Negative Impacts of Arsenic on Plants and Mitigation Strategies
Abstract
1. Introduction
2. Methodology
3. Arsenic, a Dominant Environmental Contaminant
4. Classification of Arsenic from the Biological and Toxicological Point of View
4.1. Arsenic Speciation in Soil
4.2. Inorganic and Organic Forms of Arsenic
4.3. Toxicity Assessment of the Various Arsenic Species
4.4. Factors Affecting Arsenic Speciation and Its Bio-Geochemical Properties
4.5. Soil pH Governs Arsenic Speciation and Bio-Geochemical Properties
4.6. Soil Redox Potential (Eh) Governs Arsenic Speciation and Bio-Geochemical Properties
4.7. Other Physico-Chemical Factors That Govern Arsenic Speciation and Bio-Geochemical Properties
4.8. Presence of Microorganisms Affects Speciation and Bio-Geochemical Properties
5. Perception of Arsenic by Plants
6. Phyto-Arsenic Uptake
6.1. Inorganic Arsenate Uptake
6.2. Organic As(III) Uptake in Plants
7. Arsenic Detoxification
8. Impact of Arsenic on Plants
8.1. Morphological Impact and Response
8.2. Physiological Impact
8.2.1. Arsenic Stress and Induction of Reactive Oxygen Species
8.2.2. Arsenic Stress and the Photosynthetic System
8.2.3. Arsenic Stress and Respiratory Process
8.2.4. Arsenic Stress and Damage of DNA
8.3. Biochemical Impact of Arsenic Stress
9. Mechanism Undertaken to Alleviate Arsenic-Induced Stress
9.1. Arsenic Immobilization and Compartmentalization
9.2. Triggering of Antioxidant Defense Responses
9.2.1. Upregulation of the Activity of Antioxidant Enzymes
- Superoxide dismutase (SOD)
- Catalase
- Ascorbate Peroxidase
- Glutathione Reductase
- Glutathione S-transferase
9.2.2. Increasing the Synthesis of Non-Enzymatic Antioxidants
9.2.3. Modulation of the Activation of Signaling Pathways
9.3. Cross Talk between Nitric oxide and Arsenic
9.4. Arsenic Mitigation Measures—A Step towards Good Health, Food Security and Sustainable Development
9.4.1. Simple, Semi-Efficient but Cost-Effective Human Behavior-Based Measures
9.4.2. Simple, Effective but Costly Measures
9.4.3. Adopting Decision-Making Assisting Tools
9.4.4. Arsenic Elimination Techniques
9.4.5. Nanotechnology-Based Augmentation of As Tolerance in Plants
9.4.6. NO Based As Stress Tolerance in Plants
9.4.7. Gene Editing for Developing As-Tolerant Plants Using Molecular and Traditional Plant-Breeding Techniques
10. Prospects and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Xiong, T.; Dumat, C.; Pierart, A.; Shahid, M.; Kang, Y.; Li, N.; Bertoni, G.; Laplanche, C. Measurement of Metal Bioaccessibility in Vegetables to Improve Human Exposure Assessments: Field Study of Soil–Plant–Atmosphere Transfers in Urban Areas, South China. Environ. Geochem. Health 2016, 38, 1283–1301. [Google Scholar] [CrossRef]
- Hou, D.; Ok, Y.S. Soil Pollution—Speed up Global Mapping. Nature 2019, 566, 455. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, Y.; Li, J.; Beiyuan, J.; Tsang, D.C.W.; Poon, C.; Chan, T.; Wang, W.; Li, X. Speciation, Mobilization, and Bioaccessibility of Arsenic in Geogenic Soil Profile from Hong Kong. Environ. Pollut. 2018, 232, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil Amendments for Immobilization of Potentially Toxic Elements in Contaminated Soils: A Critical Review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar Application for the Remediation of Heavy Metal Polluted Land: A Review of in Situ Field Trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar]
- Davis, H.T.; Marjorie Aelion, C.; McDermott, S.; Lawson, A.B. Identifying Natural and Anthropogenic Sources of Metals in Urban and Rural Soils Using GIS-Based Data, PCA, and Spatial Interpolation. Environ. Pollut. 2009, 157, 2378–2385. [Google Scholar] [CrossRef]
- Li, N.; Kang, Y.; Pan, W.; Zeng, L.; Zhang, Q.; Luo, J. Concentration and Transportation of Heavy Metals in Vegetables and Risk Assessment of Human Exposure to Bioaccessible Heavy Metals in Soil near a Waste-Incinerator Site, South China. Sci. Total Environ. 2015, 521–522, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Rinklebe, J.; Shaheen, S.M.; Yu, K. Release of As, Ba, Cd, Cu, Pb, and Sr under Pre-Definite Redox Conditions in Different Rice Paddy Soils Originating from the U.S.A. and Asia. Geoderma 2016, 270, 21–32. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace Elements in the Soil-Plant Interface: Phytoavailability, Translocation, and Phytoremediation—A Review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Antoniadis, V.; Golia, E.E.; Liu, Y.-T.; Wang, S.-L.; Shaheen, S.M.; Rinklebe, J. Soil and Maize Contamination by Trace Elements and Associated Health Risk Assessment in the Industrial Area of Volos, Greece. Environ. Int. 2019, 124, 79–88. [Google Scholar]
- Hou, D.; Li, F. Complexities Surrounding China’s Soil Action Plan. Land. Degrad. Dev. 2017, 28, 2315–2320. [Google Scholar] [CrossRef]
- Liu, L. Made in China: Cancer Villages. Environ. Sci. Policy Sustain. Dev. 2010, 52, 8–21. [Google Scholar] [CrossRef]
- An Estimated 12.6 Million Deaths Each Year Are Attributable to Unhealthy Environments; World Health Organization: Geneva, Switzerland, 2016.
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Saqib, Z.A.; Nawaz, M.F.; Shaheen, S.M.; Wang, H.; Tsang, D.C.W.; Bundschuh, J.; et al. Exploring the Arsenic Removal Potential of Various Biosorbents from Water. Environ. Int. 2019, 123, 567–579. [Google Scholar] [CrossRef]
- Lead Poisoning; World Health Organization: Geneva, Switzerland, 2022.
- Keller, B.; Faciano, A.; Tsega, A.; Ehrlich, J. Epidemiologic Characteristics of Children with Blood Lead Levels ≥45 Μg/DL. J. Pediatr. 2017, 180, 229–234. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ye, J.; Zhang, Y.; Ok, Y.S.; Song, Y.; Coulon, F.; Peng, T.; Tian, L. Lead-Based Paint Remains a Major Public Health Concern: A Critical Review of Global Production, Trade, Use, Exposure, Health Risk, and Implications. Environ. Int. 2018, 121, 85–101. [Google Scholar] [CrossRef]
- Yoo, J.-C.; Beiyuan, J.; Wang, L.; Tsang, D.C.W.; Baek, K.; Bolan, N.S.; Ok, Y.S.; Li, X.-D. A Combination of Ferric Nitrate/EDDS-Enhanced Washing and Sludge-Derived Biochar Stabilization of Metal-Contaminated Soils. Sci. Total Environ. 2018, 616–617, 572–582. [Google Scholar] [CrossRef]
- Beiyuan, J.; Li, J.-S.; Tsang, D.C.W.; Wang, L.; Poon, C.S.; Li, X.-D.; Fendorf, S. Fate of Arsenic before and after Chemical-Enhanced Washing of an Arsenic-Containing Soil in Hong Kong. Sci. Total Environ. 2017, 599–600, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-S.; Wang, L.; Cui, J.-L.; Poon, C.S.; Beiyuan, J.; Tsang, D.C.W.; Li, X.-D. Effects of Low-Alkalinity Binders on Stabilization/Solidification of Geogenic As-Containing Soils: Spectroscopic Investigation and Leaching Tests. Sci. Total Environ. 2018, 631–632, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Beiyuan, J.; Tsang, D.C.W.; Valix, M.; Zhang, W.; Yang, X.; Ok, Y.S.; Li, X.-D. Selective Dissolution Followed by EDDS Washing of an E-Waste Contaminated Soil: Extraction Efficiency, Fate of Residual Metals, and Impact on Soil Environment. Chemosphere 2017, 166, 489–496. [Google Scholar] [CrossRef]
- Beiyuan, J.; Tsang, D.C.W.; Valix, M.; Baek, K.; Ok, Y.S.; Zhang, W.; Bolan, N.S.; Rinklebe, J.; Li, X.-D. Combined Application of EDDS and EDTA for Removal of Potentially Toxic Elements under Multiple Soil Washing Schemes. Chemosphere 2018, 205, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tsang, D.C.W.; Poon, C.-S. Green Remediation and Recycling of Contaminated Sediment by Waste-Incorporated Stabilization/Solidification. Chemosphere 2015, 122, 257–264. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Dai, J.-G.; Wang, L.; Tsang, D.C.W.; Poon, C.S. Influence of Lead on Stabilization/Solidification by Ordinary Portland Cement and Magnesium Phosphate Cement. Chemosphere 2018, 190, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Beiyuan, J.; Tsang, D.C.W.; Ok, Y.S.; Zhang, W.; Yang, X.; Baek, K.; Li, X.-D. Integrating EDDS-Enhanced Washing with Low-Cost Stabilization of Metal-Contaminated Soil from an e-Waste Recycling Site. Chemosphere 2016, 159, 426–432. [Google Scholar] [CrossRef]
- Hou, D.; Al-Tabbaa, A. Sustainability: A New Imperative in Contaminated Land Remediation. Environ. Sci. Policy 2014, 39, 25–34. [Google Scholar] [CrossRef]
- Phosphate-Assisted Phytoremediation of Arsenic by Brassica napus and Brassica juncea: Morphological and Physiological Response. Int. J. Phytoremediat. 2017, 19, 7. Available online: https://www.tandfonline.com/doi/abs/10.1080/15226514.2016.1278427 (accessed on 25 February 2023).
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Lüttge, A. Arsenic Removal by Perilla Leaf Biochar in Aqueous Solutions and Groundwater: An Integrated Spectroscopic and Microscopic Examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef]
- Begum, M.C.; Islam, M.S.; Islam, M.; Amin, R.; Parvez, M.S.; Kabir, A.H. Biochemical and Molecular Responses Underlying Differential Arsenic Tolerance in Rice (Oryza sativa L.). Plant Physiol. Biochem. 2016, 104, 266–277. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha. Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Substance Priority List. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 18 April 2023).
- Mohan, D.; Pittman, C.U. Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Periodic Table. Arsenic-Element Information, Properties and Uses. Available online: https://www.rsc.org/periodic-table/element/33/arsenic (accessed on 18 April 2023).
