The Effect of Self-Compatibility Factors on Interspecific Compatibility in Solanum Section Petota
Abstract
1. Introduction
SC Factors Tested
2. Results
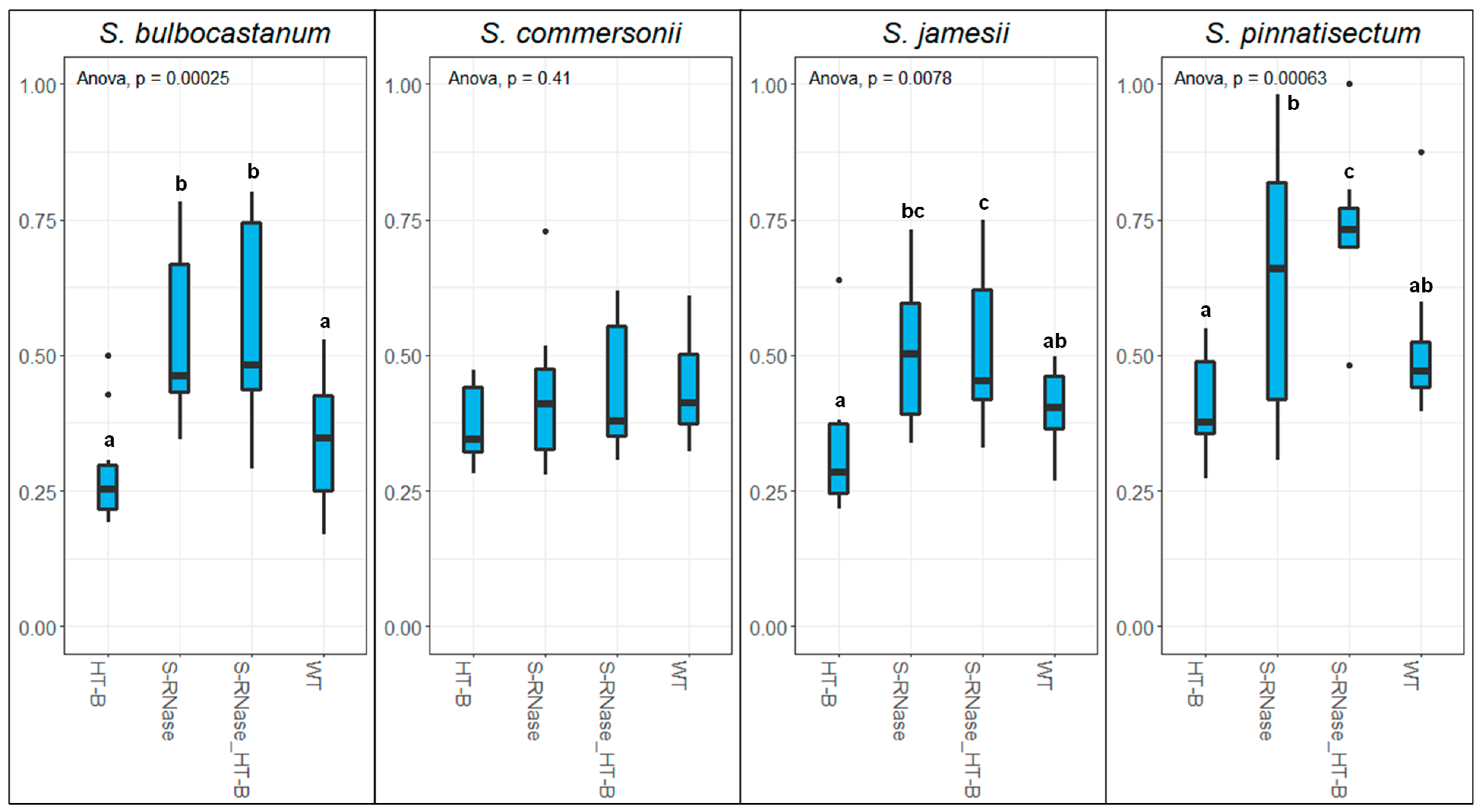
2.1. The Role of S-RNase and HT-B in Interspecific Pollen Rejection
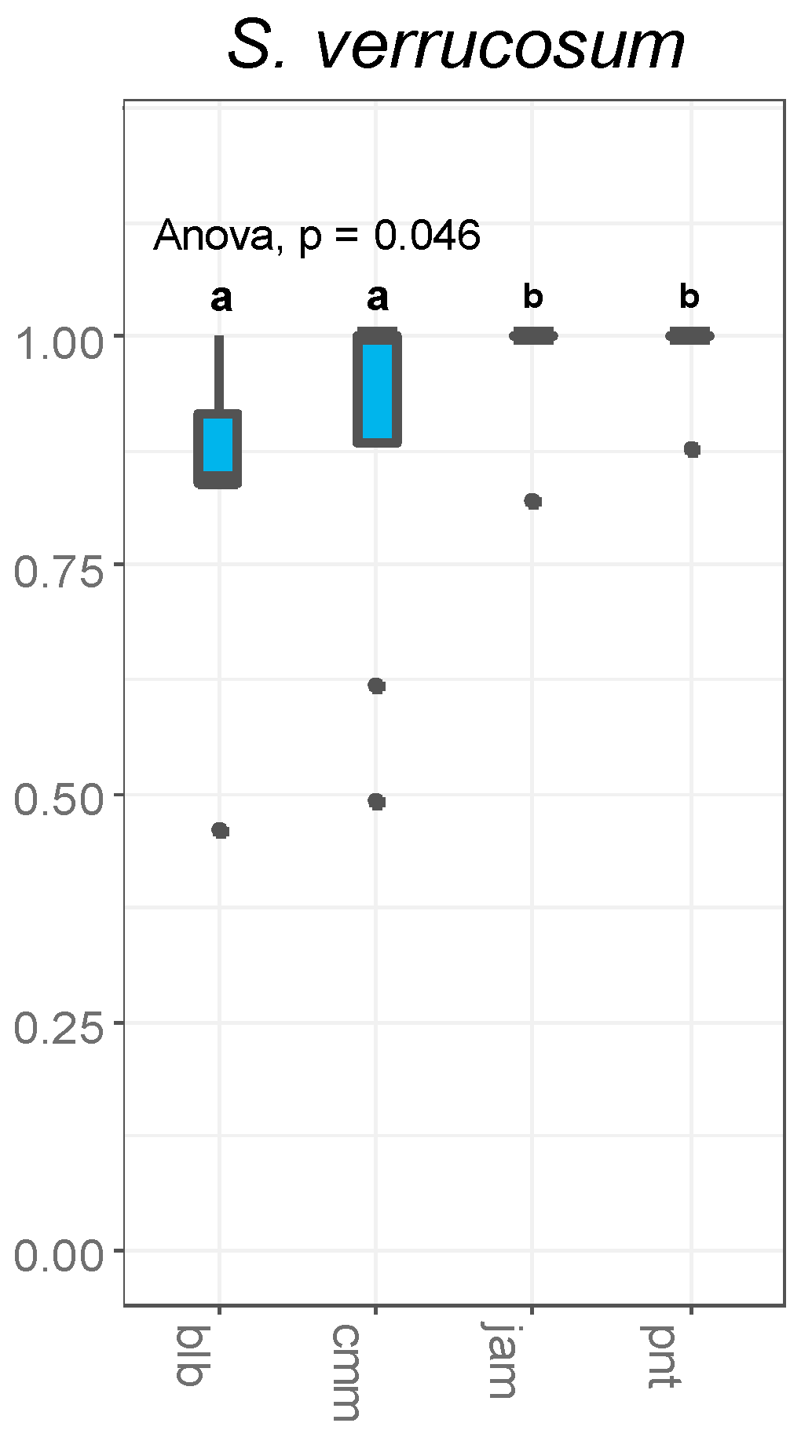
2.2. Prezygotic Interspecific Barriers in S. verrucosum
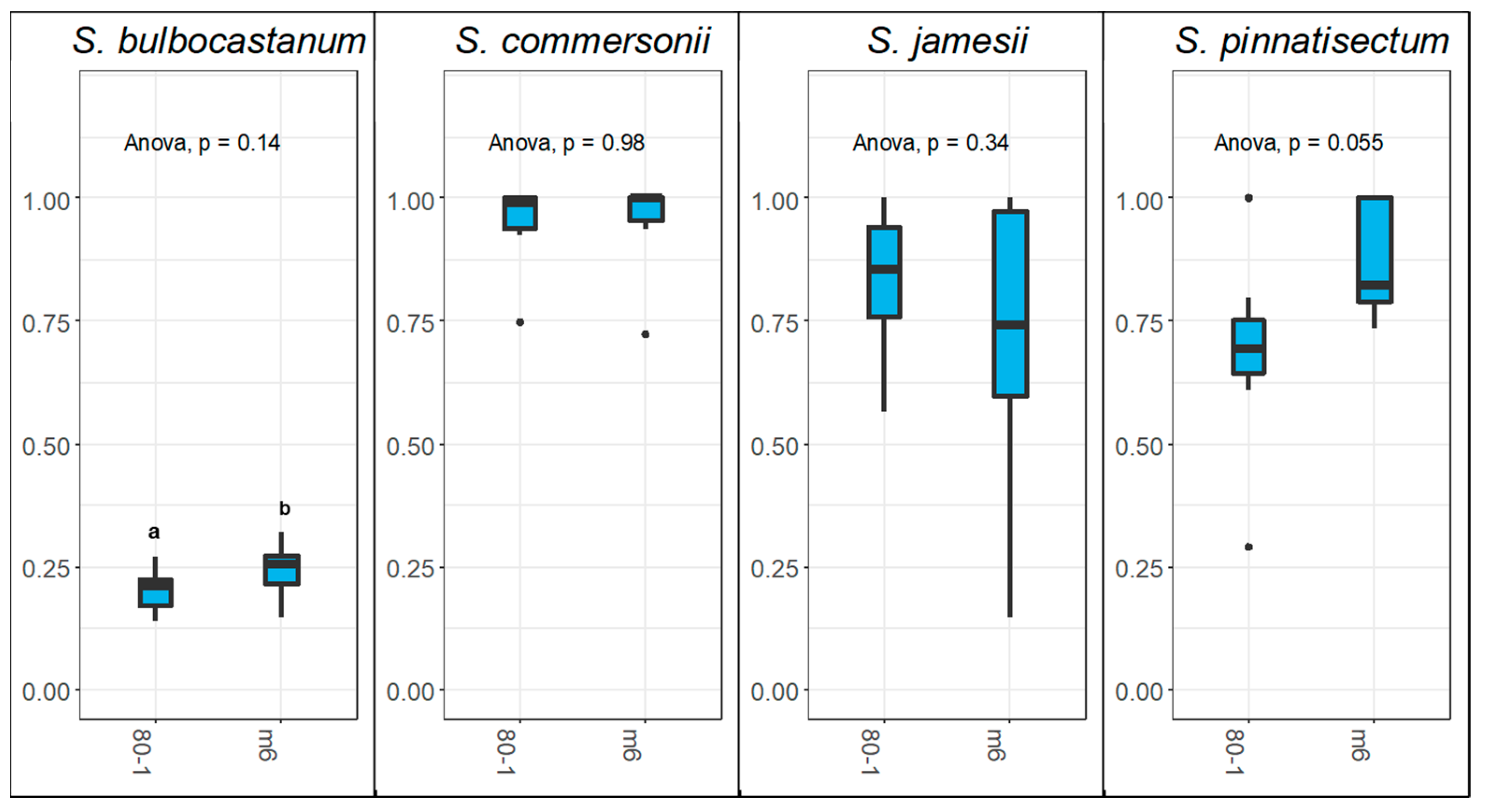
2.3. The Role of Sli in Interspecific Pollen Rejection
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Greenhouse Pollination Assays
4.3. Stylar Squash Assays
4.4. Data Collection and Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bethke, P.C.; Halterman, D.A.; Jansky, S. Are we getting better at using wild potato species in light of new tools? Crop Sci. 2017, 57, 1241–1258. [Google Scholar] [CrossRef]
- Jansky, S.; Hamernik, A. The introgression of 2× 1EBN Solanum species into the cultivated potato using Solanum verrucosum as a bridge. Genet. Resour. Crop Evol. 2009, 56, 1107–1115. [Google Scholar] [CrossRef]
- Spooner, D.M.; Bamberg, J.B. Potato genetic resources: Sources of resistance and systematics. Am. J. Potato Res. 1994, 71, 325–337. [Google Scholar] [CrossRef]
- Jansky, S.H.; Simon, R.; Spooner, D.M. A test of taxonomic predictivity: Resistance to early blight in wild relatives of cultivated potato. Phytopathology 2008, 98, 680–687. [Google Scholar] [CrossRef]
- Zlesak, D.C.; Thill, C.A. Foliar resistance to Phytophthora infestans (Mont.) de Bary (US-8) in 13 Mexican and South American Solanum species having EBNs of 1, 2, and 4 and implications for breeding. Am. J. Potato Res. 2004, 81, 421–429. [Google Scholar] [CrossRef]
- Zheng, J.; Duan, S.; Armstrong, M.R.; Duan, Y.; Xu, J.; Chen, X.; Hein, I.; Jin, L.; Li, G. New Findings on the Resistance Mechanism of an Elite Diploid Wild Potato Species JAM1-4 in Response to a Super Race Strain of Phytophthora infestans. Phytopathology 2020, 110, 1375–1387. [Google Scholar] [CrossRef]
- Novy, R.G.; Hanneman, R.E. Hybridization between Gp. Tuberosum Haploids and 1EBN wild potato species. Am. J. Potato Res. 1991, 68, 151–169. [Google Scholar] [CrossRef]
