Antioxidant and Antibacterial Activities of the Leaf and Stem Extracts of Combretum molle (R. Br. ex G. Don.) Engl. & Diels
Abstract
:1. Introduction
2. Results
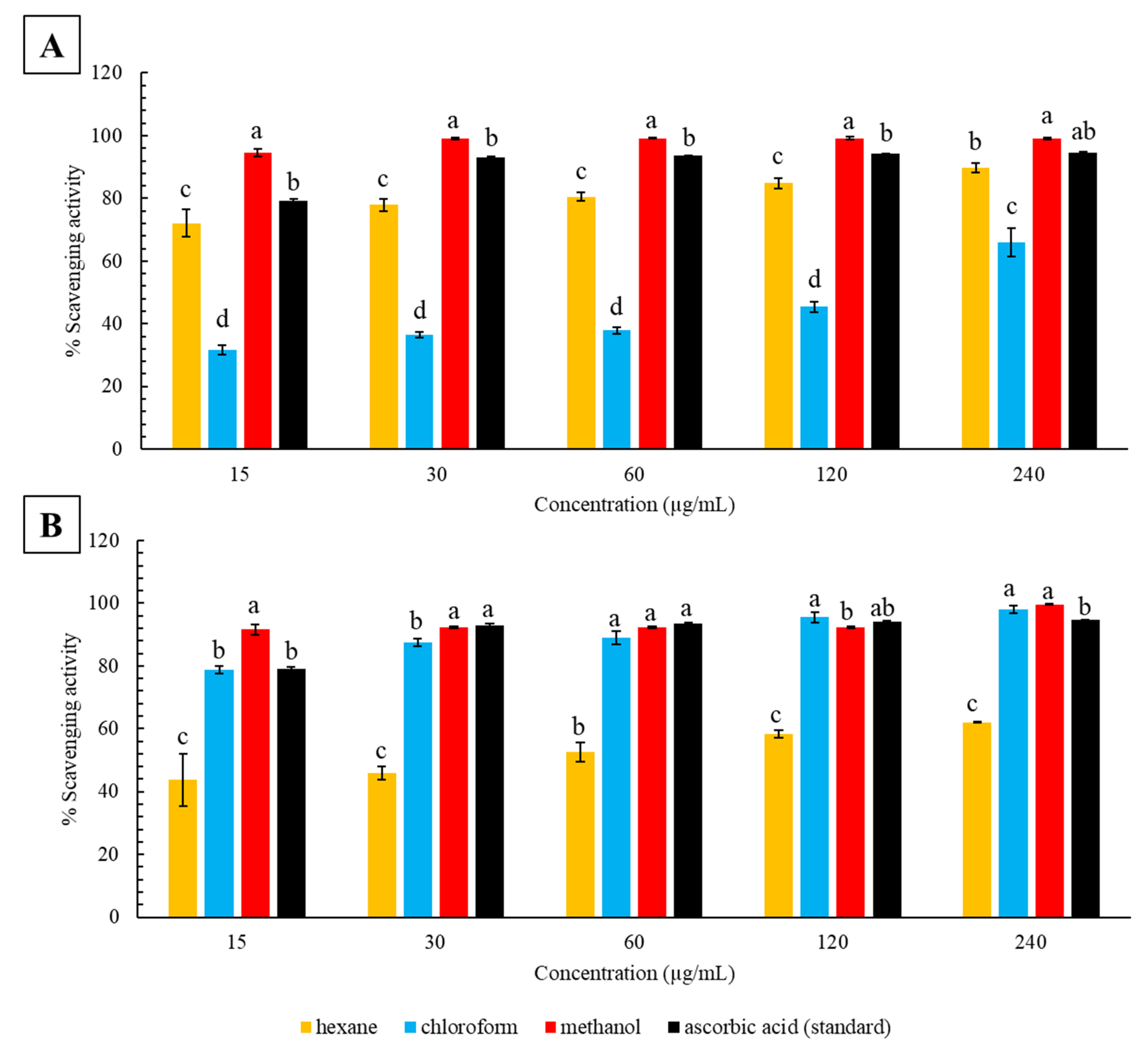
2.1. DPPH Radical Scavenging Activity
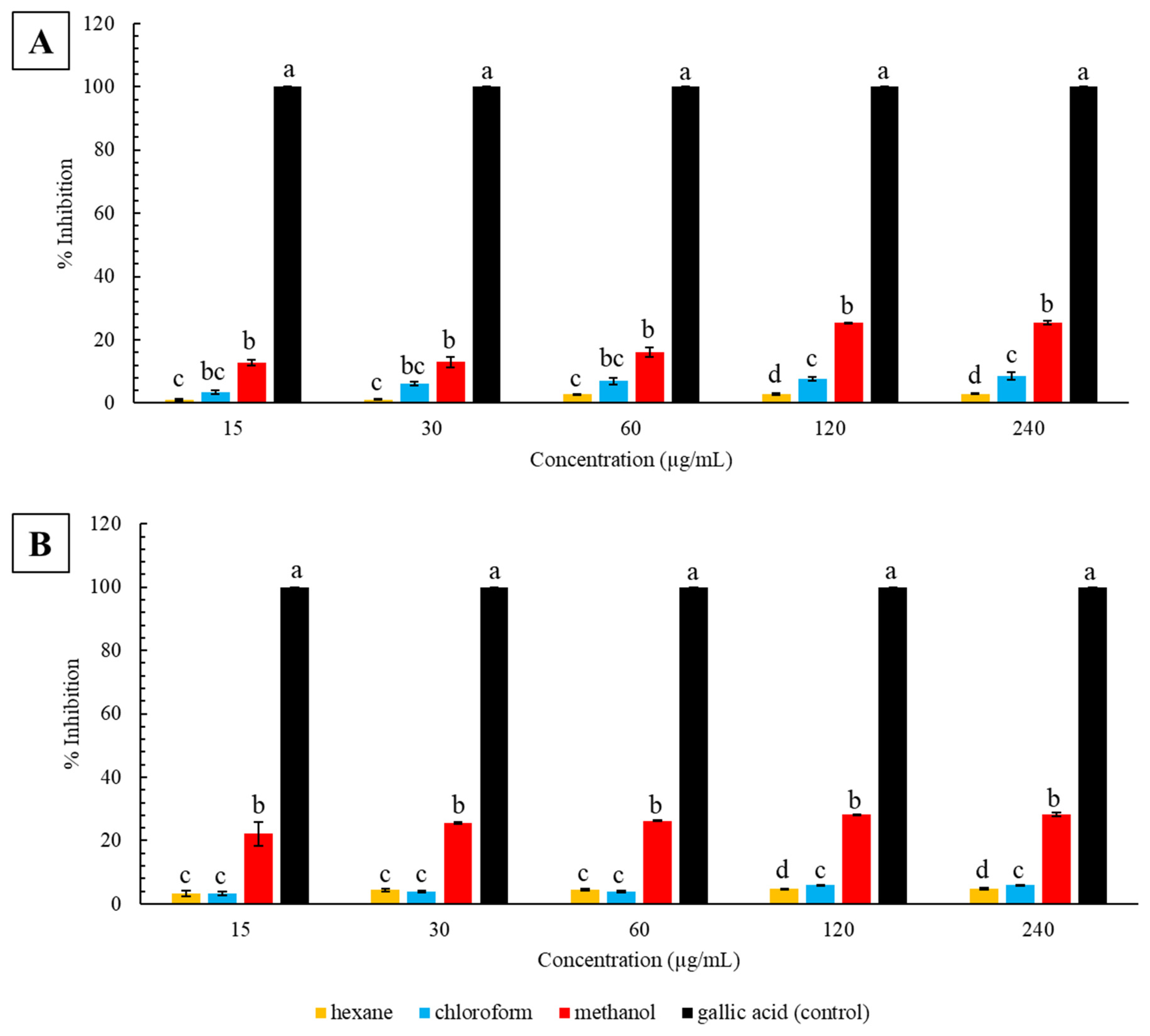
2.2. Ferric-Reducing Antioxidant Power
2.3. Antibacterial Activity
3. Discussion
3.1. Antioxidant Activity
3.2. Antibacterial Activity
4. Materials and Methods
4.1. Plant Material Collection and Extract Preparation
4.2. Antioxidant Assays
4.2.1. Assay of 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging
4.2.2. Ferric (Fe3+)-Reducing Antioxidant Power (FRAP) Assay
4.3. Antibacterial Assay
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Jalali, A.; Dabaghian, F.; Akbrialiabad, H.; Foroughinia, F.; Zarshenas, M.M. A pharmacology-based comprehensive review on medicinal plants and phytoactive constituents possibly effective in the management of COVID-19. Phytother. Res. 2021, 35, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, A.; Prinsloo, G. Medicinal plant harvesting, sustainability and cultivation in South Africa. Biol. Conserv. 2018, 227, 335–342. [Google Scholar] [CrossRef]
- Ozioma, E.-O.J.; Chinwe, O.A.N. Herbal medicines in African traditional medicine. Herb. Med. 2019, 10, 191–214. [Google Scholar]
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacol. 2015, 4, 27. [Google Scholar]
- Farooq, S.; Ngaini, Z. Natural and synthetic drugs as potential treatment for coronavirus disease 2019 (COVID-2019). Chem. Afr. 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Burman, S.; Bhattacharya, K.; Mukherjee, D.; Chandra, G. Antibacterial efficacy of leaf extracts of Combretum album Pers. against some pathogenic bacteria. BMC Complement Altern. Med. 2018, 18, 213. [Google Scholar] [CrossRef]
- Kengne, I.C.; Feugap, L.D.T.; Njouendou, A.J.; Ngnokam, C.D.J.; Djamalladine, M.D.; Ngnokam, D.; Voutquenne-Nazabadioko, L.; Tamokou, J.-D.-D. Antibacterial, antifungal and antioxidant activities of whole plant chemical constituents of Rumex abyssinicus. BMC Complement Altern. Med. 2021, 21, 164. [Google Scholar] [CrossRef]
- Daria, S.; Islam, M.R. Indiscriminate Use of Antibiotics for COVID-19 Treatment in South Asian Countries is a Threat for Future Pandemics Due to Antibiotic Resistance. Clin. Pathol. 2022, 15, 2632010X221099889. [Google Scholar] [CrossRef]
- Safari, M.; Ahmady-Asbchin, S. Evaluation of antioxidant and antibacterial activities of methanolic extract of medlar (Mespilus germanica L.) leaves. Biotechnol. Biotechnol. Equip. 2019, 33, 372–378. [Google Scholar] [CrossRef]