- Srivastava, S.; Sinha, P.; Sharma, Y.K. Status of Photosynthetic Pigments, Lipid Peroxidation and Anti-Oxidative Enzymes in Vigna mungo in Presence of Arsenic. J. Plant Nutr. 2017, 40, 298–306. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hogan, B.; Duncan, E.; Doyle, C.; Krassoi, R.; Rahman, M.M.; Naidu, R.; Lim, R.P.; Maher, W.; Hassler, C. Toxicity of Arsenic Species to Three Freshwater Organisms and Biotransformation of Inorganic Arsenic by Freshwater Phytoplankton (Chlorella Sp. CE-35). Ecotoxicol. Environ. Saf. 2014, 106, 126–135. [Google Scholar] [CrossRef]
- Drewniak, L.; Sklodowska, A. Arsenic-Transforming Microbes and Their Role in Biomining Processes. Environ. Sci. Pollut. Res. 2013, 20, 7728–7739. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K.; Suzuki, K.T. Arsenic Round the World: A Review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Imran, M.A.; Ch, M.N.; Khan, R.M.; Ali, Z.; Mahmood, T. Toxicity of Arsenic (As) on Seed Germination of Sunflower (Helianthus annuus L.). IJPS 2013, 8, 840–847. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Worldwide Occurrences of Arsenic in Ground Water. Science 2002, 296, 2143–2145. [Google Scholar] [CrossRef]
- Panda, S.K.; Upadhyay, R.K.; Nath, S. Arsenic Stress in Plants: Arsenic Stress in Plants. J. Agron. Crop. Sci. 2010, 196, 161–174. [Google Scholar] [CrossRef]
- Finnegan, P.M.; Chen, W. Arsenic Toxicity: The Effects on Plant Metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Rafiq, M.; Bakhat, H.F.; Imran, M.; Abbas, T.; Bibi, I.; Dumat, C. Arsenic Behaviour in Soil-Plant System: Biogeochemical Reactions and Chemical Speciation Influences. In Enhancing Cleanup of Environmental Pollutants: Volume 2: Non-Biological Approaches; Anjum, N.A., Gill, S.S., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 97–140. ISBN 978-3-319-55423-5. [Google Scholar]
- Duncan, E.G.; Maher, W.A.; Foster, S.D.; Krikowa, F.; O’Sullivan, C.A.; Roper, M.M. Dimethylarsenate (DMA) Exposure Influences Germination Rates, Arsenic Uptake and Arsenic Species Formation in Wheat. Chemosphere 2017, 181, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C.; Wang, H.; Pi, J.; Xu, Y. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium Speciation, Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System: A Review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, D. Balancing Roles of Reactive Oxygen Species in Plants’ Response to Metalloid Exposure. In Reactive Oxygen Species in Plants; Singh, V.P., Singh, S., Tripathi, D.K., Prasad, S.M., Chauhan, D.K., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 51–73. [Google Scholar]
- Souri, Z.; Karimi, N.; de Oliveira, L.M. Antioxidant Enzymes Responses in Shoots of Arsenic Hyperaccumulator, Isatis cappadocica Desv., under Interaction of Arsenate and Phosphate. Environ. Technol. 2018, 39, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Macnair, M.R. Suppression of the High Affinity Phosphate Uptake System: A Mechanism of Arsenate Tolerance in Holcus lanatus L. J. Exp. Bot. 1992, 43, 519–524. [Google Scholar] [CrossRef]
- Garg, N.; Singla, P. Arsenic Toxicity in Crop Plants: Physiological Effects and Tolerance Mechanisms. Environ. Chem. Lett. 2011, 9, 303–321. [Google Scholar] [CrossRef]
- Martínez-Sánchez, M.J.; Martínez-López, S.; García-Lorenzo, M.L.; Martínez-Martínez, L.B.; Pérez-Sirvent, C. Evaluation of Arsenic in Soils and Plant Uptake Using Various Chemical Extraction Methods in Soils Affected by Old Mining Activities. Geoderma 2011, 160, 535–541. [Google Scholar] [CrossRef]
- Neidhardt, H.; Kramar, U.; Tang, X.; Guo, H.; Norra, S. Arsenic Accumulation in the Roots of Helianthus annuus and Zea mays by Irrigation with Arsenic-Rich Groundwater: Insights from Synchrotron X-Ray Fluorescence Imaging. Geochemistry 2015, 75, 261–270. [Google Scholar] [CrossRef]
- Singh, N.; Ma, L.Q.; Srivastava, M.; Rathinasabapathi, B. Metabolic Adaptations to Arsenic-Induced Oxidative Stress in Pteris vittata L and Pteris ensiformis L. Plant Sci. 2006, 170, 274–282. [Google Scholar] [CrossRef]
- Rafiq, M.; Shahid, M.; Shamshad, S.; Khalid, S.; Niazi, N.K.; Abbas, G.; Saeed, M.F.; Ali, M.; Murtaza, B. A Comparative Study to Evaluate Efficiency of EDTA and Calcium in Alleviating Arsenic Toxicity to Germinating and Young Vicia faba L. Seedlings. J. Soils Sediments 2018, 18, 2271–2281. [Google Scholar] [CrossRef]
- Ghosh, P.; Rathinasabapathi, B.; Ma, L.Q. Phosphorus Solubilization and Plant Growth Enhancement by Arsenic-Resistant Bacteria. Chemosphere 2015, 134, 1–6. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.S.; McKinney, E.C.; Meagher, R.B.; Smith, A.P. Hijacking Membrane Transporters for Arsenic Phytoextraction. J. Biotechnol. 2013, 163, 1–9. [Google Scholar] [CrossRef]
- Hartley-Whitaker, J.; Ainsworth, G.; Vooijs, R.; Bookum, W.T.; Schat, H.; Meharg, A.A. Phytochelatins Are Involved in Differential Arsenate Tolerance in Holcus lanatus. Plant Physiol. 2001, 126, 299–306. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Bhattacharya, P.; Mukherjee, A.B.; Bundschuh, J.; Zevenhoven, R.; Loeppert, R.H. Arsenic in Soil and Groundwater: An Overview. In Trace Metals and other Contaminants in the Environment; Elsevier: Amsterdam, The Netherlands, 2007; pp. 3–60. [Google Scholar]
- Mishra, R.K.; Tiwari, S.; Patel, A.; Prasad, S.M. Arsenic Contamination, Speciation, Toxicity and Defense Strategies in Plants. Braz. J. Bot. 2021, 44, 1–10. [Google Scholar] [CrossRef]
- Quaghebeur, M.; Rengel, Z. Arsenic Speciation Governs Arsenic Uptake and Transport in Terrestrial Plants. Microchim. Acta 2005, 151, 141–152. [Google Scholar] [CrossRef]
- Meharg, A.A.; Hartley-Whitaker, J. Arsenic Uptake and Metabolism in Arsenic Resistant and Nonresistant Plant Species. New Phytol. 2002, 154, 29–43. [Google Scholar] [CrossRef]
- Nearing, M.M.; Koch, I.; Reimer, K.J. Complementary Arsenic Speciation Methods: A Review. Spectrochim. Acta Part B At. Spectrosc. 2014, 99, 150–162. [Google Scholar] [CrossRef]
- Caporale, A.G.; Violante, A. Chemical Processes Affecting the Mobility of Heavy Metals and Metalloids in Soil Environments. Curr. Pollut. Rep. 2016, 2, 15–27. [Google Scholar] [CrossRef]
- Lenoble, V.; Omanović, D.; Garnier, C.; Mounier, S.; Đonlagić, N.; Le Poupon, C.; Pižeta, I. Distribution and Chemical Speciation of Arsenic and Heavy Metals in Highly Contaminated Waters Used for Health Care Purposes (Srebrenica, Bosnia and Herzegovina). Sci. Total Environ. 2013, 443, 420–428. [Google Scholar] [CrossRef]
- Shahid, M.; Austruy, A.; Echevarria, G.; Arshad, M.; Sanaullah, M.; Aslam, M.; Nadeem, M.; Nasim, W.; Dumat, C. EDTA-Enhanced Phytoremediation of Heavy Metals: A Review. Soil Sediment Contam. Int. J. 2014, 23, 389–416. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Pourrut, B.; Silvestre, J.; Laplanche, C.; Pinelli, E. Influence of EDTA and Citric Acid on Lead-Induced Oxidative Stress to Vicia faba Roots. J. Soils Sediments 2014, 14, 835–843. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Pourrut, B.; Abbas, G.; Shahid, N.; Pinelli, E. Role of Metal Speciation in Lead-Induced Oxidative Stress to Vicia faba Roots. Russ. J. Plant Physiol. 2015, 62, 448–454. [Google Scholar] [CrossRef]
- Awasthi, S.; Chauhan, R.; Srivastava, S.; Tripathi, R.D. The Journey of Arsenic from Soil to Grain in Rice. Front. Plant Sci. 2017, 8, 1007. [Google Scholar] [CrossRef]
- Li, H.-B.; Li, J.; Zhu, Y.-G.; Juhasz, A.L.; Ma, L.Q. Comparison of Arsenic Bioaccessibility in Housedust and Contaminated Soils Based on Four in Vitro Assays. Sci. Total Environ. 2015, 532, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Hu, K.-N.; Decker, B. Organic Arsenic in the Soil Environment: Speciation, Occurrence, Transformation, and Adsorption Behavior. Water Air Soil Pollut. 2011, 219, 401–415. [Google Scholar] [CrossRef]
- Sadiq, M. Arsenic Chemistry in Soils: An Overview of Thermodynamic Predictions and Field Observations. Water Air Soil Pollut. 1997, 93, 117–136. [Google Scholar] [CrossRef]
- Warren, G.P.; Alloway, B.J. Reduction of Arsenic Uptake by Lettuce with Ferrous Sulfate Applied to Contaminated Soil. J. Environ. Qual. 2003, 32, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.; Dubey, B.; McBean, E.A. Human Health Risk Assessment from Arsenic Exposures in Bangladesh. Sci. Total Environ. 2015, 527–528, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Afroz, H.; Su, S.; Carey, M.; Meharg, A.A.; Meharg, C. Inhibition of Microbial Methylation via ArsM in the Rhizosphere: Arsenic Speciation in the Soil to Plant Continuum. Environ. Sci. Technol. 2019, 53, 3451–3463. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.D.; Goessler, W.; Pedersen, S.N.; Francesconi, K.A. Characterization of an Algal Extract by HPLC-ICP-MS and LC-Electrospray MS for Use in Arsenosugar Speciation Studies. J. Anal. At. Spectrom. 2000, 15, 657–662. [Google Scholar] [CrossRef]
- Ackley, K.L.; B’Hymer, C.; Sutton, K.L.; Caruso, J.A. Speciation of Arsenic in Fish Tissue Using Microwave-Assisted Extraction Followed by HPLC-ICP-MS. J. Anal. At. Spectrom. 1999, 14, 845–850. [Google Scholar] [CrossRef]
- Gómez-Ariza, J.L.; Sánchez-Rodas, D.; Giráldez, I.; Morales, E. Comparison of Biota Sample Pretreatments for Arsenic Speciation with Coupled HPLC-HG-ICP-MS. Analyst 2000, 125, 401–407. [Google Scholar] [CrossRef]
- Zeng, X.-C.; Yang, Y.; Shi, W.; Peng, Z.; Chen, X.; Zhu, X.; Wang, Y. Microbially Mediated Methylation of Arsenic in the Arsenic-Rich Soils and Sediments of Jianghan Plain. Front. Microbiol. 2018, 9, 1389. [Google Scholar] [CrossRef]
- Chen, C.; Li, L.; Huang, K.; Zhang, J.; Xie, W.-Y.; Lu, Y.; Dong, X.; Zhao, F.-J. Sulfate-Reducing Bacteria and Methanogens Are Involved in Arsenic Methylation and Demethylation in Paddy Soils. ISME J. 2019, 13, 2523–2535. [Google Scholar] [CrossRef] [PubMed]
- Cullen, W.R.; Reimer, K.J. Arsenic Speciation in the Environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef]
- Francesconi, K.A.; Kuehnelt, D. Determination of Arsenic Species: A Critical Review of Methods and Applications, 2000–2003. Analyst 2004, 129, 373–395. [Google Scholar] [CrossRef] [PubMed]
- Von Endt, D.W.; Kearney, P.C.; Kaufman, D.D. Degradation of MSMA by Soil Microorganisms. J. Agric. Food Chem. 1968, 16, 17–20. [Google Scholar] [CrossRef]
- Sohrin, Y.; Matsui, M.; Kawashima, M.; Hojo, M.; Hasegawa, H. Arsenic Biogeochemistry Affected by Eutrophication in Lake Biwa, Japan. Environ. Sci. Technol. 1997, 31, 2712–2720. [Google Scholar] [CrossRef]
- Abbas, M.H.H.; Meharg, A.A. Arsenate, Arsenite and Dimethyl Arsinic Acid (DMA) Uptake and Tolerance in Maize (Zea mays L.). Plant Soil 2008, 304, 277–289. [Google Scholar] [CrossRef]
- Sarwar, T.; Khan, S.; Muhammad, S.; Amin, S. Arsenic Speciation, Mechanisms, and Factors Affecting Rice Uptake and Potential Human Health Risk: A Systematic Review. Environ. Technol. Innov. 2021, 22, 101392. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 939161. [Google Scholar] [CrossRef]
- Abedin, M.J.; Feldmann, J.; Meharg, A.A. Uptake Kinetics of Arsenic Species in Rice Plants. Plant Physiol. 2002, 128, 1120–1128. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Yu, S.-D.; Hong, Y.-S. Environmental Source of Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [Google Scholar] [CrossRef]
- Rasul, S. Electrochemical Measurement and Speciation of Inorganic Arsenic in Groundwater of Bangladesh. Talanta 2002, 58, 33–43. [Google Scholar] [CrossRef] [PubMed]
