Phenolics Profiling by HPLC-DAD-ESI/MSn of the Scientific Unknown Polygonum hydropiperoides Michx. and Its Antioxidant and Anti-Methicillin-Resistant Staphylococcus aureus Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization
2.1.1. Phenolic Acids
2.1.2. Flavonoids
2.1.3. Other Compounds
2.2. In Vitro Antioxidant Activity
2.3. In Vitro Antibacterial Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Extraction Procedures
3.4. Chromatographic Analysis
3.5. In Vitro Antioxidant Activity
3.5.1. Phosphomolybdenum Reducing Power
3.5.2. Nitric Oxide (NO) Inhibition
3.5.3. β-Carotene Bleaching Assay
3.6. In Vitro Antibacterial Activity
Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) Determinations
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development Of New Antibiotics; WHO Press: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization (WHO). Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries: A Practical Toolkit; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Bassetti, M.; Eckmann, C.; Peghin, M.; Carnelutti, A.; Righi, E. When to switch to an oral treatment and/or to discharge a patient with skin and soft tissue infections. Curr. Opin. Infect. Dis. 2018, 31, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Mantzarlis, K.; Tsolaki, V.; Zakynthinos, E. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid. Med. Cell. Longev. 2017, 2017, 5985209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayorga, O.A.S.; da Costa, Y.F.G.; da Silva, J.B.; Scio, E.; Ferreira, A.L.P.; de Sousa, O.V.; Alves, M.S. Kalanchoe brasiliensis Cambess, a promising natural source of antioxidant and antibiotic agents against multidrug-resistant pathogens for the treatment of Salmonella gastroenteritis. Oxid. Me. Cell. Longev. 2019, 2019, 9245951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutra, R.C.; Campos, M.M.; Santos, A.R.S.; Calixto, J.B. Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol. Res. 2016, 112, 4–29. [Google Scholar] [CrossRef]
- Grandi, T.S.M. Tratado das Plantas Medicinais Mineiras, Nativas e Cultivadas, 1st ed.; Adequatio Estúdio: Belo Horizonte, Brazil, 2014. [Google Scholar]
- Macedo, J.F. Informações preliminares sobre a distribuição do gênero Polygonum L. (Polygonaceae) no estado de Minas Gerais. Daphne 1993, 3, 39–44. [Google Scholar]
- Narasimhulu, G.; Reddy, K.K.; Mohamed, J. The genus Polygonum (Polygonaceae): An ethnopharmacological and phytochemical perspectives—Review. Int. J. Pharm. Pharm. Sci. 2014, 6, 21–45. [Google Scholar]
- Datta, B.K.; Datta, S.K.; Rashid, M.A.; Sarker, S.D. Flavonoids from Polygonum stagninum (Polygonaceae). Biochem. Syst. Ecol. 2002, 30, 693–696. [Google Scholar] [CrossRef]
- Jácome, R.L.R.P.; Lopes, D.E.S.; Recio, R.A.; Macedo, J.F.; Oliveira, A.B. Caracterização farmacognóstica de Polygonum hydropiperoides Michaux e P. spectabile (Mart.) (Polygonaceae). Rev. Bras. Farmacogn. 2004, 14, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Brasil Agência Nacional De Vigilância Sanitária (ANVISA). Monografia das Espécies Polygonum Hydropiperoides e Polygonum Acre (Erva-de-Bicho); ANVISA: Brasília, Brazil, 2014.
