevedo FR, et al. Antibiotic-Potentiating Activity of the Schinus terebinthifolius Raddi Essential Oil against MDR Bacterial Strains. Plants. 2023; 12(8):1587. https://doi.org/10.3390/plants12081587
Chicago/Turabian StyleCosta da Silva, Maria Milene, José Bezerra de Araújo Neto, Antonia Thassya Lucas dos Santos, Cícera Datiane de Morais Oliveira-Tintino, Ana Carolina Justino de Araújo, Priscilla Ramos Freitas, Luiz Everson da Silva, Wanderlei do Amaral, Cícero Deschamps, Francisco Roberto de Azevedo, and et al. 2023. "Antibiotic-Potentiating Activity of the Schinus terebinthifolius Raddi Essential Oil against MDR Bacterial Strains" Plants 12, no. 8: 1587. https://doi.org/10.3390/plants12081587
APA StyleCosta da Silva, M. M., Bezerra de Araújo Neto, J., Lucas dos Santos, A. T., de Morais Oliveira-Tintino, C. D., de Araújo, A. C. J., Freitas, P. R., da Silva, L. E., do Amaral, W., Deschamps, C., de Azevedo, F. R., Gonçalves Lima, C. M., Golubkina, N., Calixto-Júnior, J. T., Ribeiro-Filho, J., Coutinho, H. D. M., Caruso, G., & Tintino, S. R. (2023). Antibiotic-Potentiating Activity of the Schinus terebinthifolius Raddi Essential Oil against MDR Bacterial Strains. Plants, 12(8), 1587. https://doi.org/10.3390/plants12081587