- B’Hymer, C.; Caruso, J.A. Arsenic and Its Speciation Analysis Using High-Performance Liquid Chromatography and Inductively Coupled Plasma Mass Spectrometry. J. Chromatogr. A 2004, 1045, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Lima, J.; Bogo, M.R.; Monserrat, J.M. Arsenic Toxicity in Mammals and Aquatic Animals: A Comparative Biochemical Approach. Ecotoxicol. Environ. Saf. 2011, 74, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Adriano, D.C. Trace Elements in Terrestrial Environments; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar] [CrossRef]
- Petrick, J.S.; Ayala-Fierro, F.; Cullen, W.R.; Carter, D.E.; Vasken Aposhian, H. Monomethylarsonous Acid (MMAIII) Is More Toxic Than Arsenite in Chang Human Hepatocytes. Toxicol. Appl. Pharmacol. 2000, 163, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-J.; McGrath, S.P.; Meharg, A.A. Arsenic as a Food Chain Contaminant: Mechanisms of Plant Uptake and Metabolism and Mitigation Strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef]
- Lakshmipathiraj, P.; Narasimhan, B.; Prabhakar, S.; Bhaskarraju, G. Adsorption of Arsenate on Synthetic Goethite from Aqueous Solutions. J. Hazard. Mater. 2006, 136, 281–287. [Google Scholar] [CrossRef]
- Stazi, S.R.; Marabottini, R.; Papp, R.; Moscatelli, M.C. Arsenic in Soil: Availability and Interactions with Soil Microorganisms. In Heavy Metal. Contamination of Soils; Sherameti, I., Varma, A., Eds.; Soil Biology; Springer International Publishing: Cham, Switzerland, 2015; Volume 44, pp. 113–126. ISBN 978-3-319-14525-9. [Google Scholar]
- Zheng, M.-Z.; Li, G.; Sun, G.-X.; Shim, H.; Cai, C. Differential Toxicity and Accumulation of Inorganic and Methylated Arsenic in Rice. Plant Soil 2013, 365, 227–238. [Google Scholar] [CrossRef]
- Mitra, A.; Chatterjee, S.; Moogouei, R.; Gupta, D. Arsenic Accumulation in Rice and Probable Mitigation Approaches: A Review. Agronomy 2017, 7, 67. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Han, Y.-H.; Chen, Y.; Zhu, L.-J.; Ma, L.Q. Arsenic Transformation and Plant Growth Promotion Characteristics of As-Resistant Endophytic Bacteria from As-Hyperaccumulator Pteris Vittata. Chemosphere 2016, 144, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Saqib, M.; Akhtar, J.; Murtaza, G.; Shahid, M.; Hussain, A. Relationship between Rhizosphere Acidification and Phytoremediation in Two Acacia Species. J. Soils Sediments 2016, 16, 1392–1399. [Google Scholar] [CrossRef]
- Adra, A.; Morin, G.; Ona-Nguema, G.; Brest, J. Arsenate and Arsenite Adsorption onto Al-Containing Ferrihydrites. Implications for Arsenic Immobilization after Neutralization of Acid Mine Drainage. Appl. Geochem. 2016, 64, 2–9. [Google Scholar] [CrossRef]
- Signes-Pastor, A.; Burló, F.; Mitra, K.; Carbonell-Barrachina, A.A. Arsenic Biogeochemistry as Affected by Phosphorus Fertilizer Addition, Redox Potential and PH in a West Bengal (India) Soil. Geoderma 2007, 137, 504–510. [Google Scholar] [CrossRef]
- Zhang, H.; Selim, H.M. Reaction and Transport of Arsenic in Soils: Equilibrium and Kinetic Modeling. Adv. Agron. 2008, 98, 45–115. [Google Scholar]
- Carbonell-Barrachina, A.A.; Jugsujinda, A.; Burlo, F.; Delaune, R.D.; Patrick, W.H. Arsenic Chemistry in Municipal Sewage Sludge as Affected by Redox Potential and PH. Water Res. 2000, 34, 216–224. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A Review of the Source, Behaviour and Distribution of Arsenic in Natural Waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Tighe, M. The Chemistry and Behaviour of Antimony in the Soil Environment with Comparisons to Arsenic: A Critical Review. Environ. Pollut. 2010, 158, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.A.; Arocena, J.M.; Faz, A. Speciation of Arsenic in Bulk and Rhizosphere Soils from Artisanal Cooperative Mines in Bolivia. Chemosphere 2015, 138, 1014–1020. [Google Scholar] [CrossRef]
- Beesley, L.; Dickinson, N. Carbon and Trace Element Mobility in an Urban Soil Amended with Green Waste Compost. J. Soils Sediments 2010, 10, 215–222. [Google Scholar] [CrossRef]
- Pongratz, R. Arsenic Speciation in Environmental Samples of Contaminated Soil. Sci. Total Environ. 1998, 224, 133–141. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Clemente, R.; Mestrot, A.; Meharg, A.A. Arsenic and Selenium Mobilisation from Organic Matter Treated Mine Spoil with and without Inorganic Fertilisation. Environ. Pollut. 2013, 173, 238–244. [Google Scholar] [CrossRef]
- Akins, M.B.; Lewis, R.J. Chemical Distribution and Gaseous Evolution of Arsenic-74 Added to Soils as DSMA-74As. Soil Sci. Soc. Am. J. 1976, 40, 655–658. [Google Scholar] [CrossRef]
- Griffin, R.A.; Shimp, N.F. Attenuation of Pollutants in Municipal Landfill Leachate by Clay Minerals; EPA 600/2-78-1570H; Environmental Protection Agency, Office of Research and Development, Municipal Environmental Research Laboratory: Washington, DC, USA, 1978. [Google Scholar]
- Kumarathilaka, P.; Seneweera, S.; Meharg, A.; Bundschuh, J. Arsenic Speciation Dynamics in Paddy Rice Soil-Water Environment: Sources, Physico-Chemical, and Biological Factors—A Review. Water Res. 2018, 140, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Nakamura, T.; Dong, D.; Takahashi, Y.; Amachi, S.; Makino, T. Arsenic Release from Flooded Paddy Soils Is Influenced by Speciation, Eh, PH, and Iron Dissolution. Chemosphere 2011, 83, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Gorny, J.; Billon, G.; Lesven, L.; Dumoulin, D.; Madé, B.; Noiriel, C. Arsenic Behavior in River Sediments under Redox Gradient: A Review. Sci. Total Environ. 2015, 505, 423–434. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Gao, S.; Tanji, K.K. Speciation and Behavior of Arsenic in Evaporation Basins, California, USA. Environ. Earth Sci. 2010, 61, 1599–1612. [Google Scholar] [CrossRef]
- Redman, A.D.; Macalady, D.L.; Ahmann, D. Natural Organic Matter Affects Arsenic Speciation and Sorption onto Hematite. Environ. Sci. Technol. 2002, 36, 2889–2896. [Google Scholar] [CrossRef]
- Gregory, S.J.; Anderson, C.W.N.; Camps Arbestain, M.; McManus, M.T. Response of Plant and Soil Microbes to Biochar Amendment of an Arsenic-Contaminated Soil. Agric. Ecosyst. Environ. 2014, 191, 133–141. [Google Scholar] [CrossRef]
- Doušová, B.; Lhotka, M.; Grygar, T.; Machovič, V.; Herzogová, L. In Situ Co-Adsorption of Arsenic and Iron/Manganese Ions on Raw Clays. Appl. Clay Sci. 2011, 54, 166–171. [Google Scholar] [CrossRef]
- Dickens, R.; Hiltbold, A.E. Movement and Persistence of Methanearsonates in Soil. Weeds 1967, 15, 299. [Google Scholar] [CrossRef]
- Kirkham, M.B. Cadmium in Plants on Polluted Soils: Effects of Soil Factors, Hyperaccumulation, and Amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Bagherifam, S.; Lakzian, A.; Fotovat, A.; Khorasani, R.; Komarneni, S. In Situ Stabilization of As and Sb with Naturally Occurring Mn, Al and Fe Oxides in a Calcareous Soil: Bioaccessibility, Bioavailability and Speciation Studies. J. Hazard. Mater. 2014, 273, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lu, C.; Xu, R.; Yang, X.; Yan, L.; Su, C. Arsenic Removal by Manganese-Doped Mesoporous Iron Oxides from Groundwater: Performance and Mechanism. Sci. Total Environ. 2022, 806, 150615. [Google Scholar] [CrossRef]
- Garau, G.; Silvetti, M.; Castaldi, P.; Mele, E.; Deiana, P.; Deiana, S. Stabilising Metal(Loid)s in Soil with Iron and Aluminium-Based Products: Microbial, Biochemical and Plant Growth Impact. J. Environ. Manag. 2014, 139, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Matzner, E. Dynamics of Organic and Inorganic Arsenic in the Solution Phase of an Acidic Fen in Germany. Geochim. Cosmochim. Acta 2006, 70, 2023–2033. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, R.D.; Singh, R.P.; Dwivedi, S.; Goutam, D.; Shri, M.; Trivedi, P.K.; Chakrabarty, D. Silicon Mediates Arsenic Tolerance in Rice (Oryza sativa L.) through Lowering of Arsenic Uptake and Improved Antioxidant Defence System. Ecol. Eng. 2013, 52, 96–103. [Google Scholar] [CrossRef]
- Mühlbachová, G. Soil Microbial Activities and Heavy Metal Mobility in Long-Term Contaminated Soils after Addition of EDTA and EDDS. Ecol. Eng. 2011, 37, 1064–1071. [Google Scholar] [CrossRef]
- Liu, C.; Luo, C.; Xu, X.; Wu, C.; Li, F.; Zhang, G. Effects of Calcium Peroxide on Arsenic Uptake by Celery (Apium graveolens L.) Grown in Arsenic Contaminated Soil. Chemosphere 2012, 86, 1106–1111. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Asghar, H.N.; Ghafoor, U.; Shahid, M. Differential Effects of Plant Growth-Promoting Rhizobacteria on Maize Growth and Cadmium Uptake. J. Plant Growth Regul. 2016, 35, 303–315. [Google Scholar] [CrossRef]
- Yamamura, S.; Amachi, S. Microbiology of Inorganic Arsenic: From Metabolism to Bioremediation. J. Biosci. Bioeng. 2014, 118, 1–9. [Google Scholar] [CrossRef]
- Vaxevanidou, K.; Giannikou, S.; Papassiopi, N. Microbial Arsenic Reduction in Polluted and Unpolluted Soils from Attica, Greece. J. Hazard. Mater. 2012, 241–242, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of Heavy Metal(Loid)s Contaminated Soils—To Mobilize or to Immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Suhadolnik, M.L.S.; Salgado, A.P.C.; Scholte, L.L.S.; Bleicher, L.; Costa, P.S.; Reis, M.P.; Dias, M.F.; Ávila, M.P.; Barbosa, F.A.R.; Chartone-Souza, E.; et al. Novel Arsenic-Transforming Bacteria and the Diversity of Their Arsenic-Related Genes and Enzymes Arising from Arsenic-Polluted Freshwater Sediment. Sci. Rep. 2017, 7, 11231. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, S.; Huang, H.; Luo, L.; Wen, B. Arsenic Accumulation and Speciation in Maize as Affected by Inoculation with Arbuscular Mycorrhizal Fungus Glomus Mosseae. J. Agric. Food Chem. 2009, 57, 3695–3701. [Google Scholar] [CrossRef]
- Feki, K.; Tounsi, S.; Mrabet, M.; Mhadhbi, H.; Brini, F. Recent Advances in Physiological and Molecular Mechanisms of Heavy Metal Accumulation in Plants. Environ. Sci. Pollut. Res. 2021, 28, 64967–64986. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy Metals, Occurrence and Toxicity for Plants: A Review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Song, W.-Y. Arsenic Uptake and Translocation in Plants. Plant Cell Physiol. 2016, 57, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hou, Q.; Yang, Z.; Zhong, C.; Zheng, G.; Yang, Z.; Li, J. Evaluation of Potential Effects of Soil Available Phosphorus on Soil Arsenic Availability and Paddy Rice Inorganic Arsenic Content. Environ. Pollut. 2014, 188, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Bucher, M. Functional Biology of Plant Phosphate Uptake at Root and Mycorrhiza Interfaces. New Phytol. 2007, 173, 11–26. [Google Scholar] [CrossRef]
- Victor Roch, G.; Maharajan, T.; Ceasar, S.A.; Ignacimuthu, S. The Role of PHT1 Family Transporters in the Acquisition and Redistribution of Phosphorus in Plants. Crit. Rev. Plant Sci. 2019, 38, 171–198. [Google Scholar] [CrossRef]
- Sun, D.; Feng, H.; Li, X.; Ai, H.; Sun, S.; Chen, Y.; Xu, G.; Rathinasabapathi, B.; Cao, Y.; Ma, L.Q. Expression of New Pteris Vittata Phosphate Transporter PvPht1;4 Reduces Arsenic Translocation from the Roots to Shoots in Tobacco Plants. Environ. Sci. Technol. 2020, 54, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Nussaume, L. Phosphate Import in Plants: Focus on the PHT1 Transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef]