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G. Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra-high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Sarnoski, P.J.; Johnson, J.V.; Reed, K.A.; Tanko, J.M.; O’keefe, S.F. Separation and characterization of proanthocyanidins in Virginia type peanut skins by LC-MSn. Food Chem. 2012, 131, 927–939. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.R.; Guo, D.A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Verardo, G.; Duse, I.; Callea, A. Analysis of underivatized oligosaccharides by liquid chromatography/electrospray ionization tandem mass spectrometry with post-column addition of formic acid. Rapid Commun. Mass Spectrom. 2009, 23, 1607–1618. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Pascoal-Neto, C.; Silvestre, A.J.D. Ultra-high performance liquid chromatography coupled to mass spectrometry applied to the identification of valuable phenolic compounds from Eucalyptus wood. J. Chromatogr. B 2013, 938, 65–74. [Google Scholar] [CrossRef]
- Nikolaeva, G.G.; Lavrent’eva, M.V.; Nikolaeva, I.G. Phenolic compounds from several Polygonum species. Chem. Nat. Compd. 2009, 45, 735–736. [Google Scholar] [CrossRef]
- Quesada-Romero, L.; Fernández-Galleguillos, C.; Bergmann, J.; Amorós, M.E.; Jiménez-Aspee, F.; González, A.; Simirgiotis, M.; Rossini, C. Phenolic fingerprinting, antioxidant, and deterrent potentials of Persicaria maculate extracts. Molecules 2020, 25, 3054. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.C.; Sousa, C.P.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complement. Altern. Med. 2019, 19, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawłowska, K.A.; Hałasa, R.; Dudek, M.K.; Majdan, M.; Jankowska, K.; Granica, S. Antibacterial and anti-inflammatory activity of bistort (Bistorta officinalis) aqueous extract and its major components. Justification of the usage of the medicinal plant material as a traditional topical agent. J. Ethnopharmacol. 2020, 260, 113077. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Murcia, A.; Butler, J.; Halliwell, B. Evaluation of the antioxidant and prooxidant actions of gallic acid and its derivatives. J. Agric. Food Chem. 1993, 41, 1880–1885. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Salgado, H.R.N. Gallic acid: Review of the methods of determination and quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Berwal, M.K.; Haldhar, S.M.; Ram, C.; Gora, J.S.; Singh, D.; Samadia, D.K. GC-MS/MS-Based phytochemical screening of therapeutic potential of Calligonum polygonoides L. flower bud against chronic diseases. Pharmacogn. Mag. 2021, 17, S68–S76. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, J.Y.; Lee, J.E.; Jung, Y.W.; Jeong, W.; Hong, S.S.; Cho, Y.R.; Choi, C.W. Skin-related properties and constituents from the aerial parts extract of Persicaria senticosa. Oxid. Med. Cell. Longev. 2020, 2020, 6627752. [Google Scholar] [CrossRef]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Comprehensive assessment of phenolics and antiradical potential of Rumex hastatus D. Don. Roots. BMC Complement. Altern. Med. 2014, 14, 47. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100-S30, 30th ed.; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Bussmann, R.W.; Malca-García, G.; Glenn, A.; Sharon, D.; Chait, G.; Díaz, D.; Pourmand, K.; Jonat, B.; Somogy, S.; Guardado, G.; et al. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J. Ethnopharmacol. 2010, 132, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Miranda, G.S.; Santana, G.S.; Machado, B.B.; Coelho, F.P.; Carvalho, C.A. Atividade antibacteriana in vitro de quatro espécies vegetais em diferentes graduações alcoólicas. Rev. Bras. Plantas Med. 2013, 15, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Bouzada, M.L.M.; Fabri, R.L.; Nogueira, M.; Konno, T.U.P.; Duarte, G.G.; Scio, E. Antibacterial, cytotoxic and phytochemical screening of some traditional medicinal plants in Brazil. Pharm. Biol. 2009, 47, 44–52. [Google Scholar] [CrossRef]
- da Silva, J.B.; de Bessa, M.E.; Mayorga, O.A.S.; Andrade, V.T.; da Costa, Y.F.G.; Mendes, R.F.; Ferreira, A.L.P.; Scio, E.; Alves, M.S. A promising antibiotic, synergistic and antibiofilm effects of Vernonia condensata Baker (Asteraceae) on Staphylococcus aureus. Microb. Pathog. 2018, 123, 385–392. [Google Scholar] [CrossRef]
- Kirmusaoğlu, S. MRSA and MSSA: The Mechanism of Methicillin Resistance and the Influence of Methicillin Resistance on Biofilm Phenotype of Staphylococcus aureus. In The Rise of Virulence and Antibiotic Resistance in Staphylococcus aureus, 1st ed.; Enany, S., Alexander, L.E.C., Eds.; Chapter 2; IntechOpen: London, UK, 2017; pp. 25–41. [Google Scholar]
- Dzoyem, J.P.; NKuete, A.H.L.; Kuete, V.; Tala, M.F.; Wabo, H.K.; Guru, S.K.; Rajput, V.S.; Sharma, A.; Tane, P.; Khan, I.A.; et al. Cytotoxicity and antimicrobial activity of the methanol extract and compounds from Polygonum limbatum. Planta Med. 2012, 78, 787–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, G.Y.; Wang, G.C.; Zhao, Y.B.; Xu, G.L.; Hao, X.Y.; Han, J.; Zhao, Q. Screening of Chinese medicinal plants for inhibition against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). J. Ethnopharmacol. 2008, 120, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Bennett, R.N.; Rosa, E.A.S. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 2009, 26, 746–757. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [Green Version]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination therapy for treatment of infections with Gram-negative bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef] [Green Version]
- World Flora Online. Polygonum hydropiperoides Michx. Available online: Worldfloraonline.org/taxon/wfo-0000489907 (accessed on 18 February 2023).
