Comparative GC-MS Analysis of Fresh and Dried Curcuma Essential Oils with Insights into Their Antioxidant and Enzyme Inhibitory Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. GC-MS Analysis of Curcuma Essential Oils in Fresh and Dried Samples Obtained by Hydrodistillation and Hexane Extraction
2.2. Assessment of the In Vitro Antioxidant Properties of Turmeric Essential Oil Using DPPH, ABTS, CUPRAC, FRAP, Phosphomolybdenum, and Ferrozine Assays
2.3. Assessment of the Enzyme Inhibitory Potential of Turmeric-Derived Essential Oils
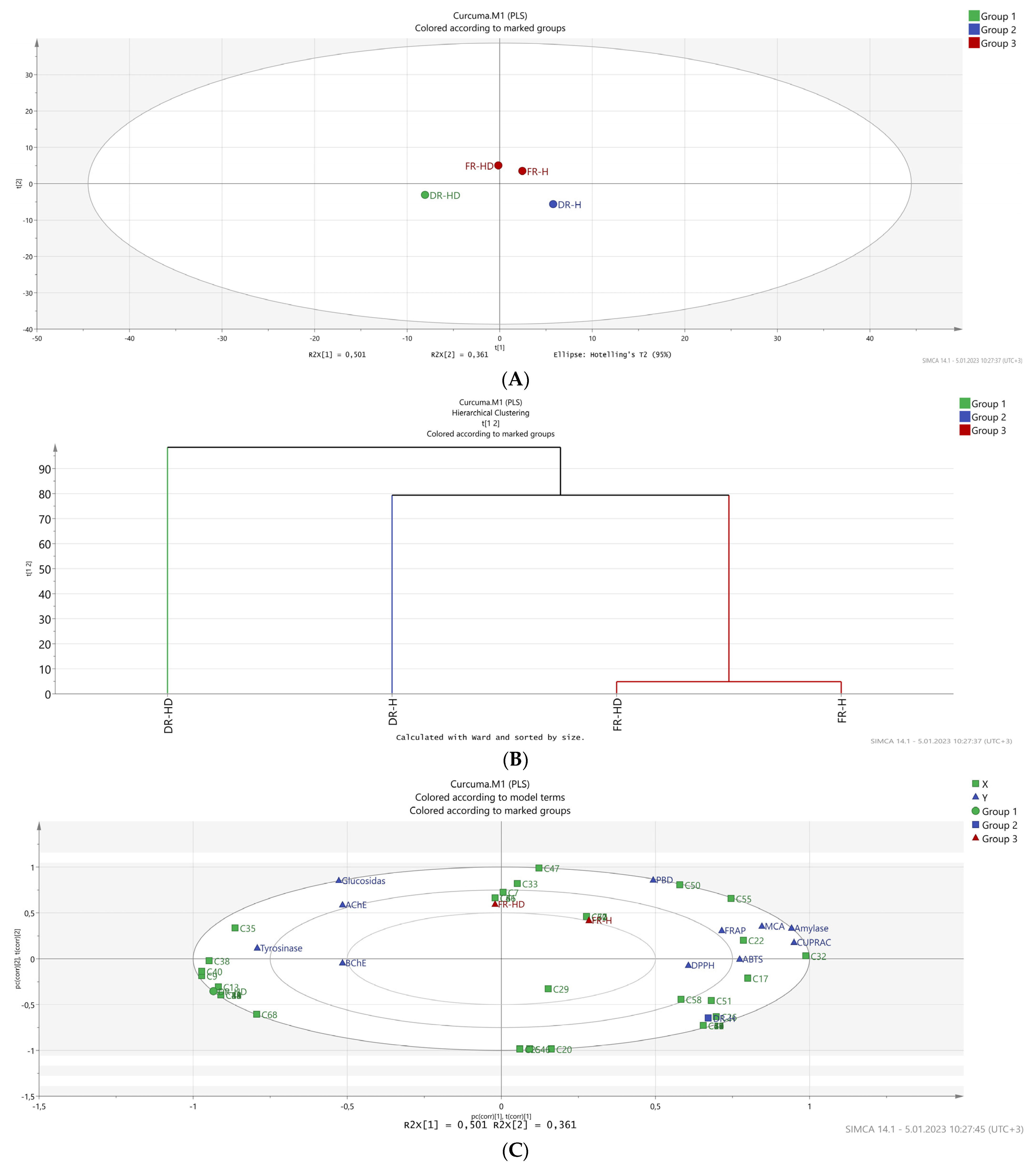
2.4. Chemometric Studies Using Multivariate Analysis
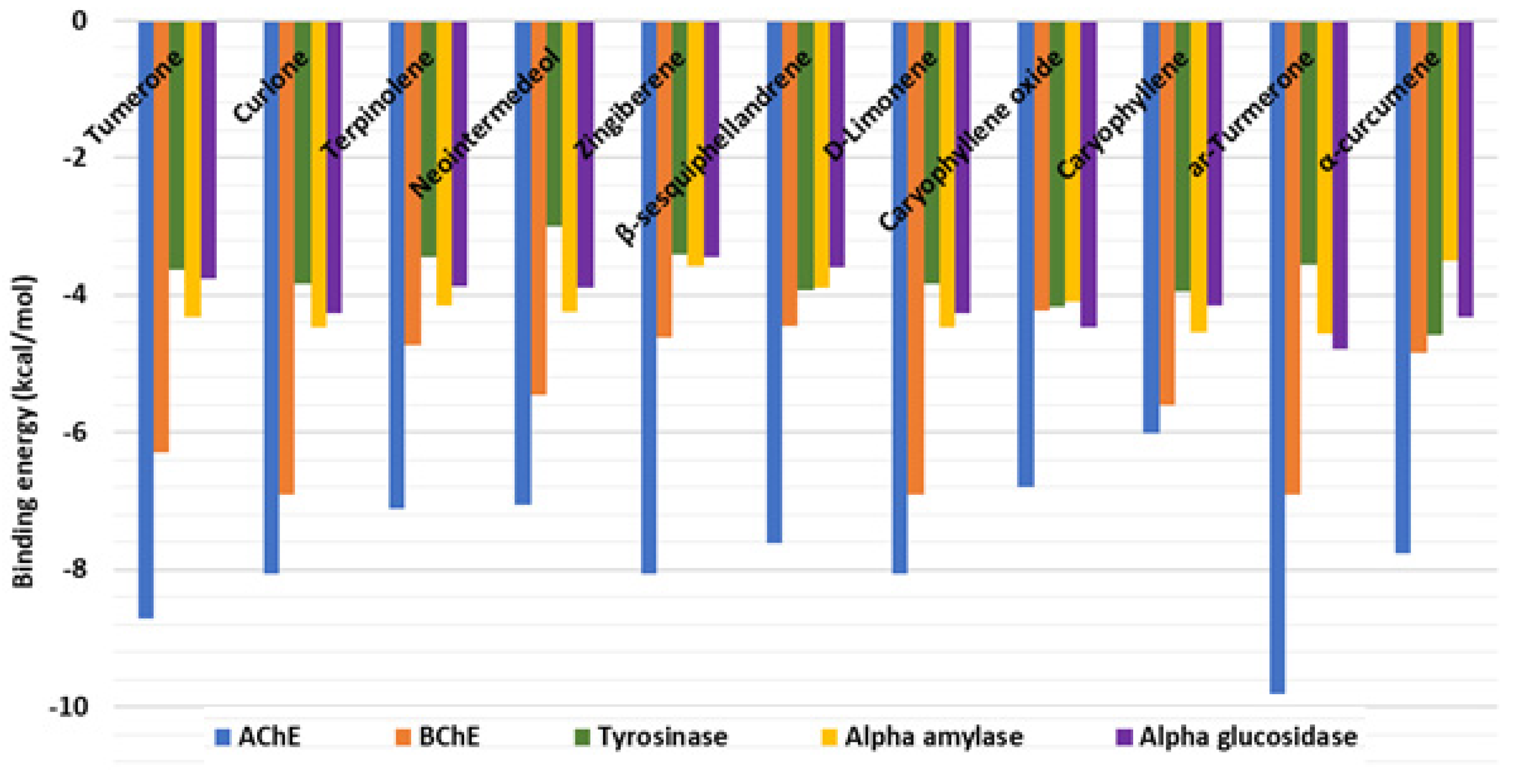
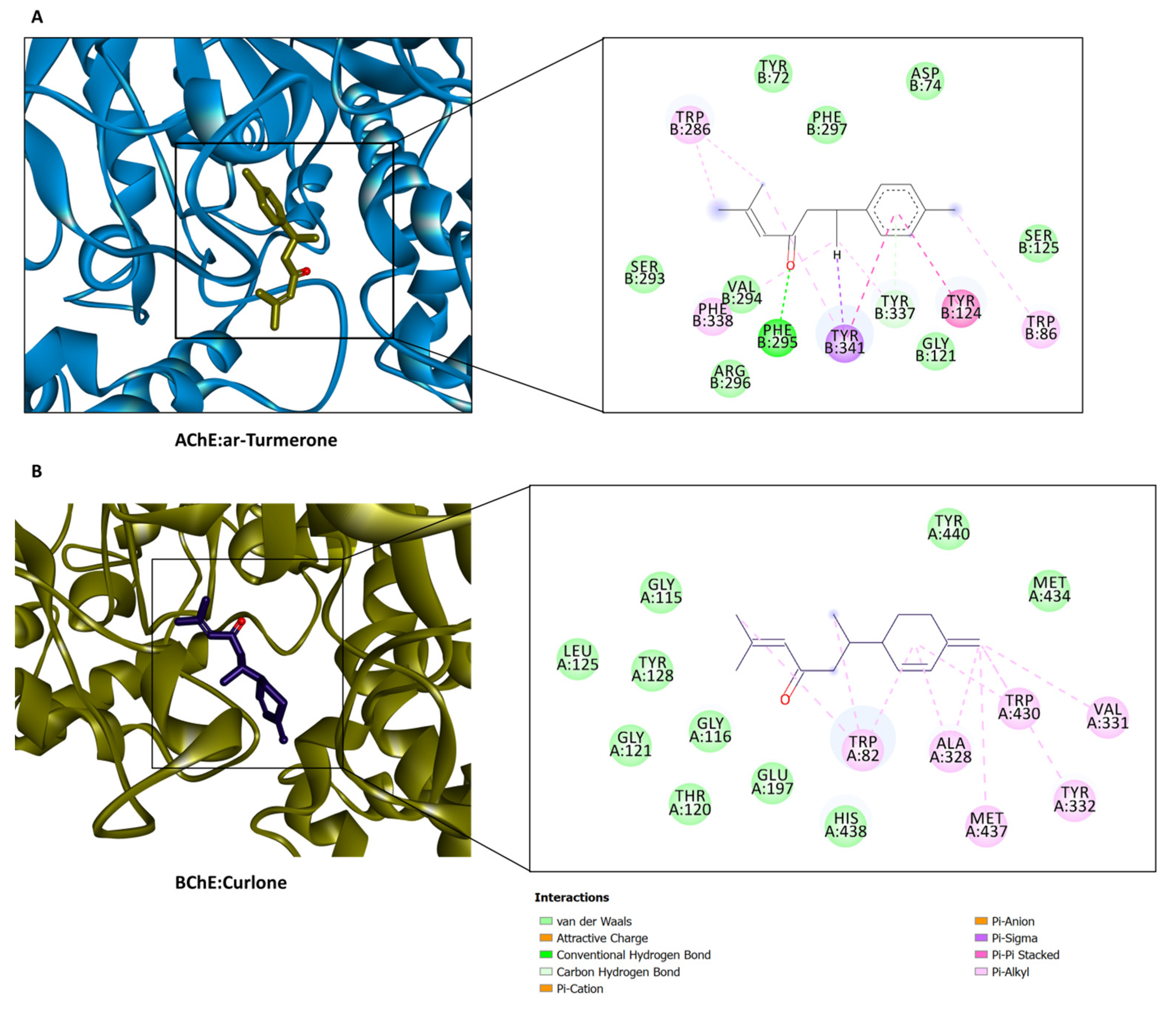
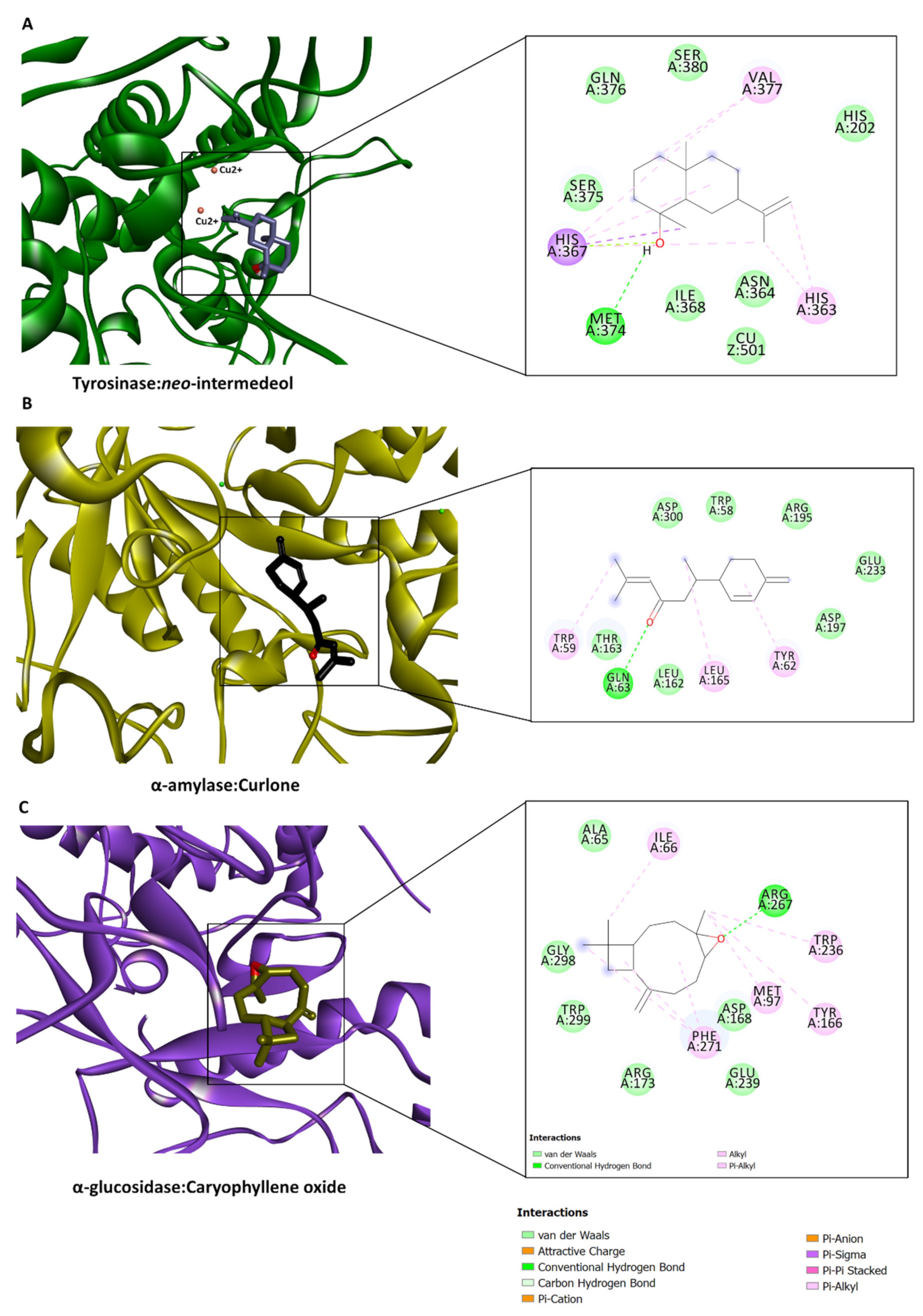
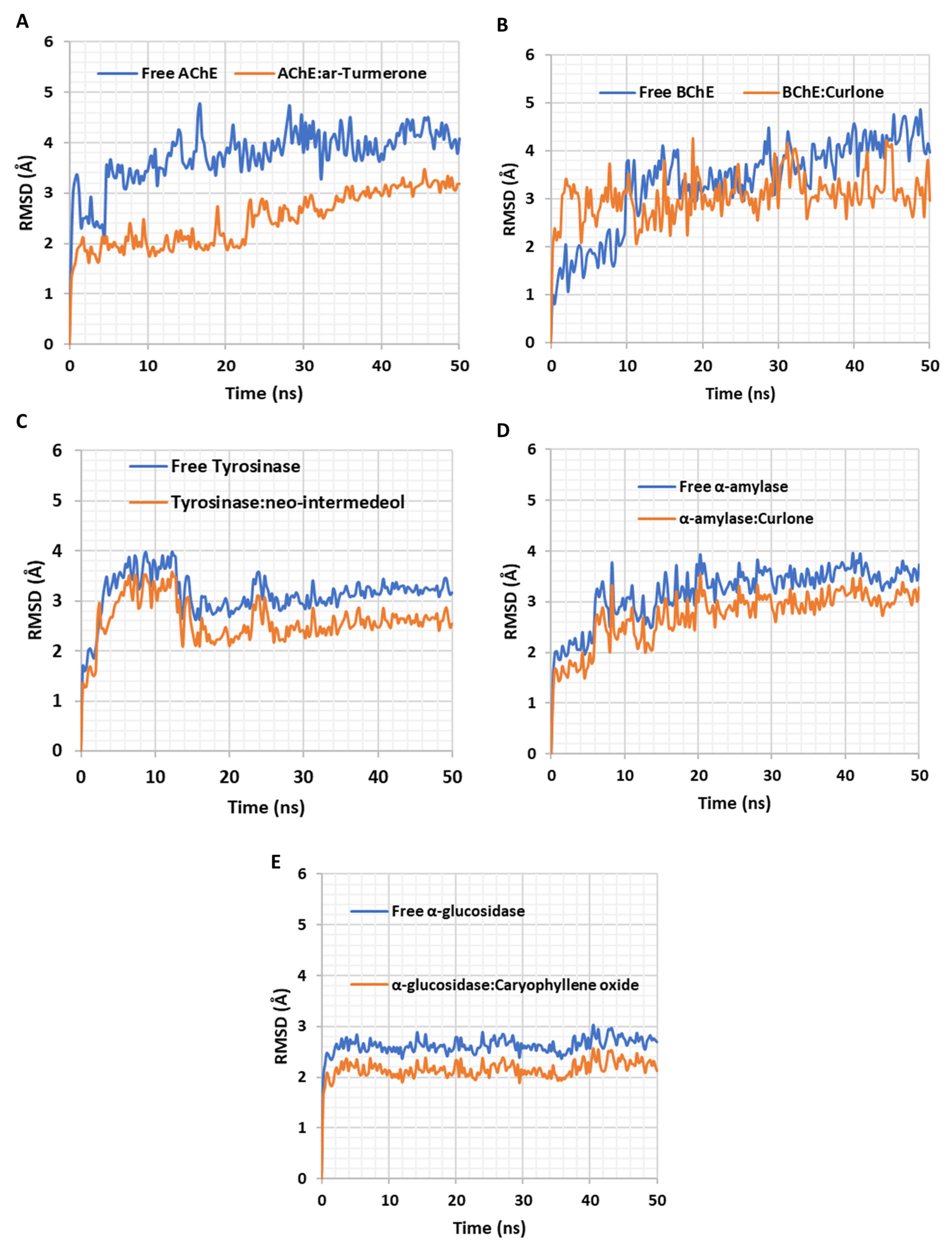
2.5. In Silico Investigations
3. Materials and Methods
3.1. Plant Collection
3.2. Processing of Turmeric Samples
3.3. Assessment of the Chemical Content of Turmeric Essential Oils Using GC-MS Analysis
3.4. In Vitro Antioxidant Analysis
3.5. Enzyme Inhibitory Analysis
3.6. Molecular Modeling and Dynamics Studies
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, W.; Li, P.; Ling, R.; Wang, Z.; Feng, X.; Liu, J.; Yang, Q.; Yan, J. Diversity of Volatile Compounds in Ten Varieties of Zingiberaceae. Molecules 2022, 27, 565. [Google Scholar] [CrossRef] [PubMed]
- Alolga, R.N.; Wang, F.; Zhang, X.; Li, J.; Tran, L.-S.P.; Yin, X. Bioactive Compounds from the Zingiberaceae Family with Known Antioxidant Activities for Possible Therapeutic Uses. Antioxidants 2022, 11, 1281. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.; Friis, I.; Balslev, H. Distribution patterns and diversity centres of Zingiberaceae in SE Asia. Biol. Skr. 2005, 55, 219–228. [Google Scholar]
- Charun, M. Two new species of Curcuma L. (Zingiberaceae) from Thailand. Biodiversitas 2021, 22, 3910–3921. [Google Scholar] [CrossRef]
- Kutti Gounder, D.; Lingamallu, J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cured turmeric (Curcuma longa) rhizomes. Ind. Crops Prod. 2012, 38, 124–131. [Google Scholar] [CrossRef]
- Bambirra, M.L.A.; Junqueira, R.G.; Glória, M.B.A. Influence of post harvest processing conditions on yield and quality of ground turmeric (Curcuma longa L.). Braz. Arch. Biol. Technol. 2002, 45, 423–429. [Google Scholar] [CrossRef]
- Parrotta, J.A. Healing Plants of Peninsular India; CABI Publishing: Wallingford, UK, 2001; pp. 726–727. [Google Scholar]
