Antioxidant Compounds of Potato Breeding Genotypes and Commercial Cultivars with Yellow, Light Yellow, and White Flesh in Iran
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Flavonoids and Phenolic Content (TFC, TPC)
2.2. Antioxidant Activity (DPPH and FRAP)
2.3. Total Carotenoids Content (TCC)
2.4. Total Ascorbic Acid (TAA)
2.5. Total Soluble Sugars (TSS)
2.6. Total Soluble Protein (TSP)
2.7. Correlation
3. Materials and Methods
3.1. Plant Material
3.2. Measurement of Flavonoids Content
3.3. Determination of Phenolic Concentration
3.4. Assay of DPPH Radical Scavenging Activity
3.5. Assay of Ferric-Reducing Antioxidant Power (FRAP)
3.6. Determination of Carotenoid Content
3.7. Determination of Ascorbic Acid (AA)
3.8. Determination of Soluble Sugars
3.9. Determination of Total Protein Content
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haan, S.; Rodriguez, F. Potato Origin and Production; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128000021. [Google Scholar]
- Machida-Hirano, R.; Niino, T. Potato genetic resources. In The Potato Genome; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Hamideldin, N.; Hussien, O.S. Morphological, Physiological and Molecular Changes in Solanum tuberosum L. in Response to Pre-sowing Tuber Irradiation by Gamma Rays. Am. J. Food. Sci. Technol. 2013, 1, 36–41. [Google Scholar]
- Mahgoub, H.A.M.; Eisa, G.S.A.; Youssef, M.A.H. Molecular, biochemical and anatomical analysis of some potato (Solanum tuberosum L.) cultivars growing in Egypt. J. Genet. Eng. Biotechnol. 2015, 13, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Patel, G.; Novy, R.; Vining, K.; Brown, C.; Holm, D.; Porter, G.; Endelman, J.; Thompson, A.; Sathuvalli, V. Evaluation of genetic diversity among Russet potato clones and varieties from breeding programs across the United States. PLoS ONE 2018, 13, e0201415. [Google Scholar] [CrossRef]
- Raigond, P.; Singh, B.; Dutt, S.; Chakrabarti, S.K. Potato: Nutrition and Food Security; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Ru, W.; Pang, Y.; Gan, Y.; Liu, Q.; Bao, J. Phenolic compounds and antioxidant activities of potato cultivars with white, yellow, red and purple flesh. Antioxidants 2019, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Nemś, A.; Pęksa, A.; Kucharska, A.Z.; Sokół-ŁĘtowska, A.; Kita, A.; Drozdz, W.; Hamouz, K. Anthocyanin and antioxidant activity of snacks with coloured potato. Food Chem. 2015, 172, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Reddivari, L.; Hale, A.L.; Miller, J.C. Determination of phenolic content, composition and their contribution to antioxidant activity in specialty potato selections. Am. J. Potato Res. 2007, 84, 275–282. [Google Scholar] [CrossRef]
