Cytoplasmic Kinase Network Mediates Defense Response to Spodoptera litura in Arabidopsis
Abstract
1. Introduction
2. Results
2.1. Molecular Interaction of PBL27 with Cytoplasmic Kinases
2.2. Central Role of the PBL27/CRK2 System in S. litura Elicitor Response
2.3. Selective Phosphorylation Ability Comparison between PBL27 and CRK2
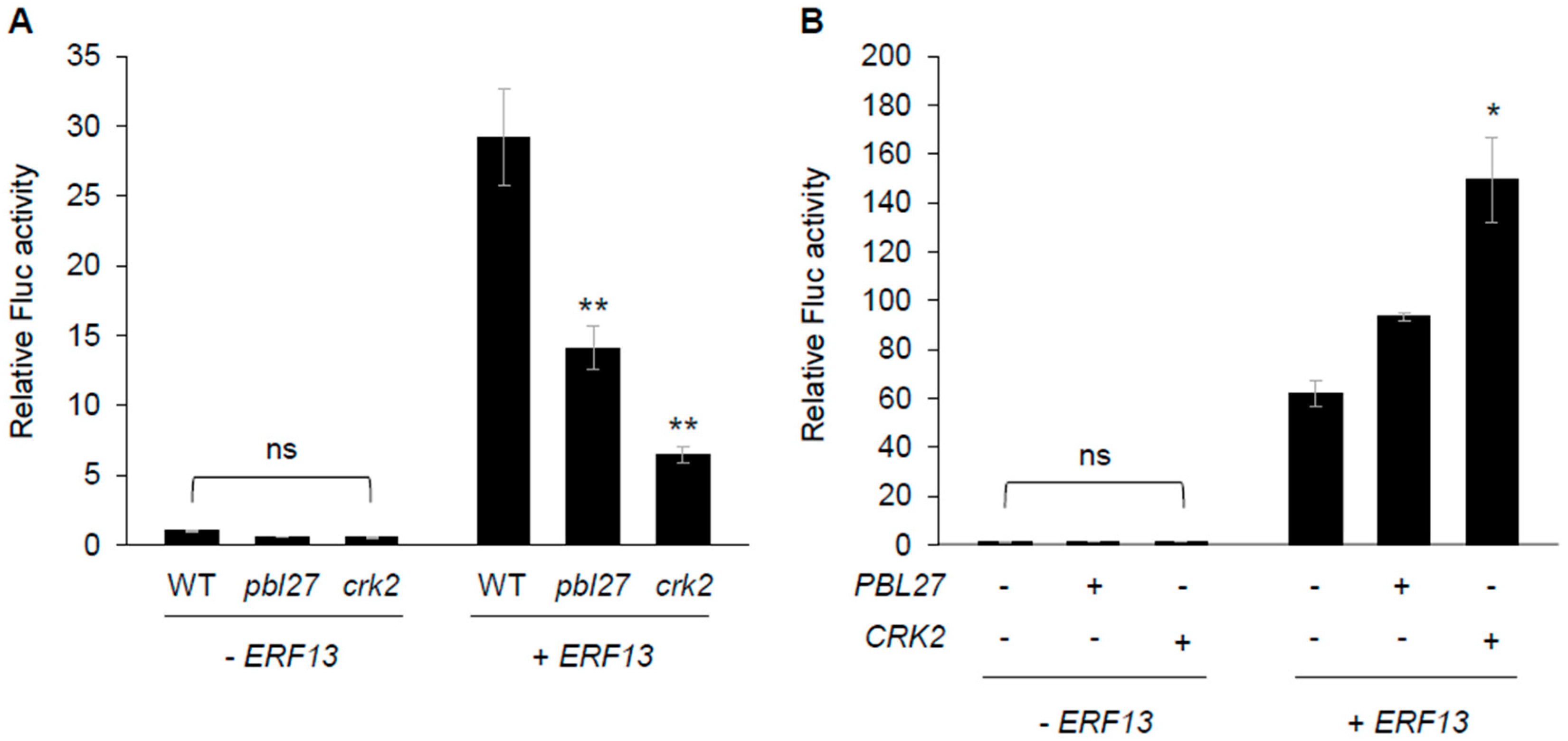
2.4. Promotion of PDF1.2 Transcription with the PBL27/CRK2/ERF13 System
2.5. Ethylene Signaling for Transcriptional Activation of ERF13
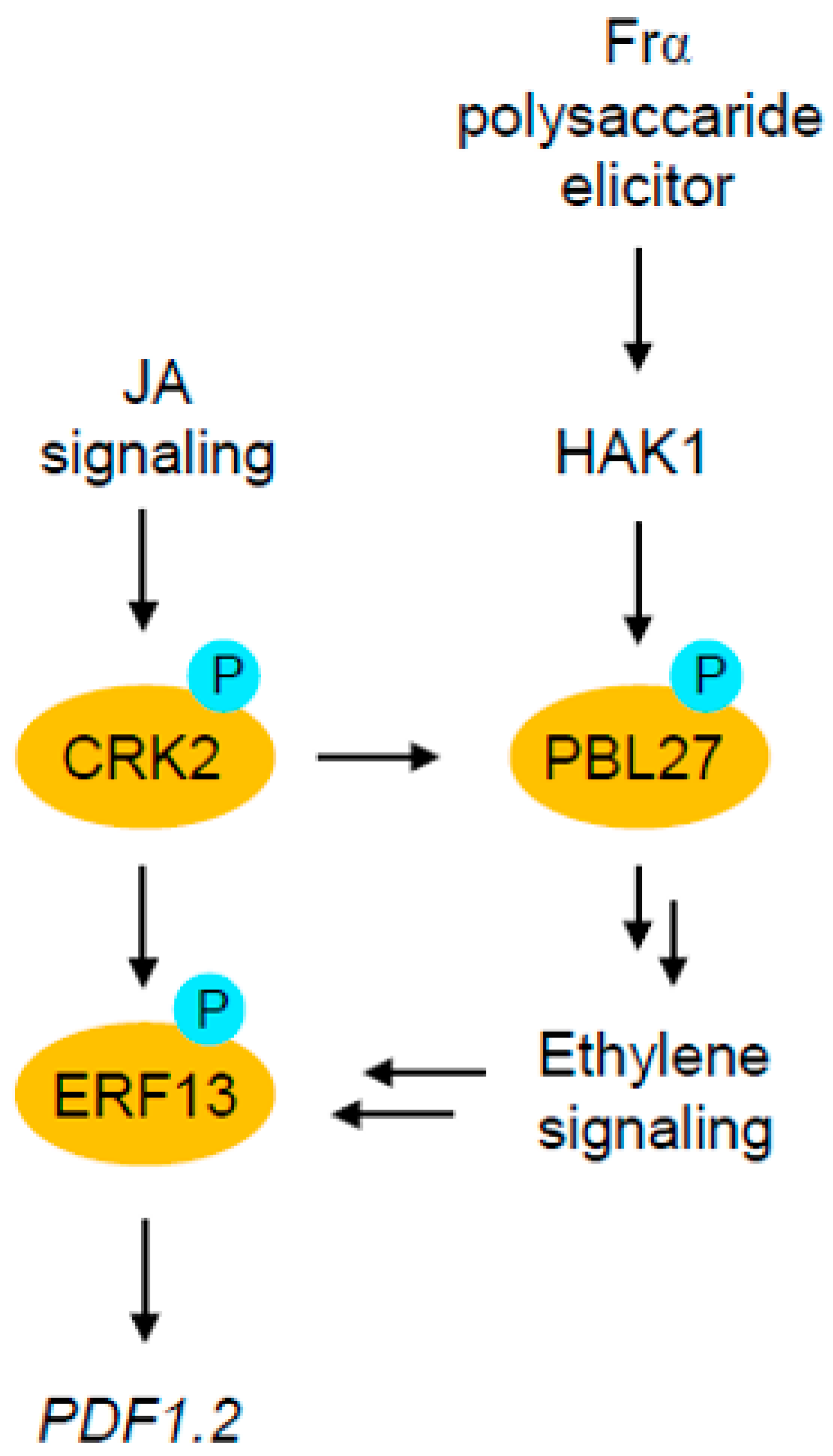
3. Discussion
4. Materials and Methods
4.1. Plants and Elicitor
4.2. Primers
4.3. Cell-Free Protein Synthesis, Immunoblotting, and AlphaScreen System
4.4. RNA Isolation, cDNA Synthesis and Quantitative Polymerase Chain Reaction (qPCR)
4.5. Co-immunoprecipitation Assay and Phosphorylation Assay
4.6. Protoplast Preparation and Transfection
4.7. LUC Assay
4.8. Statistics and Reproducibility
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Snoeck, S.; Guayazan-Palacios, N.; Steinbrenner, A.D. Molecular tug-of-war: Plant immune recognition of herbivory. Plant Cell 2022, 34, 1497–1513. [Google Scholar] [CrossRef]
- Arimura, G. Making sense of the way plants sense herbivores. Trends Plant Sci. 2021, 26, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Bricchi, I.; Occhipinti, A.; Bertea, C.M.; Zebelo, S.A.; Brillada, C.; Verrillo, F.; De Castro, C.; Molinaro, A.; Faulkner, C.; Maule, A.J.; et al. Separation of early and late responses to herbivory in Arabidopsis by changing plasmodesmal function. Plant J. 2012, 73, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Hachisu, M.; Desaki, Y.; Ito, A.; Hoshino, R.; Sano, Y.; Nozawa, A.; Mujiono, K.; Galis, I.; Yoshida, A.; et al. Soy and Arabidopsis receptor-like kinases respond to polysaccharide signals from Spodoptera species and mediate herbivore resistance. Commun. Biol. 2020, 3, 224. [Google Scholar] [CrossRef]
- Yamada, K.; Yamaguchi, K.; Shirakawa, T.; Nakagami, H.; Mine, A.; Ishikawa, K.; Fujiwara, M.; Narusaka, M.; Narusaka, Y.; Ichimura, K.; et al. The Arabidopsis CERK1-associated kinase PBL27 connects chitin perception to MAPK activation. EMBO J. 2016, 35, 2468–2483. [Google Scholar] [CrossRef] [PubMed]