- Simões, C.M.O.; Schenkel, E.; Gosmann, G.; de Mello, J.C.P.; Mentz, L.A.; Petrovick, P.R. Farmacognosia: Da Planta ao Medicamento, 5th ed; Editora da UFRGS: Porto Alegre, Brazil; Editora da UFSC: Florianópolis, Brazil, 2004. [Google Scholar]
- Gouveia-Figueira, S.C.; Castilho, P.C. Phenolic screening by HPLC–DAD–ESI/MSn and antioxidant capacity of leaves, flowers and berries of Rubus grandifolius Lowe. Ind. Crops Prod. 2015, 73, 28–40. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantification of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Clinical And Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. CLSI Document M07-A10, 10th ed.; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]
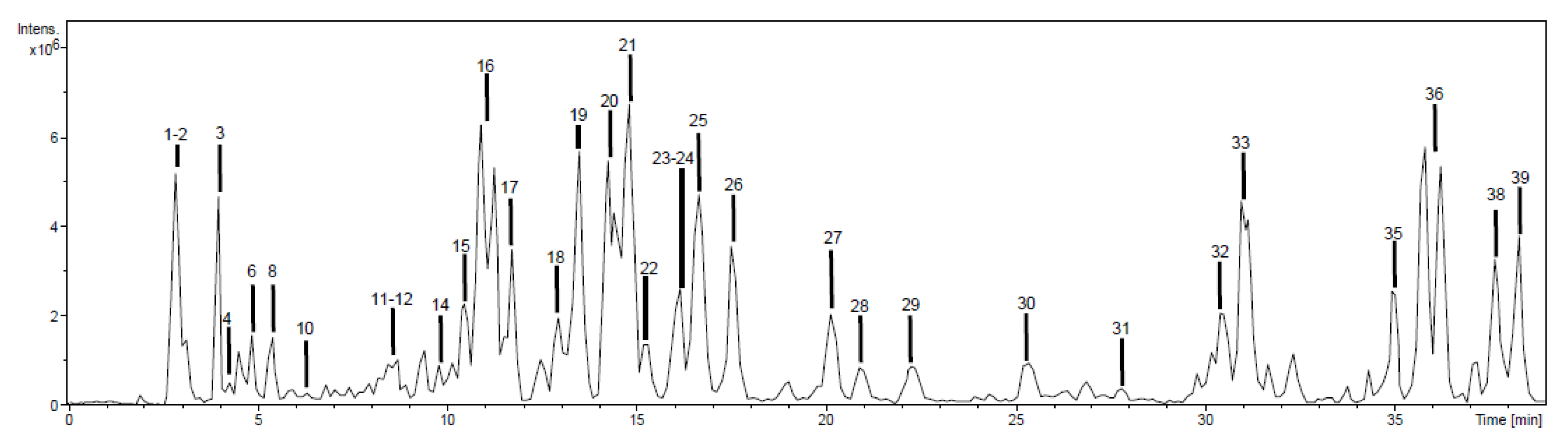
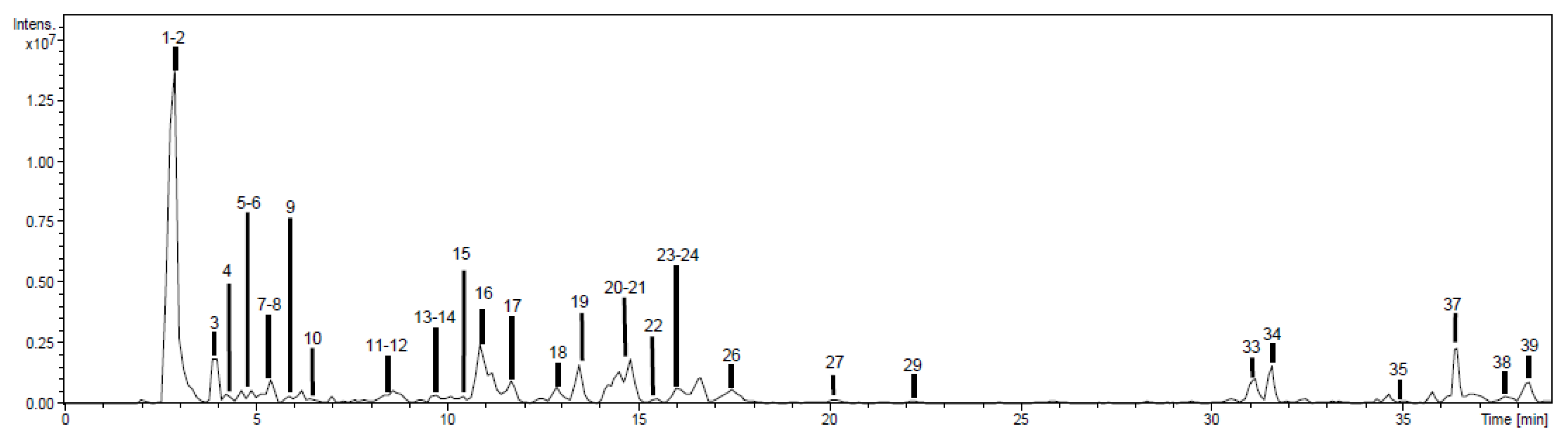
| No. | Assigned Identification | EAE-Ph | EE-Ph |
|---|---|---|---|
| Phenolic Acids | |||
| 2 | Galloyl glucose | 43.00 ± 3.00 | 48.00 ± 1.00 |
| 3 | Gallic acid | 21.00 ± 1.00 | 4.20 ± 0.01 |
| 29 | Gallic acid derivative | 0.26 ± 0.01 | -- |
| Total | 64.26 ± 4.01 | 52.20 ± 1.01 | |
| Flavonoids | |||
| 6 | Procyanidin dimer | 3.40 ± 0.10 | -- |
| 8 | Catechin | 4.10 ± 0.20 | 1.10 ± 0.10 |
| 15 | Myricetin-O-deoxyhexoside | 0.15 ± 0.02 | -- |
| 16 | Quercetin-O-hexoside | 4.00 ± 0.20 | 0.68 ± 0.01 |
| 17 | (Epi)catechin-O-gallate | 4.80 ± 0.50 | 1.07 ± 0.08 |
| 18 | Isorhamnetin-O-rutinoside | 0.38 ± 0.03 | 0.08 ± 0.01 |
| 19 | Quercetin-O-pentoside | 1.60 ± 0.10 | 0.19 ± 0.01 |
| 20 + 21 | Quercetin glycosides | 6.30 ± 0.30 | 0.73 ± 0.01 |
| 22 | Quercetin-O-acetylhexoside | 0.32 ± 0.03 | 0.01 ± 0.00 |
| 27 | Isorhamnetin-O-deoxyhexoside | 0.45 ± 0.01 | 0.01 ± 0.00 |
| 28 | Quercetin-O-acetylpentoside | 0.25 ± 0.02 | -- |
| 30 | Quercetin-O-acetylpentoside | 0.31 ± 0.03 | -- |
| 32 | Mearnsetin | 0.44 ± 0.05 | -- |
| 33 | Quercetin | 2.35 ± 0.03 | 0.18 ± 0.01 |