- Rumbaoa, R.G.O.; Cornago, D.F.; Geronimo, I.M. Phenolic content and antioxidant capacity of Philippine potato (Solanum tuberosum) tubers. J. Food Compos. Anal. 2009, 22, 546–550. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Valcarcel, J.; Reilly, K.; Gaffney, M.; O’Brien, N. Total carotenoids and l-ascorbic acid content in 60 varieties of potato (Solanum tuberosum L.) grown in Ireland. Potato Res 2015, 58, 29–41. [Google Scholar] [CrossRef]
- Brown, C.R. Antioxidants in potato. Am. J. Potato Res. 2005, 82, 163–172. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Šulc, M.; Orsák, M.; Pivec, V.; Hejtmánková, A.; Dvořák, P.; Čepl, J. Cultivar differences of total anthocyanins and anthocyanidins in red and purple-fleshed potatoes and their relation to antioxidant activity. Food Chem. 2009, 114, 836–843. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, W.; Xu, B. Profiles of phenolics, carotenoids and antioxidative capacities of thermal processed white, yellow, orange and purple sweet potatoes grown in Guilin, China. Food Sci. Hum. Wellness 2015, 4, 123–132. [Google Scholar] [CrossRef]
- Brown, C.R.; Culley, D.; Yang, C.P.; Durst, R.; Wrolstad, R. Variation of anthocyanin and carotenoid contents and associated antioxidant values in potato breeding lines. J. Am. Soc. Hortic. Sci. 2005, 130, 174–180. [Google Scholar] [CrossRef]
- Reyes, L.F.; Miller, J.C.; Cisneros-Zevallos, L. Antioxidant capacity, anthocyanins and total phenolics in purple-and red-fleshed potato (Solanum tuberosum L.) genotypes. Am. J. Potato Res. 2005, 82, 271–277. [Google Scholar] [CrossRef]
- Külen, O.; Stushnoff, C.; Holm, D.G. Effect of cold storage on total phenolics content, antioxidant activity and vitamin C level of selected potato clones. J. Sci. Food Agric. 2013, 93, 2437–2444. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.L.; Ducreux, L.; Griffiths, D.W.; Stewart, D.; Davies, H.V.; Taylor, M.A. Carotenogenesis during tuber development and storage in potato. J. Exp. Bot. 2004, 55, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Hirschberg, J. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Orsák, M.; Pivec, V.; Dvořák, P. The influence of flesh colour and growing locality on polyphenolic content and antioxidant activity in potatoes. Sci. Hortic. 2008, 117, 109–114. [Google Scholar] [CrossRef]