- Les, F.; Cásedas, G.; López, V. Bioactivity of medicinal plants and extracts. Biology 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.H. Ethnobotany of the Meskwaki Indians; Public Museum of the City of Milwaukee: Milwaukee, WI, USA, 1928. [Google Scholar]
- Ehlert, E. A History of Medicine in the Early Years; Manitowoc County Historical Society: Manitowoc, WI, USA, 1968; Volume 6. [Google Scholar]
- Rodgers, C.; Verotta, L. Chemistry and biological properties of the African Combretaceae. In Chemistry, Biological and Pharmacological Properties of African Medicinal Plants; University of Zimbabwe Publications: Harare, Zimbabwe, 1996; pp. 121–141. [Google Scholar]
- Hamza, R.Z.; Al-Baqami, N.M.; Khojah, E.; Mansour, A.M.; Al-Motaani, S.E.; Al-Salmi, F.A.; El-Megharbel, S.M. Possible antioxidant and antidiabetic effects of Combretum molle extract in a diabetes mellitus experimental model in male rats. Nat. Prod. Commun. 2021, 16, 1–10. [Google Scholar]
- Estella, O.U.; William, A.C.; Patrick, O.; Ikenna, C.; Mba, T.; Obinna, O.; Ginikachukwu, U. Evaluation of the analgesic and antipyretic activity of methanol extract of Combretum bauchiense Hutch & Dalziel (Combretaceae) leaves. Phytomed. Plus 2022, 2, 100166. [Google Scholar]
- Fyhrquist, P.; Mwasumbi, L.; Hæggström, C.-A.; Vuorela, H.; Hiltunen, R.; Vuorela, P. Ethnobotanical and antimicrobial investigation on some species of Terminalia and Combretum (Combretaceae) growing in Tanzania. J. Ethnopharmacol. 2002, 79, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Asres, K.; Mazumder, A.; Bucar, F. Antibacterial and antifungal activities of extracts of Combretum molle. Ethiop. Med. J. 2006, 44, 269. [Google Scholar] [PubMed]
- Ntshanka, N.M.; Ejidike, I.P.; Mthunzi, F.M.; Moloto, M.J.; Mubiayi, K.P. Investigation into the phytochemical profile, antioxidant and antibacterial potentials of Combretum molle and Acacia mearnsii leaf parts. Biomed. Pharmacol. J. 2020, 13, 1683–1694. [Google Scholar] [CrossRef]
- Asres, K.; Bucar, F.; Knauder, E.; Yardley, V.; Kendrick, H.; Croft, S. In vitro antiprotozoal activity of extract and compounds from the stem bark of Combretum molle. Phytother. Res. 2001, 15, 613–617. [Google Scholar] [CrossRef]
- Ahmed, B.; Al-Howiriny, T.; Passreiter, C.; Mossa, J. Combretene-A and B: Two new triterpenes from Combretum molle. Pharm. Biol. 2004, 42, 109–113. [Google Scholar] [CrossRef]
- Pegel, K.H.; Rogers, C.B. The characterisation of mollic acid 3β-D-xyloside and its genuine aglycone mollic acid, two novel 1α-hydroxycycloartenoids from Combretum molle. J. Chem. Soc. Perkin Trans. 1985, 1, 1711–1715. [Google Scholar] [CrossRef]
