Yield and Nutraceutical Value of Lettuce and Basil Improved by a Microbial Inoculum in Greenhouse Experiments
Abstract
1. Introduction
2. Results
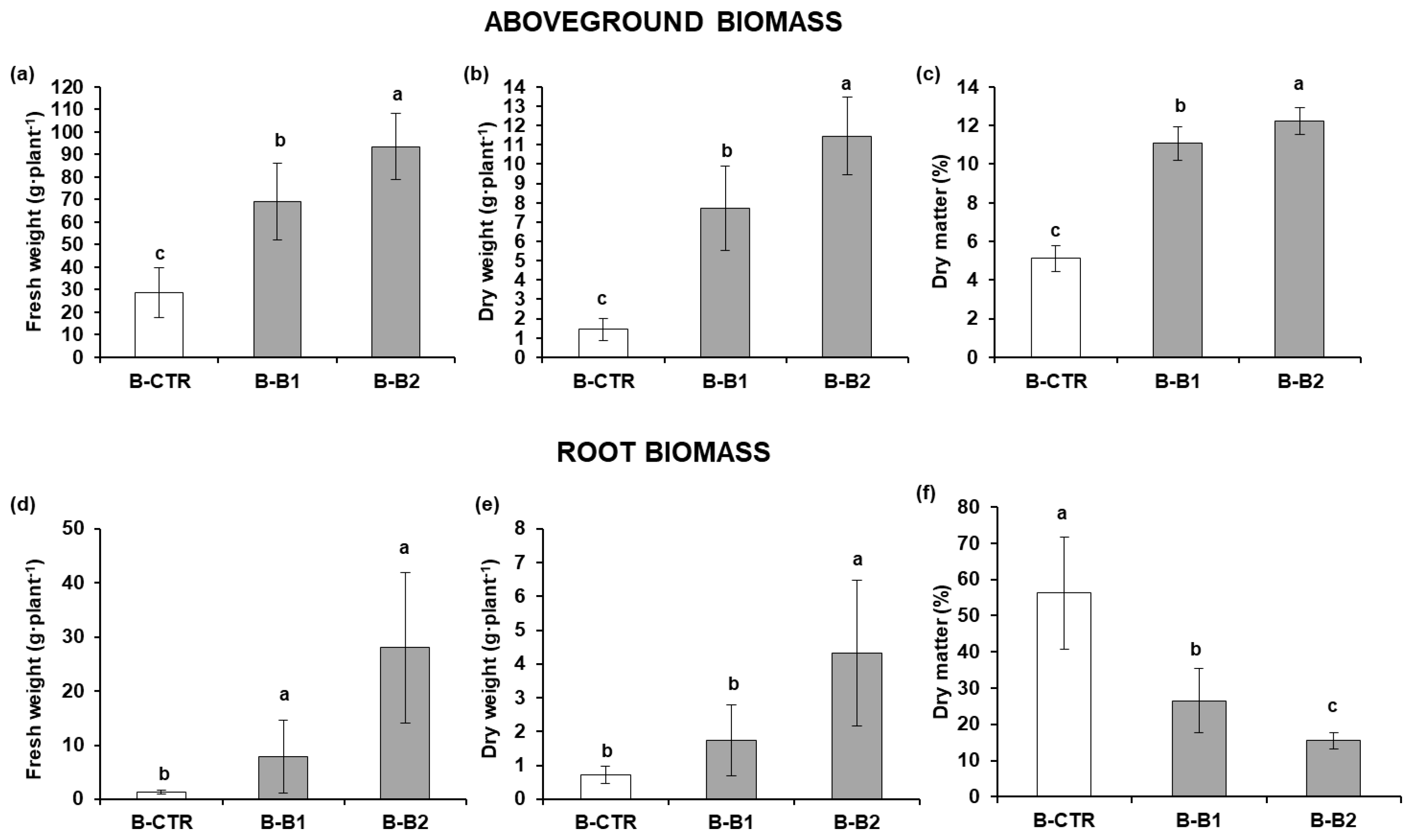
2.1. Preliminary Greenhouse Experiment
2.1.1. Growth and Yield Parameters of Lettuce
2.1.2. Mineral Uptake: N, P K, Na, Ca, Mg, Mn, Fe, Zn, Cu, B
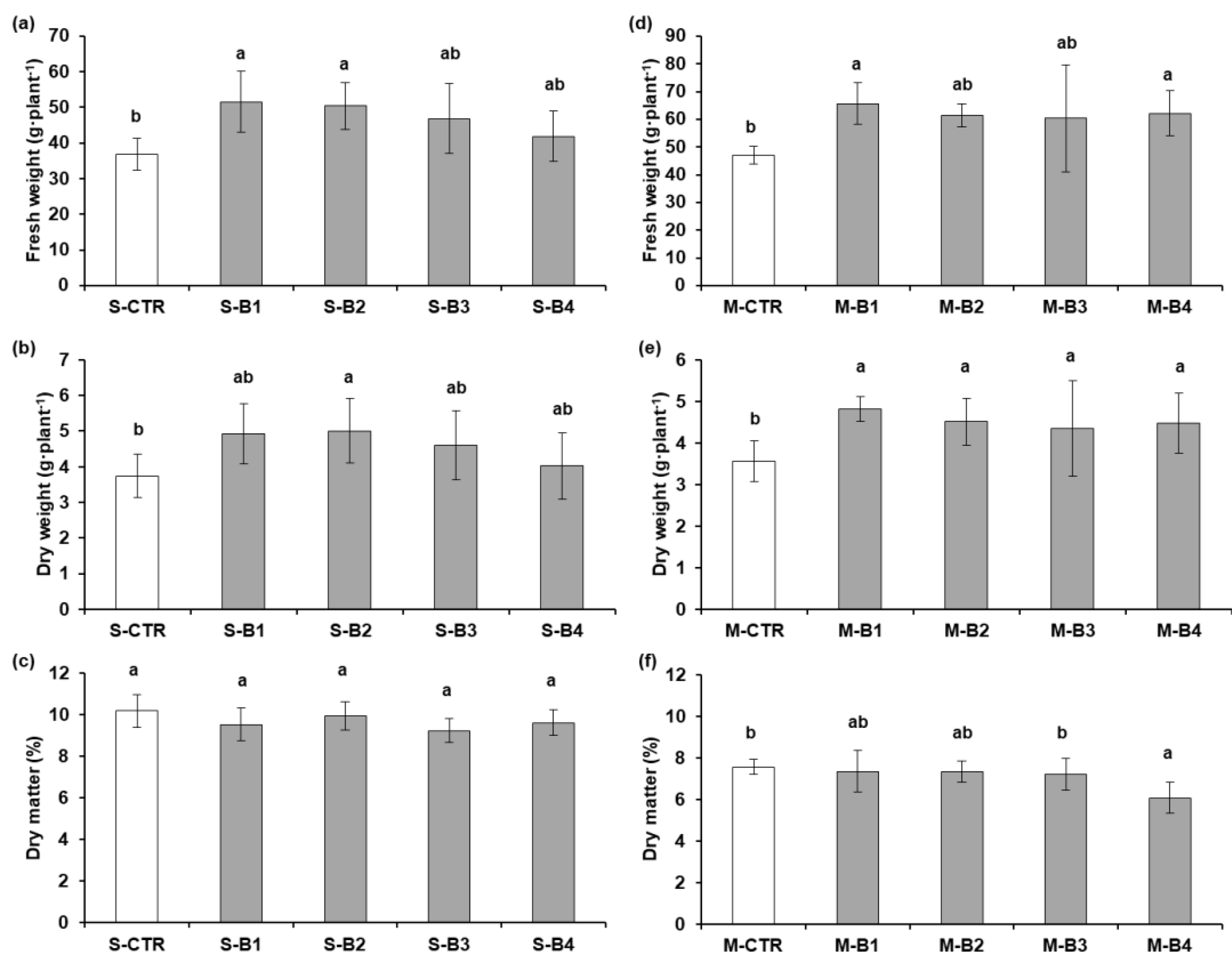
2.2. Second Greenhouse Experiment: Lettuce
2.2.1. Growth and Yield Parameters
2.2.2. Mineral Uptake: N, P K, Na, Ca, Mg, Mn, Fe, Zn, Cu, B, Cr, Mo, Se
2.3. Second Greenhouse Experiment: Basil
2.3.1. Growth and Yield Parameters
2.3.2. Mineral Uptake: N, P K, Na, Ca, Mg, Mn, Fe, Zn, Cu, B, Cr, Mo, Se
3. Discussion
4. Materials and Methods
4.1. Preliminary Assay on Lactuca sativa L.
4.2. Greenhouse Experimental Design for Lettuce and Basil
4.3. Bacterial Strain Isolation and Molecular Identification
4.4. Inoculum Setup
4.5. Sampling and Yield Assessment
4.5.1. First Experiment Design: Lettuce
4.5.2. Second Experiment Design: Lettuce and Basil
4.6. Chlorophylsl and Carotenoids Determination
4.7. Mineral Analyses
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kloepper, J.W.; Schroth, M.N. Plant growth-promoting rhizobacteria and plant growth under gnotobiotic conditions. Phytopathology 1978, 71, 642–644. [Google Scholar] [CrossRef]
- Gaggìa, F.; Baffoni, L.; Di Gioia, D.; Accorsi, M.; Bosi, S.; Marotti, I.; Biavati, B.; Dinelli, G. Inoculation with microorganisms of Lolium perenne L.: Evaluation of plant growth parameters and endophytic colonization of roots. New Biotechnol. 2013, 30, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Tontou, R.; Gaggia, F.; Baffoni, L.; Devescovi, G.; Venturi, V.; Giovanardi, D.; Stefani, E. Molecular characterisation of an endophyte showing a strong antagonistic activity against Pseudomonas syringae pv. actinidiae. PlantSoil 2016, 405, 97–106. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight into the role of PGPR in sustainable agriculture and environment. Front. Sustain. Food Syst. 2021, 5, 667150. [Google Scholar] [CrossRef]