- Suresh, D.; Manjunatha, H.; Srinivasan, K. Effect of heat processing of spices on the concentrations of their bioactive principles: Turmeric (Curcuma longa), red pepper (Capsicum annuum) and black pepper (Piper nigrum). J. Food Compos. Anal. 2007, 20, 346–351. [Google Scholar] [CrossRef]
- Llano, S.M.; Gómez, A.M.; Duarte-Correa, Y. Effect of Drying Methods and Processing Conditions on the Quality of Curcuma longa Powder. Processes 2022, 10, 702. [Google Scholar] [CrossRef]
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. Changes in curcuminoids and chemical components of turmeric (Curcuma longa L.) under freeze-drying and low-temperature drying methods. Food Chem. 2021, 339, 128121. [Google Scholar] [CrossRef]
- Ray, A.; Mohanty, S.; Jena, S.; Sahoo, A.; Acharya, L.; Panda, P.C.; Sial, P.; Duraisamy, P.; Nayak, S. Drying methods affects physicochemical characteristics, essential oil yield and volatile composition of turmeric (Curcuma longa L.). J. Appl. Res. Med. Aromat. Plants 2022, 26, 100357. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, M.; Mujumdar, A.S.; Chen, J. Valorization of turmeric (Curcuma longa L.) rhizome: Effect of different drying methods on antioxidant capacity and physical properties. Dry. Technol. 2022, 40, 1609–1619. [Google Scholar] [CrossRef]
- Liao, W.; Chen, Y.; Zhu, Z.; Chen, J.; Gao, T.; Limsila, B.; Techadamrongsin, Y.; Wang, L.; Yu, J.; Fu, C.; et al. Vinegar-processed Curcuma phaeocaulis promotes anti-angiogenic activity and reduces toxicity in zebrafish and rat models. Pharm. Biol. 2021, 59, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Green, C.E.; Hibbert, S.L.; Bailey-Shaw, Y.A.; Williams, L.A.D.; Mitchell, S.; Garraway, E. Extraction, Processing, and Storage Effects on Curcuminoids and Oleoresin Yields from Curcuma longa L. Grown in Jamaica. J. Agric. Food Chem. 2008, 56, 3664–3670. [Google Scholar] [CrossRef]
- Chowdhury, J.U.; Nandi, N.C.; Bhuiyan, M.N.I.; Mobarok, M.H. Essential Oil Constituents of The Rhizomes of Two Types of Curcuma longa of Bangladesh. Bangladesh J. Sci. Ind. Res. 2008, 43, 259–266. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.; Singh, P.; de Heluani, C.S.; de Lampasona, M.P.; Catalan, C.A. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn.). Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 1026–1031. [Google Scholar] [CrossRef]
- Banerjee, S.; Bose, S.; Mandal, S.C.; Dawn, S.; Sahoo, U.; Ramadan, M.A.; Mandal, S.K. Pharmacological Property of Pentacyclic Triterpenoids. Egypt. J. Chem. 2019, 62, 13–35. [Google Scholar] [CrossRef]