- Ponou, B.K.; Barboni, L.; Teponno, R.B.; Mbiantcha, M.; Nguelefack, T.B.; Park, H.-J.; Lee, K.-T.; Tapondjou, L.A. Polyhydroxyoleanane-type triterpenoids from Combretum molle and their anti-inflammatory activity. Phytochem. Lett. 2008, 1, 183–187. [Google Scholar] [CrossRef]
- Masoko, P.; Picard, J.; Eloff, J. Antifungal activities of six south African Terminalia species (Combretaceae). J. Ethnopharmacol. 2005, 99, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gruz, J.; Ayaz, F.A.; Torun, H.; Strnad, M. Phenolic acid content and radical scavenging activity of extracts from medlar (Mespilus germanica L.) fruit at different stages of ripening. Food Chem. 2011, 124, 271–277. [Google Scholar] [CrossRef]
- Muhtadi, M.; Wiyono, A.A.F. Testing antioxidant activity of Plumeria Alba and Plumeria Rubra ethanolic extracts using DPPH and Frap methods and determining their total flavonoid and phenolic levels. J. Nutrac. Herb. Med. 2020, 3, 38–50. [Google Scholar]
- Palipoch, S. A review of oxidative stress in acute kidney injury: Protective role of medicinal plants-derived antioxidants. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 88–93. [Google Scholar] [CrossRef]
- Koevi, K.-K.A.; Millogo, V.; Fokou, J.B.H.; Sarr, A.; Ouedraogo, G.A.; Bassene, E. Phytochemical analysis and antioxidant activities of Combretum molle and Pericopsis laxiflora. Int. J. Biol. Chem. Sci. 2015, 9, 2423–2431. [Google Scholar] [CrossRef]
- Babatunde, S.; Moyinoluwa, O.; Oluwatosin, A.; Eigege, W.; Shreyans, J. Bioguided isolation of an antioxidant compound from Combretum racemosum P. Beav leaf. J. Biol. Chem. Sci. 2014, 8, 2339–2346. [Google Scholar]
- Bhuiya, N.M.A. Investigation on antioxidant and antimicrobial properties of methanolic extract of Combretum indicum leaf. Int. J. Green Pharm. 2020, 14, 169–174. [Google Scholar]
- Rajalingam, D.; Varadharajan, R.; Palani, S. Evaluation of hepatoprotective and antioxidant effect of Combretum albidum G. don against CCl4 induced hepatotoxicity in rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 218–223. [Google Scholar] [CrossRef]
- Stanković, N.; Mihajilov-Krstev, T.; Zlatković, B.; Stankov-Jovanović, V.; Mitić, V.; Jović, J.; Čomić, L.; Kocić, B.; Bernstein, N. Antibacterial and antioxidant activity of traditional medicinal plants from the Balkan Peninsula. NJAS -Wagening. J. Life Sci. 2016, 78, 21–28. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Van Staden, J. In vitro antibacterial activity of Combretum edwardsii, Combretum krausii, and Maytenus nemorosa and their synergistic effects in combination with antibiotics. Fron. Pharmacol. 2016, 7, 208–217. [Google Scholar] [CrossRef]
