Unlocking Therapeutic Potential: Comprehensive Extraction, Profiling, and Pharmacological Evaluation of Bioactive Compounds from Eclipta alba (L.) Hassk. for Dermatological Applications
Abstract
:1. Introduction
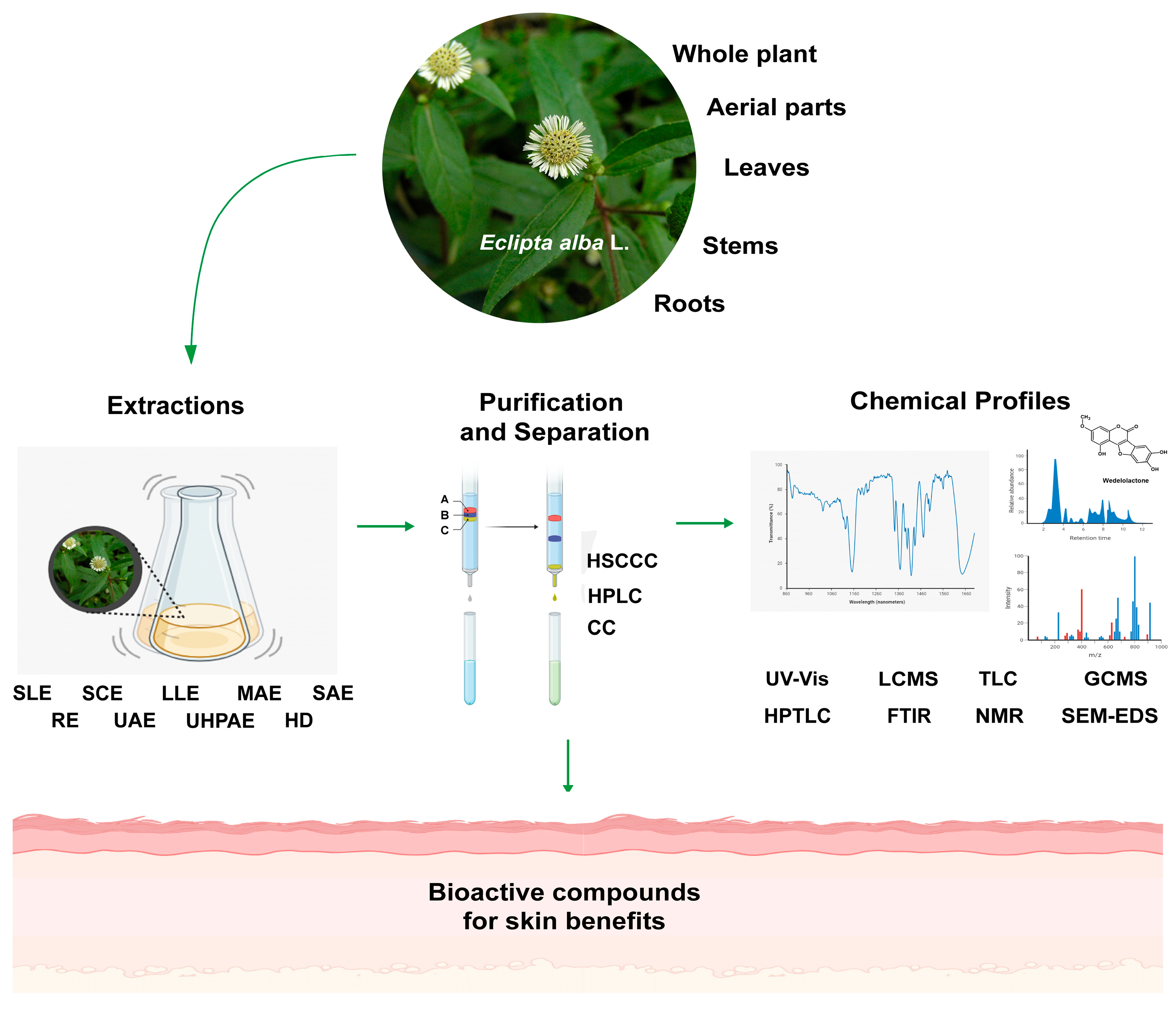
2. Materials and Methods
3. Phytochemicals of E. alba
3.1. Coumestans
3.2. Flavonoids
3.3. Thiopenes
3.4. Triterpenes
3.5. Other Compounds
4. Pharmacological Activities of Phytochemicals Found in E. alba for Skin Care and Therapeutics
4.1. Coumestans
4.1.1. Wedelolactone
4.1.2. Demethyl Wedelolactone
4.2. Flavonoids
4.2.1. Luteolin
4.2.2. Luteolin-7-O-Glucoside
4.3. Thiopenes
Ecliprostin
4.4. Triterpenes and Saponins
4.4.1. Ursolic Acid
4.4.2. Eclalbasaponin
5. Extraction Methods
5.1. Conventional Methods for Extracting Bioactive Compounds from E. alba
5.1.1. Solid–Liquid Extraction: Maceration, Agitation, and Percolation
5.1.2. Solid–Liquid Continuous Extraction: Soxhlet Extraction
5.1.3. Solid–Liquid Continuous Extraction: Reflux Extraction
5.1.4. Liquid–Liquid Extraction: Aqueous Two-Phase Extraction (ATPE)
5.1.5. Hydrodistillation (HD)
5.2. Unconventional Extraction Methods for Extracting Bioactive Compounds from E. alba
5.2.1. Ultrasound-Assisted Extraction (UAE)
5.2.2. Supercritical Fluid Extraction (SFE)
5.2.3. Microwave-Assisted Extraction (MAE)
5.2.4. Ultrahigh Pressure-Assisted Extraction (UHPE)
5.3. Combinatorial Processes for Extracting Bioactive Compounds from E. alba
5.3.1. Maceration-Percolation
5.3.2. Ultrasound-Assisted Microwave Extraction (UAME)
5.3.3. Combination of Other Extraction Methods
6. Separation and Purification Technologies
6.1. Column Chromatography (CC)
6.2. High-Performance Liquid Chromatography (HPLC)
6.3. High-Speed Counter-Current Chromatography (HSCCC)
6.4. Other Methods for Separation and Purification of Bioactive Compounds
7. Characterization Techniques
7.1. Ultraviolet–Visible Spectroscopy (UV–Vis)
7.2. Thin Layer Chromatography (TLC) and High-Performance Thin Layer Chromatography (HPTLC)
7.3. Fourier Transform Infrared Spectroscopy (FT–IR)
7.4. Scanning Electron Microscopy with Energy-Dispersive X-Ray Spectroscopy (SEM–EDS)
7.5. Nuclear Magnetic Resonance Spectroscopy (NMR)
7.6. Liquid Chromatography Coupled with Mass Spectrometry (LC–MS)
7.7. Gas Chromatography Coupled with Mass Spectrometry (GC–MS)
8. Strengths and Limitations of the Current Review
9. Conclusion and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Swe, K.N.N.; Soe, S.W.M.L. Screening of Phytochemical Constituents and Some Pharmacological Activities of Eclipta alba Hassk. Plant (Trailing eclipta). 2nd Myanmar Korea Conf. Res. J. 2019, 2, 57–63. [Google Scholar]
- Bhalerao, S.A.; Verma, D.R.; Teli, N.C.; Murukate, V.R. Eclipta alba (L): An overview. Int. J. Bioassays 2013, 2, 1443–1447. [Google Scholar]
- Timalsina, D.; Devkota, H.P. Eclipta prostrata (L.) L. (Asteraceae): Ethnomedicinal uses, chemical constituents, and biological activities. Biomolecules 2021, 11, 1738. [Google Scholar] [CrossRef]
- Rafif, K.A.; Intan, S.T.; Muhammad, A.A.N.; Mulyo, R.H. A review on phytochemistry and pharmacology of Eclipta alba L.: A 748 valuable medicinal plant. Res. J. Biotechnol. 2022, 17, 134–139. [Google Scholar]
- Upadhyay, R.; Pandey, M.; Jha, R.; Pandey, V. Eclalbatin, a triterpene saponin from Eclipta alba. J. Asian Nat. Prod. Res. 2001, 3, 213–217. [Google Scholar] [CrossRef]
- Jahan, R.; Al-Nahain, A.; Majumder, S.; Rahmatullah, M. Ethnopharmacological significance of Eclipta alba (L.) hassk. (Asteraceae). Int. Sch. Res. Notices 2014, 2014, 385969. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility Secretariat (GBIF) Eclipta prostrata (L.) L. 2023. Available online: https://www.gbif.org/species/5384950 (accessed on 13 December 2023).
- Liu, Q.-M.; Zhao, H.-Y.; Zhong, X.-K.; Jiang, J.-G. Eclipta prostrata L. phytochemicals: Isolation, structure elucidation, and their antitumor activity. Food Chem. Toxicol. 2012, 50, 4016–4022. [Google Scholar] [CrossRef]
- Yu, S.-J.; Yu, J.-H.; He, F.; Bao, J.; Zhang, J.-S.; Wang, Y.-Y.; Zhang, H. New antibacterial thiophenes from Eclipta prostrata. Fitoterapia 2020, 142, 104471. [Google Scholar] [CrossRef]