- Kröber, M.; Wibberg, D.; Grosch, R.; Eikmeyer, F.; Verwaaijen, B.; Chowdhury, S.P.; Hartmann, A.; Pühler, A.; Schlüter, A. Effect of the strain Bacillus amyloliquefaciens FZB42 on the microbial community in the rhizosphere of lettuce under field conditions analyzed by whole metagenome sequencing. Front. Microbiol. 2014, 5, 252. [Google Scholar]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Satapute, P.; Kamble, M.V.; Adhikari, S.S.; Jogaiah, S. Influence of triazole pesticides on tillage soil microbial populations and metabolic changes. Sci. Total Environ. 2019, 651, 2334–2344. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Response of soil microorganisms and enzymes to the foliar application of Helicur 250 EW fungicide on Horderum vulgare L. Chemosphere 2020, 242, 125163. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, X.F.; Chaparro, J.M.; Badri, D.V.; Manter, D.K.; Vivanco, J.M.; Guo, J. Root and bacterial secretions regulate the interaction between plants and PGPR leading to distinct plant growth promotion effects. Plant Soil 2016, 401, 259–272. [Google Scholar] [CrossRef]
- Sansinenea, E. Bacillus spp.: As plant growth-promoting bacteria. Second. Metab. Plant Growth Promot. Rhizomicroorg. 2019, 2019, 225–237. [Google Scholar]
- Govindasamy, V.; Senthilkumar, M.; Magheshwaran, V.; Kumar, U.; Bose, P.; Sharma, V.; Annapurna, K. Bacillus and Paenibacillus spp.: Potential PGPR for sustainable agriculture. Plant Growth Health Prom. Bac. 2010, 2010, 333–364. [Google Scholar]
- Piggot, P.J.; Hilbert, D.W. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 2004, 7, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Angelina, E.; Papatheodorou, E.M.; Demirtzoglou, T.; Monokrousos, N. Effects of Bacillus subtilis and Pseudomonas fluorescens inoculation on attributes of the lettuce (Lactuca sativa L.) soil rhizosphere microbial community: The role of the management system. Agronomy 2020, 10, 1428. [Google Scholar] [CrossRef]
- Van Gerrewey, T.; Vandecruys, M.; Ameloot, N.; Perneel, M.; Van Labeke, M.C.; Boon, N.; Geelen, D. Microbe-plant growing media interactions modulate the effectiveness of bacterial amendments on lettuce performance inside a plant factory with artificial lighting. Agronomy 2020, 10, 1456. [Google Scholar] [CrossRef]
- Comite, E.; El-Nakhel, C.; Rouphael, Y.; Ventorino, V.; Pepe, O.; Borzacchiello, A.; Vinale, F.; Rigano, D.; Staropoli, A.; Lorito, M.; et al. Bioformulations with beneficial microbial consortia, a bioactive compound and plant biopolymers modulate sweet basil productivity, photosynthetic activity and metabolites. Pathogens 2021, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- Tahami, M.K.; Jahan, M.; Khalilzadeh, H.; Mehdizadeh, M. Plant growth promoting rhizobacteria in an ecological cropping system: A study on basil (Ocimum basilicum L.) essential oil production. Ind. Crops Prod. 2017, 107, 97–104. [Google Scholar] [CrossRef]
