Low-Arsenic Accumulating Cabbage Possesses Higher Root Activities against Oxidative Stress of Arsenic
Abstract
:1. Introduction
2. Results
2.1. Biomass and Root Length
2.2. Arsenic Concentration and Speciation
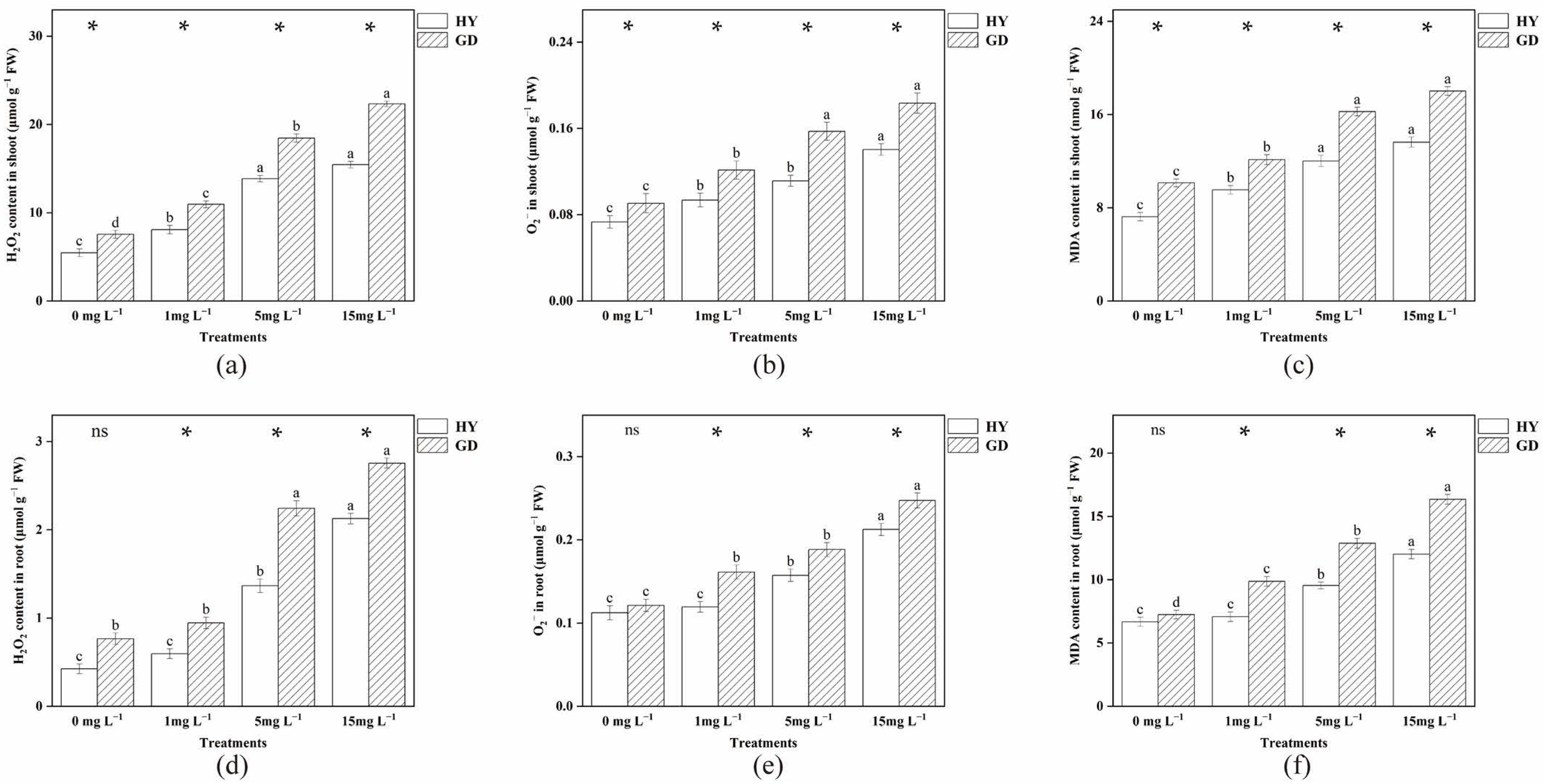
2.3. Accumulation of O2−, H2O2 and MDA Content
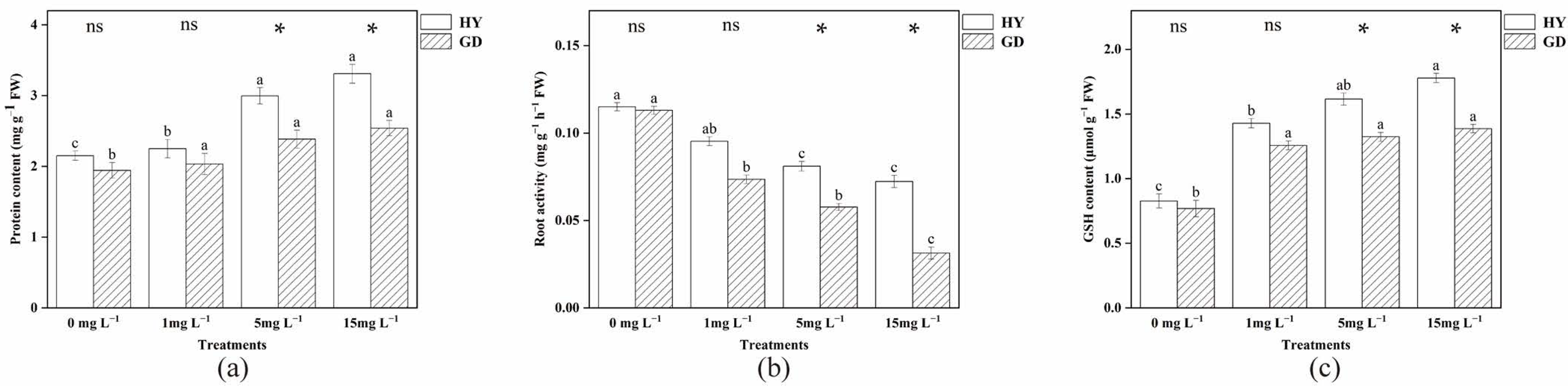
2.4. Analysis of Root Protein Content, Root Activity, and Glutathione (GSH)
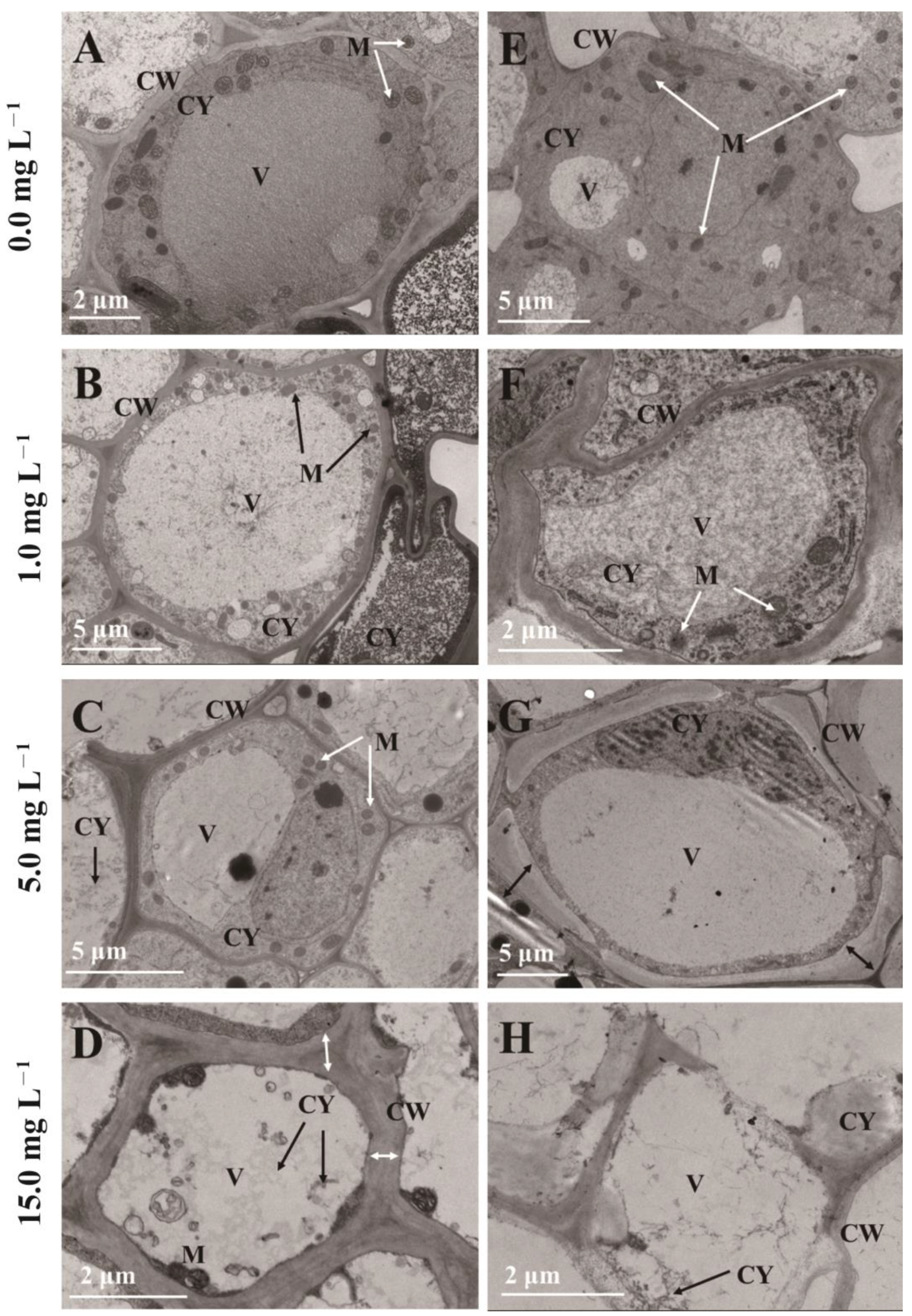
2.5. Ultrastructure of Root Cells
2.6. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Harvesting and Sampling
4.3. Measurement of Arsenic Concentration
4.4. Analysis of Root Protein Content, GSH Content, and Root Activity
4.5. Analysis of MDA Content and Reactive Oxygen Species (ROS)
4.6. Investigation of Root Ultrastructure
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Ma, L.Q.; Santos, J.; Rathinasabapathi, B.; Stamps, B.; Littell, R.C. Effects of arsenic species and concentrations on arsenic accumulation by different fern species in a hydroponic system. Int. J. Phytorem. 2005, 7, 231–240. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Marzaioli, R.; De Crescenzo, S.; Trifuoggi, M. Human health risk from consumption of two common crops grown in polluted soils. Sci. Total Environ. 2019, 691, 195–204. [Google Scholar] [CrossRef]
- Xu, X.W.; Chen, C.; Wang, P.; Kretzschmar, R.; Zhao, F.J. Control of arsenic mobilization in paddy soils by manganese and iron oxides. Environ. Pollut. 2017, 231, 37–47. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Meharg, A.A. Arsenic in rice—Understanding a new disaster for South-East Asia. Trends Plant Sci. 2004, 9, 415–417. [Google Scholar] [CrossRef]
- Islam, S.; Rahman, M.M.; Islam, M.R.; Naidu, R. Effect of irrigation and genotypes towards reduction in arsenic load in rice. Sci. Total Environ. 2017, 609, 311–318. [Google Scholar] [CrossRef]
- Wang, J.L.; Yuan, J.G.; Yang, Z.Y.; Huang, B.F.; Zhou, Y.H.; Xin, J.L.; Gong, Y.L.; Yu, H. Variation in Cadmium Accumulation among 30 Cultivars and Cadmium Subcellular Distribution in 2 Selected Cultivars of Water Spinach (Ipomoea aquatica Forsk.). J. Agric. Food. Chem. 2009, 57, 8942–8949. [Google Scholar] [CrossRef]