- Abdur, R.; Noor, J. Natural products as a potential enzyme inhibitors from medicinal plants. In Enzyme Inhibitors and Activators; Murat, S., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 7. [Google Scholar]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.-J. Aromatic-turmerone inhibits α-MSH and IBMX-induced melanogenesis by inactivating CREB and MITF signaling pathways. Arch. Dermatol. Res. 2011, 303, 737–744. [Google Scholar] [CrossRef]
- El-Kersh, D.M.; Mostafa, N.M.; Fayez, S.; Al-Warhi, T.; Abourehab, M.A.S.; Eldehna, W.M.; Salem, M.A. GC-MS metabolites profiling of anethole-rich oils by different extraction techniques: Antioxidant, cytotoxicity and in-silico enzymes inhibitory insights. J. Enzym. Inhib. Med. Chem. 2022, 37, 1974–1986. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Elhady, S.S.; Bannan, D.F.; Malatani, R.T.; Gad, H.A. Citrus reticulata Leaves Essential Oil as an Antiaging Agent: A Comparative Study between Different Cultivars and Correlation with Their Chemical Compositions. Plants 2022, 11, 3335. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol Stream 2005, 16, 65–120. [Google Scholar]
- Gamal El-Din, M.; Youssef, F.; Ashour, M.; Eldahshan, O.; Singab, A.N. Comparative Analysis of Volatile Constituents of Pachira aquatica Aubl. and Pachira glabra Pasq., their Anti-Mycobacterial and Anti-Helicobacter pylori Activities and their Metabolic Discrimination using Chemometrics. J. Essent. Oil Bear. Plants 2019, 21, 1550–1567. [Google Scholar] [CrossRef]
- Abd-El-Ghffar, E.; El-Nashar, H.; Eldahshan, O.; Singab, A.N. GC-MS analysis and hepatoprotective activity of the n-hexane extract of Acrocarpus fraxinifolius leaves against paracetamol-induced hepatotoxicity in male albino rats. Pharm. Biol. 2017, 55, 441–449. [Google Scholar] [CrossRef]
- Younis, M.; Ayoub, I.; Mostafa, N.; Elhassab, M.; Eldehna, W.; Eldahshan, O. GC/MS Profiling, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase Activities of a Stenocarpus sinuatus Leaves Extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Ashmawy, A.; Mostafa, N.; Eldahshan, O. GC/MS Analysis and Molecular Profiling of Lemon Volatile Oil against Breast Cancer. J. Essent. Oil Bear. Plants 2019, 22, 903–916. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and Enzyme Inhibitory Potential of Two Potentilla species (P. speciosa L. and P. reptans Willd.) and Their Chemical Composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Gerlits, O.; Ho, K.-Y.; Cheng, X.; Blumenthal, D.; Taylor, P.; Kovalevsky, A.; Radić, Z. A new crystal form of human acetylcholinesterase for exploratory room-temperature crystallography studies. Chem.-Biol. Interact. 2019, 309, 108698. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, T.; Brazzolotto, X.; Macdonald, I.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef]