- Shinya, T.; Yamaguchi, K.; Desaki, Y.; Yamada, K.; Narisawa, T.; Kobayashi, Y.; Maeda, K.; Suzuki, M.; Tanimoto, T.; Takeda, J.; et al. Selective regulation of the chitin-induced defense response by the Arabidopsis receptor-like cytoplasmic kinase PBL27. Plant J. 2014, 79, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Bi, G.; Zhou, Z.; Wang, W.; Li, L.; Rao, S.; Wu, Y.; Zhang, X.; Menke, F.L.H.; Chen, S.; Zhou, J.M. Receptor-like cytoplasmic kinases directly link diverse pattern recognition receptors to the activation of mitogen-activated protein kinase cascades in Arabidopsis. Plant Cell 2018, 30, 1543–1561. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Zhou, Z.; Miao, P.; Bi, G.; Hu, M.; Wu, Y.; Feng, F.; Zhang, X.; Zhou, J.M. Roles of receptor-like cytoplasmic kinase VII members in pattern-triggered immune signaling. Plant Physiol. 2018, 177, 1679–1690. [Google Scholar] [CrossRef]
- Nagamangala Kanchiswamy, C.; Takahashi, H.; Quadro, S.; Maffei, M.E.; Bossi, S.; Bertea, C.; Atsbaha Zebelo, S.; Muroi, A.; Ishihama, N.; Yoshioka, H.; et al. Regulation of Arabidopsis defense responses against Spodoptera littoralis by CPK-mediated calcium signaling. BMC Plant Biol. 2010, 10, 97. [Google Scholar] [CrossRef]
- Ludwig, A.A.; Saitoh, H.; Felix, G.; Freymark, G.; Miersch, O.; Wasternack, C.; Boller, T.; Jones, J.D.; Romeis, T. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc. Natl. Acad. Sci. USA 2005, 102, 10736–10741. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ohura, I.; Kawakita, K.; Yokota, N.; Fujiwara, M.; Shimamoto, K.; Doke, N.; Yoshioka, H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 2007, 19, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Ichikawa, T.; Nomura, H.; Kobayashi, M.; Kamiyoshihara, Y.; Mori, H.; Kadota, Y.; Zipfel, C.; Jones, J.D.G.; Yoshioka, H. The variable domain of a plant calcium-dependent protein kinase (CDPK) confers subcellular localization and substrate recognition for NADPH oxidase. J. Biol. Chem. 2013, 288, 14332–14340. [Google Scholar] [CrossRef] [PubMed]
- Nagamangala Kanchiswamy, C.; Muroi, A.; Maffei, M.E.; Yoshioka, H.; Sawasaki, T.; Arimura, G. Ca2+-dependent protein kinases and their substrate HsfB2a are differently involved in the heat response signaling pathway in Arabidopsis. Plant Biotechnol. 2010, 27, 469–473. [Google Scholar] [CrossRef]