- Zhao, H.M.; Du, H.; Xiang, L.; Chen, Y.L.; Lu, L.A.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H. Variations in phthalate ester (PAE) accumulation and their formation mechanism in Chinese flowering cabbage (Brassica parachinensis L.) cultivars grown on PAE-contaminated soils. Environ. Pollut. 2015, 206, 95–103. [Google Scholar]
- Zhou, Q.; Yang, Y.C.; Yang, Z.Y. Molecular dissection of cadmium-responsive transcriptome profile in a low cadmium-accumulating cultivar of Brassica parachinensis. Ecotox. Environ. Safe. 2019, 176, 85–94. [Google Scholar] [CrossRef]
- Mi, B.; Liu, F.; Xie, L.; Zhou, H.; Wu, F.; Dai, X. Evaluation of the uptake capacities of heavy metals in Chinese cabbage. Ecotox. Environ. Safe. 2019, 171, 511–517. [Google Scholar] [CrossRef]
- Huang, W.X.; Chen, X.W.; Wu, L.; Yu, Z.S.; Gao, M.Y.; Zhao, H.M.; Mo, C.H.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; et al. Root cell wall chemistry remodelling enhanced arsenic fixation of a cabbage cultivar. J. Hazard. Mater. 2021, 420, 126165. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, Y.; Zhang, H.; Wang, L.; Sun, G.Q.; Sun, X.; Erinle, K.O.; Feng, C.C.; Song, Q.X.; Li, M. Effect of di-n-butyl phthalate on root physiology and rhizosphere microbial community of cucumber seedlings. J. Hazard. Mater. 2015, 289, 9–17. [Google Scholar] [CrossRef]
- Kummerova, M.; Zezulka, S.; Babula, P.; Vanova, L. Root response in Pisum sativum and Zea mays under fluoranthene stress: Morphological and anatomical traits. Chemosphere 2013, 90, 665–673. [Google Scholar] [CrossRef]
- Kubo, K.; Watanabe, Y.; Matsunaka, H.; Seki, M.; Fujita, M.; Kawada, N.; Hatta, K.; Nakajima, T. Differences in Cadmium Accumulation and Root Morphology in Seedlings of Japanese Wheat Varieties with Distinctive Grain Cadmium Concentration. Plant Prod. Sci. 2011, 14, 148–155. [Google Scholar] [CrossRef]
- Kofronova, M.; Hrdinova, A.; Maskova, P.; Soudek, P.; Tremlova, J.; Pinkas, D.; Lipavska, H. Strong antioxidant capacity of horseradish hairy root cultures under arsenic stress indicates the possible use of Armoracia rusticana plants for phytoremediation. Ecotox. Environ. Safe. 2019, 174, 295–304. [Google Scholar] [CrossRef]
- Tylová, E.; Pecková, E.; Blascheová, Z.; Soukup, A. Casparian bands and suberin lamellae in exodermis of lateral roots: An important trait of roots system response to abiotic stress factors. Ann. Bot. 2017, 120, 71–85. [Google Scholar] [CrossRef]