- Teng, W.; Zhao, Y.-Y.; Zhao, X.-Q.; He, X.; Ma, W.-Y.; Deng, Y.; Chen, X.-P.; Tong, Y.-P. Genome-Wide Identification, Characterization, and Expression Analysis of PHT1 Phosphate Transporters in Wheat. Front. Plant Sci. 2017, 8, 543. [Google Scholar] [CrossRef]
- Ye, Y.; Yuan, J.; Chang, X.; Yang, M.; Zhang, L.; Lu, K.; Lian, X. The Phosphate Transporter Gene OsPht1;4 Is Involved in Phosphate Homeostasis in Rice. PLoS ONE 2015, 10, e0126186. [Google Scholar] [CrossRef] [PubMed]
- Roch, G.V.; Maharajan, T.; Krishna, T.P.A.; Ignacimuthu, S.; Ceasar, S.A. Expression of PHT1 Family Transporter Genes Contributes for Low Phosphate Stress Tolerance in Foxtail Millet (Setaria italica) Genotypes. Planta 2020, 252, 98. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Shin, H.-S.; Dewbre, G.R.; Harrison, M.J. Phosphate Transport in Arabidopsis: Pht1;1 and Pht1;4 Play a Major Role in Phosphate Acquisition from Both Low- and High-Phosphate Environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef]
- Catarecha, P.; Segura, M.D.; Franco-Zorrilla, J.M.; García-Ponce, B.; Lanza, M.; Solano, R.; Paz-Ares, J.; Leyva, A. A Mutant of the Arabidopsis Phosphate Transporter PHT1;1 Displays Enhanced Arsenic Accumulation. Plant Cell 2007, 19, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Luan, M.; Wang, Y.; Zhang, C.; Zhang, B.; Shi, J.; Zhao, F.-G.; Lan, W.; Luan, S. A Vacuolar Phosphate Transporter Essential for Phosphate Homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, E6571–E6578. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Liu, J.; Liu, Y.; Han, X.; Sun, G.; Lan, W.; Luan, S. Vacuolar Phosphate Transporter 1 (VPT1) Affects Arsenate Tolerance by Regulating Phosphate Homeostasis in Arabidopsis. Plant Cell Physiol. 2018, 59, 1345–1352. [Google Scholar] [CrossRef]
- Puckett, E.E.; Serapiglia, M.J.; DeLeon, A.M.; Long, S.; Minocha, R.; Smart, L.B. Differential Expression of Genes Encoding Phosphate Transporters Contributes to Arsenic Tolerance and Accumulation in Shrub Willow (Salix Spp.). Environ. Exp. Bot. 2012, 75, 248–257. [Google Scholar] [CrossRef]
- Kamiya, T.; Islam, R.; Duan, G.; Uraguchi, S.; Fujiwara, T. Phosphate Deficiency Signaling Pathway Is a Target of Arsenate and Phosphate Transporter OsPT1 Is Involved in As Accumulation in Shoots of Rice. Soil Sci. Plant Nutr. 2013, 59, 580–590. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, W.; Mao, C.; Xu, G.; Zhao, F.-J. The Role of OsPT8 in Arsenate Uptake and Varietal Difference in Arsenate Tolerance in Rice. J. Exp. Bot. 2016, 67, 6051–6059. [Google Scholar] [CrossRef]
- Ye, Y.; Li, P.; Xu, T.; Zeng, L.; Cheng, D.; Yang, M.; Luo, J.; Lian, X. OsPT4 Contributes to Arsenate Uptake and Transport in Rice. Front. Plant Sci. 2017, 8, 2197. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, D.; Ai, H.; Mei, H.; Liu, X.; Sun, S.; Xu, G.; Liu, Y.; Chen, Y.; Ma, L.Q. Knocking Out OsPT4 Gene Decreases Arsenate Uptake by Rice Plants and Inorganic Arsenic Accumulation in Rice Grains. Environ. Sci. Technol. 2017, 51, 12131–12138. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.-Y.; Tian, Z.-H.; Yang, X.-L.; Liu, B.-H.; Yang, J.; Lin, H.-H. The Role of OsNLA1 in Regulating Arsenate Uptake and Tolerance in Rice. J. Plant Physiol. 2019, 236, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Chen, J.; He, S.; Dai, Z.; Liu, X.; Cao, Y.; Ma, L.Q. Arsenic-Induced up-Regulation of P Transporters PvPht1;3–1;4 Enhances Both As and P Uptake in As-Hyperaccumulator Pteris vittata. J. Hazard. Mater. 2022, 438, 129430. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Feng, H.; Sun, D.; Xu, G.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Heterologous Expression of Pteris vittata Phosphate Transporter PvPht1;3 Enhances Arsenic Translocation to and Accumulation in Tobacco Shoots. Environ. Sci. Technol. 2019, 53, 10636–10644. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.-H.; Wang, X.-F.; Li, Z.-D.; Zhang, X.; Li, X.-G.; Gu, W.; Zhang, F.; Yu, J.; He, S. A Panax Notoginseng Phosphate Transporter, PnPht1;3, Greatly Contributes to Phosphate and Arsenate Uptake. Funct. Plant Biol. 2022, 49, 259–271. [Google Scholar] [CrossRef]
- Su, Y.-H.; McGrath, S.P.; Zhao, F.-J. Rice Is More Efficient in Arsenite Uptake and Translocation than Wheat and Barley. Plant Soil 2010, 328, 27–34. [Google Scholar] [CrossRef]
- Salt, D.E. Would the Real Arsenate Reductase Please Stand Up? New Phytol. 2017, 215, 926–928. [Google Scholar] [CrossRef]
- Ellis, D.R.; Gumaelius, L.; Indriolo, E.; Pickering, I.J.; Banks, J.A.; Salt, D.E. A Novel Arsenate Reductase from the Arsenic Hyperaccumulating Fern Pteris vittata. Plant Physiol. 2006, 141, 1544–1554. [Google Scholar] [CrossRef]
- Fischer, S.; Sánchez-Bermejo, E.; Xu, X.; Flis, P.; Ramakrishna, P.; Guerinot, M.L.; Zhao, F.-J.; Salt, D.E. Targeted Expression of the Arsenate Reductase HAC1 Identifies Cell Type Specificity of Arsenic Metabolism and Transport in Plant Roots. J. Exp. Bot. 2021, 72, 415–425. [Google Scholar] [CrossRef]
- Shi, S.; Wang, T.; Chen, Z.; Tang, Z.; Wu, Z.; Salt, D.E.; Chao, D.-Y.; Zhao, F.-J. OsHAC1;1 and OsHAC1;2 Function as Arsenate Reductases and Regulate Arsenic Accumulation. Plant Physiol. 2016, 172, 1708–1719. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, F.-J. The Roles of Membrane Transporters in Arsenic Uptake, Translocation and Detoxification in Plants. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2449–2484. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Yamaji, N.; Zhao, F.-J.; Ma, J.F. The Aromatic/Arginine Selectivity Filter of NIP Aquaporins Plays a Critical Role in Substrate Selectivity for Silicon, Boron, and Arsenic. J. Exp. Bot. 2011, 62, 4391–4398. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.-Y.; Su, Y.-H.; McGrath, S.P.; Zhao, F.-J. Transporters of Arsenite in Rice and Their Role in Arsenic Accumulation in Rice Grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [PubMed]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of Aquaporins in Plants under Stress. Biol. Res. 2018, 51, 4. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-K.; Chen, Y.; Che, J.; Konishi, N.; Tang, Z.; Miller, A.J.; Ma, J.F.; Zhao, F.-J. Decreasing Arsenic Accumulation in Rice by Overexpressing OsNIP1;1 and OsNIP3;3 through Disrupting Arsenite Radial Transport in Roots. New Phytol. 2018, 219, 641–653. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, S.-K.; Tang, Z.; Liu, G.; Moore, K.L.; Maathuis, F.J.M.; Miller, A.J.; McGrath, S.P.; Zhao, F.-J. The Nodulin 26-like Intrinsic Membrane Protein OsNIP3;2 Is Involved in Arsenite Uptake by Lateral Roots in Rice. J. Exp. Bot. 2017, 68, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Thorsen, M.; Schüssler, M.D.; Nilsson, H.R.; Wagner, A.; Tamás, M.J.; Jahn, T.P. A Subgroup of Plant Aquaporins Facilitate the Bi-Directional Diffusion of As(OH)3 and Sb(OH)3across Membranes. BMC Biol. 2008, 6, 26. [Google Scholar] [CrossRef]
- Kamiya, T.; Tanaka, M.; Mitani, N.; Ma, J.F.; Maeshima, M.; Fujiwara, T. NIP1;1, an Aquaporin Homolog, Determines the Arsenite Sensitivity of Arabidopsis Thaliana. J. Biol. Chem. 2009, 284, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dai, W.; Yan, H.; Li, S.; Shen, H.; Chen, Y.; Xu, H.; Sun, Y.; He, Z.; Ma, M. Arabidopsis NIP3;1 Plays an Important Role in Arsenic Uptake and Root-to-Shoot Translocation under Arsenite Stress Conditions. Mol. Plant 2015, 8, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J.M. The Arabidopsis thaliana Aquaglyceroporin AtNIP7;1 Is a Pathway for Arsenite Uptake. FEBS Lett. 2008, 582, 1625–1628. [Google Scholar] [CrossRef] [PubMed]
- Katsuhara, M.; Sasano, S.; Horie, T.; Matsumoto, T.; Rhee, J.; Shibasaka, M. Functional and Molecular Characteristics of Rice and Barley NIP Aquaporins Transporting Water, Hydrogen Peroxide and Arsenite. Plant Biotechnol. 2014, 31, 213–219. [Google Scholar] [CrossRef]
- De Paolis, A.; De Caroli, M.; Rojas, M.; Curci, L.M.; Piro, G.; Di Sansebastiano, G.-P. Evaluation of Dittrichia viscosa Aquaporin Nip1.1 Gene as Marker for Arsenic-Tolerant Plant Selection. Plants 2022, 11, 1968. [Google Scholar] [CrossRef]
- Mosa, K.A.; Kumar, K.; Chhikara, S.; Mcdermott, J.; Liu, Z.; Musante, C.; White, J.C.; Dhankher, O.P. Members of Rice Plasma Membrane Intrinsic Proteins Subfamily Are Involved in Arsenite Permeability and Tolerance in Plants. Transgenic Res. 2012, 21, 1265–1277. [Google Scholar] [CrossRef]
- Modareszadeh, M.; Bahmani, R.; Kim, D.; Hwang, S. Decreases in Arsenic Accumulation by the Plasma Membrane Intrinsic Protein PIP2;2 in Arabidopsis and Yeast. Environ. Pollut. 2021, 275, 116646. [Google Scholar] [CrossRef]
- Schmöger, M.E.V.; Oven, M.; Grill, E. Detoxification of Arsenic by Phytochelatins in Plants. Plant Physiol. 2000, 122, 793–802. [Google Scholar] [CrossRef]
- Song, W.-Y.; Park, J.; Mendoza-Cózatl, D.G.; Suter-Grotemeyer, M.; Shim, D.; Hörtensteiner, S.; Geisler, M.; Weder, B.; Rea, P.A.; Rentsch, D.; et al. Arsenic Tolerance in Arabidopsis Is Mediated by Two ABCC-Type Phytochelatin Transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 21187–21192. [Google Scholar] [CrossRef]
- Song, W.-Y.; Yamaki, T.; Yamaji, N.; Ko, D.; Jung, K.-H.; Fujii-Kashino, M.; An, G.; Martinoia, E.; Lee, Y.; Ma, J.F. A Rice ABC Transporter, OsABCC1, Reduces Arsenic Accumulation in the Grain. Proc. Natl. Acad. Sci. USA 2014, 111, 15699–15704. [Google Scholar] [CrossRef]
- Indriolo, E.; Na, G.; Ellis, D.; Salt, D.E.; Banks, J.A. A Vacuolar Arsenite Transporter Necessary for Arsenic Tolerance in the Arsenic Hyperaccumulating Fern Pteris vittata Is Missing in Flowering Plants. Plant Cell 2010, 22, 2045–2057. [Google Scholar] [CrossRef]
- Duan, G.-L.; Hu, Y.; Schneider, S.; McDermott, J.; Chen, J.; Sauer, N.; Rosen, B.P.; Daus, B.; Liu, Z.; Zhu, Y.-G. Inositol Transporters AtINT2 and AtINT4 Regulate Arsenic Accumulation in Arabidopsis Seeds. Nat. Plants 2016, 2, 15202. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Sharma, D.; Dwivedi, S.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression in Arabidopsis and Cellular Localization Reveal Involvement of Rice NRAMP, OsNRAMP1, in Arsenic Transport and Tolerance. Plant Cell Environ. 2014, 37, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Chen, Y.; Miller, A.J.; Zhao, F.-J. The C-Type ATP-Binding Cassette Transporter OsABCC7 Is Involved in the Root-to-Shoot Translocation of Arsenic in Rice. Plant Cell Physiol. 2019, 60, 1525–1535. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, Y.; Chen, F.; Ji, Y.; Zhao, F.-J. OsPTR7 (OsNPF8.1), a Putative Peptide Transporter in Rice, Is Involved in Dimethylarsenate Accumulation in Rice Grain. Plant Cell. Physiol. 2017, 58, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Bhattacharya, S.; Bhattacharyya, S.; Maiti, M.K. Expression of Rice MATE Family Transporter OsMATE2 Modulates Arsenic Accumulation in Tobacco and Rice. Plant Mol. Biol. 2018, 98, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Kidwai, M.; Dutta, P.; Narayan, S.; Gautam, N.; Chawda, K.; Shirke, P.A.; Mishra, A.K.; Chakrabarty, D. A Tau Class Glutathione-S-Transferase (OsGSTU5) Confers Tolerance against Arsenic Toxicity in Rice by Accumulating More Arsenic in Root. J. Hazard. Mater. 2022, 426, 128100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, R.; Cui, D.; Cao, Q.; Shan, Z.; Jiao, Z. Physio-Biochemical and Molecular Mechanism Underlying the Enhanced Heavy Metal Tolerance in Highland Barley Seedlings Pre-Treated with Low-Dose Gamma Irradiation. Sci. Rep. 2017, 7, 14233. [Google Scholar] [CrossRef]