- Städler, T.; Florez-Rueda, A.M.; Roth, M. A revival of effective ploidy: The asymmetry of parental roles in endosperm-based hybridization barriers. Curr. Opin. Plant Biol. 2021, 61, 102015. [Google Scholar] [CrossRef]
- Carson, G.; Howard, H. Self-incompatibility in certain diploid potato species. Nature 1942, 150, 290. [Google Scholar] [CrossRef]
- Whalen, M.D.; Anderson, G.J. Distribution of gametophytic self-incompatibility and infrageneric classification in Solanum. Taxon 1981, 30, 761–767. [Google Scholar] [CrossRef]
- Fujii, S.; Kubo, K.; Takayama, S. Non-self- and self-recognition models in plant self-incompatibility. Nat. Plants 2016, 2, 16130. [Google Scholar] [CrossRef] [PubMed]
- Enciso-Rodriguez, F.; Manrique-Carpintero, N.C.; Nadakuduti, S.S.; Buell, C.R.; Zarka, D.; Douches, D. Overcoming self-incompatibility in diploid potato using CRISPR-Cas9. Front. Plant Sci. 2019, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Entani, T.; Takara, A.; Wang, N.; Fields, A.M.; Hua, Z.; Toyoda, M.; Kawashima, S.-I.; Ando, T.; Isogai, A.; et al. Collaborative non-self recognition system in S-RNase based self-incompatibility. Science 2010, 330, 796–799. [Google Scholar] [CrossRef]
- McClure, B.; Cruz-García, F.; Romero, C. Compatibility and incompatibility in S-RNase-based systems. Ann. Bot. 2011, 108, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.S.; Covey, P.A.; Petersen, J.J.; Chetelat, R.T.; McClure, B.; Bedinger, P.A. Testing the SI × SC rule: Pollen–pistil interactions in interspecific crosses between members of the tomato clade (Solanum section Lycopersicon, Solanaceae). Am. J. Bot. 2015, 102, 302–311. [Google Scholar] [CrossRef]
- Bedinger, P.A.; Chetelat, R.T.; McClure, B.; Moyle, L.C.; Rose, J.K.; Stack, S.M.; Kumar, A.; Van Der Knaap, E.; Baek, Y.S.; Lopez-Casado, G.; et al. Interspecific reproductive barriers in the tomato clade: Opportunities to decipher mechanisms of reproductive isolation. Sex. Plant Reprod. 2011, 24, 171–187. [Google Scholar] [CrossRef]