- Xi, F.-M.; Li, C.-T.; Mi, J.-L.; Wu, Z.-J.; Chen, W.-S. Three new olean-type triterpenoid saponins from aerial parts of Eclipta prostrata (L.). Nat. Prod. Res. 2014, 28, 35–40. [Google Scholar] [CrossRef]
- Chung, I.-M.; Rajakumar, G.; Lee, J.-H.; Kim, S.-H.; Thiruvengadam, M. Ethnopharmacological uses, phytochemistry, biological activities, and biotechnological applications of Eclipta prostrata. Appl. Microbiol. Biotechnol. 2017, 101, 5247–5257. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Benzie, I. An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs. In Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Gorelick, J.; Bernstein, N. Elicitation: An underutilized tool in the development of medicinal plants as a source of therapeutic secondary metabolites. Adv. Agron. 2014, 124, 201–230. [Google Scholar]
- Shiponi, S.; Bernstein, N. The highs and lows of P supply in medical cannabis: Effects on cannabinoids, the ionome, and morpho-physiology. Front. Plant Sci. 2021, 12, 657323. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S.; Cheenpracha, S.; Karalai, C. HIV-1 protease and HIV-1 integrase inhibitory substances from Eclipta prostrata. Phytother. Res. 2007, 21, 1092–1095. [Google Scholar] [CrossRef]
- Morel, L.J.F.; Azevedo, B.C.; Carmona, F.; Contini, S.H.T.; Teles, A.M.; Ramalho, F.S.; Bertoni, B.W.; França, S.C.; Borges, M.C.; Pereira, A.M.S. A standardized methanol extract of Eclipta prostrata (L.) L. (Asteraceae) reduces bronchial hyperresponsiveness and production of Th2 cytokines in a murine model of asthma. J. Ethnopharmacol. 2017, 198, 226–234. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, H.M.; Ryu, B.; Lee, J.-S.; Choi, J.-H.; Jang, D.S. Constituents of the aerial parts of Eclipta prostrata and their cytotoxicity on human ovarian cancer cells in vitro. Arch. Pharm. Res. 2015, 38, 1963–1969. [Google Scholar] [CrossRef]
- Xi, F.-M.; Li, C.-T.; Han, J.; Yu, S.-S.; Wu, Z.-J.; Chen, W.-S. Thiophenes, polyacetylenes and terpenes from the aerial parts of Eclipata prostrata. Bioorg. Med. Chem. 2014, 22, 6515–6522. [Google Scholar] [CrossRef]
- Lee, M.K.; Ha, N.R.; Yang, H.; Sung, S.H.; Kim, Y.C. Stimulatory constituents of Eclipta prostrata on mouse osteoblast differentiation. Phytother. Res. 2009, 23, 129–131. [Google Scholar] [CrossRef]
- Nakbanpote, W.; Ruttanakorn, M.; Sukadeetad, K.; Sakkayawong, N.; Damrianant, S. Effects of drying and extraction methods on phenolic compounds and in vitro assays of Eclipta prostrata Linn leaf extracts. Sci. Asia 2019, 45, 127–137. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Bahler, B.D.; Malone, S.; Werkhoven, M.C.; van Troon, F.D.; Wisse, J.H.; Bursuker, I.; Neddermann, K.M.; Mamber, S.W.; Kingston, D.G.; et al. DNA-damaging steroidal alkaloids from Eclipta alba from the suriname rainforest. J. Nat. Prod. 1998, 61, 1202–1208. [Google Scholar] [CrossRef]
- Muruganantham, S.; Anbalagan, G.; Ramamurthy, N. FT-IR and SEM-EDS comparative analysis of medicinal plants, Eclipta alba Hassk and Eclipta prostrata Linn. Rom. J. Biophys. 2009, 19, 285–294. [Google Scholar]
- Tu, Y.; Yang, Y.; Li, Y.; He, C. Naturally occurring coumestans from plants, their biological activities and therapeutic effects on human diseases. Pharmacol. Res. 2021, 169, 105615. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Abedinifar, F.; Babazadeh Rezaei, E.; Biglar, M.; Larijani, B.; Hamedifar, H.; Ansari, S.; Mahdavi, M. Recent strategies in the synthesis of thiophene derivatives: Highlights from the 2012–2020 literature. Mol. Divers. 2021, 25, 2571–2604. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Omar, A.M.; Bagalagel, A.A.; Diri, R.M.; Noor, A.O.; Almasri, D.M.; Mohamed, S.G.A.; Mohamed, G.A. Thiophenes—Naturally Occurring Plant Metabolites: Biological Activities and In Silico Evaluation of Their Potential as Cathepsin D Inhibitors. Plants 2022, 11, 539. [Google Scholar] [CrossRef]
- Oleszek, W.; Kapusta, I.; Stochmal, A. 20TLC of Triterpenes (Including Saponins). In Thin Layer Chromatography in Phytochemistry; CRC Press/Taylor & Francis Group: New York, NY, USA, 2008; p. 519. [Google Scholar]
- Metelmann, H.-R.; Brandner, J.M.; Schumann, H.; Bross, F.; Fimmers, R.; Bfttger, K.; Scheffler, A.; Podmelle, F. Accelerated Reepithelialization by Triterpenes: Proof of Concept in the Healing of Surgical Skin Lesions. Skin Pharmacol. Physiol. 2014, 28, 1–11. [Google Scholar] [CrossRef]