- Maurus, R.; Begum, A.; Williams, L.K.; Fredriksen, J.R.; Zhang, R.; Withers, S.G.; Brayer, G.D. Alternative Catalytic Anions Differentially Modulate Human α-Amylase Activity and Specificity. Biochemistry 2008, 47, 3332–3344. [Google Scholar] [CrossRef] [PubMed]
- Ielo, L.; Deri, B.; Germanò, M.P.; Vittorio, S.; Mirabile, S.; Gitto, R.; Rapisarda, A.; Ronsisvalle, S.; Floris, S.; Pazy, Y.; et al. Exploiting the 1-(4-fluorobenzyl)piperazine fragment for the development of novel tyrosinase inhibitors as anti-melanogenic agents: Design, synthesis, structural insights and biological profile. Eur. J. Med. Chem. 2019, 178, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Karade, S.S.; Hill, M.L.; Kiappes, J.L.; Manne, R.; Aakula, B.; Zitzmann, N.; Warfield, K.L.; Treston, A.M.; Mariuzza, R.A. N-Substituted Valiolamine Derivatives as Potent Inhibitors of Endoplasmic Reticulum α-Glucosidases I and II with Antiviral Activity. J. Med. Chem. 2021, 64, 18010–18024. [Google Scholar] [CrossRef] [PubMed]
- Omer, H.A.A.; Caprioli, G.; Abouelenein, D.; Mustafa, A.M.; Uba, A.I.; Ak, G.; Ozturk, R.B.; Zengin, G.; Yagi, S. Phenolic Profile, Antioxidant and Enzyme Inhibitory Activities of Leaves from Two Cassia and Two Senna Species. Molecules 2022, 27, 5590. [Google Scholar] [CrossRef]
- Martínez-Rosell, G.; Giorgino, T.; De Fabritiis, G. PlayMolecule ProteinPrepare: A Web Application for Protein Preparation for Molecular Dynamics Simulations. J. Chem. Inf. Model. 2017, 57, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Ruiz-Medina, A.; Zengin, G.; Ak, G.; Jugreet, S.; Mahomoodally, M.F.; Emre, G.; Orlando, G.; Libero, M.L.; Nilofar. New Biological and Chemical Evidences of Two Lamiaceae Species (Thymbra capitata and Thymus sipyleus subsp. rosulans): In Vitro, In Silico and Ex Vivo Approaches. Molecules 2022, 27, 9029. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Uba, A.I.; Yelekçi, K. Pharmacophore-based virtual screening for identification of potential selective inhibitors of human histone deacetylase 6. Comput. Biol. Chem. 2018, 77, 318–330. [Google Scholar] [CrossRef]
- Weako, J.; Uba, A.I.; Keskin, Ö.; Gürsoy, A.; Yelekçi, K. Identification of potential inhibitors of human methionine aminopeptidase (type II) for cancer therapy: Structure-based virtual screening, ADMET prediction and molecular dynamics studies. Comput. Biol. Chem. 2020, 86, 107244. [Google Scholar] [CrossRef] [PubMed]