- Ortega-Villasante, C.; Rellán-Álvarez, R.; Del Campo, F.F.; Carpena-Ruiz, R.O.; Hernández, L.E. Cellular Damage Induced by Cadmium and Mercury in Medicago sativa. J. Exp. Bot. 2005, 56, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Ahmed, S.; Malik, A.; Naheed, K.; Hussain, S.; Yasin, N.A.; Javad, S.; Siddiqui, M.H.; Ali, H.M.; Ali, A.; et al. Potassium Silicate and Zinc Oxide Nanoparticles Modulate Antioxidant System, Membranous H+-ATPase and Nitric Oxide Content in Faba Bean (Vicia faba) Seedlings Exposed to Arsenic Toxicity. Funct. Plant Biol. 2022, 50, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Thapar Kapoor, R.; Ingo Hefft, D.; Ahmad, A. Nitric Oxide and Spermidine Alleviate Arsenic-Incited Oxidative Damage In. Funct. Plant Biol. 2021, 50, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Husain, T.; Kushwaha, B.K.; Suhel, M.; Fatima, A.; Mishra, V.; Singh, S.K.; Bhatt, J.A.; Rai, M.; Prasad, S.M.; et al. Regulation of Ascorbate-Glutathione Cycle by Exogenous Nitric Oxide and Hydrogen Peroxide in Soybean Roots under Arsenate Stress. J. Hazard. Mater. 2021, 409, 123686. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.; Kushwaha, B.K.; Singh, V.P.; Siddiqui, M.H.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M. Ascorbate and Glutathione Independently Alleviate Arsenate Toxicity in Brinjal but Both Require Endogenous Nitric Oxide. Physiol. Plant. 2021, 173, 276–286. [Google Scholar] [CrossRef]
- Alamri, S.; Siddiqui, M.H.; Kushwaha, B.K.; Singh, V.P.; Ali, H.M. Mitigation of Arsenate Toxicity by Indole-3-Acetic Acid in Brinjal Roots: Plausible Association with Endogenous Hydrogen Peroxide. J. Hazard. Mater. 2021, 405, 124336. [Google Scholar] [CrossRef]
- Kashyap, L.; Garg, N. Arsenic Toxicity in Crop Plants: Responses and Remediation Strategies. In Mechanisms of Arsenic Toxicity and Tolerance in Plants; Hasanuzzaman, M., Nahar, K., Fujita, M., Eds.; Springer: Singapore, 2018; pp. 129–169. ISBN 9789811312922. [Google Scholar]
- Chandrakar, V.; Pandey, N.; Keshavkant, S. Plant Responses to Arsenic Toxicity: Morphology and Physiology. In Mechanisms of Arsenic Toxicity and Tolerance in Plants; Hasanuzzaman, M., Nahar, K., Fujita, M., Eds.; Springer: Singapore, 2018; pp. 27–48. ISBN 9789811312922. [Google Scholar]
- Monteiro, C.; Santos, C.; Pinho, S.; Oliveira, H.; Pedrosa, T.; Dias, M.C. Cadmium-Induced Cyto- and Genotoxicity Are Organ-Dependent in Lettuce. Chem. Res. Toxicol. 2012, 25, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Goel, S.; Sandhir, R.; Nayyar, H. Uptake and Distribution of Arsenic in Chickpea: Effects on Seed Yield and Seed Composition. Commun. Soil. Sci. Plant Anal. 2011, 42, 1728–1738. [Google Scholar] [CrossRef]
- Vromman, D.; Lutts, S.; Lefèvre, I.; Somer, L.; De Vreese, O.; Šlejkovec, Z.; Quinet, M. Effects of Simultaneous Arsenic and Iron Toxicities on Rice (Oryza sativa L.) Development, Yield-Related Parameters and As and Fe Accumulation in Relation to As Speciation in the Grains. Plant Soil 2013, 371, 199–217. [Google Scholar] [CrossRef]
- Nath, S.; Panda, P.; Mishra, S.; Dey, M.; Choudhury, S.; Sahoo, L.; Panda, S.K. Arsenic Stress in Rice: Redox Consequences and Regulation by Iron. Plant Physiol. Biochem. 2014, 80, 203–210. [Google Scholar] [CrossRef]
- Talukdar, D. Effect of Arsenic-Induced Toxicity on Morphological Traits of Trigonella foenum-Graecum L. and Lathyrus sativus L. During Germination and Early Seedling Growth. Curr. Res. J. Biol. Sci. 2011, 3, 116–123. [Google Scholar]
- Reichman, S.M. Probing the Plant Growth-Promoting and Heavy Metal Tolerance Characteristics of Bradyrhizobium japonicum CB1809. Eur. J. Soil Biol. 2014, 63, 7–13. [Google Scholar] [CrossRef]
- Bustingorri, C.; Lavado, R.S. Soybean as Affected by High Concentrations of Arsenic and Fluoride in Irrigation Water in Controlled Conditions. Agric. Water Manag. 2014, 144, 134–139. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Villasuso, A.L.; Travaglia, C.; Racagni, G.E.; Reinoso, H.; Agostini, E. Arsenic Stress Induces Changes in Lipid Signalling and Evokes the Stomata Closure in Soybean. Plant Physiol. Biochem. 2016, 103, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Milton, N.M.; Ager, C.M.; Eiswerth, B.A.; Power, M.S. Arsenic- and Selenium-Induced Changes in Spectral Reflectance and Morphology of Soybean Plants. Remote Sens. Environ. 1989, 30, 263–269. [Google Scholar] [CrossRef]
- Jiang, J.; Bauer, I.; Paul, A.; Kappler, A. Arsenic Redox Changes by Microbially and Chemically Formed Semiquinone Radicals and Hydroquinones in a Humic Substance Model Quinone. Environ. Sci. Technol. 2009, 43, 3639–3645. [Google Scholar] [CrossRef]
- Kaur, S.; Chowhan, N.; Sharma, P.; Rathee, S.; Singh, H.P.; Batish, D.R. β-Pinene Alleviates Arsenic (As)-Induced Oxidative Stress by Modulating Enzymatic Antioxidant Activities in Roots of Oryza Sativa. Ecotoxicol. Environ. Saf. 2022, 229, 113080. [Google Scholar] [CrossRef]
- Sharma, I. Arsenic Induced Oxidative Stress in Plants. Biologia 2012, 67, 447–453. [Google Scholar] [CrossRef]
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The Roles of Mitochondrial Reactive Oxygen Species in Cellular Signaling and Stress Response in Plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.-J.; Shaki, F.; Ghazi-Khansari, M.; Pourahmad, J. Toxicity of Arsenic (III) on Isolated Liver Mitochondria: A New Mechanistic Approach. Iran. J. Pharm. Res. 2013, 12, 121–138. [Google Scholar] [PubMed]
- Thakur, M.; Rachamalla, M.; Niyogi, S.; Datusalia, A.K.; Flora, S.J.S. Molecular Mechanism of Arsenic-Induced Neurotoxicity Including Neuronal Dysfunctions. Int. J. Mol. Sci. 2021, 22, 10077. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sharma, P.M. Arsenic Toxicity Induced Endothelial Dysfunction and Dementia: Pharmacological Interdiction by Histone Deacetylase and Inducible Nitric Oxide Synthase Inhibitors. Toxicol. Appl. Pharmacol. 2013, 273, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Zargari, F.; Rahaman, M.S.; KazemPour, R.; Hajirostamlou, M. Arsenic, Oxidative Stress and Reproductive System. J. Xenobiotics 2022, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc Oxide Nanoparticles Application Alleviates Arsenic (As) Toxicity in Soybean Plants by Restricting the Uptake of as and Modulating Key Biochemical Attributes, Antioxidant Enzymes, Ascorbate-Glutathione Cycle and Glyoxalase System. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Stevanović, B.; Mitrović, M.; Pavlović, P. Phytoremediation Potential, Photosynthetic and Antioxidant Response to Arsenic-Induced Stress of Dactylis glomerata L. Sown on Fly Ash Deposits. Plants 2020, 9, 657. [Google Scholar] [CrossRef]
- Zhang, J.; Hamza, A.; Xie, Z.; Hussain, S.; Brestic, M.; Tahir, M.A.; Ulhassan, Z.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Arsenic Transport and Interaction with Plant Metabolism: Clues for Improving Agricultural Productivity and Food Safety. Environ. Pollut. 2021, 290, 117987. [Google Scholar] [CrossRef]
- Mishra, S.; Alfeld, M.; Sobotka, R.; Andresen, E.; Falkenberg, G.; Küpper, H. Analysis of Sublethal Arsenic Toxicity to Ceratophyllum demersum: Subcellular Distribution of Arsenic and Inhibition of Chlorophyll Biosynthesis. J. Exp. Bot. 2016, 67, 4639–4646. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N.; Jones, S.E.; Gonzalez-Blanco, P. Silencing of δ-Aminolevulinic Acid Dehydratase via Virus Induced Gene Silencing Promotes Callose Deposition in Plant Phloem. Plant Signal. Behav. 2022, 17, 2024733. [Google Scholar] [CrossRef]
- Bhadauria, S.; Flora, S.J.S. Arsenic Induced Inhibition of Delta-Aminolevulinate Dehydratase Activity in Rat Blood and Its Response to Meso 2,3-Dimercaptosuccinic Acid and Monoisoamyl DMSA. Biomed. Environ. Sci. 2004, 17, 101–108. [Google Scholar]
- Flora, S.J.S. Metal Poisoning: Threat and Management. Al Ameen J. Med. Sci. 2009, 2, 4–26. [Google Scholar]
- Andrade, H.M.; Oliveira, J.A.; Farnese, F.S.; Ribeiro, C.; Silva, A.A.; Campos, F.V.; Neto, J.L. Arsenic Toxicity: Cell Signalling and the Attenuating Effect of Nitric Oxide in Eichhornia Crassipes. Biol. Plant. 2016, 60, 173–180. [Google Scholar] [CrossRef]
- Onuki, J.; Chen, Y.; Teixeira, P.C.; Schumacher, R.I.; Medeiros, M.H.G.; Van Houten, B.; Di Mascio, P. Mitochondrial and Nuclear DNA Damage Induced by 5-Aminolevulinic Acid. Arch. Biochem. Biophys. 2004, 432, 178–187. [Google Scholar] [CrossRef]
- Nahar, K.; Rhaman, M.S.; Parvin, K.; Bardhan, K.; Marques, D.N.; García-Caparrós, P.; Hasanuzzaman, M. Arsenic-Induced Oxidative Stress and Antioxidant Defense in Plants. Stresses 2022, 2, 13. [Google Scholar] [CrossRef]
- Zavaleta-Mancera, H.; Ortega-Ramírez, L.; Jiménez-García, L.; Sánchez-Viveros, G.; Alarcón, A. Effect of Arsenic on Chloroplast Ultrastructure in Azolla filliculoides Lam. Microsc. Microanal. 2016, 22, 1206–1207. [Google Scholar] [CrossRef]
- Li, W.-X.; Chen, T.-B.; Huang, Z.-C.; Lei, M.; Liao, X.-Y. Effect of Arsenic on Chloroplast Ultrastructure and Calcium Distribution in Arsenic Hyperaccumulator Pteris vittata L. Chemosphere 2006, 62, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, A.K.; Singh, B.; Suprasanna, P.; D’souza, S.F. The Effect of Arsenic on Pigment Composition and Photosynthesis in Hydrilla verticillata. Biol. Plant. 2013, 57, 385–389. [Google Scholar] [CrossRef]
- Van Aken, O. Mitochondrial Redox Systems as Central Hubs in Plant Metabolism and Signaling. Plant Physiol. 2021, 186, 36–52. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Finkemeier, I. Mitochondrial Energy and Redox Signaling in Plants. Antioxid. Redox Signal. 2013, 18, 2122–2144. [Google Scholar] [CrossRef]
- Zhao, R.-Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Ohnishi, T.; Ohnishi, S.T.; Salerno, J.C. Five Decades of Research on Mitochondrial NADH-Quinone Oxidoreductase (Complex I). Biol. Chem. 2018, 399, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, H.; Kuppusamy, P.; Zweier, J.; Trush, M. Mitochondrial Electron Transport Chain-Derived Superoxide Exits Macrophages: Implications for Mononuclear Cell-Mediated Pathophysiological Processes. React. Oxyg. Species 2016, 1, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Bleier, L.; Dröse, S. Superoxide Generation by Complex III: From Mechanistic Rationales to Functional Consequences. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2013, 1827, 1320–1331. [Google Scholar] [CrossRef]
- Palmieri, L.; Picault, N.; Arrigoni, R.; Besin, E.; Palmieri, F.; Hodges, M. Molecular Identification of Three Arabidopsis thaliana Mitochondrial Dicarboxylate Carrier Isoforms: Organ Distribution, Bacterial Expression, Reconstitution into Liposomes and Functional Characterization. Biochem. J. 2008, 410, 621–629. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, H.; McGrath, S.P.; Wu, P.; Zhao, F.-J. Investigating the Contribution of the Phosphate Transport Pathway to Arsenic Accumulation in Rice. Plant Physiol. 2011, 157, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Strawn, D.G. Review of Interactions between Phosphorus and Arsenic in Soils from Four Case Studies. Geochem. Trans. 2018, 19, 10. [Google Scholar] [CrossRef]
- Shrivastava, A.; Ghosh, D.; Dash, A.; Bose, S. Arsenic Contamination in Soil and Sediment in India: Sources, Effects, and Remediation. Curr. Pollut. Rep. 2015, 1, 35–46. [Google Scholar] [CrossRef]