- Sharma, R.K.; Bibi, S.; Chopra, H.; Khan, M.S.; Aggarwal, N.; Singh, I.; Ahmad, S.U.; Hasan, M.M.; Moustafa, M.; Al-Shehri, M.; et al. In Silico and In Vitro Screening Constituents of Eclipta alba Leaf Extract to Reveal Antimicrobial Potential. Evid. Based Complement. Altern. Med. 2022, 2022, 3290790. [Google Scholar] [CrossRef]
- Kaneria, M.J.; Rakholiya, K.D.; Chanda, S.V. Chapter 15—Role of Medicinal Plants and Bioactive Compounds Against Skin Disease–Causing Microbes, with Special Emphasis on Their Mechanisms of Action. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Kon, K., Rai, M., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 2, pp. 255–269. [Google Scholar]
- Reuter, J.; Merfort, I.; Schempp, C.M. Botanicals in Dermatology. Am. J. Clin. Dermatol. 2010, 11, 247–267. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Kim, H.-M.; Lee, H.; Lee, D.-S.; An, H.-J. Anti-inflammatory effects of Eclipta prostrata Linné on house dust mite-induced atopic dermatitis in vivo and in vitro. J. Ethnopharmacol. 2022, 292, 115233. [Google Scholar] [CrossRef]
- Dalal, S.; Rana, S.; Sastry, K.; Kataria, S. Wedelolactone as an Antibacterial agent extracted from Eclipta alba. Internet J. Micro 2009, 7, 1–11. [Google Scholar]
- Lenza, V.A.; Morel, L.J.; Coppede, J.S.; Fernandes, V.C.; Martinez-Rossi, N.M.; Franca, S.C.; Beleboni, R.O.; Pereira, P.S.; Fachin, A. Antimicrobial activities of ethanol extract and coumestans from Eclipta alba (L.) Hassk. (Asteraceae). Lat. Am. J. Pharm. 2009, 28, 863–868. [Google Scholar]
- Syed, S.D.; Deepak, M.; Yogisha, S.; Chandrashekar, A.P.; Muddarachappa, K.A.; D’Souza, P.; Agarwal, A.; Venkataraman, B.V. Trypsin inhibitory effect of wedelolactone and demethylwedelolactone. Phytother. Res. 2003, 17, 420–421. [Google Scholar] [CrossRef]
- Ali, F.; Khan, B.A.; Sultana, S. Wedelolactone mitigates UVB induced oxidative stress, inflammation and early tumor promotion events in murine skin: Plausible role of NFkB pathway. Eur. J. Pharmacol. 2016, 786, 253–264. [Google Scholar] [CrossRef]
- Wölfle, U.; Esser, P.R.; Simon-Haarhaus, B.; Martin, S.F.; Lademann, J.; Schempp, C.M. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med. 2011, 50, 1081–1093. [Google Scholar] [CrossRef]
- Park, C.M.; Song, Y.-S. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. NRP 2013, 7, 423–429. [Google Scholar] [CrossRef]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef]
- Wan, D.; Fu, Y.; Le, Y.; Zhang, P.; Ju, J.; Wang, B.; Zhang, G.; Wang, Z.; Su, H.; Wang, L.; et al. Luteolin-7-glucoside Promotes Human Epidermal Stem Cell Proliferation by Upregulating β-Catenin, c-Myc, and Cyclin Expression. Stem Cells Int. 2019, 2019, 1575480. [Google Scholar] [CrossRef]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- Park, H.J.; Jo, D.S.; Choi, D.S.; Bae, J.-E.; Park, N.Y.; Kim, J.-B.; Chang, J.H.; Shin, J.J.; Cho, D.-H. Ursolic acid inhibits pigmentation by increasing melanosomal autophagy in B16F1 cells. Biochem. Biophys. Res. Commun. 2020, 531, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Soo Lee, Y.; Jin, D.-Q.; Beak, S.-M.; Lee, E.-S.; Kim, J.-A. Inhibition of ultraviolet-A-modulated signaling pathways by asiatic acid and ursolic acid in HaCaT human keratinocytes. Eur. J. Pharmacol. 2003, 476, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Prasad, N.R. Effect of ursolic acid, a triterpenoid antioxidant, on ultraviolet-B radiation-induced cytotoxicity, lipid peroxidation and DNA damage in human lymphocytes. Chem. Biol. Interact. 2008, 176, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Bharali, P.; Konwar, B.K. Mode of Antibacterial Activity of Eclalbasaponin Isolated from Eclipta alba. Appl. Biochem. Biotechnol. 2013, 171, 2003–2019. [Google Scholar] [CrossRef] [PubMed]
- Rasul, M.G. Conventional extraction methods use in medicinal plants, their advantages and disadvantages. Int. J. Basic Sci. Appl. Comput 2018, 2, 10–14. [Google Scholar]
- Bitwell, C.; Sen, I.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Handa, S. An overview of extraction techniques for medicinal and aromatic plants. Extr. Technol. Med. Aromat. Plants 2008, 1, 21–40. [Google Scholar]