- Breitbaupt, D.E.; Bamedi, A. Carotenoids and carotenoid esters in potatoes (Solanum tuberosum L.): New insights into an ancient vegetable. J. Agric. Food Chem. 2002, 50, 7175–7181. [Google Scholar] [CrossRef]
- Dale, M.F.B.; Griffiths, D.W.; Todd, D.T. Effects of genotype, environment, and postharvest storage on the total ascorbate content of potato (Solanum tuberosum) tubers. J. Agric. Food Chem. 2003, 51, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.Ł.; Olejnik, A.; Białas, W.; Rybicka, I.; Zielińska-Dawidziak, M.; Siger, A.; Kubiak, P.; Lewandowicz, G. The nutritional value and biological activity of concentrated protein fraction of potato juice. Nutrients 2019, 11, 1523. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, W.N.; Soladoye, O.P. Towards potato protein utilisation: Insights into separation, functionality and bioactivity of patatin. Int. J. Food Sci. 2020, 55, 2314–2322. [Google Scholar] [CrossRef]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Mu, T.H.; Sun, H.N.; Chen, J.W.; Zhang, M. Comparative study of potato protein concentrates extracted using ammonium sulfate and isoelectric precipitation. Int. J. Food Prop. 2017, 20, 2113–2127. [Google Scholar] [CrossRef]
- Saar-Reismaa, P.; Kotkas, K.; Rosenberg, V.; Kulp, M.; Kuhtinskaja, M.; Vaher, M. Analysis of Total Phenols, Sugars, and Mineral Elements in Colored Tubers of Solanum tuberosum L. Foods 2020, 9, 1862. [Google Scholar] [CrossRef] [PubMed]
- Ly, D.N.P.; Iqbal, S.; Fosu-Nyarko, J.; Milroy, S.; Jones, M.G. Multiplex CRISPR-Cas9 Gene-Editing Can Deliver Potato Cultivars with Reduced Browning and Acrylamide. Plants 2023, 12, 379. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B.P.; Kumar, P. An overview of the factors affecting sugar content of potatoes. Ann. Appl. Biol. 2004, 145, 247–256. [Google Scholar] [CrossRef]
- Jarienė, E.; Vaitkevičienė, N.; Danilčenko, H.; Tajner-Czopek, A.; Rytel, E.; Kucharska, A.; Sokół-Łętowska, A.; Gertchen, M.; Jeznach, M. Effect of biodynamic preparations on the phenolic antioxidants in potatoes with coloured-flesh. Biol. Agric. Hortic. 2017, 33, 172–182. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Lai, S.; Cao, H.; Guan, Y.; San Cheang, W.; Liu, B.; Zhao, K.; Miao, S.; Riviere, C.; et al. Effects of domestic cooking process on the chemical and biological properties of dietary phytochemicals. Trends Food Sci. Technol. 2019, 85, 55–66. [Google Scholar] [CrossRef]