- Jahan, M.; Nassiri Mahallati, M.; Amiri, M.B.; Tahami, M.K. The effects of winter cover crops and plant growth promoting rhizobacteria on fertility of soil and crop yield in an organic production system of Ocimum basilicum. In Proceedings of the 13th Congress of the International Society for Ethnopharmacology, Graz, Austria, 2–6 September 2012. [Google Scholar]
- Yilmaz, A.; Karik, Ü. AMF and PGPR enhance yield and secondary metabolite profile of basil (Ocimum basilicum L.). Ind. Crops Prod. 2022, 176, 114327. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Pannico, A.; Giordano, M.; Colla, G.; Rouphael, Y. Foliar and root applications of vegetal-derived protein hydrolysates differentially enhance the yield and qualitative attributes of two lettuce cultivars grown in floating system. Agronomy 2021, 11, 1194. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Comp. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Bączek, K.; Kosakowska, O.; Gniewosz, M.; Gientka, I.; Węglarz, Z. Sweet basil (Ocimum basilicum L.) productivity and raw material quality from organic cultivation. Agronomy 2019, 9, 279. [Google Scholar] [CrossRef]
- Rubab, S.; Hussain, I.; Khan, B.A.; Unar, A.A.; Abbas, K.A.; Khichi, Z.H.; Khan, M.; Khanum, S.; Ur Rehman, K.; Khan, H. Biomedical Description of Ocimu basilicum L. J. Isl. Inter. Med. Col. 2017, 12, 59–67. [Google Scholar]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral biofortification of vegetables as a tool to improve human diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Soni, S.K.; Patel, R.P.; Kalra, A. Technology for improving essential oil yield of Ocimum basilicum L.(sweet basil) by application of bioinoculant colonized seeds under organic field conditions. Ind. Crops Prod. 2013, 45, 335–342. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Akinrinlola, R.J.; Yuen, G.Y.; Drijber, R.A.; Adesemoye, A.O. Evaluation of Bacillus strains for plant growth promotion and predictability of efficacy by in vitro physiological traits. Int. J. Microbiol. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dubey, R.C.; Maheshwari, D.K. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 2012, 167, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Moccaldi, L.A. Application Method and Efficacy of Bacillus spp. in Mitigating Abiotic and Biotic Stresses and Enhancing Plant Performance. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol. Bacilli in Climate Resilient Agriculture and Bioprospecting; Islam, M., Rahman, M., Pandey, P., Boehme, M., Haesaert, G., Eds.; Springer: Cham, Switzerland, 2019; pp. 267–289. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hort. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Fu, J.; Xiao, Y.; Wang, Y.F.; Liu, Z.H.; Yang, K.J. Trichoderma affects the physiochemical characteristics and bacterial community composition of saline–alkaline maize rhizosphere soils in the cold-region of Heilongjiang Province. Plant Soil 2019, 436, 211–227. [Google Scholar] [CrossRef]
- Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of bacterial inoculum and fertigation management on nursery and field production of lettuce plants. Agronomy 2020, 10, 1477. [Google Scholar] [CrossRef]
- Kasozi, N.; Kaiser, H.; Wilhelmi, B. Effect of Bacillus spp. on lettuce growth and root associated bacterial community in a small-scale aquaponics system. Agronomy 2021, 11, 947. [Google Scholar] [CrossRef]
- Pishchik, V.N.; Vorobyov, N.I.; Walsh, O.S.; Surin, V.G.; Khomyakov, Y.V. Estimation of synergistic effect of humic fertilizer and Bacillus subtilis on lettuce plants by reflectance measurements. J. Plant. Nutr. 2016, 39, 1074–1086. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Dietel, K.; Rändler, M.; Schmid, M.; Junge, H.; Borriss, R.; Hartmann, A.; Grosch, R. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 2013, 8, e68818. [Google Scholar] [CrossRef]
- Gomes, A.; Mariano, R.L.; Silveira, E.B.; Mesquita, J.C. Isolation, selection of bacteria, and effect of Bacillus spp. in the production of organic lettuce seedlings. Hortic. Brasil. 2003, 21, 699–703. [Google Scholar] [CrossRef]
- Vivas, A.; Marulanda, A.; Ruiz-Lozano, J.M.; Barea, J.M.; Azcón, R. Influence of a Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi and on plant responses to PEG-induced drought stress. Mycorrhiza 2003, 13, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Banchio, E.; Xie, X.; Zhang, H.; Pare, P.W. Soil bacteria elevate essential oil accumulation and emissions in sweet basil. J. Agric. Food Chem. 2009, 57, 653–657. [Google Scholar] [CrossRef]