- Hong, S.; Pedersen, P.L. ATP Synthase and the Actions of Inhibitors Utilized to Study Its Roles in Human Health, Disease, and Other Scientific Areas. Microbiol. Mol. Biol. Rev. 2008, 72, 590–641. [Google Scholar] [CrossRef]
- Ralph, S.J. Arsenic-Based Antineoplastic Drugs and Their Mechanisms of Action. Met. Based Drugs 2008, 260146. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Arsenic: A Review on a Great Health Issue Worldwide. Appl. Sci. 2022, 12, 6184. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S. Is It Possible to Avert Arsenic Effects on Cells and Tissues Bypassing Its Toxicity and Suppressive Consequences of Energy Production? A Hypothesis. BLDE Univ. J. Health Sci. 2017, 2, 91. [Google Scholar] [CrossRef]
- Bergquist, E.R.; Fischer, R.J.; Sugden, K.D.; Martin, B.D. Inhibition by Methylated Organo-Arsenicals of the Respiratory 2-Oxo-Acid Dehydrogenases. J. Organomet. Chem. 2009, 694, 973–980. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Guzhova, I.V.; Margulis, B.A. Glyceraldehyde-3-Phosphate Dehydrogenase Is a Multifaceted Therapeutic Target. Pharmaceutics 2020, 12, 416. [Google Scholar] [CrossRef]
- Blake, C.C.; Rice, D.W. Phosphoglycerate Kinase. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1981, 293, 93–104. [Google Scholar] [CrossRef]
- Chen, J.; Yoshinaga, M.; Garbinski, L.D.; Rosen, B.P. Synergistic Interaction of Glyceraldehydes-3-Phosphate Dehydrogenase and ArsJ, a Novel Organoarsenical Efflux Permease, Confers Arsenate Resistance. Mol. Microbiol. 2016, 100, 945–953. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Gan, R.; Tong, T.; Bian, H.; Li, Z.; Du, S.; Deng, Z.; Chen, S. Signature Arsenic Detoxification Pathways in Halomonas Sp. Strain GFAJ-1. mBio 2018, 9, e00515-18. [Google Scholar] [CrossRef] [PubMed]
- Faita, F.; Cori, L.; Bianchi, F.; Andreassi, M.G. Arsenic-Induced Genotoxicity and Genetic Susceptibility to Arsenic-Related Pathologies. Int. J. Environ. Res. Public Health 2013, 10, 1527–1546. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Mellone, B.G. Chromatin Assembly: Journey to the CENter of the Chromosome. J. Cell Biol. 2016, 214, 13–24. [Google Scholar] [CrossRef]
- Mariño-Ramírez, L.; Kann, M.G.; Shoemaker, B.A.; Landsman, D. Histone Structure and Nucleosome Stability. Expert Rev. Proteom. 2005, 2, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Sadakierska-Chudy, A.; Filip, M. A Comprehensive View of the Epigenetic Landscape. Part II: Histone Post-Translational Modification, Nucleosome Level, and Chromatin Regulation by NcRNAs. Neurotox. Res. 2015, 27, 172–197. [Google Scholar] [CrossRef]
- Demetriadou, C.; Koufaris, C.; Kirmizis, A. Histone N-Alpha Terminal Modifications: Genome Regulation at the Tip of the Tail. Epigenetics Chromatin 2020, 13, 29. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone Deacetylases and Mechanisms of Regulation of Gene Expression. Crit. Rev. Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef]
- Liu, D.; Wu, D.; Zhao, L.; Yang, Y.; Ding, J.; Dong, L.; Hu, L.; Wang, F.; Zhao, X.; Cai, Y.; et al. Arsenic Trioxide Reduces Global Histone H4 Acetylation at Lysine 16 through Direct Binding to Histone Acetyltransferase HMOF in Human Cells. PLoS ONE 2015, 10, e0141014. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, H.; Ellen, T.P.; Chen, H.; Costa, M. Arsenite Alters Global Histone H3 Methylation. Carcinogenesis 2008, 29, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Loaeza-Loaeza, J.; Beltran, A.S.; Hernández-Sotelo, D. DNMTs and Impact of CpG Content, Transcription Factors, Consensus Motifs, LncRNAs, and Histone Marks on DNA Methylation. Genes 2020, 11, 1336. [Google Scholar] [CrossRef]
- Reichard, J.F.; Puga, A. Effects of Arsenic Exposure on DNA Methylation and Epigenetic Gene Regulation. Epigenomics 2010, 2, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.B.; Dubey, R.S. Carbohydrate Metabolism in Growing Rice Seedlings under Arsenic Toxicity. J. Plant Physiol. 2004, 161, 867–872. [Google Scholar] [CrossRef]
- De Souza, P.M.; Magalhães, P.D.O.E. Application of Microbial α-Amylase in Industry—A Review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Shan, X.; Zhu, Y.-G. Toxicity of Arsenate and Arsenite on Germination, Seedling Growth and Amylolytic Activity of Wheat. Chemosphere 2005, 61, 293–301. [Google Scholar] [CrossRef]
- Rathore, R.S.; Garg, N.; Garg, S.; Kumar, A. Starch Phosphorylase: Role in Starch Metabolism and Biotechnological Applications. Crit. Rev. Biotechnol. 2009, 29, 214–224. [Google Scholar] [CrossRef]
- Robyt, J.F. Chapter 7-Enzymes and Their Action on Starch. In Starch, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2009; pp. 237–292. ISBN 978-0-12-746275-2. [Google Scholar]
- Choudhury, B.; Mitra, S.; Biswas, A.K. Regulation of Sugar Metabolism in Rice (Oryza sativa L.) Seedlings under Arsenate Toxicity and Its Improvement by Phosphate. Physiol. Mol. Biol. Plants 2010, 16, 59–68. [Google Scholar] [CrossRef]
- Talukdar, D. Arsenic-Induced Oxidative Stress in the Common Bean Legume, Phaseolus vulgaris L. Seedlings and Its Amelioration by Exogenous Nitric Oxide. Physiol. Mol. Biol. Plants 2013, 19, 69–79. [Google Scholar] [CrossRef]
- de Dios Alché, J. A Concise Appraisal of Lipid Oxidation and Lipoxidation in Higher Plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Luo, Y.; Liao, B.; Xie, L.; Chen, L.; Xiao, S.; Li, J.; Hu, S.; Shu, W. Comparative Transcriptome Analysis of Transporters, Phytohormone and Lipid Metabolism Pathways in Response to Arsenic Stress in Rice (Oryza sativa). New Phytol. 2012, 195, 97–112. [Google Scholar] [CrossRef]
- Jha, A.B.; Dubey, R.S. Arsenic Exposure Alters Activity Behaviour of Key Nitrogen Assimilatory Enzymes in Growing Rice Plants. Plant Growth Regul. 2004, 43, 259–268. [Google Scholar] [CrossRef]
- Mótyán, J.A.; Tóth, F.; Tőzsér, J. Research Applications of Proteolytic Enzymes in Molecular Biology. Biomolecules 2013, 3, 923–942. [Google Scholar] [CrossRef]
- Mishra, S.; Dubey, R.S. Inhibition of Ribonuclease and Protease Activities in Arsenic Exposed Rice Seedlings: Role of Proline as Enzyme Protectant. J. Plant Physiol. 2006, 163, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein Carbonylation as a Major Hallmark of Oxidative Damage: Update of Analytical Strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Sghaier, D.B.; Pedro, S.; Diniz, M.S.; Duarte, B.; Caçador, I.; Sleimi, N. Tissue Localization and Distribution of As and Al in the Halophyte Tamarix Gallica under Controlled Conditions. Front. Mar. Sci. 2016, 3, 274. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon Uptake and Accumulation in Higher Plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Zia, Z.; Fahad, S.; Abbas, S.; Hammad, H.M.; Shahzad, A.N.; Abbas, F.; Alharby, H.; Shahid, M. Arsenic Uptake, Accumulation and Toxicity in Rice Plants: Possible Remedies for Its Detoxification: A Review. Environ. Sci. Pollut. Res. 2017, 24, 9142–9158. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Kong, Y.-H.; Chen, Y.; Duan, J.-Y.; Wu, W.-H.; Chen, Y.-F. Arabidopsis WRKY45 Transcription Factor Activates PHOSPHATE TRANSPORTER1;1 Expression in Response to Phosphate Starvation. Plant Physiol. 2014, 164, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yan, H.; Chen, Y.; Shen, H.; Xu, W.; Zhang, H.; Shi, L.; Zhu, Y.-G.; Ma, M. An Aquaporin PvTIP4;1 from Pteris Vittata May Mediate Arsenite Uptake. New Phytol. 2016, 209, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.-M.; Scheckel, K.G.; Lombi, E.; Newville, M.; Choi, Y.; Norton, G.J.; Charnock, J.M.; Feldmann, J.; Price, A.H.; Meharg, A.A. Grain Unloading of Arsenic Species in Rice. Plant Physiol. 2009, 152, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Kadohashi, K.; Maki, T.; Hasegawa, H. Transport of DMAA and MMAA into Rice (Oryza sativa L.) Roots. Environ. Exp. Bot. 2011, 72, 41–46. [Google Scholar] [CrossRef]
- Krzesłowska, M. The Cell Wall in Plant Cell Response to Trace Metals: Polysaccharide Remodeling and Its Role in Defense Strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A Review of the Biochemistry of Heavy Metal Biosorption by Brown Algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef] [PubMed]
- Pelloux, J.; Rusterucci, C.; Mellerowicz, E. New Insights into Pectin Methylesterase Structure and Function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef]
- Wu, H.-C.; Bulgakov, V.P.; Jinn, T.-L. Pectin Methylesterases: Cell Wall Remodeling Proteins Are Required for Plant Response to Heat Stress. Front. Plant Sci. 2018, 9, 1612. [Google Scholar] [CrossRef]
- Huang, W.X.; Chen, X.W.; Wu, L.; Yu, Z.S.; Gao, M.Y.; Zhao, H.M.; Mo, C.H.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; et al. Root Cell Wall Chemistry Remodelling Enhanced Arsenic Fixation of a Cabbage Cultivar. J. Hazard. Mater. 2021, 420, 126165. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic Uptake and Metabolism in Plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Dai, X.; Xu, W.; He, Z.; Ma, M. Evidence of Vacuolar Compartmentalization of Arsenic in the Hyperaccumulator Pteris Vittata. Chin. Sci. Bull. 2009, 54, 4229–4233. [Google Scholar] [CrossRef]
- Farooq, M.A.; Islam, F.; Ali, B.; Najeeb, U.; Mao, B.; Gill, R.A.; Yan, G.; Siddique, K.H.M.; Zhou, W. Arsenic Toxicity in Plants: Cellular and Molecular Mechanisms of Its Transport and Metabolism. Environ. Exp. Bot. 2016, 132, 42–52. [Google Scholar] [CrossRef]
- Mirza, N.; Mahmood, Q.; Maroof Shah, M.; Pervez, A.; Sultan, S. Plants as Useful Vectors to Reduce Environmental Toxic Arsenic Content. Sci. World J. 2014, 921581. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A Fern That Hyperaccumulates Arsenic. Nature 2001, 411, 438. [Google Scholar] [CrossRef]
- Ye, W.-L.; Wood, B.A.; Stroud, J.L.; Andralojc, P.J.; Raab, A.; McGrath, S.P.; Feldmann, J.; Zhao, F.-J. Arsenic Speciation in Phloem and Xylem Exudates of Castor Bean. Plant Physiol. 2010, 154, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- De Caroli, M.; Furini, A.; DalCorso, G.; Rojas, M.; Di Sansebastiano, G.-P. Endomembrane Reorganization Induced by Heavy Metals. Plants 2020, 9, 482. [Google Scholar] [CrossRef]
- Socha, A.L.; Guerinot, M.L. Mn-Euvering Manganese: The Role of Transporter Gene Family Members in Manganese Uptake and Mobilization in Plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef]
- Barozzi, F.; Papadia, P.; Stefano, G.; Renna, L.; Brandizzi, F.; Migoni, D.; Fanizzi, F.P.; Piro, G.; Di Sansebastiano, G.-P. Variation in Membrane Trafficking Linked to SNARE AtSYP51 Interaction With Aquaporin NIP1;1. Front. Plant Sci. 2019, 9, 1949. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the Mechanisms of Plant Tolerance to Manganese Toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J.; Mimura, T. Vacuolar Compartmentalization as Indispensable Component of Heavy Metal Detoxification in Plants: Vacuolar Functions in HM Detoxification. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A.; Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the Life of Heavy Metal-Stressed Plants a Little Easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Srivastava, M.; Ma, L.Q.; Singh, N.; Singh, S. Antioxidant Responses of Hyper-Accumulator and Sensitive Fern Species to Arsenic. J. Exp. Bot. 2005, 56, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.P. RS An Overview of the Relationship between Oxidative Stress and Mercury and Arsenic. Toxic. Subst. Mech. 1996, 15, 151–181. [Google Scholar]