- Lewis, D.; Crowe, L. Unilateral interspecific incompatibility in flowering plants. Heredity 1958, 12, 233–256. [Google Scholar] [CrossRef]
- Murfett, J.; Strabala, T.J.; Zurek, D.M.; Mou, B.; Beecher, B.; McClure, B.A. S RNase and Interspecific Pollen Rejection in the Genus Nicotiana: Multiple Pollen-Rejection Pathways Contribute to Unilateral Incompatibility between Self-Incompatible and Self-Compatible Species. Plant Cell 1996, 8, 943–958. [Google Scholar] [CrossRef]
- Pandey, K.K. Interspecific incompatibility in Solanum species. Am. J. Bot. 1962, 49, 874–882. [Google Scholar] [CrossRef]
- Tovar-Méndez, A.; Lu, L.; McClure, B. HT proteins contribute to S-RNase-independent pollen rejection in Solanum. Plant J. 2017, 89, 718–729. [Google Scholar] [CrossRef]
- Markova, D.N.; Petersen, J.J.; Qin, X.; Short, D.R.; Valle, M.J.; Tovar-Méndez, A.; McClure, B.A.; Chetelat, R.T. Mutations in two pollen self-incompatibility factors in geographically marginal populations of Solanum habrochaites impact mating system transitions and reproductive isolation. Am. J. Bot. 2016, 103, 1847–1861. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.H.; Fields, A.; Kao, T. Biochemical models for S-RNase-based self-incompatibility. Mol. Plant 2008, 1, 575–585. [Google Scholar] [CrossRef]
- Tovar-Méndez, A.; Kumar, A.; Kondo, K.; Ashford, A.; Baek, Y.S.; Welch, L.; Bedinger, P.A.; McClure, B.A. Restoring pistil-side self-incompatibility factors recapitulates an interspecific reproductive barrier between tomato species. Plant J. 2014, 77, 727–736. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Kapfer, C.; Major, G.; Laurin, M.; Bertrand, C.; Kondo, K.; Kowyama, Y.; Matton, D.P. Molecular analysis of the stylar-expressed Solanum chacoense small asparagine-rich protein family related to the HT modifier of gametophytic self-incompatibility in Nicotiana. Plant J. 2002, 32, 985–996. [Google Scholar] [CrossRef]