| 39 | Isorhamnetin | 2.11 ± 0.05 | 0.17 ± 0.01 |
| Total | 30.96 ± 1.67 | 4.22 ± 0.24 | |
| TIPC | 95.22 ± 5.68 | 56.42 ± 1.25 | |
| Sample | Phosphomolybdenum Reducing Power | Inhibition of Nitric Oxide Production (%) | β-Carotene | ||||
|---|---|---|---|---|---|---|---|
| AAE (µg/mg) | 400 µg/mL | 200 µg/mL | 100 µg/mL | 50 µg/mL | 25 µg/mL | Inhibition of Lipid Peroxidation (%) | |
| EAE-Ph | 214.57 ± 23.99 a,*,# | 67.96 ± 3.22 †,‡ | 66.30 ± 3.33 †,‡ | 56.86 ± 3.11 †,‡ | 45.06 ± 5.58 e | 37.48 ± 9.13 g | 74.44 ± 5.17 ¤,Φ,i |
| EE-Ph | 230.82 ± 4.60 a,*,# | 47.56 ± 1.71 b | 43.05 ± 1.60 c | 33.73 ± 2.35 d | 22.36 ± 1.49 f | 15.31 ± 2.95 h | 45.56 ± 8.62 Φ,¥,j |
| Gallic acid | 160.78 ± 17.04 | 37.62 ± 7.25 b | 33.96 ± 2.43 c | 31.94 ± 1.33 d | 29.70 ± 0.11 e,f | 25.99 ± 1.87 g,h | - |
| Quercetin | 424.70 ± 10.99 | - | - | - | - | - | 43.33 ± 5.47 j |
| Trolox | - | 42.43 ± 8.69 b | 37.25 ± 9.25 c | 32.79 ± 7.68 d | 29.93 ± 10.69 e,f | 26.92 ± 8.73 g,h | - |
| Rutin | - | - | - | - | - | - | 27.78 ± 7.43 |
| BHT | - | - | - | - | - | - | 61.11 ± 5.33 i |
| Bacterial Strain | MIC (µg/mL) | MBC (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| HE-Ph | EAE-Ph | EE-Ph | AMP | CHL | HE-Ph | EAE-Ph | EE-Ph | |
| Staphylococcus aureus (ATCC 6538) | >5000 | 1250 2 | 1250 2 | <4 a | 8 c | >5000 | 5000 | 5000 |
| Staphylococcus aureus (ATCC 29213) | >5000 | 1250 2 | 1250 2 | <4 a | 16 c | >5000 | 5000 | 5000 |
| Staphylococcus aureus (ATCC 25923) | >5000 | 1250 2 | 1250 2 | <4 a | 16 c | >5000 | >5000 | 5000 |
| Escherichia coli (ATCC 10536) | >5000 | 5000 2 | >5000 | <4 b | 4 c | >5000 | >5000 | >5000 |
| Escherichia coli (ATCC 25922) | >5000 | 5000 2 | >5000 | <4 b | 4 c | >5000 | >5000 | >5000 |
| Salmonella Choleraesuis (ATCC 10708) | >5000 | 5000 2 | 5000 2 | <4 b | 4 c | >5000 | >5000 | >5000 |
| Salmonella Typhimurium (ATCC 13311) | >5000 | 5000 2 | 5000 2 | <4 b | 4 c | >5000 | >5000 | >5000 |