- Lewis, C.E.; Walker, J.R.; Lancaster, J.E.; Sutton, K.H. Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Coloured cultivars of Solanum tuberosum L. J. Sci. Food Agric. 1998, 77, 45–57. [Google Scholar] [CrossRef]
- Kammoun, M.; Jeddou, K.B.; Rokka, V.M.; Pihlava, J.M.; Hellström, J.; Gutiérrez-Quequezana, L.; Nouri-Ellouz, O. Determination of bioactive compounds, antioxidant capacities and safety of the somatic hybrid potatoes. Potato Res. 2022, 65, 881–902. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.; Lv, F.; Chen, S.; Chen, J.; Liu, D.; Ye, X. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chem. 2016, 197, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Haynes, K.; Wiley, E.; Clevidence, B. Carotenoid content and color in diploid potatoes. J. Am. Soc. Hortic. Sci. 2001, 126, 722–726. [Google Scholar] [CrossRef]
- Haynes, K.G.; Clevidence, B.A.; Rao, D.; Vinyard, B.T. Inheritance of carotenoid content in tetraploid× diploid potato crosses. J. Am. Soc. Hortic. Sci. 2011, 136, 265–272. [Google Scholar] [CrossRef]
- Love, S.L.; Salaiz, T.; Shafii, B.; Price, W.J.; Mosley, A.R.; Thornton, R.E. Stability of expression and concentration of ascorbic acid in North American potato germplasm. Hortscience 2004, 39, 156–160. [Google Scholar] [CrossRef]
- Davies, C.S.; Ottman, M.J.; Peloquin, S.J. Can germplasm resources be used to increase the ascorbic acid content of stored potatoes? Am. J. Potato Res. 2002, 79, 295–299. [Google Scholar] [CrossRef]
- Hamouz, K.; Bečka, D.; Capouchová, I. Ascorbic acid content in potato tubers with coloured flesh as affected by genotype, environment and storage. Plant Soil Environ. 2018, 64, 605–611. [Google Scholar] [CrossRef]
- Cebulak, T.; Krochmal-Marczak, B.; Stryjecka, M.; Krzysztofik, B.; Sawicka, B.; Danilčenko, H.; Jarienè, E. Phenolic Acid Content and Antioxidant Properties of Edible Potato (Solanum tuberosum L.) with Various Tuber Flesh Colours. Foods 2023, 12, 100. [Google Scholar] [CrossRef]
- Duarte-Delgado, D.; Ñústez-López, C.E.; Narváez-Cuenca, C.E.; Restrepo-Sánchez, L.P.; Melo, S.E.; Sarmiento, F.; Kushalappa, A.C.; Mosquera-Vásquez, T. Natural variation of sucrose, glucose and fructose contents in Colombian genotypes of Solanum tuberosum Group Phureja at harvest. J. Sci. Food Agric. 2016, 96, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, D. Potato Composition, Carbohydrate Components of the Potato Tuber. J. Agric. Food Chem. 1956, 4, 543–545. [Google Scholar] [CrossRef]