- Eijlander, R.; Ter Laak, W.; Hermsen, J.G.; Ramanna, M.S.; Jacobsen, E. Occurrence of self-compatibility, self-incompatibility and unilateral incompatibility after crossing diploid S. tuberosum (SI) with S. verrucosum (SC): I. Expression and inheritance of self-compatibility. Euphytica 2000, 115, 127–139. [Google Scholar] [CrossRef]
- Lee, S. Generation of HT-B and HT-B Plus S-RNase Knockout Lines to Understand Self-Compatibility in Diploid Potato. Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2021. [Google Scholar]
- Ma, L.; Zhang, C.; Zhang, B.; Tang, F.; Li, F.; Liao, Q.; Tang, D.; Peng, Z.; Jia, Y.; Gao, M.; et al. A nonS-locus F-box gene breaks self-incompatibility in diploid potatoes. Nat. Commun. 2021, 12, 4142. [Google Scholar] [CrossRef] [PubMed]
- Eggers, E.J.; van der Burgt, A.; van Heusden, S.A.; de Vries, M.E.; Visser, R.G.; Bachem, C.W.; Lindhout, P. Neofunctionalisation of the Sli gene leads to self-compatibility and facilitates precision breeding in potato. Nat. Commun. 2021, 12, 267. [Google Scholar] [CrossRef]
- Sanetomo, R.; Akino, S.; Suzuki, N.; Hosaka, K. Breakdown of a hybridization barrier between Solanum pinnatisectum Dunal and potato using the S locus inhibitor gene (Sli). Euphytica 2014, 197, 119–132. [Google Scholar] [CrossRef]
- Yermishin, A.P.; Polyukhovich, Y.V.; Voronkova, E.V.; Savchuk, A.V. Production of hybrids between the 2EBN bridge species Solanum verrucosum and 1EBN diploid potato species. Am. J. Potato Res. 2014, 91, 610–617. [Google Scholar] [CrossRef]
- Eijlander, R. Mechanisms of self-incompatibility and unilateral incompatibility in diploid potato (Solanum tuberosum L.). Ph.D. Thesis, Wageningen Agricultural University, Wageningen, The Netherlands, 1998. [Google Scholar]
- Kaiser, N.R.; Jansky, S.; Coombs, J.J.; Collins, P.; Alsahlany, M.; Douches, D.S. Assessing the Contribution of Sli to Self-Compatibility in North American Diploid Potato Germplasm Using KASP™ Markers. Am. J. Potato Res. 2021, 98, 104–113. [Google Scholar] [CrossRef]
- Qin, X.; Chetelat, R.T. Ornithine decarboxylase genes contribute to S-RNase-independent pollen rejection. Plant Physiol. 2021, 186, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Jansky, S.H.; Chung, Y.S.; Kittipadukal, P. M6: A diploid potato inbred line for use in breeding and genetics research. J. Plant Regist. 2014, 8, 195–199. [Google Scholar] [CrossRef]