- Singh, J. Maceration, percolation and infusion techniques for the extraction of medicinal and aromatic plants. Extr. Technol. Med. Aromat. Plants 2008, 67, 32–35. [Google Scholar]
- Savita, K.; Prakashchandra, K. Optimization of extraction conditions and development of a sensitive HPTLC method for estimation of wedelolactone in different extracts of Eclipta alba. Int. J. Pharmaceut. Sci. Drug Res. 2011, 3, 56–61. [Google Scholar]
- Gharat, N.N.; Rathod, V.K. Response surface methodology for the extraction of wedelolactone from Eclipta alba using aqueous two-phase extraction. Prep. Biochem. Biotechnol. 2020, 50, 827–833. [Google Scholar] [CrossRef]
- Charpe, T.; Rathod, V. Kinetics of ultrasound assisted extraction of wedelolactone from Eclipta alba. Braz. J. Chem. Eng 2016, 33, 1003–1010. [Google Scholar] [CrossRef]
- Lee, M.K.; Ha, N.R.; Yang, H.; Sung, S.H.; Kim, G.H.; Kim, Y.C. Antiproliferative activity of triterpenoids from Eclipta prostrata on hepatic stellate cells. Phytomedicine 2008, 15, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Mors, W.B.; Do Nascimento, M.C.; Parente, J.; Da Silva, M.H.; Melo, P.A.; Suarez-Kurtz, G. Neutralization of lethal and myotoxic activities of South American rattlesnake venom by extracts and constituents of the plant Eclipta prostrata (Asteraceae). Toxicon 1989, 27, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-F.; Huang, W.-Y.; Guo, H.-Y.; Wang, B.R. Potent antioxidative and UVB protective effect of water extract of Eclipta prostrata L. Sci. World J. 2014, 2014, 759039. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Mehta, J.; Sharma, B.; Joshi, P. Phytochemical Screening of Hydroalcoholic Extract and Antimicrobial Activity of Active Principles of Eclipta alba (L.). Asian J. Emerg. Res. 2020, 2, 146–151. [Google Scholar]
- Shi, D.; Ding, H.; Xu, S. Optimization of microwave-assisted extraction of wedelolactone from Eclipta alba using response surface methodology. Front. Chem. Sci. Eng. 2014, 8, 34–42. [Google Scholar] [CrossRef]
- Yi, J.; Wu, J.-G.; Wu, Y.-B.; Lin, X.-H.; Wu, J.-Z. Ultrasound and microwave assisted extraction of luteolin from Eclipta prostrata. Afr. J. Pharm. Pharmacol. 2013, 7, 114–124. [Google Scholar] [CrossRef]
- Fang, X.; Wang, J.; Wang, Y.; Li, X.; Zhou, H.; Zhu, L. Optimization of ultrasonic-assisted extraction of wedelolactone and antioxidant polyphenols from Eclipta prostrate L using response surface methodology. Sep. Purif. Technol. 2014, 138, 55–64. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, S.; Zhang, L.; Dong, H.; Zhang, Y.; Wang, X. Ultra-high-pressure-assisted extraction of wedelolactone and isodemethylwedelolactone from Ecliptae Herba and purification by high-speed counter-current chromatography. Biomed. Chromatogr. 2019, 33, e4497. [Google Scholar] [CrossRef]
- Patil, A.; Sachin, B.; Shinde, D.; Wakte, P. Optimization of sample preparation variables for wedelolactone from Eclipta alba using Box-Behnken experimental design followed by HPLC identification. Ann. Pharm. Fr. 2013, 71, 249–259. [Google Scholar] [CrossRef]
- Shanshol, Z.A.; Alaubydi, M.A.; Sadik, A. The extraction and partial purification of wedelolactone from local plant. Iraqi J. Sci. 2013, 54, 1084–1089. [Google Scholar]
- Lin, X.-H.; Wu, Y.-B.; Lin, S.; Zeng, J.-W.; Zeng, P.-Y.; Wu, J.-Z. Effects of volatile components and ethanolic extract from Eclipta prostrata on proliferation and differentiation of primary osteoblasts. Molecules 2010, 15, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Guo, Y.Y.; Zhou, Q.F.; Zhong, X.K.; Zhu, L.; Piao, J.H.; Chen, J.; Jiang, J.G. Optimization of ultrasonic-assisted extraction of total saponins from Eclipta prostrasta L. using response surface methodology. J. Food Sci. 2012, 77, C975–C982. [Google Scholar] [CrossRef] [PubMed]
- López-Bascón, M.; De Castro, M.L. Soxhlet Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–354. [Google Scholar]
- Goti, D.; Dasgupta, S. A comprehensive review of conventional and non-conventional solvent extraction techniques. J. Pharmacogn. Phytochem. 2023, 12, 202–211. [Google Scholar] [CrossRef]
- Rajesh, Y.; Khan, N.M.; Shaikh, A.R.; Mane, V.S.; Daware, G.; Dabhade, G. Investigation of geranium oil extraction performance by using soxhlet extraction. Mater. Today Proc. 2023, 72, 2610–2617. [Google Scholar] [CrossRef]