- Baroja-Fernández, E.; Muñoz, F.J.; Montero, M.; Etxeberria, E.; Sesma, M.T.; Ovecka, M.; Pozueta-Romero, J. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADPglucose and UDPglucose and total yield. Plant Cell Physiol. 2009, 50, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Gavira, J.M.; Pont, S.D.; Morris, J.A.; Hedley, P.E.; Stewart, D.; Taylor, M.A.; Hancock, R.D. Senescent sweetening in potato (Solanum tuberosum) tubers is associated with a reduction in plastidial glucose-6-phosphate/phosphate translocator transcripts. Postharvest Biol. Technol. 2021, 181, 111637. [Google Scholar] [CrossRef]
- Viklund, G.Å.; Olsson, K.M.; Sjöholm, I.M.; Skog, K.I. Variety and storage conditions affect the precursor content and amount of acrylamide in potato crisps. J. Sci. Food Agric. 2008, 88, 305–312. [Google Scholar] [CrossRef]
- Pęksa, A.; Miedzianka, J. Potato Industry By-Products as a Source of Protein with Beneficial Nutritional, Functional, Health-Promoting and Antimicrobial Properties. Appl. Sci. 2021, 11, 3497. [Google Scholar] [CrossRef]
- Ortiz-Medina, E.; Donnelly, D.J. Concentration and distribution of total soluble protein in fresh and stored potato tubers. In Proceedings of the XXVI International Horticultural Congress: Potatoes, Healthy Food for Humanity: International Developments in Breeding, Toronto, ON, Canada, 11–17 August 2002; Volume 619, pp. 323–328. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, L.; Xiao, P.; Lu, Y.; Bao, J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J. Cereal. Sci. 2009, 49, 106–111. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Phenolic acids, anthocyanins, and antioxidant capacity in rice (Oryza sativa L.) grains at four stages of development after flowering. Food Chem. 2014, 143, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Heydari, H.R.; Chamani, E.; Esmaielpour, B. Cell line selection through gamma irradiation combined with multi-walled carbon nanotubes elicitation enhanced phenolic compounds accumulation in Salvia nemorosa cell culture. PCTOC 2020, 142, 353–367. [Google Scholar] [CrossRef]
- Terada, M.; Watanabe, Y.; Kunitomo, M.; Hayashi, E. Differential rapid analysis of ascorbic acid and ascorbic acid 2-sulfate by dinitrophenylhydrazine method. Anal. Biochem. 1978, 84, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Liu, R.H.; Nock, J.F.; Holliday, D.; Watkins, C.B. Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry. Postharvest Biol. Technol. 2007, 45, 349–357. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
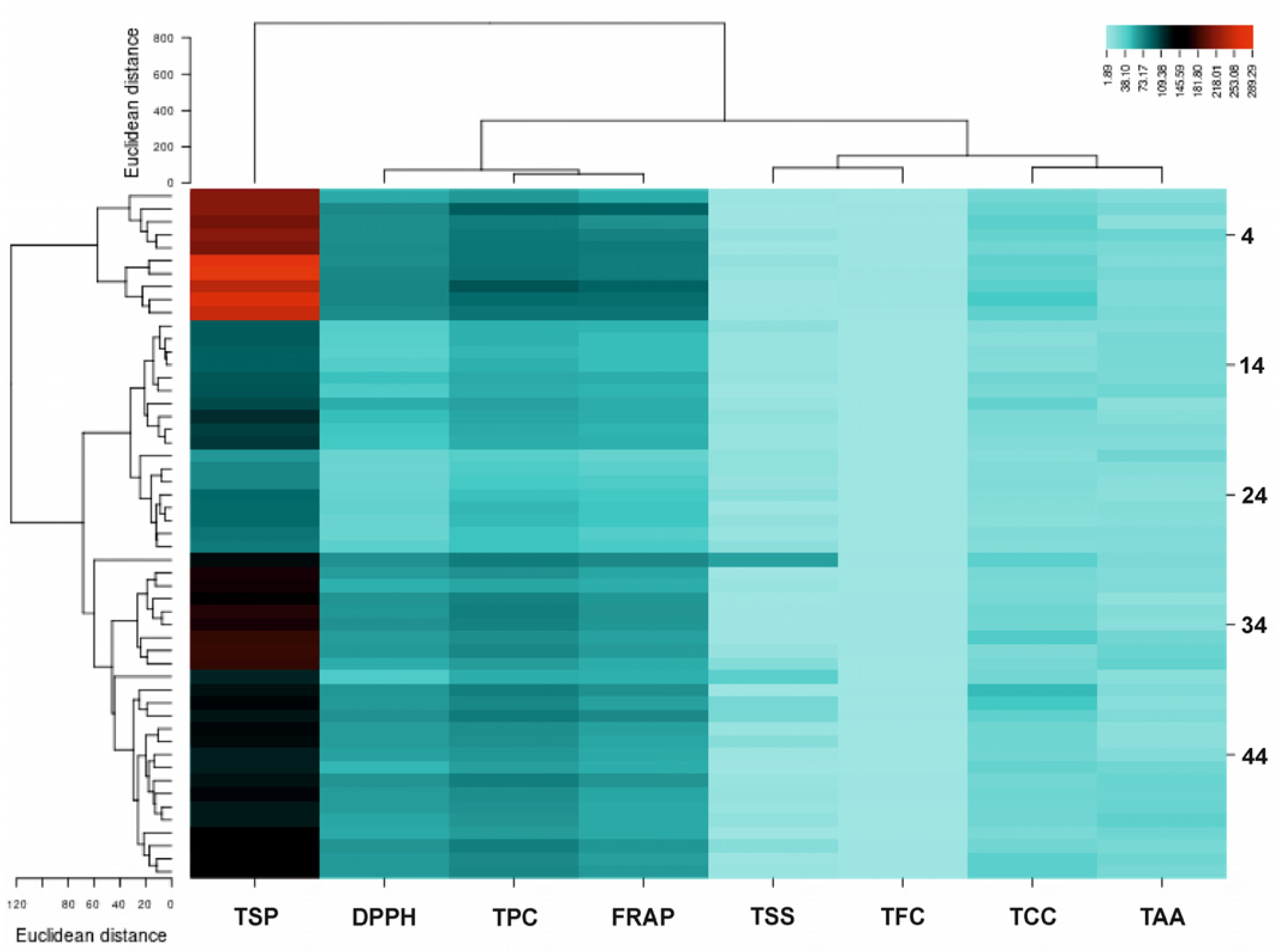