- Bari, M.A.; Akther, M.S.; Abu Reza, M.; Kabir, A.H. Cadmium tolerance is associated with the root-driven coordination of cadmium sequestration, iron regulation, and ROS scavenging in rice. Plant Physiol. Biochem. 2019, 136, 22–33. [Google Scholar] [CrossRef]
- Li, T.Q.; Tao, Q.; Shohag, M.J.I.; Yang, X.E.; Sparks, D.L.; Liang, Y.C. Root cell wall polysaccharides are involved in cadmium hyperaccumulation in Sedum alfredii. Plant Soil. 2015, 389, 387–399. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gill, R.A.; Ali, B.; Wang, J.; Islam, F.; Ali, S.; Zhou, W.J. Subcellular distribution, modulation of antioxidant and stress-related genes response to arsenic in Brassica napus L. Ecotoxicology. 2016, 25, 350–366. [Google Scholar] [CrossRef]
- Chmielowska-Bak, J.; Gzyl, J.; Rucinska-Sobkowiak, R.; Arasimowicz-Jelonek, M.; Deckert, J. The new insights into cadmium sensing. Front. Plant Sci. 2014, 5, 245. [Google Scholar] [PubMed]
- Armendariz, A.L.; Talano, M.A.; Travaglia, C.; Reinoso, H.; Oller, A.L.W.; Agostini, E. Arsenic toxicity in soybean seedlings and their attenuation mechanisms. Plant Physiol. Biochem. 2016, 98, 119–127. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Hu, C.X.; Wang, X.; Qing, X.J.; Wang, P.; Zhang, Y.; Zhang, X.; Zhao, X.H. Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotox. Environ. Safe. 2019, 173, 314–321. [Google Scholar]
- Hu, Y.X.; Li, J.; Lou, B.; Wu, R.R.; Wang, G.; Lu, C.W.; Wang, H.H.; Pi, J.B.; Xu, Y.Y. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules. 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Chandrakar, V.; Yadu, B.; Meena, R.K.; Dubey, A.; Keshavkant, S. Arsenic-induced genotoxic responses and their amelioration by diphenylene iodonium, 24-epibrassinolide and proline in Glycine max L. Plant Physiol. Biochem. 2017, 112, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Li, L.; Ali, B.; Gill, R.A.; Wang, J.; Ali, S.; Gill, M.B.; Zhou, W.J. Oxidative injury and antioxidant enzymes regulation in arsenic-exposed seedlings of four Brassica napus L. cultivars. Environ. Sci. Pollut. Res. 2015, 22, 10699–10712. [Google Scholar] [CrossRef]
- Cao, H.B.; Jiang, Y.; Chen, J.J.; Zhang, H.; Huang, W.; Li, L.; Zhang, W.S. Arsenic accumulation in Scutellaria baicalensis Georgi and its effects on plant growth and pharmaceutical components. J. Hazard. Mater. 2009, 171, 508–513. [Google Scholar] [CrossRef]