- Chong, K.Y.; Stefanova, R.; Zhang, J.; Brooks, M.S.-L. Aqueous two-phase extraction of bioactive compounds from haskap leaves (Lonicera caerulea): Comparison of salt/ethanol and sugar/propanol systems. Sep. Purif. Technol. 2020, 252, 117399. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Da Silva, R.F.; Carneiro, C.N.; de Sousa, C.B.d.C.; Gomez, F.J.; Espino, M.; Boiteux, J.; Fernández, M.d.l.Á.; Silva, M.F.; Dias, F.d.S. Sustainable extraction bioactive compounds procedures in medicinal plants based on the principles of green analytical chemistry: A review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.d.M. Extraction Systems and Analytical Techniques for Food Phenolic Compounds: A Review. Foods 2022, 11, 3671. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Alvi, T.; Asif, Z.; Khan, M.K.I. Clean label extraction of bioactive compounds from food waste through microwave-assisted extraction technique—A review. Food Biosci. 2022, 46, 101580. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Montero, L.; Mendiola, J.A.; Herrero, M.; Ibáñez, E. Novel Extraction Techniques for Bioactive Compounds from Herbs and Spices. In Herbs, Spices and Medicinal Plants: Processing, Health Benefits and Safety; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 95–128. [Google Scholar]
- Mediani, A.; Kamal, N.; Lee, S.Y.; Abas, F.; Farag, M.A. Green extraction methods for isolation of bioactive substances from coffee seed and spent. Sep. Purif. Rev. 2023, 52, 24–42. [Google Scholar] [CrossRef]
- Han, L.-f.; Zhao, J.; Zhang, Y.; Kojo, A.; Liu, E.-w.; Wang, T. Chemical Constituents from Dried Aerial Parts of Eclipta prostrata. Chin. Herb. Med. 2013, 5, 313–316. [Google Scholar] [CrossRef]
- Kumar, S.; Dhanani, T. Development and validation of a rapid high performance liquid chromatography-photodiode array detection method for estimation of a bioactive compound wedelolactone in extracts of Eclipta alba. Braz. J. Pharm. Sci. 2013, 49, 57–63. [Google Scholar] [CrossRef]
- Tambe, R.; Patil, A.; Jain, P.; Sancheti, J.; Somani, G.; Sathaye, S. Assessment of luteolin isolated from Eclipta alba leaves in animal models of epilepsy. Pharm. Biol. 2017, 55, 264–268. [Google Scholar] [CrossRef]
- Ali, A.H. High-Performance Liquid Chromatography (HPLC): A review. Ann. Adv. Chem. 2022, 6, 010–020. [Google Scholar]
- Mendes, F.P.; De Morais, S.; Silva, M.V.; Vieira, I.; Rodrigues, P.; Beliz Ariodiniz, D.; Vieira, Í. Determination of wedelolactone and demethylwedelolactone in Eclipta Alba (L) Hassk By HPLC. Int. J. Pharm. Pharm. Sci. 2014, 6, 403–406. [Google Scholar]
- Kumar, B.A.; Rao, V.K.; Bindu, K.H.; Rohini, M.R.; Shivakumar. Development, validation and application of RP-HPLC method for quantitative estimation of wedelolactone in different accessions and plant parts of Eclipta alba (L.). J. Plant Biochem. Biotechnol. 2022, 31, 788–802. [Google Scholar] [CrossRef]
- Ma, Y.; Ito, Y. Chiral high-speed counter-current chromatography: Future strategies for chiral selector development. Curr. Chromatogr. 2014, 1, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Xu, P.; Qiu, H.; Wen, W.; Zhang, A.; Tong, S. Two-dimensional chromatography in screening of bioactive components from natural products. Phytochem. Anal. 2022, 33, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Coskun, O. Separation techniques: Chromatography. North. Clin. Istanb. 2016, 3, 156. [Google Scholar] [PubMed]
- Ingle, K.P.; Deshmukh, A.G.; Padole, D.A.; Dudhare, M.S.; Moharil, M.P.; Khelurkar, V.C. Phytochemicals: Extraction methods, identification and detection of bioactive compounds from plant extracts. J. Plant Biochem. Biotechnol. 2017, 6, 32–36. [Google Scholar]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Avram, I.; Gatea, F.; Vamanu, E. Functional Compounds from Banana Peel Used to Decrease Oxidative Stress Effects. Processes 2022, 10, 248. [Google Scholar] [CrossRef]
- Jain, A.K.; Jain, A.; Jain, A. Development and characterization of soya phosphatidylcholine complex of coumestans from Eclipta alba for the effective management of hepatotoxicity. Int. J. Pharmacogn. 2019, 6, 6–14. [Google Scholar]