| Sample | F/S Colour | TFC (µg mg−1 FW) | TPC (µg mg−1 FW) | DPPH (%) | FRAP (µg mg−1 FW) | TCC (µg mg−1 FW) | TAA (µg mg−1 FW) | TSS (µg mg−1 FW) | TSP (µg mg−1 FW) |
|---|---|---|---|---|---|---|---|---|---|
| 6 | Y/Y | 2.80 c–i | 75.40 a–g | 63.90 a–d | 67.20 a–i | 50.20 a | 18.30 d–m | 3.07 n–q | 127.70 g–n |
| 7 | Y/Y | 2.53 c–j | 70.70 a–i | 62.80 a–d | 60.80 a–k | 37.43 b–e | 26.83 a–f | 4.34 l–q | 140.30f–m |
| 8 | Y/Y | 2.43 d–l | 71.90 a–h | 62.90 a–d | 60.00 a–k | 44.52 ab | 14.34 i–m | 23.90 c | 133.80 g–n |
| 30 | Y/Y | 2.14 e–o | 61.90 c–k | 57.80 a–g | 57.42 c–k | 20.66 j–r | 27.16 a–e | 2.95 n–q | 148.30 e–l |
| 43 | Y/Y | 2.42 d–m | 68.30 b–j | 62.00 a–e | 59.20 a–k | 26.50 g–l | 13.30 j–m | 5.31 j–q | 133.10 g–n |
| 49 | Y/Y | 2.48 d–k | 68.80 b–i | 62.10 a–e | 58.30 b–k | 26.98 g–k | 27.51 a–e | 7.61 h–k | 133.80 g–n |
| 61 | Y/Y | 2.05 f–o | 60.40 c–k | 51.14 c–k | 55.60 c–k | 30.91 e–i | 25.21 a–h | 3.08 m–q | 120.90 g–n |
| Burren | Y/Y | 4.61 a | 96.80 a | 73.50 a | 89.40 a | 36.81 c–e | 18.60 d–m | 2.82 o–q | 241.30 a–d |
| Jelly | Y/Y | 3.01 c–e | 76.60 a–f | 68.62 ab | 67.40 a–i | 36.91 c–e | 12.83 k–m | 3.36 l–q | 205.80 b–f |
| Impala | Y/Y | 2.40 d–m | 67.30 b–j | 61.98 a–e | 58.90 a–k | 26.51 g–l | 13.76 i–m | 15.53 de | 131.40 g–n |
| Milva | Y/Y | 1.91 h–o | 55.60 d–k | 41.56 h–n | 54.33 c–k | 25.03 h–o | 14.43 i–m | 36.1 b | 117.60 g–n |
| 1 | LY/Y | 2.91 c–h | 75.80 a–g | 64.63 a–d | 65.20 a–j | 27.07 g–k | 16.53 g–m | 3.40 l–q | 173.00 d–h |
| 4 | LY/Y | 2.53 c–j | 51.30 e–k | 36.10 k–n | 49.00 e–k | 18.26 l–r | 21.83 c–l | 5.64 j–p | 91.10 k–n |
| 10 | LY/Y | 1.72 j–o | 53.70 d–k | 38.70 i–n | 49.50 e–k | 16.25 o–r | 21.71 c–l | 5.69 j–o | 90.90 k–n |
| 15 | LY/Y | 2.75 c–i | 74.38 a–h | 65.15 a–d | 63.60 a–j | 23.00 i–q | 10.26 m | 2.454 q | 157.30 e–k |
| 19 | LY/Y | 2.00 g–o | 57.44 d–k | 48.40 d–k | 54.50c–k | 26.19 g–m | 20.72 c–l | 6.45 i–m | 95.70 i–n |
| 20 | LY/Y | 2.03 f–o | 58.58 c–k | 49.60 c–k | 54.60 c–k | 21.38 j–r | 14.91 i–m | 9.63 gh | 112.40 g–n |
| 26 | LY/Y | 4.20 ab | 86.10 a–c | 73.17 a | 84.20 a–c | 44.04 a–c | 18.72 d–m | 3.4 l–q | 261.40 ab |
| 42 | LY/Y | 2.47 d–k | 68.30 b–j | 62.08 a–e | 59.70 a–k | 39.96 b–d | 25.14 a–h | 2.53 pq | 180.90 c–g |
| Satina | LY/Y | 2.80 c–i | 74.39 a–h | 66.13 a–c | 63.70 a–j | 26.20 g–m | 14.09 i–m | 3.36 l–q | 166.90 e–j |
| Maradona | LY/Y | 2.07 f–o | 59.65 c–k | 54.68 b–i | 54.80 c–k | 32.83 d–h | 12.23 l–m | 5.09 k–q | 101.53 e–j |
| Kayzer | LY/Y | 1.87 i–o | 53.93 d–k | 38.90 i–n | 53.10 d–k | 18.90 k–r | 18.46 d–m | 11.30 fg | 93.40 j–n |