- Kaiser, N.; Manrique-Carpintero, N.C.; DiFonzo, C.; Coombs, J.; Douches, D. Mapping Solanum chacoense mediated Colorado potato beetle (Leptinotarsa decemlineata) resistance in a self-compatible F2 diploid population. Theor. Appl. Genet. 2020, 133, 2583–2603. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, K.; Hanneman, R.E. Genetics of self-compatibility in a self-incompatible wild diploid potato species Solanum chacoense. 1. Detection of an S locus inhibitor (Sli) gene. Euphytica 1998, 99, 191–197. [Google Scholar] [CrossRef]
- Hosaka, K.; Hanneman, R.E. Genetics of self-compatibility in a self-incompatible wild diploid potato species Solanum chacoense. 2. Localization of an S locus inhibitor (Sli) gene on the potato genome using DNA markers. Euphytica 1998, 103, 265–271. [Google Scholar] [CrossRef]
- Johnston, S.A.; Den Nijs, T.P.M.; Peloquin, S.J.; Hanneman, R.E. The significance of genic balance to endosperm development in interspecific crosses. Theor. Appl. Genet. 1980, 57, 5–9. [Google Scholar] [CrossRef]
- Köhler, C.; Dziasek, K.; Del Toro-De León, G. Postzygotic reproductive isolation established in the endosperm: Mechanisms, drivers and relevance. Philos. Trans. R. Soc. B 2021, 376, 20200118. [Google Scholar] [CrossRef]
- Li, W.; Chetelat, R.T. Unilateral incompatibility gene ui1. 1 encodes an S-locus F-box protein expressed in pollen of Solanum species. Proc. Natl. Acad. Sci. USA 2015, 112, 4417–4422. [Google Scholar] [CrossRef]
- McCormick, S. Unilateral incompatibility is linked to reduced pollen expression of a farnesyl pyrophosphate synthase. Plant J. 2018, 93, 415–416. [Google Scholar] [CrossRef]
- Canady, M.A.; Meglic, V.; Chetelat, R.T. A library of Solanum lycopersicoides introgression lines in cultivated tomato. Genome 2005, 48, 685–697. [Google Scholar] [CrossRef]