- Romeis, T.; Herde, M. From local to global: CDPKs in systemic defense signaling upon microbial and herbivore attack. Curr. Opin. Plant Biol. 2014, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Uemura, T.; Nemoto, K.; Daito, M.; Nozawa, A.; Sawasaki, T.; Arimura, G. Tyrosine kinase-dependent defense responses against herbivory in Arabidopsis. Front. Plant Sci. 2019, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, K.; Takemori, N.; Seki, M.; Shinozaki, K.; Sawasaki, T. Members of the plant CRK superfamily are capable of trans- and autophosphorylation of tyrosine residues. J. Biol. Chem. 2015, 290, 16665–16677. [Google Scholar] [CrossRef]
- Yamada, Y.; Sato, F. Tyrosine phosphorylation and protein degradation control the transcriptional activity of WRKY involved in benzylisoquinoline alkaloid biosynthesis. Sci. Rep. 2016, 6, 31988. [Google Scholar] [CrossRef]
- Nemoto, K.; Ramadan, A.; Arimura, G.; Imai, K.; Tomii, K.; Shinozaki, K.; Sawasaki, T. Tyrosine phosphorylation of the GARU E3 ubiquitin ligase promotes gibberellin signalling by preventing GID1 degradation. Nat. Commun. 2017, 8, 1004. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, C.K. Preparation of ethylene gas and comparison of ethylene responses induced by ethylene, ACC, and ethephon. Plant Physiol. Biochem. 2010, 48, 45–53. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, J.M. Receptor-like cytoplasmic kinases: Central players in plant receptor kinase-mediated signaling. Annu. Rev. Plant Biol. 2018, 69, 267–299. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Petersen, M.; Mundy, J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010, 61, 621–649. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 2004, 16, 3386–3399. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, G.J.; Yang, K.Y.; Mao, G.; Wang, R.; Liu, Y.; Zhang, S. Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J 2010, 64, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Maierhofer, T.; Rybak, K.; Sklenar, J.; Breakspear, A.; Johnston, M.G.; Fliegmann, J.; Huang, S.; Roelfsema, M.R.G.; Felix, G.; et al. Anion channel SLAH3 is a regulatory target of chitin receptor-associated kinase PBL27 in microbial stomatal closure. Elife 2019, 8, e44474. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Sumioka, H.; Takiguchi, M.; Uemura, T.; Kihara, Y.; Shinya, T.; Galis, I.; Arimura, G. Phytohormone-dependent plant defense signaling orchestrated by oral bacteria of the herbivore Spodoptera litura. New Phytol. 2021, 231, 2029–2038. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Li, S.; Zhang, L.; Zou, C.; Yu, D. The role of WRKY transcription factors in plant abiotic stresses. Biochim. Biophys. Acta 2012, 1819, 120–128. [Google Scholar] [CrossRef]