- Mishra, P.; Sharma, R.K.; Chauhan, N. In-vitro Antioxidant, Antibacterial and Phytochemical Properties of Various Solvents Extracts of Eclipta alba Against Isolated ESBL Uropathogens. J. Mountain Res. 2022, 17, 257–267. [Google Scholar] [CrossRef]
- Han, L.; Liu, E.; Kojo, A.; Zhao, J.; Li, W.; Zhang, Y.; Wang, T.; Gao, X. Qualitative and quantitative analysis of Eclipta prostrata L. by LC/MS. Sci. World J. 2015, 2015, 980890. [Google Scholar] [CrossRef]
- Sahoo, S.; Singh, S.; Dash, M.; Jena, S.; Sahoo, A.; Nayak, S.; Kar, B. Electron ionization based detection of volatile constituents of aerial parts of Eclipta prostrata (Linn.) by one dimensional Gas Chromatography and Mass Spectrometry. J. Essent. Oil-Bear. Plants 2020, 23, 559–566. [Google Scholar] [CrossRef]
- Wyson, J.W.; Deventhiran, M.; Saravanan, P.; Anand, D.; Rajarajan, S. Phytochemical analysis of leaf extract of Eclipta prostrata (L.) by GC-MS method. Int. J. Pharm. Sci. Res. 2016, 7, 272. [Google Scholar]
- Chauhan, N.; Singh, D.; Painuli, R. Screening of bioprotective properties and phytochemical analysis of various extracts of Eclipta alba whole plant. Int. J. Pharm. Pharmaceut. Sci. 2012, 4, 554–560. [Google Scholar]
- Anand, D.; Wyson, W.J.; Saravanan, P.; Rajarajan, S. Phytochemical analysis of leaf extract of Eclipta alba (L.) Hassk. by GC-MS method. Int. J. Pharmacogn. Phytochemical. Res. 2014, 6, 562–566. [Google Scholar]
- Thambiratnam, K.; Reduan, S.A.; Tiu, Z.C.; Ahmad, H. Application of Two-Dimensional Materials in Fiber Laser Systems. In Nano-Optics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 227–264. [Google Scholar]
- Pratiwi, R.A.; Nandiyanto, A.B.D. How to read and interpret UV-VIS spectrophotometric results in determining the structure of chemical compounds. Indo. J. Edu. Res. Technol. 2022, 2, 1–20. [Google Scholar] [CrossRef]
- Peng, L.; Gao, X.; Wang, L.; Zhu, A.; Cai, X.; Li, P.; Li, W. Design of experiment techniques for the optimization of chromatographic analysis conditions: A review. Electrophoresis 2022, 43, 1882–1898. [Google Scholar] [CrossRef]
- Urbain, A.; Simões-Pires, C.A. Thin-Layer Chromatography for the Detection and Analysis of Bioactive Natural Products. Phytochem. Lett. 2020, 11, 445–454. [Google Scholar]
- Pramod, S.; Andola, H.; Rawat, M.; Pant, G.; Purohit, V. Fourier transform infrared (FT-IR) spectroscopy in an-overview. Res. J. Med. Plant 2011, 5, 127–135. [Google Scholar]
- Bates, J. Fourier Transform Infrared Spectroscopy: The basic principles and current applications of a rapidly expanding technique are reviewed. Science 1976, 191, 31–37. [Google Scholar] [CrossRef]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman fingerprints of flavonoids—A review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef]
- Mohammed, A.; Abdullah, A. Scanning electron microscopy (SEM): A review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 7–9 November 2018; pp. 7–9. [Google Scholar]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Hatzakis, E. Nuclear magnetic resonance (NMR) spectroscopy in food science: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 189–220. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.H. Spin Dynamics: Basics of Nuclear Magnetic Resonance; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Emwas, A.-H.; Szczepski, K.; Poulson, B.G.; Chandra, K.; McKay, R.T.; Dhahri, M.; Alahmari, F.; Jaremko, L.; Lachowicz, J.I.; Jaremko, M. NMR as a “gold standard” method in drug design and discovery. Molecules 2020, 25, 4597. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.J. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem. Rev. 2009, 30, 19. [Google Scholar]
- Stashenko, E.; Martínez, J.R. Gas Chromatography-Mass Spectrometry. In Advances in Gas Chromatography; IntechOpen: London, UK, 2014; pp. 1–38. [Google Scholar]
- Karasek, F.W.; Clement, R.E. Basic Gas Chromatography-Mass Spectrometry: Principles and Techniques; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]

| Parts of Plant | Types | Compounds | References |
|---|---|---|---|
| Whole plants | Triterpenes | Eclalbasaponin, ursolic acid, α-amyrin, eclalbatin | [7] |
| Flavonoids | Orobol, oroboside | [17,18] | |
| Thiopenes | 5-Hydroxymethyl-terthienyl tiglate, hydroxymethyl-terthienyl acetate, ecliptal hydroxymethyl-terthienyl agelate | [17] | |