- Savarino, G.; Corsello, A.; Corsello, G. Macronutrient balance and micronutrient amounts through growth and development. It. J. Ped. 2021, 47, 1–14. [Google Scholar] [CrossRef]
- Dal Cortivo, C.; Barion, G.; Ferrari, M.; Visioli, G.; Dramis, L.; Panozzo, A.; Vamerali, T. Effects of field inoculation with VAM and bacteria consortia on root growth and nutrients uptake in common wheat. Sustainability 2018, 10, 3286. [Google Scholar] [CrossRef]
- de Aquino, G.S.; Ventura, M.U.; Alexandrino, R.P.; Michelon, T.A.; de Araujo Pescador, P.G.; Nicio, T.T.; Watanabe, V.S.; Diniz, T.G.; de Oliveira, A.L.M.; Hata, F.T. Plant-promoting rhizobacteria Methylobacterium komagatae increases crambe yields, root system and plant height. Ind. Crops Prod. 2018, 121, 277–281. [Google Scholar] [CrossRef]
- Rojas-Padilla, J.; de-Bashan, L.E.; Parra-Cota, F.I.; Rocha-Estrada, J.; de Los Santos-Villalobos, S. Microencapsulation of Bacillus Strains for Improving Wheat (Triticum turgidum Subsp. durum) Growth and Development. Plants 2022, 11, 2920. [Google Scholar] [CrossRef]
- Lim, E.F.; Costa Neto, P.; Araujo, J.M.; Alcântara Neto, F.; Bonifacio, A.; Rodrigues, A.C. Varieties of lima bean shows different growth responses when inoculated with Bacillus sp., a plant growth-promoting bacteria. Bioscience 2016, 32, 1221–1233. [Google Scholar] [CrossRef]
- Medeiros, C.A.A.; Bettiol, W. Multifaceted intervention of Bacillus spp. against salinity stress and Fusarium wilt in tomato. J. Appl. Microbiol. 2021, 131, 2387–2401. [Google Scholar] [CrossRef] [PubMed]
- Muday, G.K.; Rahman, A.; Binder, B.M. Auxin and ethylene: Collaborators or competitors? Trends Plant. Sci. 2012, 17, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Heidari, M.; Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2012, 11, 57–61. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, C.T.; Steinberg, C.; Alabouvette, C.; Moenne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant. Soil 2021, 321, 341–361. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant. Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Ngoroyemoto, N.; Gupta, S.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Effect of organic biostimulants on the growth and biochemical composition of Amaranthus hybridus L. S. Afr. J. Bot. 2019, 124, 87–93. [Google Scholar] [CrossRef]
- He, A.; Niu, S.; Yang, D.; Ren, W.; Zhao, L.; Sun, Y.; Meng, L.; Zhao, Q.; Paré, P.W.; Zhang, J. Two PGPR strains from the rhizosphere of Haloxylon ammodendron promoted growth and enhanced drought tolerance of ryegrass. Plant. Physiol. Biochem. 2021, 161, 74–85. [Google Scholar] [CrossRef]