- Mogashoa, M.; Masoko, P.; Eloff, J. Different Combretum molle (Combretaceae) leaf extracts contain several different antifungal and antibacterial compounds. S. Afr. J. Bot. 2019, 126, 322–327. [Google Scholar] [CrossRef]
- Mu, J.; Uehara, T.; Li, J.; Furuno, T. Identification and evaluation of antioxidant activities of bamboo extracts. For. Stud. China. 2004, 6, 1–5. [Google Scholar] [CrossRef]
- Agbor, A.M.; Naidoo, S. Knowledge and practice of traditional healers in oral health in the Bui Division, Cameroon. J. Ethnobiol. Ethnomedicine. 2011, 7, 6. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Braca, A.; Sortino, C.; Politi, M.; Morelli, I.; Mendez, J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002, 79, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food. Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Rademan, S.; Anantharaju, P.G.; Madhunapantula, S.V.; Lall, N. The anti-proliferative and antioxidant activity of four indigenous South African plants. Afr. J. Tradit. Complement. Altern. Med. 2019, 16, 13–23. [Google Scholar] [CrossRef]
- Njoya, E.M. Medicinal plants, antioxidant potential, and cancer. In Cancer, 2nd ed.; Academic Press: Cambridge, MA, USA; Elsevier: New York, NY, USA, 2021; pp. 349–357. [Google Scholar]
- Mulaw, T.; Wubetu, M.; Dessie, B.; Demeke, G.; Molla, Y. Evaluation of antimalarial activity of the 80% methanolic stem bark extract of Combretum molle against Plasmodium berghei in mice. J. Evid.-Based Integr. Med. 2019, 24, 1–9. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, S. Comparative assessment of total phenolic content and in vitro antioxidant activities of bark and leaf methanolic extracts of Manilkara hexandra (Roxb.) Dubard. J. King Saud Univ. Sci. 2020, 32, 643–647. [Google Scholar] [CrossRef]
- Naidoo, C.M.; Naidoo, Y.; Dewir, Y.H.; Singh, M.; Daniels, A.N.; El-Ramady, H. In Vitro investigation of the antioxidant and cytotoxic potential of Tabernaemontana ventricosa hochst. Ex A. DC. leaf, stem, and latex extracts. Horticulturae 2022, 8, 91. [Google Scholar] [CrossRef]
- Aderogba, M.; Kgatle, D.T.; McGaw, L.J.; Eloff, J.N. Isolation of antioxidant constituents from Combretum apiculatum subsp. apiculatum. S. Afr. J. Bot. 2012, 79, 125–131. [Google Scholar] [CrossRef]