| Pseudomonas aeruginosa (ATCC 9027) | >5000 | 5000 1 | 5000 2 | >500 | 8 | >5000 | 5000 | >5000 |
| Pseudomonas aeruginosa (ATCC 27853) | >5000 | >5000 | >5000 | >500 | 8 | >5000 | >5000 | >5000 |
| MRSA 1485279 | >5000 | 1250 2 | 1250 2 | >500 a | 125 c | >5000 | 2500 | 2500 |
| MRSA 1605677 | >5000 | 1250 2 | 1250 2 | >500 a | 8 c | >5000 | 5000 | 5000 |
| MRSA 1664534 | >5000 | 625 2 | 1250 2 | 32 a | 16 c | >5000 | 2500 | 5000 |
| MRSA 1688441 | >5000 | 1250 2 | 1250 2 | 500 a | 8 c | >5000 | 5000 | 2500 |
| MRSA 1830466 | >5000 | 1250 2 | 1250 2 | 250 a | 16 c | >5000 | 2500 | 5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, Y.F.G.; Llorent-Martínez, E.J.; Fernandes, L.S.; de Freitas, P.H.S.; Scio, E.; de Sousa, O.V.; Castilho, P.C.; Alves, M.S. Phenolics Profiling by HPLC-DAD-ESI/MSn of the Scientific Unknown Polygonum hydropiperoides Michx. and Its Antioxidant and Anti-Methicillin-Resistant Staphylococcus aureus Activities. Plants 2023, 12, 1606. https://doi.org/10.3390/plants12081606
da Costa YFG, Llorent-Martínez EJ, Fernandes LS, de Freitas PHS, Scio E, de Sousa OV, Castilho PC, Alves MS. Phenolics Profiling by HPLC-DAD-ESI/MSn of the Scientific Unknown Polygonum hydropiperoides Michx. and Its Antioxidant and Anti-Methicillin-Resistant Staphylococcus aureus Activities. Plants. 2023; 12(8):1606. https://doi.org/10.3390/plants12081606
Chicago/Turabian Styleda Costa, Ygor Ferreira Garcia, Eulogio José Llorent-Martínez, Laura Silva Fernandes, Pedro Henrique Santos de Freitas, Elita Scio, Orlando Vieira de Sousa, Paula Cristina Castilho, and Maria Silvana Alves. 2023. "Phenolics Profiling by HPLC-DAD-ESI/MSn of the Scientific Unknown Polygonum hydropiperoides Michx. and Its Antioxidant and Anti-Methicillin-Resistant Staphylococcus aureus Activities" Plants 12, no. 8: 1606. https://doi.org/10.3390/plants12081606