- Emmanuel, O.C.; Babalola, O.O. Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microb. Res. 2020, 239, 126569. [Google Scholar] [CrossRef]
- Mizzi, J.; Gaggìa, F.; Cionci, N.B.; Di Gioia, D.; Attard, E. Selection of Acetic Acid Bacterial Strains and Vinegar Production From Local Maltese Food Sources. Front. Microbiol. 2022, 13, 897825. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J.; Thompson, W.A.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta (BBA)-Bioenerg. 1983, 975, 384–394. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Kingston, H.M.; Jassie, L.B. Monitoring and predicting parameters in microwave dissolution. In Introduction to Microwave Sample Preparation: Theory and Practice; American Chemical Society: Washington, DC, USA, 1988. [Google Scholar]

| Single Inoculum | Multiple Inoculum | ||||||
|---|---|---|---|---|---|---|---|
| N | P | K | N | P | K | ||
| (mg·Plant−1) | (mg·Plant−1) | ||||||
| S-CTR | 90.92 ± 6.47 a | 8.96 ± 3.22 a | 57.18 ± 19.28 a | M-CTR | 116.97 ± 38.79 b | 12.99 ± 3.62 b | 57.15 ± 13.87 b |
| S-B1 | 99.37 ± 21.79 a | 14.67 ± 5.12 a | 91.30 ± 26.72 a | M-B1 | 165.66 ± 19.87 a | 34.49 ± 4.66 a | 136.41 ± 11.50 a |
| S-B2 | 97.63 ± 11.30 a | 14.98 ± 3.74 a | 95.27 ± 27.69 a | M-B2 | 164.52 ± 11.60 a | 30.32 ± 5.86 a | 117.42 ± 0.51 a |
| S-B3 | 99.75 ± 19.14 a | 13.21 ± 3.81 a | 86.61 ± 30.66 a | M-B3 | 158.88 ± 26.13 a | 26.97 ± 5.97 a | 118.46 ± 39.89 a |
| S-B4 | 97.54 ± 11.89 a | 12.71 ± 2.29 a | 78.33 ± 14.27 a | M-B4 | 170.49 ± 18.39 a | 26.81 ± 2.17 a | 129.05 ± 19.26 a |
| s | ns | ns | ns | ** | *** | *** | |
| Single Inoculum (mg·plant−1) | ||||||||
| Na | Ca | Mg | Mn | Fe | Zn | Cu | B | |
| S-CTR | 10.56 ± 3.48 b | 14.63 ± 5.29 a | 6.06 ± 2.15 a | 0.78 ± 0.25 a | 0.29 ± 0.06 a | 0.14 ± 0.06 a | 0.017 ± 0.005 a | 0.18 ± 0.05 a |
| S-B1 | 16.05 ± 4.86 a | 24.67 ± 7.51 a | 9.76 ± 3.10 a | 1.31 ± 0.39 a | 0.36 ± 0.12 a | 0.20 ± 0.08 a | 0.022 ± 0.04 a | 0.26 ± 0.07 a |
| S-B2 | 14.59 ± 1.92 ab | 25.88 ± 8.46 a | 9.74 ± 2.09 a | 1.40 ± 0.62 a | 0.37 ± 0.12 a | 0.20 ± 0.06 a | 0.025 ± 0.07 a | 0.25 ± 0.06 a |
| S-B3 | 12.75 ± 2.09 ab | 22.52 ± 7.06 a | 8.50 ± 1.98 a | 1.26 ± 0.42 a | 0.43 ± 0.10 a | 0.22 ± 0.07 a | 0.026 ± 0.09 a | 0.21 ± 0.05 a |