| Coumestans | Isodemethylwedelolactone, demethylwedelolactone Wedelolactone, | [17,18] | |
| Aerial parts | Triterpenes | 3-O-(2-O-Acetyl-β-D-glucopyranosyl) oleanolic acid-28-O-(β-d-glucopyranosyl) ester, 3-O-(β-D-glucopyranosyl) oleanolic acid-28-O-(6-O-acetyl-β-D-glucopyranosyl) ester, and 3-O-(6-O-acetyl-β-D-glucopyranosyl) oleanolic acid-28-O-(β-D-glucopyranosyl) ester (three new triterpenes), eclalbasaponin, echinocystic acid, 3-oxo-16α-hydroxy-olean-12-en-28-oic acid | [12,19,20] |
| Saponin | Echinocystic acid-3-O-(6-O-acetyl)-β-D-glucopyranoside | [19] | |
| Flavonoids | Apigenin, luteolin, pratensein, pratensein-7-O-β-D-glucopyranoside, diosmetin, 3′-hydroxybiochanin A, 3′-O-methylorobol | [19,21] | |
| Thiopenes | 5′-Isovaleryloxymethyl-5-(4-isovaleryloxybut-1-ynyl)-2,2′-bithiophene, 5-methoxymethyl-2,2′:5′,2″-terthiophene, 3′-hydroxy-2,2′:5′,2″-terthiophene3′-O-β-D-glucopyranoside | [19,20] | |
| Coumestans | Wedelolactone, demethyl wedelolactone | [19] | |
| Leaves | Coumestans | Wedelolactone, dimethylwedelolactone sulfate | [22] |
| Triterpenes | Eclalbasaponin | [22] | |
| Phenolic | Gallic acid, protocatechuic acid, chlorogenic acid, protocatechualdehyde | [22] | |
| Flavonoids | Luteolin, luteolin-7-O-glucoside, 3-hydroxybiochanin A | [22] | |
| Steroidal alkaloids | Ecliptalbine, verazine, hydroxyverazine, epi-verazine | [23] | |
| Stems | Coumestans | Wedelolactone | [24] |
| Roots | Coumestans | Wedelolactone | [24] |
| Extraction Technique | Part of the Plant | Extraction Solvent | Optimal Extraction Parameters | Phytochemical Content | Identification Procedure | Main Results | References |
|---|---|---|---|---|---|---|---|
| Solid–liquid extraction | Roots | Petroleum ether-water system | 50 g of powder using hydroalcoholic solvent for 48 h | Phytochemicals | Phytochemical screening | Yield (2.5% w/v), phenols, saponin, diterpene | [59] |
| Leaves | Ethanol | Freeze-dried sample, the solid–liquid ratio (SLR) of 1:100, shaking at 150 rpm for 24 h at room temperature | Total phenolics | LC–ESI–QTOF–MS/MS | TPC (20.3 ± 2.4 mg GA/g), wedelolactone, luteolin, other phenolic compounds | [22] | |
| Aerial parts | Ethanol–water system | SLR of 1:33 of 90% ethanol in water for 24 h | Wedelolactone | HPLC | Wedelolactone yield (67.79 ± 2.59%) | [60] | |
| Leaves | Methanol | SLR of 1:40 at 50 °C, 600 rpm for 15 h | Wedelolactone | HPLC | Wedelolactone yield (5.1 mg/g) | [54] | |
| Leaves | Methanol | SLR of 1:80 at 70 °C, 400 rpm for 90 min | Wedelolactone | HPLC | Wedelolactone yield (0.41 mg/g) | [55] | |
| Aerial parts | Water | SLR of 1:2 at 100 °C for 20 min | Total phenolics | HPLC | TPC (176.45 ± 11.56 mg GAE/g sample), CGA (1.75 ± 0.01 mg/g sample) | [58] | |
| Whole plants | Methanol | SLR of 1:5 for 3 h | Wedelolactone | HPTLC | Wedelolactone yield (0.48% w/w) | [53] | |
| Leaves | Ethanol | Freeze-dried sample, SLR of 1:100, the flow rate at 0.1–0.2 mL/min using disposal syringe | Total phenolics | LC–ESI–QTOF–MS/MS | TPC (20.3 ± 0.9 mg GA/g), wedelolactone, luteolin, other phenolic compounds | [22] | |
| Reflux extraction | Aerial parts | Ethanol–water system | SLR of 1:150 of 80% ethanol in water, containing 1.8 mL hydrochloric acid at 80 ± 2 °C for 60 min | Luteolin | HPLC | Luteolin (0.689 mg/g) | [61] |
| Aerial parts | Ethanol–water system | SLR of 1:40 of 70% ethanol in water for 90 min | Total phenolics, wedelolactone | HPLC | Wedelolactone yield (3.89 ± 0.11 mg/g), TPC (18.20 ± 0.51 mg/g) | [62] | |
| Aerial parts | Ethanol–water system | SLR of 1:75 of 80% ethanol in water at 80 ± 2 °C for 120 min | Luteolin | HPLC | Luteolin (0.676 mg/g) | [61] | |
| Aerial parts | Ethanol–water system | SLR of 1:33 of 90% ethanol in water for 5 h | Wedelolactone | HPLC | Wedelolactone yield (62.93 ± 0.82%) | [60] | |
| Aerial parts | Methanol–water system | SLR of 1:20 using 80% methanol in water at 85 °C for 60 min | Wedelolactone | HPLC | Wedelolactone yield (2.8 mg/g) isodemethylwedelolactone yield (0.8 mg/g) | [63] | |
| Soxhlet assisted extraction | Whole plants | Methanol | SLR of 1:30 for 24 h | Wedelolactone | HPLC | Wedelolactone (13.71 ± 0.82 mg/100 g E. alba) | [64] |