- Manga, F.N.; El Khattabi, C.; Fontaine, J.; Berkenboom, G.; Duez, P.; Nzunzu, J.L.; Pochet, S. Vascular effects and antioxidant activity of two Combretum species from Democratic Republic of Congo. J. Ethnopharmacol. 2012, 142, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Kpemissi, M.; Eklu-Gadegbeku, K.; Veerapur, V.P.; Potârniche, A.-V.; Adi, K.; Vijayakumar, S.; Banakar, S.M.; Thimmaiah, N.; Metowogo, K.; Aklikokou, K. Antioxidant and nephroprotection activities of Combretum micranthum: A phytochemical, in-vitro and ex-vivo studies. Heliyon 2019, 5, e01365. [Google Scholar] [CrossRef] [PubMed]
- Henkel, S.; Misuraca, M.C.; Troselj, P.; Davidson, J.; Hunter, C.A. Polarisation effects on the solvation properties of alcohols. Chem. Sci 2018, 9, 88–99. [Google Scholar] [CrossRef]
- Roopashree, K.; Naik, D. Advanced method of secondary metabolite extraction and quality analysis. J. Pharmacogn. Phytochem. 2019, 8, 1829–1842. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Fernandes, R.d.P.; Trindade, M.; Tonin, F.; Lima, C.; Pugine, S.; Munekata, P.; Lorenzo, J.; De Melo, M. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef]
- Bashir, M. Ameliorative Potential of Ethyl Acetate and Aqueous Fractions of Methanol Leaf Extract of Combretum micranthum against Free Radicals. Saudi J. Med. Pharm. Sci. 2022, 8, 12–20. [Google Scholar] [CrossRef]
- Amos-Tautua, B.; Oluwafemi, O.; Ajileye, O.; Alayande, K.; Olawuni, I.; Bamidele, F.; Onigbinde, A.; Songca, S. Antimicrobial, antioxidant activities in vitro and polyphenol contents of the leaf extract of a versatile medicinal plant. Asian J. Appl. Sci. 2017, 5, 1057–1067. [Google Scholar]
- Müller, F.; Rapp, J.; Hacker, A.-L.; Feith, A.; Takors, R.; Blombach, B. CO2/HCO3− accelerates iron reduction through phenolic compounds. MBio 2020, 11, e00085-20. [Google Scholar] [CrossRef] [PubMed]
- Chirumamilla, P.; Taduri, S. Assessment of in vitro anti-inflammatory, antioxidant and antidiabetic activities of Solanum khasianum Clarke. Vegetos 2022, 35, 1–8. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- Perez, C. Antibiotic assay by agar-well diffusion method. Acta Biol. Med. Exp. 1990, 15, 113–115. [Google Scholar]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart Jr, C.N. The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Adamski, Z.; Blythe, L.L.; Milella, L.; Bufo, S.A. Biological activities of alkaloids: From toxicology to pharmacology. Toxins 2020, 12, 210. [Google Scholar] [CrossRef]
- Martini, N.; Katerere, D.; Eloff, J. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J. Ethnopharmacol. 2004, 93, 207–212. [Google Scholar] [CrossRef]
- Marquardt, P.; Seide, R.; Vissiennon, C.; Schubert, A.; Birkemeyer, C.; Ahyi, V.; Fester, K. Phytochemical characterization and in vitro anti-inflammatory, antioxidant and antimicrobial activity of Combretum collinum Fresen leaves extracts from Benin. Molecules 2020, 25, 288. [Google Scholar] [CrossRef]