| S-B4 | 13.60 ± 2.32 ab | 21.51 ± 4.94 a | 8.63 ± 1.64 a | 1.18 ± 0.35 a | 0.35 ± 0.07 a | 0.20 ± 0.04 a | 0.022 ± 0.03 a | 0.21 ± 0.04 a |
| s | * | ns | ns | ns | ns | ns | ns | ns |
| Multiple Inoculum (mg·plant−1) | ||||||||
| Na | Ca | Mg | Mn | Fe | Zn | Cu | B | |
| M-CTR | 9.01 ± 2.11 c | 18.47 ± 3.23 b | 7.26 ± 1.60 c | 0.74 ± 0.14 c | 0.30 ± 0.06 b | 0.20 ± 0.06 b | 0.024 ± 0.046 b | 0.14 ± 0.03 c |
| M-B1 | 17.82 ± 1.60 a | 33.93 ± 4.41 a | 14.63 ± 1.73 ab | 1.72 ± 0.27 ab | 0.60 ± 0.08 a | 0.41 ± 0.06 a | 0.046 ± 0.006 a | 0.29 ± 0.04 a |
| M-B2 | 14.40 ± 2.35 b | 28.94 ± 5.08 a | 12.75 ± 2.38 ab | 1.60 ± 0.32 ab | 0.54 ± 0.13 a | 0.36 ± 0.07 a | 0.040 ± 0.008 a | 0.25 ± 0.05 ab |
| M-B3 | 13.30 ± 2.13 b | 27.70 ± 11.76 a | 12.05 ± 3.39 b | 1.27 ± 0.41 bc | 0.50 ± 0.16 ab | 0.33 ± 0.07 a | 0.040 ± 0.008 a | 0.22 ± 0.04 b |
| M-B4 | 15.60 ± 1.76 ab | 37.48 ± 3.90 a | 15.98 ± 1.58 a | 1.81 ± 0.47 a | 0.62 ± 0.10 a | 0.43 ± 0.04 a | 0.046 ± 0.005 a | 0.30 ± 0.03 a |
| s | *** | *** | *** | *** | *** | *** | *** | *** |
| Chlorophyll-a (µg·g−1) | Chlorophyll-b (µg·g−1) | Carotenoids (µg·g−1) | |
|---|---|---|---|
| L-CTR | 42.29 ± 4.42 b | 18.54 ± 9.20 b | 4310.03 ± 101.84 b |
| L-B1 | 103.18 ± 13.68 b | 46.15 ± 5.50 b | 7147.74 ± 533.33 b |
| L-B2 | 335.76 ± 135.55 a | 172.23 ± 37.93 a | 21174.75 ± 9445.08 a |
| s | ** | *** | * |
| N | P | K | Na | Ca | Mg | |
| (mg·plant−1) | ||||||
| L-CTR | 168.45 ± 11.45 b | 18.18 ± 5.19 b | 78.88 ± 36.29 b | 11.12 ± 7.56 b | 21.46 ± 5.92 b | 8.24 ± 2.56 c |
| L-B1 | 235.40 ± 30.02 a | 35.35 ± 2.65 a | 181.09 ± 23.06 a | 19.14 ± 7.28 a | 34.33 ± 6.23 a | 13.37 ± 1.23 b |
| L-B2 | 242.38 ± 46.26 a | 32.40 ± 3.18 a | 183.38 ± 21.27 a | 20.12 ± 7.18 a | 37.24 ± 3.28 a | 15.38 ± 1.56 a |
| s | *** | *** | ** | *** | *** | *** |
| Mn | Fe | Zn | Cu | B | Cr | |
| L-CTR | 0.80 ± 0.33 c | 0.40 ± 0.10 b | 0.24 ± 0.08 b | 0.028 ± 0.008 b | 0.16 ± 0.04 b | 0.0040 ± 0.0012 b |
| L-B1 | 1.66 ± 0.25 b | 0.75 ± 0.15 a | 0.44 ± 0.05 a | 0.051 ± 0.009 a | 0.28 ± 0.03 a | 0.0058 ± 0.0011 a |
| L-B2 | 1.86 ± 0.09 a | 0.74 ± 0.14 a | 0.42 ± 0.06 a | 0.049 ± 0.009 a | 0.32 ± 0.03 a | 0.0060 ± 0.0010 a |
| s | *** | *** | ** | *** | *** | *** |
| Mo | Se | |||||
| L-CTR | 0.0011 ± 0.0009 b | 0.0036 ± 0.0008 b | ||||
| L-B1 | 0.0019 ± 0.0007 ab | 0.0055 ± 0.00021 a | ||||
| L-B2 | 0.0021 ± 0.0015 a | 0.0051 ± 0.0015 a | ||||
| s | * | ** | ||||
| Chlorophyll-a (µg·g−1) | Chlorophyll-b (µg·g−1) | Carotenoids (µg·g−1) | |