- Wang, H.; Lemke, R.; Goddard, T.; Sprout, C. Tillage and root heat stress in wheat in central Alberta. Can. J. Soil Sci. 2007, 87, 3–10. [Google Scholar] [CrossRef]
- Chen, W.H.; Chi, Y.J.; Taylor, N.L.; Lambers, H.; Finnegan, P.M. Disruption of ptLPD1 or ptLPD2, Genes That Encode Isoforms of the Plastidial Lipoamide Dehydrogenase, Confers Arsenate Hypersensitivity in Arabidopsis. Plant Physiol. 2010, 153, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.; Lamb, D.T.; Megharaj, M.; Naidu, R. Sorption parameters as a predictor of arsenic phytotoxicity in Australian soils. Geoderma. 2016, 265, 103–110. [Google Scholar] [CrossRef]
- Prum, C.; Dolphen, R.; Thiravetyan, P. Enhancing arsenic removal from arsenic-contaminated water by Echinodorus cordifolius−endophytic Arthrobacter creatinolyticus interactions. J. Environ. Manag. 2018, 213, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Studzinska, S.; Buszewski, B. Study of toxicity of imidazolium ionic liquids to watercress (Lepidium sativum L.). Anal. Bioanal. Chem. 2009, 393, 983–990. [Google Scholar] [CrossRef]
- Bari, E.; Daryaei, M.G.; Karim, M.; Bahmani, M.; Schmidt, O.; Woodward, S.; Ghanbary, M.A.T.; Sistani, A. Decay of Carpinus betulus wood by Trametes versicolor—An anatomical and chemical study. Int. Biodeterior. Biodegrad. 2019, 137, 68–77. [Google Scholar] [CrossRef]
- Chigbo, C.; Batty, L. Effect of EDTA and citric acid on phytoremediation of Cr- B a P-co-contaminated soil. Environ. Sci. Pollut. Res. 2013, 20, 8955–8963. [Google Scholar] [CrossRef]
- Zhao, H.M.; Du, H.; Xiang, L.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Cao, G.; Wong, M.H. Physiological differences in response to di-n-butyl phthalate (DBP) exposure between low- and high-DBP accumulating cultivars of Chinese flowering cabbage (Brassica parachinensis L.). Environ. Pollut. 2016, 208, 840–849. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Zheng, W.; Fei, Y.; Huang, Y. Soluble protein and acid phosphatase exuded by ectomycorrhizal fungi and seedlings in response to excessive Cu and Cd. J. Environ. Sci. 2009, 21, 1667–1672. [Google Scholar] [CrossRef]