- Ji, Y.; Pertuzé, R.; Chetelat, R.T. Genome differentiation by GISH in interspecific and intergeneric hybrids of tomato and related nightshades. Chromosome Res. 2004, 12, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Carputo, D.; Barone, A.; Cardi, T.; Sebastiano, A.; Frusciante, L.; Peloquin, S.J. Endosperm balance number manipulation for direct in vivo germplasm introgression to potato from a sexually isolated relative (Solanum commersonii Dun.). Proc. Natl. Acad. Sci. USA 1997, 94, 12013–12017. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, B. Pollen Viability Assessment; International Potato Center (CIP): Lima, Peru, 2014; pp. 1–8. [Google Scholar]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]


| Line ID | Species | PI * | Gene Edit |
|---|---|---|---|
| SBGG505-A | S. bulbocastanum | 275197 | - |
| M6 | S. chacoense | BS 228 | - |
| USDA8380-1 | S. chacoense | 458310 | - |
| Scmm320266-02 | S. commersonii | 320266 | - |
| SPGG544-A | S. jamesii | 592417 | - |
| SJGG520-A | S. pinnatisectum | 275232 | - |
| SV607845.02 | S. verrucosum | 607845 | - |
| DRH195 | S. tuberosum | - | - |
| DRH195.158 | S. tuberosum | - | S-RNase KO |
| DRH195.121_009 | S. tuberosum | - | HT-B KO |
| DRH195.124_001 | S. tuberosum | - | S-RNase/HT-B KO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behling, W.L.; Douches, D.S. The Effect of Self-Compatibility Factors on Interspecific Compatibility in Solanum Section Petota. Plants 2023, 12, 1709. https://doi.org/10.3390/plants12081709
Behling WL, Douches DS. The Effect of Self-Compatibility Factors on Interspecific Compatibility in Solanum Section Petota. Plants. 2023; 12(8):1709. https://doi.org/10.3390/plants12081709
Chicago/Turabian StyleBehling, William L., and David S. Douches. 2023. "The Effect of Self-Compatibility Factors on Interspecific Compatibility in Solanum Section Petota" Plants 12, no. 8: 1709. https://doi.org/10.3390/plants12081709
APA StyleBehling, W. L., & Douches, D. S. (2023). The Effect of Self-Compatibility Factors on Interspecific Compatibility in Solanum Section Petota. Plants, 12(8), 1709. https://doi.org/10.3390/plants12081709