|---|---|---|---|
| B-CTR | 42.26 ± 1.91 b | 30.33 ± 0.62 b | 3.127 ± 145 c |
| B-B1 | 120.08 ± 2.52 a | 100.62 ± 33.15 a | 13.183 ± 896 b |
| B-B2 | 122.89 ± 4.04 a | 127.38 ± 7.20 a | 15.292 ± 628 a |
| s | *** | ** | *** |
| N | P | K | Na | Ca | Mg | |
| (mg·plant−1) | ||||||
| B-CTR | 39.47 ± 5.18 c | 9.92 ± 0.99 b | 62.40 ± 12.22 c | 0.15 ± 0.25 c | 15.34 ± 3.99 c | 3.90 ± 1.41 c |
| B-B1 | 231.15 ± 43.83 b | 19.80 ± 4.86 a | 304.99 ± 65.36 b | 1.32 ± 0.82 b | 135.72 ± 40.37 b | 26.69 ± 9.10 b |
| B-B2 | 372.84 ± 34.84 a | 21.02 ± 3.22 a | 374.41 ± 76.94 a | 7.06 ± 2.05 a | 196.72 ± 39.64 a | 38.63 ± 8.84 a |
| s | *** | *** | ** | *** | *** | *** |
| Mn | Fe | Zn | Cu | B | Cr | |
| (mg·plant−1) | ||||||
| B-CTR | 0.05 ± 0.2 c | 0.16 ± 0.20 c | 0.08 ± 0.02 c | 0.003 ± 0.001 b | 0.03 ± 0.01 c | 0.0003 ± 0.00006 b |
| B-B1 | 2.55 ± 0.72 b | 1.50 ± 0.54 b | 0.46 ± 0.15 b | 0.095 ± 0.025 a | 0.17 ± 0.04 b | 0.0186 ± 0.0147 ab |
| B-B2 | 3.62 ± 1.05 a | 2.47 ± 1.38 a | 0.75 ± 0.30 a | 0.118 ± 0.039 a | 0.24 ± 0.05 a | 0.0366 ± 0.0422 a |
| s | *** | *** | *** | *** | *** | ** |
| Mo | Se | |||||
| (mg·plant−1) | ||||||
| B-CTR | 0.016 ± 0.007 b | 0.0030 ± 0.002 c | ||||
| B-B1 | 0.016 ± 0.007 b | 0.0071 ± 0.003 b | ||||
| B-B2 | 0.054 ± 0.017 a | 0.0160 ± 0.003 a | ||||
| s | *** | *** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagliarini, E.; Gaggìa, F.; Quartieri, M.; Toselli, M.; Di Gioia, D. Yield and Nutraceutical Value of Lettuce and Basil Improved by a Microbial Inoculum in Greenhouse Experiments. Plants 2023, 12, 1700. https://doi.org/10.3390/plants12081700
Pagliarini E, Gaggìa F, Quartieri M, Toselli M, Di Gioia D. Yield and Nutraceutical Value of Lettuce and Basil Improved by a Microbial Inoculum in Greenhouse Experiments. Plants. 2023; 12(8):1700. https://doi.org/10.3390/plants12081700
Chicago/Turabian StylePagliarini, Elia, Francesca Gaggìa, Maurizio Quartieri, Moreno Toselli, and Diana Di Gioia. 2023. "Yield and Nutraceutical Value of Lettuce and Basil Improved by a Microbial Inoculum in Greenhouse Experiments" Plants 12, no. 8: 1700. https://doi.org/10.3390/plants12081700
APA StylePagliarini, E., Gaggìa, F., Quartieri, M., Toselli, M., & Di Gioia, D. (2023). Yield and Nutraceutical Value of Lettuce and Basil Improved by a Microbial Inoculum in Greenhouse Experiments. Plants, 12(8), 1700. https://doi.org/10.3390/plants12081700