- Porcher, A.; Guérin, V.; Leduc, N.; Lebrec, A.; Lothier, J.; Vian, A. Ascorbate–glutathione pathways mediated by cytokinin regulate H2O2 levels in light-controlled rose bud burst. Plant Physiol. 2021, 186, 910–928. [Google Scholar] [CrossRef]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox Signaling. 2006, 8, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ngo, H.H.; Khanal, S.; Larroche, C.; Kim, S.-H.; Pandey, A. Efficiency of transporter genes and proteins in hyperaccumulator plants for metals tolerance in wastewater treatment: Sustainable technique for metal detoxification. Environ. Technol. Innovation. 2021, 23, 101725. [Google Scholar] [CrossRef]
- Daud, M.K.; Ali, S.; Variath, M.T.; Zhu, S.J. Differential physiological, ultramorphological and metabolic responses of cotton cultivars under cadmium stress. Chemosphere 2013, 93, 2593–2602. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sharma, Y.K. Arsenic occurrence and accumulation in soil and water of eastern districts of Uttar Pradesh, India. Environ. Monit. Assess. 2013, 185, 4995–5002. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, J.-W.; Guan, D.-X.; Cao, Y.; Luo, J.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Arsenic Induced Phytate Exudation, and Promoted FeAsO4 Dissolution and Plant Growth in As-Hyperaccumulator Pteris vittata. Environ. Sci. Technol. 2016, 50, 9070–9077. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, S.; Zhao, J.; Wang, F.; Du, Y.; Zou, S.; Li, H.; Wen, D.; Huang, Y. Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2017, 133, 1–11. [Google Scholar]
- Li, F.; Li, Y.; Li, S.; Wu, G.; Niu, X.; Shen, A. Green light promotes healing and root regeneration in double-root-cutting grafted tomato seedlings. Sci. Hortic. 2021, 289, 110503. [Google Scholar] [CrossRef]
- Chomkitichai, W.; Chumyam, A.; Rachtanapun, P.; Uthaibutra, J.; Saengnil, K. Reduction of reactive oxygen species production and membrane damage during storage of ‘Daw’ longan fruit by chlorine dioxide. Sci. Hortic. 2014, 170, 143–149. [Google Scholar] [CrossRef]
- Wang, W.; Wan, Q.; Li, Y.; Ge, J.; Feng, F.; Yu, X. Application of an Endophyte Enterobacter sp. TMX13 to Reduce Thiamethoxam Residues and Stress in Chinese Cabbage (Brassica chinensis L.). J. Agric. Food. Chem. 2020, 68, 9180–9187. [Google Scholar]