- Mitani, T.; Ota, K.; Inaba, N.; Kishida, K.; Koyama, H.A. Antimicrobial activity of the phenolic compounds of Prunus mume against Enterobacteria. Biol. Pharm. Bull. 2018, 41, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Fankam, A.G.; Kuiate, J.R.; Kuete, V. Antibacterial and antibiotic resistance modifying activity of the extracts from Allanblackia gabonensis, Combretum molle and Gladiolus quartinianus against Gram-negative bacteria including multi-drug resistant phenotypes. BMC Complement Altern. Med. 2015, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saidu, T.; Abdullahi, M. Phytochemical determinations and antibacterial activities of the leaf extracts of Combretum molle and Gossypium arboretum. Bayero J. Pure Appl. Sci. 2011, 4, 132–136. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Cock, I.; Van Vuuren, S. A comparison of the antimicrobial activity and toxicity of six Combretum and two Terminalia species from Southern Africa. Pharmacogn. Mag. 2015, 11, 208. [Google Scholar] [CrossRef]
- Fanoro, O.T.; Parani, S.; Maluleke, R.; Lebepe, T.C.; Varghese, J.R.; Mavumengwana, V.; Oluwafemi, O.S. Facile Green, Room-Temperature Synthesis of Gold Nanoparticles Using Combretum erythrophyllum Leaf Extract: Antibacterial and Cell Viability Studies against Normal and Cancerous Cells. Antibiotics 2021, 10, 893. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J. Inflamm. Res. 2018, 11, 307–317. [Google Scholar] [CrossRef]
- Bungau, S.; Tit, D.M.; Behl, T.; Aleya, L.; Zaha, D.C. Aspects of excessive antibiotic consumption and environmental influences correlated with the occurrence of resistance to antimicrobial agents. Curr. Opin. Environ. Sci. Health. 2021, 19, 100224. [Google Scholar] [CrossRef]
- Abdullahi, A.A. Trends and challenges of traditional medicine in Africa. Afr. J. Tradit Complement. Altern. Med. 2011, 8, 115–123. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231. [Google Scholar] [CrossRef]
- Mathur, P.; Sanyal, D.; Callahan, D.L.; Conlan, X.A.; Pfeffer, F.M. Treatment technologies to mitigate the harmful effects of recalcitrant fluoroquinolone antibiotics on the environment and human health. Environ. Pollut. 2021, 291, 118233. [Google Scholar] [CrossRef] [PubMed]
- Regassa, F.; Araya, M. In vitro antimicrobial activity of Combretum molle (Combretaceae) against Staphylococcus aureus and Streptococcus agalactiae isolated from crossbred dairy cows with clinical mastitis. Trop. Anim. Health Prod. 2012, 44, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.-M.; Oosthuizen, C.B.; Fibrich, B.D.; Twilley, D.; Lambrechts, I.A.; de Canha, M.N.; Rademan, S.; Lall, N. Traditional medicine: The ancient roots of modern practice. In Medicinal Plants for Holistic Health and Well-Being; Academic Press: Cambridge, MA, USA, 2018; pp. 1–11. [Google Scholar]