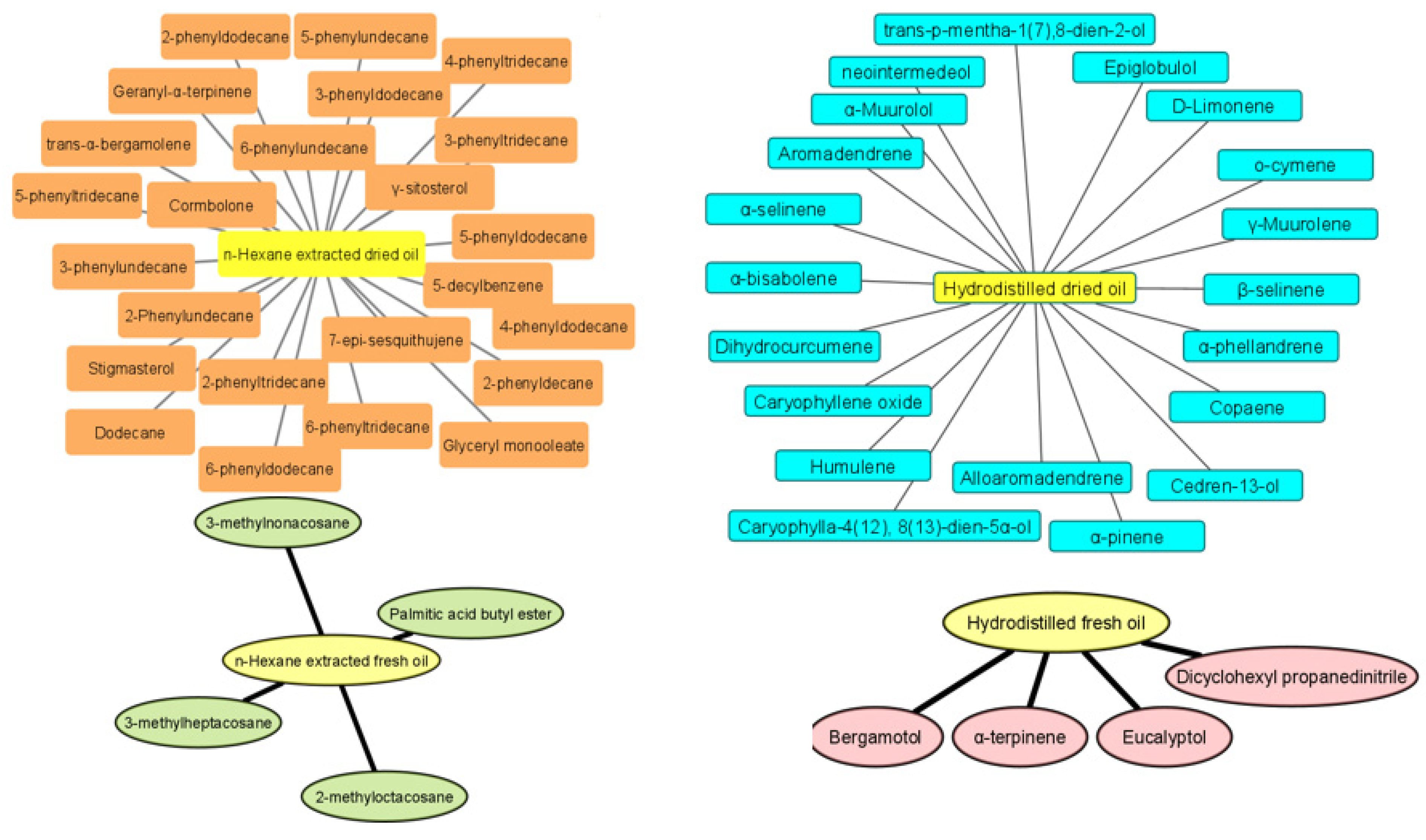
| No. | tR (min) | Compound Name | Molecular Formula | KIexp a | KIrep b | Peak Area (%) in Fresh Curcuma | Peak Area (%) in Dried Curcuma | Chemical Class | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Hydrodistilled | Hexane- Extracted | Hydrodistilled | Hexane- Extracted | |||||||
| 1. | 7.16 | α-Pinene | C10H16 | 931 | 931 | - | - | 0.62 | - | Monoterpene hydrocarbon |
| 2. | 9.28 | α-Phellandrene | 1003 | 1003 | - | - | 1.58 | - | ||
| 3. | 9.67 | α-Terpinene | 1016 | 1016 | 0.18 | - | - | - | ||
| 4. | 9.91 | o-Cymene | C10H14 | 1023 | 1023 | - | - | 2.26 | - | |
| 5. | 10.05 | D-Limonene | C10H16 | 1028 | 1028 | - | - | 23.21 | - | |
| 6. | 10.10 | Eucalyptol | C10H18O | 1029 | 1029 | 1.92 | - | - | - | Monoterpene ether |
| 7. | 11.91 | Terpinolene | C10H16 | 1088 | 1088 | 4.47 | 0.43 | - | - | Monoterpene hydrocarbon |
| 8. | 14.98 | trans-p-Mentha-1(7),8-dien-2-ol | C10H16O | 1188 | 1185 | - | - | 0.33 | - | Monoterpene alcohol |
| 9. | 15.07 | α-Terpineol | C10H18O | 1191 | 1191 | 0.17 | - | 0.51 | - | |
| 10. | 15.50 | Dodecane | C12H26 | 1189 | 1200 | - | - | - | 0.08 | Aliphatic hydrocarbon |
| 11. | 20.43 | Copaene | C15H24 | 1378 | 1378 | - | - | 0.33 | - | Sesquiterpene hydrocarbon |
| 12. | 21.47 | 7-epi-Sesquithujene | 1396 | 1391 | - | - | - | 0.08 | ||
| 13. | 21.64 | Caryophyllene | 1423 | 1423 | 0.35 | 0.97 | 4.58 | 0.16 | ||
| 14. | 22.30 | trans-α-Bergamotene | 1428 | 1428 | - | - | - | 0.04 | ||
| 15. | 22.15 | Aromandendrene | 1443 | 1441 | - | - | 0.20 | - | ||
| 16. | 22.54 | Humulene | 1458 | 1458 | - | - | 0.62 | - | ||
| 17. | 22.54 | β-Farnesene | 1458 | 1458 | - | 0.25 | - | 0.24 | ||
| 18. | 22.74 | Alloaromadendrene | 1465 | 1465 | - | - | 0.20 | - | ||
| 19. | 23.11 | γ-Muurolene | 1479 | 1479 | - | - | 0.21 | - | ||
| 20. | 23.25 | α-Curcumene | C15H22 | 1485 | 1485 | - | 0.77 | 2.32 | 3.73 | |
| 21. | 23.41 | β-Selinene | C15H24 | 1491 | 1491 | - | - | 2.36 | - | |
| 22. | 23.58 | Zingiberene | 1497 | 1497 | 1.57 | 5.90 | - | 3.73 | ||
| 23. | 23.63 | α-Selinene | 1499 | 1499 | - | - | 2.82 | - | ||
| 24. | 23.75 | α-Bisabolene | 1504 | 1504 | - | - | 0.53 | - | ||
| 25. | 23.93 | β-Bisabolene | 1511 | 1511 | 0.15 | 0.57 | 1.11 | 1.41 | ||
| 26. | 24.31 | β-Sesquiphellandrene | 1527 | 1527 | 1.04 | 4.28 | 2.31 | 6.74 | ||
| 27. | 24.88 | 5-Decylbenzene | C16H26 | 1529 | 1535 | - | - | - | 0.64 | |