- Shen, G.; Niu, J.; Deng, Z. Abscisic acid treatment alleviates cadmium toxicity in purple flowering stalk (Brassica campestris L. ssp. chinensis var. purpurea Hort.) seedlings. Plant Physiol. Biochem. 2017, 118, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, A.Y.; Wu, Y.; Wu, L.; Xiang, L.; Zhao, H.M.; Cai, Q.Y.; Li, Y.W.; Mo, C.H.; Wong, M.H.; et al. Applying beta-cyclodextrin to amaranth inoculated with white-rot fungus for more efficient remediation of soil co-contaminated with Cd and BDE-209. Sci. Total Environ. 2018, 634, 417–426. [Google Scholar] [CrossRef]
- Meck, M.L.; Mudimbu, D.; Davies, T.C. Accumulation of potentially harmful elements in edible parts of vegetables grown on two different geological substrates in Zimbabwe. J. Geochem. Explor. 2020, 208, 106392. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Yu, Y.; Li, X.; Tan, H. Integrated proteomics, metabolomics and physiological analyses for dissecting the toxic effects of halosulfuron-methyl on soybean seedlings (Glycine max merr.). Plant Physiol. Biochem. 2020, 157, 303–315. [Google Scholar] [CrossRef] [PubMed]


| Organ | As Concentration (mg L−1) | Treatments | As Speciation (mg kg−1) | Total As (mg kg−1) | |||
|---|---|---|---|---|---|---|---|
| As(III) | MMA | DMA | As (V) | ||||
| Shoot | 0 | HY | 0.18 ± 0.02 a | 0.14 ± 0.03 a | 0.027 ± 0.002 a | 0.074 ± 0.03 a | 0.50 ± 0.043 a |
| GD | 0.18 ± 0.02 a | 0.15 ± 0.02 a | 0.023 ± 0.002 a | 0.072 ± 0.02 a | 0.50 ± 0.064 a | ||
| 1 | HY | 2.26 ± 0.21 b | 1.70 ± 0.02 b | 0.29 ± 0.02 a | 0.86 ± 0.06 b | 5.51 ± 0.22 b | |
| GD | 3.08 ± 0.27 a | 2.50 ± 0.21 a | 0.34 ± 0.04 a | 1.08 ± 0.09 a | 7.12 ± 0.26 a | ||
| 5 | HY | 4.45 ± 0.42 b | 2.61 ± 0.25 b | 0.70 ± 0.05 b | 1.21 ± 0.08 a | 9.31 ± 0.46 b | |
| GD | 5.95 ± 0.48 a | 3.34 ± 0.39 a | 0.87 ± 0.05 a | 1.30 ± 0.09 a | 12.94 ± 0.75 a | ||
| 15 | HY | 7.34 ± 0.78 a | 2.83 ± 0.21 a | 1.48 ± 0.09 a | 1.28 ± 0.09 a | 13.31 ± 0.56 b | |
| GD | 7.67 ± 0.58 a | 2.81 ± 0.26 a | 1.51 ± 0.11 a | 1.32 ± 0.09 a | 14.84 ± 0.85 a | ||
| Root | 0 | HY | 0.88 ± 0.02 a | 0.63 ± 0.03 a | 0.19 ± 0.002 a | 0.35 ± 0.03 a | 2.41 ± 0.14 a |
| GD | 0.92 ± 0.02 a | 0.72 ± 0.02 a | 0.17 ± 0.002 a | 0.37 ± 0.02 a | 2.58 ± 0.17 a | ||
| 1 | HY | 5.49 ± 0.20 b | 4.14 ± 0.02 b | 0.71 ± 0.02 b | 2.12 ± 0.06 b | 13.45 ± 1.33 b | |
| GD | 11.35 ± 0.27 a | 9.10 ± 0.21 a | 1.04 ± 0.04 a | 4.03 ± 0.09 a | 25.93 ± 2.54 a | ||