- Akwu, N.; Naidoo, Y.; Singh, M.; Nundkumar, N.; Lin, J. Phytochemical screening, in vitro evaluation of the antimicrobial, antioxidant and cytotoxicity potentials of Grewia lasiocarpa E. Mey. ex Harv. S. Afr. J. Bot. 2019, 123, 180–192. [Google Scholar] [CrossRef]
| Extract | Solvent | IC50 (µg/mL) |
|---|---|---|
| Leaf | Hexane | 42.57 ± 9.88 b |
| Chloroform | 118.12 ± 28.07 b | |
| Methanol | 1.52 × 10−7 ± 2.93 × 10−8 b | |
| Stem | Hexane | 1373.30 ± 479.44 a |
| Chloroform | 0.22 ± 0.09 b | |
| Methanol | 2.46 × 10−14 ± 8.53 × 10−15 b | |
| Ascorbic acid (standard) | 0.01 ± 0.00 b | |
| Extract | Solvent | IC50 (µg/mL) |
|---|---|---|
| Leaf | Hexane | 6292.55 ± 795.31 cd |
| Chloroform | 2948.11 ± 868.55 cd | |
| Methanol | 663.77 ± 74.71 bd | |
| Stem | Hexane | 8358.53 ± 5514.41 ac |
| Chloroform | 2602.06 ± 1856.93 cd | |
| Methanol | 1368.11 ± 424.54 cd | |
| Gallic acid (control) | 0.53 ± 0.16 bd | |
| Bacterial Strain | Extract Concentration (mg/mL) | Positive Control (10 µg/mL) | ||||
|---|---|---|---|---|---|---|
| 0.625 | 1.25 | 2.5 | 5 | 10 | ||
| Leaf | ||||||
| E. coli | 6.67 ± 0.24 * | 7.33 ± 1.18 * | 7.50 ± 1.08 *† | 10.00 ± 1.22 | 12.00 ± 0.00 § | 12.33 ± 0.47 |
| S. aureus | 7.00 ± 0.00 * | 7.00 ± 0.00 * | 8.67 ± 0.24 * | 11.00 ± 0.00 | 14.50 ± 1.08 § | 12.33 ± 1.25 |
| Stem | ||||||
| E. coli | 6.33 ± 0.24 * | 7.00 ± 0.00 * | 7.00 ± 0.41 *† | 9.67 ± 0.47* | 11.83 ± 0.62 | 12.67 ± 0.47 |
| S. aureus | 7.17 ± 0.62 * | 7.17 ± 0.62 * | 7.50 ± 1.08 * | 10.33 ± 0.47* | 12.33 ± 0.47 | 13.33 ± 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parusnath, M.; Naidoo, Y.; Singh, M.; Kianersi, F.; Dewir, Y.H. Antioxidant and Antibacterial Activities of the Leaf and Stem Extracts of Combretum molle (R. Br. ex G. Don.) Engl. & Diels. Plants 2023, 12, 1757. https://doi.org/10.3390/plants12091757
Parusnath M, Naidoo Y, Singh M, Kianersi F, Dewir YH. Antioxidant and Antibacterial Activities of the Leaf and Stem Extracts of Combretum molle (R. Br. ex G. Don.) Engl. & Diels. Plants. 2023; 12(9):1757. https://doi.org/10.3390/plants12091757
Chicago/Turabian StyleParusnath, Myuri, Yougasphree Naidoo, Moganavelli Singh, Farzad Kianersi, and Yaser Hassan Dewir. 2023. "Antioxidant and Antibacterial Activities of the Leaf and Stem Extracts of Combretum molle (R. Br. ex G. Don.) Engl. & Diels" Plants 12, no. 9: 1757. https://doi.org/10.3390/plants12091757