| 28. | 25.27 | Epiglobulol | C15H26O | 1565 | 1564 | - | - | 0.48 | - | Sesquiterpene alcohol |
| 29. | 25.72 | aR-Turmerol (Bisacumol) | C15H22O | 1584 | 1584 | 1.28 | - | 0.72 | 1.33 | |
| 30. | 25.77 | 2-Phenyl-decane | C16H26 | 1589 | 1588 | - | - | - | 1.26 | Aromatic hydrocarbon |
| 31. | 25.87 | Caryophyllene oxide | C15H24O | 1590 | 1590 | - | - | 5.74 | - | Sesquiterpene ether |
| 32. | 25.96 | trans-Sesquisabinene hydrate | C15H26O | 1593 | 1590 | 0.47 | 0.49 | - | 0.71 | Sesquiterpene alcohol |
| 33. | 26.31 | β-Curcumene | C15H22 | 1608 | 1517 | 1.76 | 0.44 | - | - | Sesquiterpene hydrocarbon |
| 34. | 26.35 | Dihydrocurcumene | C15H24 | 1609 | 1692 | - | - | 1.67 | - | |
| 35. | 26.54 | cis-Sesquisabinene hydrate | C15H26O | 1618 | 1620 | 0.72 | 0.94 | 1.33 | - | Sesquiterpene alcohol |
| 36. | 26.72 | 6-Phenylundecane | C17H28 | 1621 | 1628 | - | - | - | 1.50 | Aromatic hydrocarbon |
| 37. | 26.81 | 5-Phenylundecane | 1625 | 1633 | - | - | - | 2.19 | ||
| 38. | 26.96 | Zingiberenol | C15H26O | 1636 | 1620 | 0.67 | - | 1.18 | - | Sesquiterpene alcohol |
| 39. | 27.05 | 4-Phenylundecane | C17H28 | 1635 | 1643 | - | - | 2.55 | Aromatic hydrocarbon | |
| 40. | 27.06 | trans-Longi pinocarveol | C15H24O | 1640 | 1634 | 0.49 | - | 1.21 | - | Sesquiterpene alcohol |
| 41. | 27.07 | Bergamotol | 1640 | 1657 | 0.73 | - | - | - | ||
| 42. | 27.16 | Caryophylla-4(12),8(13)-dien-5-α-ol | 1644 | 1640 | - | - | 3.82 | - | ||
| 43. | 27.37 | α-Muurolol | C15H26O | 1653 | 1653 | - | - | 0.71 | - | |
| 44. | 27.53 | 3-Phenylundecane | C17H28 | 1656 | 1667 | - | - | 2.16 | Aromatic hydrocarbon | |
| 45. | 27.59 | neo-intermedeol | C15H26O | 1662 | 1660 | - | - | 3.28 | - | Sesquiterpene alcohol |
| 46. | 27.79 | ar-Turmerone | C15H20O | 1671 | 1672 | - | - | 16.27 | 26.24 | Sesquiterpene ketone |
| 47. | 28.01 | Tumerone | C15H22O | 1680 | 1680 | 60.80 | 51.65 | 6.07 | - | |
| 48. | 28.30 | Cedren-13-ol | C15H24O | 1693 | 1690 | - | 1.48 | - | Sesquiterpene alcohol | |
| 49. | 28.88 | 2-Phenylundecane | C17H28 | 1695 | 1703 | - | - | - | 3.50 | Aromatic hydrocarbon |
| 50. | 29.00 | Curlone | C15H22O | 1709 | 1701 | 15.61 | 17.00 | 5.72 | 10.40 | Sesquiterpene ketone |
| 51. | 29.27 | α-Atlantone | 1712 | 1722 | 0.25 | - | - | 0.57 | ||
| 52. | 29.46 | 6-Phenyldodecane | C18H30 | 1720 | 1726 | - | - | - | 1.45 | Aromatic hydrocarbon |
| 53. | 29.57 | 5-Phenyldodecane | 1724 | 1730 | - | - | - | 1.55 | ||
| 54. | 29.84 | 4-Phenyldodecane | 1736 | 1742 | - | - | - | 1.41 | ||