| Khavaran | LY/Y | 1.81 i–o | 53.92 d–k | 38.40 j–n | 49.90 e–k | 13.84 r | 23.78 b–i | 4.90 k–q | 93.20 j–n |
| javid | LY/Y | 2.05 f–o | 58.62 c–k | 54.46 b–j | 54.50 c–k | 21.68 j–r | 18.47 d–m | 5.49 j–q | 165.00 e–j |
| 12 | LY/P | 3.08 c–e | 79.00 a–e | 68.60 ab | 74.80 a–g | 31.07 e–i | 27.83 a–d | 6.23 i–m | 215.20 b–e |
| 22 | LY/P | 3.15 cd | 79.70 a–d | 68.70 ab | 76.40 a–g | 33.90 d–h | 19.35 c–m | 8.26 h–j | 273.60 ab |
| 33 | LY/P–Y | 1.50 k–o | 48.61 g–k | 31.66 l–n | 45.30 g–k | 16.60 o–r | 11.72 l–m | 12.85 ef | 86.20 k–n |
| 16 | W/W | 3.50 bc | 79.80 a–d | 69.41 ab | 77.40 a–e | 26.13 g–n | 20.98 c–l | 3.55 l–q | 207.80b–f |
| 35 | W/W | 1.42 m–o | 43.50 i–k | 28.80 mn | 41.00 h–k | 18.589 l–r | 14.16 i–m | 6.04 j–n | 72.40 mn |
| 39 | W/W | 1.38 no | 46.84 h–k | 29.65 mn | 39.60 i–k | 18.43 l–r | 18.83 d–m | 5.03 k–q | 80.90 l–n |
| 54 | W/W | 1.92 h–o | 54.90 d–k | 42.00 g–n | 53.20c–k | 22.016 j–r | 26.87 a–e | 3.34 m–q | 96.50 i–n |
| 57 | W/P | 2.26 d–o | 66.10 b–j | 61.60 a–e | 57.70 c–k | 25.358 h–o | 29.18 a–c | 5.37 j–q | 123.20 g–n |
| 68 | W/P | 4.03 ab | 81.00 a–d | 71.90 a | 76.90 a–g | 30.8 e–i | 23.46 b–j | 5.50 j–q | 289.86 a |
| 387 | W/P | 2.15 e–o | 65.29 b–k | 58.00 a–g | 57.40 c–k | 25.63 h–o | 34.37 a | 8.95 g–i | 123.40 g–n |
| sp 11 13 | W/P–W C/P–Y C/P–Y | 1.34 o 3.08 c–e 3.09 c–e | 38.10 k 77.72 a–f 75.10 a–g | 28.40 mn 66.30 a–c 65.01 a–d | 31.70 k 70.80 a–h 64.20 a–j | 15.94 p–r 34.10 d–g 26.06 g–n | 26.19 a–g 19.06 d–m 21.85 c–l | 9.84 gh 21.92 c 15.20 de | 64.10 n 125.60 g–n 147.01 e–l |
| 14 | W/P–Y | 2.76 c–i | 75.33 a–g | 65.07 a–d | 64.70 a–j | 24.41 i–p | 29.16 a–c | 5.08 k–q | 126.30 g–n |
| 23 | W/P–Y | 1.98 g–o | 57.00d–k | 46.20 e–l | 53.30 c–k | 20.29 k–r | 20.46 c–l | 5.46 j–q | 105.70 h–n |
| 31 | W/P–Y | 2.16 d–o | 64.11 c–k | 59.60 a–f | 57.10 c–k | 25.54 h–o | 17.55 e–m | 3.81 l–q | 120.30 g–n |
| 36 | W/P–Y | 2.11 e–o | 64.23 c–k | 57.12 a–h | 56.40 c–k | 24.06 i–q | 17.57 e–m | 3.83 l–q | 213.00 b–e |
| 40 | W/P–Y | 2.56 c–j | 71.90 a–h | 63.30 a–d | 63.40 a–j | 36.4 c–f | 23.33 b–j | 4.86 k–q | 150.50 e–l |
| Fontane | W/P–Y | 2.93 c–g | 76.32 a–g | 66.00 a–c | 70.60 a–i | 35.61 d–f | 19.81 c–m | 59.73 a | 131.20 g–n |
| 21 | W/Y | 1.50 k–o | 51.25 e–k | 32.30 l–n | 45.50 g–k | 15.42 qr | 16.52 g–m | 4.42 l–q | 85.80 k–n |
| 27 | W/Y | 1.32 o | 41.10 jk | 27.90 n | 36.10 j–k | 17.71 n–r | 16.69 f–m | 10.19 gh | 71.90 mn |
| 28 | W/Y | 1.96 g–o | 56.60 d–k | 44.50 f–m | 53.40 c–k | 17.82 m–r | 17.53 e–m | 5.32 j–q | 108.30 g–n |