- Koch, I.; Wang, L.; Ollson, C.A.; Cullen, W.R.; Reimer, K.J. The Predominance of Inorganic Arsenic Species in Plants from Yellowknife, Northwest Territories, Canada. Environ. Sci. Technol. 2000, 34, 22–26. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K.; Arora, K. Arsenic-Induced Root Growth Inhibition in Mung Bean (Phaseolus aureus Roxb.) Is Due to Oxidative Stress Resulting from Enhanced Lipid Peroxidation. Plant Growth Regul. 2007, 53, 65–73. [Google Scholar] [CrossRef]
- Mascher, R.; Lippmann, B.; Holzinger, S.; Bergmann, H. Arsenate Toxicity: Effects on Oxidative Stress Response Molecules and Enzymes in Red Clover Plants. Plant Sci. 2002, 163, 961–969. [Google Scholar] [CrossRef]
- Shri, M.; Kumar, S.; Chakrabarty, D.; Trivedi, P.K.; Mallick, S.; Misra, P.; Shukla, D.; Mishra, S.; Srivastava, S.; Tripathi, R.D.; et al. Effect of Arsenic on Growth, Oxidative Stress, and Antioxidant System in Rice Seedlings. Ecotoxicol. Environ. Saf. 2009, 72, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Requejo, R.; Tena, M. Proteome Analysis of Maize Roots Reveals That Oxidative Stress Is a Main Contributing Factor to Plant Arsenic Toxicity. Phytochemistry 2005, 66, 1519–1528. [Google Scholar] [CrossRef]
- Mylona, P.V.; Polidoros, A.N.; Scandalios, J.G. Modulation of Antioxidant Responses by Arsenic in Maize. Free. Radic. Biol. Med. 1998, 25, 576–585. [Google Scholar] [CrossRef]
- Abercrombie, J.M.; Halfhill, M.D.; Ranjan, P.; Rao, M.R.; Saxton, A.M.; Yuan, J.S.; Stewart, C.N. Transcriptional Responses of Arabidopsis thaliana Plants to As (V) Stress. BMC Plant Biol. 2008, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kondo, N.; Sugahara Yatabe, I.; Mitsuhashi, H. Further Evidence for Inactivation of Fructose-1,6-Bisphosphatase at the Beginning of SO2 Fumigation: Increase in Fructose-1,6-Bisphosphate and Decrease in Fructose-6-Phosphate in SO2-Fumigated Spinach Leaves. Plant Cell Physiol. 1982, 23, 1467–1470. [Google Scholar]
- Mehlhorn, H. Ethylene-Promoted Ascorbate Peroxidase Activity Protects Plants against Hydrogen Peroxide, Ozone and Paraquat. Plant Cell Environ. 1990, 13, 971–976. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Stoeva, N.; Berova, M.; Vassilev, A.; Zlatev, Z. Effect of Exogenous Polyamine Diethylenetriamine on Oxidative Changes and Photosynthesis in As-Treated Maize Plants (Zea mays L.). J. Cent. Eur. Agric. 2005, 6, 367–374. [Google Scholar]
- Miteva, E.; Peycheva, S. Arsenic accumulation and effect on peroxidase activity in green bean and tomatoes. Bulg. J. Agric. Sci. 1999, 5, 737–740. [Google Scholar]
- Gomes-Junior, R.A.; Gratão, P.L.; Gaziola, S.A.; Mazzafera, P.; Lea, P.J.; Azevedo, R.A.; Gomes-Junior, R.A.; Gratão, P.L.; Gaziola, S.A.; Mazzafera, P.; et al. Selenium-Induced Oxidative Stress in Coffee Cell Suspension Cultures. Funct. Plant Biol. 2007, 34, 449–456. [Google Scholar] [CrossRef]
- Ghelfi, A.; Gaziola, S.A.; Cia, M.C.; Chabregas, S.M.; Falco, M.C.; Kuser-Falcão, P.R.; Azevedo, R.A. Cloning, Expression, Molecular Modelling and Docking Analysis of Glutathione Transferase from Saccharum officinarum. Ann. Appl. Biol. 2011, 159, 267–280. [Google Scholar] [CrossRef]
- Mokgalaka-Matlala, N.S.; Flores-Tavizón, E.; Castillo-Michel, H.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Arsenic Tolerance in Mesquite (Prosopis Sp.): Low Molecular Weight Thiols Synthesis and Glutathione Activity in Response to Arsenic. Plant Physiol. Biochem. 2009, 47, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Czech, V.C.P.; Fodor, J.; Bóka, K.; Fodor, F.; Cseh, E. Investigation of Arsenate Phytotoxicity in Cucumber Plants. Acta Biol. Szeged. 2008, 52, 79–80. [Google Scholar]
- Shen, S.; Li, X.-F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic Binding to Proteins. Chem. Rev. 2013, 113, 7769–7792. [Google Scholar] [CrossRef]
- Rosen, B.P. Biochemistry of Arsenic Detoxification. FEBS Lett. 2002, 529, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mishra, S.; Tripathi, R.D.; Dwivedi, S.; Trivedi, P.K.; Tandon, P.K. Phytochelatins and Antioxidant Systems Respond Differentially during Arsenite and Arsenate Stress in Hydrilla verticillata (L.f.) Royle. Environ. Sci. Technol. 2007, 41, 2930–2936. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.L.; Kang, J.-H.; Howe, G.A. Jasmonate-Triggered Plant Immunity. J. Chem. Ecol. 2014, 40, 657–675. [Google Scholar] [CrossRef]
- Kappler, A.; Amstaetter, K.; Borch, T.; Larese-Casanova, P.; Jiang, J.; Bauer, I.; Paul, A. Arsenic Redox Transformation by Humic Substances and Fe Minerals. Appl. Geochem. 2011, 26, S317. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Antioxidants as Modulators of Arsenic-Induced Oxidative Stress Tolerance in Plants: An Overview. J. Hazard. Mater. 2022, 427, 127891. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Nitro-Oxidative Stress vs Oxidative or Nitrosative Stress in Higher Plants. New Phytol. 2013, 199, 633–635. [Google Scholar] [CrossRef]
- Guo, F.-Q.; Okamoto, M.; Crawford, N.M. Identification of a Plant Nitric Oxide Synthase Gene Involved in Hormonal Signaling. Science 2003, 302, 100–103. [Google Scholar] [CrossRef]
- Crawford, N.M. Mechanisms for Nitric Oxide Synthesis in Plants. J. Exp. Bot. 2006, 57, 471–478. [Google Scholar] [CrossRef]
- Gupta, K.J.; Kaiser, W.M. Production and Scavenging of Nitric Oxide by Barley Root Mitochondria. Plant Cell Physiol. 2010, 51, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Li, X.-C.; Zhu-Ge, Q.; Jiang, X.; Wang, W.-D.; Fang, W.-P.; Chen, X.; Li, X.-H. Nitric Oxide Participates in Cold-Inhibited Camellia Sinensis Pollen Germination and Tube Growth Partly via CGMP In Vitro. PLoS ONE 2012, 7, e52436. [Google Scholar] [CrossRef] [PubMed]
- Mengel, A.; Chaki, M.; Shekariesfahlan, A.; Lindermayr, C. Effect of Nitric Oxide on Gene Transcription—S-Nitrosylation of Nuclear Proteins. Front. Plant Sci. 2013, 4, 293. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Lindermayr, C. Nitric Oxide-Dependent Posttranslational Modification in Plants: An Update. Int. J. Mol. Sci. 2012, 13, 15193–15208. [Google Scholar] [CrossRef]
- Wang, Y.; Loake, G.J.; Chu, C. Cross-Talk of Nitric Oxide and Reactive Oxygen Species in Plant Programed Cell Death. Front. Plant Sci. 2013, 4, 314. [Google Scholar] [CrossRef]
- Fan, Q.-J.; Liu, J.-H. Nitric Oxide Is Involved in Dehydration/Drought Tolerance in Poncirus trifoliata Seedlings through Regulation of Antioxidant Systems and Stomatal Response. Plant Cell Rep. 2012, 31, 145–154. [Google Scholar] [CrossRef]
- Arc, E.; Sechet, J.; Corbineau, F.; Rajjou, L.; Marion-Poll, A. ABA Crosstalk with Ethylene and Nitric Oxide in Seed Dormancy and Germination. Front. Plant Sci. 2013, 4, 63. [Google Scholar] [CrossRef]
- Kolbert, Z.; Ördög, A. Involvement of Nitric Oxide (NO) in Plant Responses to Metalloids. J. Hazard. Mater. 2021, 420, 126606. [Google Scholar] [CrossRef]
- Leterrier, M.; Airaki, M.; Palma, J.M.; Chaki, M.; Barroso, J.B.; Corpas, F.J. Arsenic Triggers the Nitric Oxide (NO) and S-Nitrosoglutathione (GSNO) Metabolism in Arabidopsis. Environ. Pollut. 2012, 166, 136–143. [Google Scholar] [CrossRef]
- Piacentini, D.; Ronzan, M.; Fattorini, L.; Della Rovere, F.; Massimi, L.; Altamura, M.M.; Falasca, G. Nitric Oxide Alleviates Cadmium- but Not Arsenic-Induced Damages in Rice Roots. Plant Physiol. Biochem. 2020, 151, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.S.; Mani, A. Analysis of BHLH Coding Genes of Cicer arietinum during Heavy Metal Stress Using Biological Network. Physiol. Mol. Biol. Plants 2019, 25, 113–121. [Google Scholar] [CrossRef]
- Singh, P.K.; Chakrabarty, D.; Dwivedi, S.; Kumar, A.; Singh, S.P.; Sinam, G.; Niranjan, A.; Singh, P.C.; Chatterjee, S.; Majumdar, D.; et al. Nitric Oxide-Mediated Alleviation of Arsenic Stress Involving Metalloid Detoxification and Physiological Responses in Rice (Oryza sativa L.). Environ. Pollut. 2022, 297, 118694. [Google Scholar] [CrossRef]
- Singh, A.P.; Dixit, G.; Kumar, A.; Mishra, S.; Singh, P.K.; Dwivedi, S.; Trivedi, P.K.; Chakrabarty, D.; Mallick, S.; Pandey, V.; et al. Nitric Oxide Alleviated Arsenic Toxicity by Modulation of Antioxidants and Thiol Metabolism in Rice (Oryza sativa L.). Front. Plant Sci. 2016, 6, 1272. [Google Scholar] [CrossRef]
- Praveen, A.; Gupta, M. Nitric Oxide Confronts Arsenic Stimulated Oxidative Stress and Root Architecture through Distinct Gene Expression of Auxin Transporters, Nutrient Related Genes and Modulates Biochemical Responses in Oryza sativa L. Environ. Pollut. 2018, 240, 950–962. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Mukherjee, S.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedhi, B.M.A.; Ali, H.M.; Kalaji, H.M.; Fahad, S.; Rajput, V.D.; et al. Molybdenum and Hydrogen Sulfide Synergistically Mitigate Arsenic Toxicity by Modulating Defense System, Nitrogen and Cysteine Assimilation in Faba Bean (Vicia faba L.) Seedlings. Environ. Pollut. 2021, 290, 117953. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic Acid-Induced Nitric Oxide Enhances Arsenic Toxicity Tolerance in Maize Plants by Upregulating the Ascorbate-Glutathione Cycle and Glyoxalase System. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Costa, M. Arsenic: A Global Environmental Challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef]
- Gardner, R.; Hamadani, J.; Grandér, M.; Tofail, F.; Nermell, B.; Palm, B.; Kippler, M.; Vahter, M. Persistent Exposure to Arsenic via Drinking Water in Rural Bangladesh Despite Major Mitigation Efforts. Am. J. Public Health 2011, 101, S333–S338. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U. Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 304524. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.S.; Mahanta, C. Groundwater Flow and Arsenic Contamination Transport Modeling for a Multi Aquifer Terrain: Assessment and Mitigation Strategies. J. Environ. Manag. 2019, 231, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Leupin, O.X.; Hug, S.J. Oxidation and Removal of Arsenic (III) from Aerated Groundwater by Filtration through Sand and Zero-Valent Iron. Water Res. 2005, 39, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. National Primary Drinking Water Regulations; Arsenic and Clarifications to Compliance and New Source Contaminants Monitoring. Fed. Regist. 2001, 66, 6975–7066. [Google Scholar]
- Penke, Y.K.; Anantharaman, G.; Ramkumar, J.; Kar, K.K. Redox Synergistic Mn-Al-Fe and Cu-Al-Fe Ternary Metal Oxide Nano Adsorbents for Arsenic Remediation with Environmentally Stable As(0) Formation. J. Hazard. Mater. 2019, 364, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Nriagu, J. Oxidation of Arsenite in Groundwater Using Ozone and Oxygen. Sci. Total Environ. 2000, 247, 71–79. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Lee, J.H. Oxidation Mechanism of As(III) in the UV/TiO2 System: Evidence for a Direct Hole Oxidation Mechanism. Environ. Sci. Technol. 2005, 39, 9695–9701. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Wang, P.; Kretzschmar, R.; Zhao, F.-J. Control of Arsenic Mobilization in Paddy Soils by Manganese and Iron Oxides. Environ. Pollut. 2017, 231, 37–47. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zouboulis, A.I. Application of Biological Processes for the Removal of Arsenic from Groundwaters. Water Res. 2004, 38, 17–26. [Google Scholar] [CrossRef]