| 55. | 29.99 | (6R,7R)-Bisabolone | C15H24O | 1748 | 1747 | 1.56 | 1.49 | 0.32 | 1.16 | Sesquiterpene ketone |
| 56. | 30.07 | Dicyclohexyl-propanedinitrile | C15H22N2 | 1766 | 1769 | 0.55 | - | - | - | Nitrile |
| 57. | 30.32 | 3-Phenyldodecane | C18H30 | 1757 | 1755 * | - | - | - | 2.12 | Aromatic hydrocarbon |
| 58. | 30.62 | E-Atlantone | C15H22O | 1774 | 1773 | 1.24 | 0.37 | 0.56 | 1.92 | Sesquiterpene ketone |
| 59. | 31.17 | 2-Phenyldodecane | C18H30 | 1794 | 1791 * | - | - | - | 3.08 | Aromatic hydrocarbon |
| 60. | 31.62 | 6-phenyltridecane | C19H32 | 1815 | 1819 | - | - | - | 1.82 | |
| 61. | 31.76 | 5-phenyltridecane | 1822 | 1821 * | - | - | - | 1.13 | ||
| 62. | 32.04 | 4-phenyltridecane | 1835 | 1840 | - | - | - | 1.08 | ||
| 63. | 32.53 | 3-phenyltridecane | 1859 | 1856 * | - | - | - | 1.25 | ||
| 64. | 33.18 | Corymbolone | C15H24O2 | 1890 | 1898 | - | - | - | 0.16 | Sesquiterpene ketone |
| 65. | 33.25 | Geranyl-α-terpinene | C20H32 | 1939 | 1952 | - | - | - | 0.11 | Diterpene hydrocarbon |
| 66. | 33.36 | 2-Phenyltridecane | C19H32 | 1898 | 1916 | - | - | - | 1.95 | Aromatic hydrocarbon |
| 67. | 38.36 | Palmitic acid butyl ester | C20H40O2 | 2186 | 2188 | - | 0.40 | - | - | Fatty acid ester |
| 68. | 44.31 | Palmitic acid β-monoglyceride | C19H38O4 | 2497 | 2498 | - | - | 0.33 | 0.08 | Glyceryl ester |
| 69. | 47.17 | Glyceryl monooleate | C21H40O4 | 2682 | 2714 | - | - | - | 0.10 | |
| 70. | 47.86 | 3-Methyl heptacosane | C28H58 | 2771 | 2771 | - | 0.14 | - | - | Aliphatic hydrocarbon |
| 71. | 49.12 | 2-Methyloctacosane | C29H60 | 2860 | 2859 | - | 0.19 | - | - | |
| 72. | 50.67 | 3-Methylnonacosane | C30H62 | 2971 | 2972 | - | 0.27 | - | - | |
| 73. | 55.54 | Stigmasterol | C29H48O | 3220 | 3170 | - | - | - | 0.19 | Sterol |
| 74. | 56.55 | γ-Sitosterol | C29H50O | 3285 | 3290 | - | - | - | 0.64 | |
| Monoterpene hydrocarbons (%) | 4.65 | 0.43 | 27.67 | - | ||||||
| Oxygenated monoterpenes (%) | 2.09 | - | 0.84 | - | ||||||
| Sesquiterpene hydrocarbons (%) | 4.87 | 13.18 | 19.26 | 16.13 | ||||||
| Oxygenated sesquiterpenes (%) | 83.82 | 71.94 | 48.89 | 42.49 | ||||||
| Diterpene hydrocarbon (%) | - | - | - | 0.11 | ||||||
| Others (%) | 0.73 | 1 | 0.33 | 31.73 | ||||||
| Total identified (%) | 95.98 | 86.55 | 96.99 | 90.46 | ||||||
| Samples | Methods | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | MCA (mg EDTAE/g) | PBD (mmol TE/g) |
|---|---|---|---|---|---|---|---|
| Dried | Hydrodistilled | 3.63 ± 0.59 c | 17.58 ± 0.77 d | 37.97 ± 0.99 d | 34.75 ± 0.82 d | 6.65 ± 0.26 c | 3.44 ± 0.09 d |