| 5 | HY | 33.15 ± 0.42 b | 9.58 ± 0.25 b | 5.21 ± 0.05 a | 9.1 ± 0.08 a | 70.27 ± 5.44 a | |
| GD | 35.71 ± 0.40 a | 19.22 ± 0.39 a | 3.50 ± 0.05 b | 7.38 ± 0.09 b | 68.82 ± 2.35 a | ||
| 15 | HY | 97.81 ± 0.78 b | 36.14 ± 0.21 b | 19.21 ± 0.09 b | 16.85 ± 0.09 b | 177.56 ± 10.44 b | |
| GD | 128.2 ± 0.58 a | 46.31 ± 0.26 a | 24.37 ± 0.11 a | 19.71 ± 0.09 a | 238.35 ± 15.35 a | ||
| HY | GD | |||||
|---|---|---|---|---|---|---|
| Root Biomass | Root Length | As Concentration in Root | Root Biomass | Root Length | As Concentration in Root | |
| As concentration in root | −0.68 * | −0.62 * | −0.91 ** | −0.8 ** | ||
| H2O2 in root | −0.64 * | −0.7 * | 0.9 ** | −0.84 ** | −0.75 ** | 0.86 ** |
| O2− in root | −0.8 ** | −0.66 * | 0.98 ** | −0.92 ** | −0.9 ** | 0.92 ** |
| Protein content | NS | NS | 0.84 ** | −0.63 * | −0.58 * | 0.62 * |
| Root activity | 0.86 ** | 0.67 * | −0.8 ** | 0.87 ** | 0.81 ** | −0.84 ** |
| MDA in root | −0.89 ** | −0.80 ** | 0.98 ** | −0.88 ** | −0.91 ** | 0.93 ** |
| GSH content | −0.87 ** | −0.87 ** | 0.79 ** | −0.76 ** | −0.84 ** | 0.68 * |
| Root Biomass | Root Length | As Concentration | H2O2 in Root | O2− in Root | Protein Content | Root Activity | |
|---|---|---|---|---|---|---|---|
| Cultivar | p < 0.05 | p < 0.05 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.01 | p < 0.001 |
| As concentration | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Cultivar × As concentration | NS | NS | p < 0.001 | NS | p < 0.05 | p < 0.05 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, Y.; Li, X.; Chen, X.W.; Chen, A.; Wu, L.; Wong, M.H.; Li, H. Low-Arsenic Accumulating Cabbage Possesses Higher Root Activities against Oxidative Stress of Arsenic. Plants 2023, 12, 1699. https://doi.org/10.3390/plants12081699
Li H, Li Y, Li X, Chen XW, Chen A, Wu L, Wong MH, Li H. Low-Arsenic Accumulating Cabbage Possesses Higher Root Activities against Oxidative Stress of Arsenic. Plants. 2023; 12(8):1699. https://doi.org/10.3390/plants12081699
Chicago/Turabian StyleLi, Hanhao, Yongtao Li, Xing Li, Xun Wen Chen, Aoyu Chen, Li Wu, Ming Hung Wong, and Hui Li. 2023. "Low-Arsenic Accumulating Cabbage Possesses Higher Root Activities against Oxidative Stress of Arsenic" Plants 12, no. 8: 1699. https://doi.org/10.3390/plants12081699