- Gustave, W.; Yuan, Z.-F.; Sekar, R.; Chang, H.-C.; Zhang, J.; Wells, M.; Ren, Y.-X.; Chen, Z. Arsenic Mitigation in Paddy Soils by Using Microbial Fuel Cells. Environ. Pollut. 2018, 238, 647–655. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Singh, N.K.; Singh, R.; Rai, U.N. Amelioration of Arsenic Toxicity in Rice: Comparative Effect of Inoculation of Chlorella vulgaris and Nannochloropsis Sp. on Growth, Biochemical Changes and Arsenic Uptake. Ecotoxicol. Environ. Saf. 2016, 124, 68–73. [Google Scholar] [CrossRef]
- Preetha, J.S.Y.; Arun, M.; Vidya, N.; Kowsalya, K.; Halka, J.; Ondrasek, G. Biotechnology Advances in Bioremediation of Arsenic: A Review. Molecules 2023, 28, 1474. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Su, Y.; Li, Q.; Gao, S.; Shang, J.K. Exceptional Arsenic (III,V) Removal Performance of Highly Porous, Nanostructured ZrO2 Spheres for Fixed Bed Reactors and the Full-Scale System Modeling. Water Res. 2013, 47, 6258–6268. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Singh, A.K. Nitric Oxide Mitigates Arsenic-Induced Oxidative Stress and Genotoxicity in Vicia faba L. Environ. Sci. Pollut. Res. 2015, 22, 13881–13891. [Google Scholar] [CrossRef]
- Praveen, A.; Pandey, A.; Gupta, M. Nitric Oxide Alters Nitrogen Metabolism and PIN Gene Expressions by Playing Protective Role in Arsenic Challenged Brassica juncea L. Ecotoxicol. Environ. Saf. 2019, 176, 95–107. [Google Scholar] [CrossRef]
- Bhat, J.A.; Ahmad, P.; Corpas, F.J. Main Nitric Oxide (NO) Hallmarks to Relieve Arsenic Stress in Higher Plants. J. Hazard. Mater. 2021, 406, 124289. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Pishkar, L.; Roodbari, N.; Pehlivan, N.; Wu, C. Nitric Oxide Could Allay Arsenic Phytotoxicity in Tomato (Solanum lycopersicum L.) by Modulating Photosynthetic Pigments, Phytochelatin Metabolism, Molecular Redox Status and Arsenic Sequestration. Plant Physiol. Biochem. 2021, 167, 337–348. [Google Scholar] [CrossRef]
- Singh, V.P.; Srivastava, P.K.; Prasad, S.M. Nitric Oxide Alleviates Arsenic-Induced Toxic Effects in Ridged Luffa Seedlings. Plant Physiol. Biochem. 2013, 71, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Verma, P.K.; Chakrabarty, D. Potential Biotechnological Strategies to Improve Quality and Productivity of Rice Under Arsenic Stress. In Rice Research for Quality Improvement: Genomics and Genetic Engineering: Volume 1: Breeding Techniques and Abiotic Stress Tolerance; Roychoudhury, A., Ed.; Springer: Singapore, 2020; pp. 357–371. ISBN 9789811541209. [Google Scholar]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An Efflux Transporter of Silicon in Rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef]
- Kumar, S.; Asif, M.H.; Chakrabarty, D.; Tripathi, R.D.; Dubey, R.S.; Trivedi, P.K. Expression of a Rice Lambda Class of Glutathione S-Transferase, OsGSTL2, in Arabidopsis Provides Tolerance to Heavy Metal and Other Abiotic Stresses. J. Hazard. Mater. 2013, 248–249, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Kuramata, M.; Abe, T.; Takagi, H.; Ozawa, K.; Ishikawa, S. Phytochelatin Synthase OsPCS1 Plays a Crucial Role in Reducing Arsenic Levels in Rice Grains. Plant J. 2017, 91, 840–848. [Google Scholar] [CrossRef]
- Ali, W.; Isner, J.-C.; Isayenkov, S.V.; Liu, W.; Zhao, F.-J.; Maathuis, F.J.M. Heterologous Expression of the Yeast Arsenite Efflux System ACR3 Improves Arabidopsis thaliana Tolerance to Arsenic Stress. New Phytol. 2012, 194, 716–723. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Shen, H.; Yan, H.; Xu, W.; He, Z.; Ma, M. Engineering Arsenic Tolerance and Hyperaccumulation in Plants for Phytoremediation by a PvACR3 Transgenic Approach. Environ. Sci. Technol. 2013, 47, 9355–9362. [Google Scholar] [CrossRef]
- Chen, Y.; Hua, C.-Y.; Jia, M.-R.; Fu, J.-W.; Liu, X.; Han, Y.-H.; Liu, Y.; Rathinasabapathi, B.; Cao, Y.; Ma, L.Q. Heterologous Expression of Pteris vittata Arsenite Antiporter PvACR3;1 Reduces Arsenic Accumulation in Plant Shoots. Environ. Sci. Technol. 2017, 51, 10387–10395. [Google Scholar] [CrossRef]
- Nahar, N.; Rahman, A.; Nawani, N.N.; Ghosh, S.; Mandal, A. Phytoremediation of Arsenic from the Contaminated Soil Using Transgenic Tobacco Plants Expressing ACR2 Gene of Arabidopsis thaliana. J. Plant Physiol. 2017, 218, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shi, S.; Wang, L.; Tang, Z.; Lv, T.; Zhu, X.; Ding, X.; Wang, Y.; Zhao, F.-J.; Wu, Z. OsHAC4 Is Critical for Arsenate Tolerance and Regulates Arsenic Accumulation in Rice. New Phytol. 2017, 215, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, M.-X.; Hu, H.; Ding, X.-M.; Lin, H.-W.; Wang, L.; Xu, J.-M.; Mao, C.-Z.; Zhao, F.-J.; Wu, Z.-C. OsCLT1, a CRT-like Transporter 1, Is Required for Glutathione Homeostasis and Arsenic Tolerance in Rice. New Phytol. 2016, 211, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Sandhi, A.; Greger, M.; Landberg, T.; Jacks, G.; Bhattacharya, P. Arsenic Concentrations in Local Aromatic and High-Yielding Hybrid Rice Cultivars and the Potential Health Risk: A Study in an Arsenic Hotspot. Environ. Monit. Assess. 2017, 189, 184. [Google Scholar] [CrossRef]
- Das, N.; Bhattacharya, S.; Bhattacharyya, S.; Maiti, M.K. Identification of Alternatively Spliced Transcripts of Rice Phytochelatin Synthase 2 Gene OsPCS2 Involved in Mitigation of Cadmium and Arsenic Stresses. Plant Mol. Biol. 2017, 94, 167–183. [Google Scholar] [CrossRef]
- Gasic, K.; Korban, S.S. Transgenic Indian Mustard (Brassica juncea) Plants Expressing an Arabidopsis Phytochelatin Synthase (AtPCS1) Exhibit Enhanced As and Cd Tolerance. Plant Mol. Biol. 2007, 64, 361–369. [Google Scholar] [CrossRef]
- Sundaram, S.; Wu, S.; Ma, L.Q.; Rathinasabapathi, B. Expression of a Pteris Vittata Glutaredoxin PvGRX5 in Transgenic Arabidopsis thaliana Increases Plant Arsenic Tolerance and Decreases Arsenic Accumulation in the Leaves. Plant Cell Environ. 2009, 32, 851–858. [Google Scholar] [CrossRef]
- Verma, P.K.; Verma, S.; Pande, V.; Mallick, S.; Deo Tripathi, R.; Dhankher, O.P.; Chakrabarty, D. Overexpression of Rice Glutaredoxin OsGrx_C7 and OsGrx_C2.1 Reduces Intracellular Arsenic Accumulation and Increases Tolerance in Arabidopsis Thaliana. Front. Plant Sci. 2016, 7, 740. [Google Scholar] [PubMed]
- Wang, F.-Z.; Chen, M.-X.; Yu, L.-J.; Xie, L.-J.; Yuan, L.-B.; Qi, H.; Xiao, M.; Guo, W.; Chen, Z.; Yi, K.; et al. OsARM1, an R2R3 MYB Transcription Factor, Is Involved in Regulation of the Response to Arsenic Stress in Rice. Front. Plant Sci. 2017, 8, 1868. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, X.; Tang, Z.; Zhang, W.; Huang, X.-Y.; Zhao, F.-J. OsWRKY28 Regulates Phosphate and Arsenate Accumulation, Root System Architecture and Fertility in Rice. Front. Plant Sci. 2018, 9, 1330. [Google Scholar] [CrossRef]
- Kidwai, M.; Dhar, Y.V.; Gautam, N.; Tiwari, M.; Ahmad, I.Z.; Asif, M.H.; Chakrabarty, D. Oryza Sativa Class III Peroxidase (OsPRX38) Overexpression in Arabidopsis thaliana Reduces Arsenic Accumulation Due to Apoplastic Lignification. J. Hazard. Mater. 2019, 362, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.K.; Verma, S.; Tripathi, R.D.; Chakrabarty, D. A Rice Glutaredoxin Regulate the Expression of Aquaporin Genes and Modulate Root Responses to Provide Arsenic Tolerance. Ecotoxicol. Environ. Saf. 2020, 195, 110471. [Google Scholar] [CrossRef]
- Manuka, R.; Saddhe, A.A.; Srivastava, A.K.; Kumar, K.; Penna, S. Overexpression of Rice OsWNK9 Promotes Arsenite Tolerance in Transgenic Arabidopsis Plants. J. Biotechnol. 2021, 332, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Gautam, S.; Michael, R.; Shukla, T.; Trivedi, P.K. Arsenic-Induced Galactinol Synthase1 Gene, AtGolS1, Provides Arsenic Stress Tolerance in Arabidopsis thaliana. Environ. Exp. Bot. 2023, 207, 105217. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Genetic Engineering in Plants for Enhancing Arsenic Tolerance. In Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids; Prasad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 463–475. [Google Scholar]


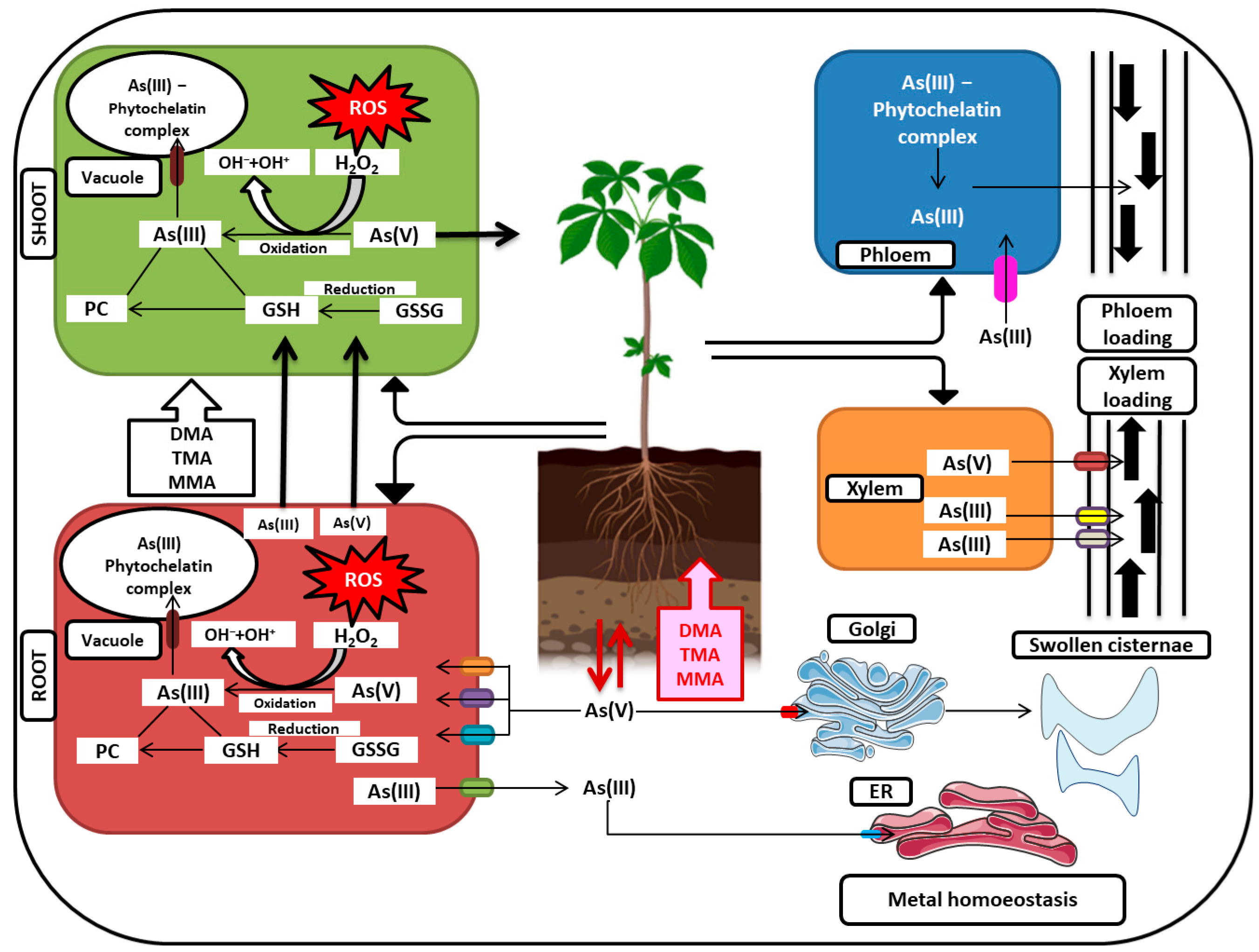

| S.No. | Characteristic Feature | Arsenic |
|---|---|---|
| 1. | Atomic Number | 33 |
| 2. | Atomic Weight | 74.9 |
| 3. | Specific Gravity | 5.73 |
| 4. | Melting Point | 817 °C (at 28 atm), |
| 5. | Boiling Point | 613 °C, |
| 6. | Vapor Pressure | 1 mm Hg at 372 °C |
| 7. | Nature | Brittle Crystalline Solid |
| Oxidation State of Arsenic | Species Present | pH | Reference |
|---|---|---|---|
| As(III) | H3AsO3 | At pH 0–9, the most dominant form (53–100%) | [44] |
| H2AsO3− HAsO32− | pH > 9 | ||
| As(V) | H3AsO4 | pH 1–3 | [44,106] |
| H2AsO4− | pH 3–6 | ||
| HAsO42− | pH 7–11 | ||
| AsO43− | pH 12–14 | ||
| As(V) | H2AsO4− HAsO42− | pH 4–9 | [107,108] |
| As(V) | H2AsO4− (96%) | pH 3–4 | [109] |
| HAsO42− (73%) | pH 6–7 |
| Redox Condition | Reducing Conditions Low Eh Values −200 to 0 mV | Moderately Reducing Conditions Intermediate Eh Value 0 to 100 mV | Oxidizing Conditions High Eh Value 100–200 mV |
|---|---|---|---|
| Arsenic speciation | As(V) is reduced to As(III) | Partial dissolution of As(V) | As(V) predominates and is co-precipitated with iron and manganese oxides |
| Reference | [44,85] | ||
| Category of Transporter | Name of the Transporter | Plant Source | Function | Reference |
|---|---|---|---|---|
| Nodulin 26-like intrinsic proteins | OsNIP2;1/OsLsi1 | Oryza sativa | Enhanced As(III) uptake | [167] |
| OsNIP2;2/OsLsi6 | As(III) transport | [167] | ||
| OsNIP1;1 and OsNIP3;3 | As(III) loading in xylem and root-shoot translocation | [169] | ||
| OsNIP3;2 | As(III) uptake | [170] | ||
| LjNIP5;1 and LjNIP6;1 | Lotus japonicas | As(III) transport | [171] | |
| AtNIP5;1 and AtNIP6;1 | Arabidopsis thaliana | As(III) transport | [171] | |
| AtNIP1;1, AtNIP1;2 | As(III) uptake | [172] | ||
| AtNIP3;1 | As(III) uptake and root-shoot translocation | [173] | ||
| AtNIP7;1 | As(III) uptake | [174] | ||
| HvNIP1;2 | Hordeum vulgare | As(III) transport | [175] | |
| - | DvNip1 | Dittrichia viscosa | As(III) tolerance | [176] |
| Silicon transporter | OsLsi2 | Oryza sativa | As(III) uptake | [167] |
| Plasma membrane intrinsic proteins | OsPIP2;4, OsPIP2;6, and OsPIP2;7 | Oryza sativa | Bi-directional permease | [177] |
| AtPIP2;2 | Arabidopsis thaliana | As(III) efflux | [178] | |
| Tonoplast intrinsic protein | PvTIP4;1 | Pteris vittate | As(III) uptake | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha, D.; Datta, S.; Mishra, R.; Agarwal, P.; Kumari, T.; Adeyemi, S.B.; Kumar Maurya, A.; Ganguly, S.; Atique, U.; Seal, S.; et al. Negative Impacts of Arsenic on Plants and Mitigation Strategies. Plants 2023, 12, 1815. https://doi.org/10.3390/plants12091815
Sinha D, Datta S, Mishra R, Agarwal P, Kumari T, Adeyemi SB, Kumar Maurya A, Ganguly S, Atique U, Seal S, et al. Negative Impacts of Arsenic on Plants and Mitigation Strategies. Plants. 2023; 12(9):1815. https://doi.org/10.3390/plants12091815
Chicago/Turabian StyleSinha, Dwaipayan, Soumi Datta, Reema Mishra, Preeti Agarwal, Tripti Kumari, Sherif Babatunde Adeyemi, Arun Kumar Maurya, Sharmistha Ganguly, Usman Atique, Sanchita Seal, and et al. 2023. "Negative Impacts of Arsenic on Plants and Mitigation Strategies" Plants 12, no. 9: 1815. https://doi.org/10.3390/plants12091815
APA StyleSinha, D., Datta, S., Mishra, R., Agarwal, P., Kumari, T., Adeyemi, S. B., Kumar Maurya, A., Ganguly, S., Atique, U., Seal, S., Kumari Gupta, L., Chowdhury, S., & Chen, J.-T. (2023). Negative Impacts of Arsenic on Plants and Mitigation Strategies. Plants, 12(9), 1815. https://doi.org/10.3390/plants12091815