| hexane | 15.09 ± 0.51 b | 52.93 ± 0.85 b | 161.14 ± 3.39 b | 70.20 ± 1.47 b | 26.88 ± 0.50 a | 7.82 ± 0.27 c | |
| Fresh | Hydrodistilled | na | 22.25 ± 0.73 c | 112.35 ± 2.10 c | 53.11 ± 0.69 c | 28.91 ± 2.23 a | 15.36 ± 0.61 a |
| hexane | 23.53 ± 0.74 a | 66.24 ± 0.50 a | 172.49 ± 3.63 a | 103.40 ± 2.51 a | 22.05 ± 1.23 b | 12.83 ± 0.40 b | |
| p value | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Samples | Processing | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|---|
| Dried | Hydrodistilled | 2.46 ± 0.02 b | 5.27 ± 0.73 ab | 51.54 ± 0.87 a | 0.19 ± 0.01 c | 1.27 ± 0.01 d |
| Hexane | 2.17 ± 0.01 c | 4.82 ± 0.77 b | 22.73 ± 0.92 b | 1.05 ± 0.02 a | 2.15 ± 0.06 c | |
| Fresh | Hydrodistilled | 2.72 ± 0.09 a | 6.35 ± 0.04 a | 49.83 ± 7.60 a | 0.94 ± 0.01 b | 2.45 ± 0.04 a |
| Hexane | 2.25 ± 0.07 c | 3.23 ± 0.31 c | 17.37 ± 1.78 b | 1.08 ± 0.01 a | 2.26 ± 0.03 b | |
| p value | 0.0001 | 0.001 | 0.0001 | 0.0001 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahmy, N.M.; Fayez, S.; Uba, A.I.; Shariati, M.A.; Aljohani, A.S.M.; El-Ashmawy, I.M.; Batiha, G.E.-S.; Eldahshan, O.A.; Singab, A.N.; Zengin, G. Comparative GC-MS Analysis of Fresh and Dried Curcuma Essential Oils with Insights into Their Antioxidant and Enzyme Inhibitory Activities. Plants 2023, 12, 1785. https://doi.org/10.3390/plants12091785
Fahmy NM, Fayez S, Uba AI, Shariati MA, Aljohani ASM, El-Ashmawy IM, Batiha GE-S, Eldahshan OA, Singab AN, Zengin G. Comparative GC-MS Analysis of Fresh and Dried Curcuma Essential Oils with Insights into Their Antioxidant and Enzyme Inhibitory Activities. Plants. 2023; 12(9):1785. https://doi.org/10.3390/plants12091785
Chicago/Turabian StyleFahmy, Nouran M., Shaimaa Fayez, Abdullahi Ibrahim Uba, Mohammad Ali Shariati, Abdullah S. M. Aljohani, Ibrahim M. El-Ashmawy, Gaber El-Saber Batiha, Omayma A. Eldahshan, Abdel Nasser Singab, and Gokhan Zengin. 2023. "Comparative GC-MS Analysis of Fresh and Dried Curcuma Essential Oils with Insights into Their Antioxidant and Enzyme Inhibitory Activities" Plants 12, no. 9: 1785. https://doi.org/10.3390/plants12091785
APA StyleFahmy, N. M., Fayez, S., Uba, A. I., Shariati, M. A., Aljohani, A. S. M., El-Ashmawy, I. M., Batiha, G. E.-S., Eldahshan, O. A., Singab, A. N., & Zengin, G. (2023). Comparative GC-MS Analysis of Fresh and Dried Curcuma Essential Oils with Insights into Their Antioxidant and Enzyme Inhibitory Activities. Plants, 12(9), 1785. https://doi.org/10.3390/plants12091785