- Meena, M.K.; Prajapati, R.; Krishna, D.; Divakaran, K.; Pandey, Y.; Reichelt, M.; Mathew, M.K.; Boland, W.; Mithöfer, A.; Vadassery, J. The Ca2+ channel CNGC19 regulates Arabidopsis defense against Spodoptera herbivory. Plant Cell 2019, 31, 1539–1562. [Google Scholar] [CrossRef]
- Klimecka, M.; Muszyńska, G. Structure and functions of plant calcium-dependent protein kinases. Acta Biochim. Pol. 2007, 54, 219–233. [Google Scholar] [CrossRef]
- Yano, T.; Takeda, H.; Uematsu, A.; Yamanaka, S.; Nomura, S.; Nemoto, K.; Iwasaki, T.; Takahashi, H.; Sawasaki, T. AGIA tag system based on a high affinity rabbit monoclonal antibody against human dopamine receptor D1 for protein analysis. PLoS ONE 2016, 11, e0156716. [Google Scholar] [CrossRef]
- Wu, F.H.; Shen, S.C.; Lee, L.Y.; Lee, S.H.; Chan, M.T.; Lin, C.S. Tape-Arabidopsis Sandwich—A simpler Arabidopsis protoplast isolation method. Plant Methods 2009, 5, 16. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Luehrsen, K.R.; Dewet, J.R.; Walbot, V. Transient expression analysis in plants using firefly luciferase reporter gene. Methods Enzymol. 1992, 216, 397–414. [Google Scholar] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desaki, Y.; Morishima, M.; Sano, Y.; Uemura, T.; Ito, A.; Nemoto, K.; Nozawa, A.; Sawasaki, T.; Arimura, G.-i. Cytoplasmic Kinase Network Mediates Defense Response to Spodoptera litura in Arabidopsis. Plants 2023, 12, 1747. https://doi.org/10.3390/plants12091747
Desaki Y, Morishima M, Sano Y, Uemura T, Ito A, Nemoto K, Nozawa A, Sawasaki T, Arimura G-i. Cytoplasmic Kinase Network Mediates Defense Response to Spodoptera litura in Arabidopsis. Plants. 2023; 12(9):1747. https://doi.org/10.3390/plants12091747
Chicago/Turabian StyleDesaki, Yoshitake, Minami Morishima, Yuka Sano, Takuya Uemura, Ayaka Ito, Keiichirou Nemoto, Akira Nozawa, Tatsuya Sawasaki, and Gen-ichiro Arimura. 2023. "Cytoplasmic Kinase Network Mediates Defense Response to Spodoptera litura in Arabidopsis" Plants 12, no. 9: 1747. https://doi.org/10.3390/plants12091747
APA StyleDesaki, Y., Morishima, M., Sano, Y., Uemura, T., Ito, A., Nemoto, K., Nozawa, A., Sawasaki, T., & Arimura, G.-i. (2023). Cytoplasmic Kinase Network Mediates Defense Response to Spodoptera litura in Arabidopsis. Plants, 12(9), 1747. https://doi.org/10.3390/plants12091747