| 32 | W/Y | 2.63 c–i | 72.40 a–h | 63.44 a–d | 61.70 a–k | 19.76 k–r | 29.27 a–c | 6.47 i–l | 181.50 c–g |
| 34 | W/Y | 1.66 j–o | 50.10 f–k | 29.65 mn | 46.00 f–k | 14.48 r | 15.81 h–m | 8.21 h–j | 85.40 k–n |
| 47 | W/Y | 2.08 f–o | 61.50 c–k | 54.77 b–i | 56.30 c–k | 22.86 i–q | 32.00 ab | 16.88 d | 179.80 d–g |
| 48 | W/Y | 2.33 d–n | 66.80 b–j | 61.60 a–e | 58.90 a–k | 23.20 i–q | 18.23 d–m | 2.58 o–q | 167.33 e–i |
| 52 | W/Y | 1.45 l–o | 47.20 h–k | 35.90 k–n | 42.50 h–k | 16.49 o–r | 18.87 d–m | 12.95 ef | 77.60l–n |
| 58 | W/Y | 4.27 ab | 92.64 ab | 70.30 ab | 88.60 ab | 28.65 f–j | 23.02 b–k | 3.36 l–q | 214.70 b–e |
| 67 | W/Y | 3.48 bc | 81.38 a–d | 69.70 ab | 80.90 a–d | 33.90 d–g | 21.39 c–l | 4.68 l–q | 249.70 a–c |
| TFC | TPC | DPPH | FRAP | TCC | TAA | TSS | TSP | |
| TFC | ||||||||
| TPC | 0.945 *** | |||||||
| DPPH | 0.848 *** | 0.947 *** | ||||||
| FRAP | 0.961 *** | 0.972 *** | 0.892 *** | |||||
| TCC | 0.641 *** | 0.712 *** | 0.729 *** | 0.677 *** | ||||
| TAA | 0.083 | 0.099 | 0.150 | 0.093 | −0.009 | |||
| TSS | −0.060 | −0.030 | −0.033 | −0.018 | 0.101 | −0.102 | ||
| TSP | 0.837 *** | 0.823 *** | 0.800 *** | 0.850 *** | 0.546 *** | 0.113 | −0.165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahadori, S.; Giglou, M.T.; Esmaielpour, B.; Dehdar, B.; Estaji, A.; Hano, C.; Gohari, G.; Vergine, M.; Vita, F. Antioxidant Compounds of Potato Breeding Genotypes and Commercial Cultivars with Yellow, Light Yellow, and White Flesh in Iran. Plants 2023, 12, 1707. https://doi.org/10.3390/plants12081707
Bahadori S, Giglou MT, Esmaielpour B, Dehdar B, Estaji A, Hano C, Gohari G, Vergine M, Vita F. Antioxidant Compounds of Potato Breeding Genotypes and Commercial Cultivars with Yellow, Light Yellow, and White Flesh in Iran. Plants. 2023; 12(8):1707. https://doi.org/10.3390/plants12081707
Chicago/Turabian StyleBahadori, Somayeh, Mousa Torabi Giglou, Behrooz Esmaielpour, Bahram Dehdar, Asghar Estaji, Christophe Hano, Gholamreza Gohari, Marzia Vergine, and Federico Vita. 2023. "Antioxidant Compounds of Potato Breeding Genotypes and Commercial Cultivars with Yellow, Light Yellow, and White Flesh in Iran" Plants 12, no. 8: 1707. https://doi.org/10.3390/plants12081707
APA StyleBahadori, S., Giglou, M. T., Esmaielpour, B., Dehdar, B., Estaji, A., Hano, C., Gohari, G., Vergine, M., & Vita, F. (2023). Antioxidant Compounds of Potato Breeding Genotypes and Commercial Cultivars with Yellow, Light Yellow, and White Flesh in Iran. Plants, 12(8), 1707. https://doi.org/10.3390/plants12081707