| Aerial parts | Methanol–water system | SLR of 1:100 of 70% methanol for 180 min | Total phenolics, wedelolactone | HPLC | Wedelolactone yield (4.01 ± 0.08 mg/g), TPC (13.79 ± 0.40 mg/g) | [62] | |
| Leaves | Ethanol | Freeze–dried sample, SLR of 1:100 (w/v), 10 h at 80 °C | Total phenolics | LC–ESI–QTOF–MS/MS, HPTLC | TPC (30.7 ± 1.1 mg GAE/g), wedelolactone, luteolin, other phenolic compounds | [22] | |
| Aerial parts | Methanol, hexane, ethanol, water | SLR of 1:9 of solvent at 50 °C for 36 h | Wedelolactone | TLC, HPLC | Best yield (76% with methanol) | [65] | |
| Leaves | Methanol | SLR of 1:150 at 90 °C for 6 h | Wedelolactone | HPLC | Wedelolactone yield (5.05 mg/g) | [54] | |
| Leaves | Methanol | SLR 1:100 at 90 °C for 6 h | Wedelolactone | HPLC | Wedelolactone yield (0.7 mg/g) | [55] | |
| Whole plants | Methanol | SLR of 1:6 for 12 h | Wedelolactone | HPTLC | Wedelolactone yield (0.48% w/w) | [53] | |
| Liquid–liquid extraction (Aqueous two-phase extraction) | Leaves | Polyethylene glycol, sodium citrate | SLR of 1:40 using PEG 6000,18% (w/v), PEG concentration, Sodium citrate salt concentration, 17.96% (w/v), and pH 7 for 2 h | Wedelolactone | HPLC | Wedelolactone yield (6.52 mg/g) | [54] |
| Hydrodistillation | Aerial parts | Water | SLR of 1:10, soaked for 12 h, distilled for 3 h | Volatile compounds | GC–MS | Heptadecane, n-hexadecanoic acid, pentadecane, and other 52 volatile compounds | [66] |
| Supercritical carbon dioxide extraction | Whole plants | Liquid carbon dioxide | 25 MPa using cosolvent of 9.44% at 56 °C for 60 min | Wedelolactone | HPLC | Wedelolactone (15.37 ± 0.63 mg/100 g E. alba) | [64] |
| Whole plants | Liquid carbon dioxide | CO2 flow rate—23.98 mL/min, Pressure—4000–6000 psi, at 40–50 °C | Wedelolactone | HPTLC | Wedelolactone yield (0.002–0.013% w/w) | [53] | |
| Ultrasound-assisted extraction | Aerial parts | Ethanol–water system | SLR of 1:50 using 48% of ethanol in water temperature at 40 °C and 90 W for 11 min | Total phenolics, wedelolactone | HPLC | Wedelolactone yield (3.90 ± 0.10 mg/g), TPC (22.57 ± 0.90 mg/g) | [62] |
| Aerial parts | Ethanol–water system | SLR of 1:33 of 90% ethanol in water using frequency of 40 kHz for 26.5 min | Wedelolactone | HPLC | Wedelolactone yield (63.16 ± 0.10%) | [60] | |
| Leaves | Methanol | SLR of 1:60 at 50 °C using 60% duty cycle for 45 min | Wedelolactone | HPLC | Wedelolactone yield (0.62 mg/g) | [55] | |
| Whole plants | Methanol | SLR of 1:5, for 45 min | Wedelolactone | HPTLC | Wedelolactone yield (0.36% w/w) | [53] | |
| Leaves | Ethanol–water system | SLR of 1:14 using 70% of ethanol in water at 70 °C for 3 h | Total saponins | - | Saponins content (2.096%) | [67] | |
| Microwave-assisted extraction | Aerial parts | Ethanol–water system | SLR of 1:33 of 90% ethanol in water at 208 W for 26.5 min | Wedelolactone | HPLC | Wedelolactone yield (82.67 ± 0.16%) | [60] |
| Whole plants | Methanol | SLR of 1:5, 100 W for 15 min | Wedelolactone | HPTLC | Wedelolactone yield (0.27% w/w) | [53] | |
| Ultrahigh pressure-assisted extraction | Aerial parts | Methanol–water system | SLR of 1:20 using 80% methanol in water at 100 MPa pressure for 3 min | Wedelolactone | HPLC | Wedelolactone yield (2.4 mg/g) isodemethylwedelolactone yield (0.7 mg/g) | [63] |
| Maceration-percolation | Whole plants | Methanol | SLR of 100 g/500 mL for 24 h, followed by percolation until the percolate was colorless | Wedelolactone | HPTLC | Wedelolactone yield (0.38% w/w) | [53] |
| Ultrasound and microwave-assisted extraction | Aerial parts | Ethanol–water system | SLR of sample, 80% ethanol in water, and hydrochloric acid:1: 50: 0.3 (w/v/v), microwave power: 40 W, for 9 min (3 min × 3 cycles) | Luteolin | RP–HPLC | Luteolin (0.690 mg/g) | [61] |
| Part of the Plant | Procedure | Operating Parameters | Targeted Compounds | References | ||
|---|---|---|---|---|---|---|
| Stationary Phase | Detector | Mobile Phases and Conditions | ||||
| Whole plants | HPLC | C18 column | PDA detector at 352 nm | Mobile phase (MP)—methanol: water: acetic acid, 0.6 mL/min, the injection volume (IV)—10 µL | Wedelolactone | [83] |
| CC, HPTLC | silica gel CC (60–120 mesh) | Eluted with toluene, HPTLC—a pre-activated silica gel HPTLC plate, mobile phase toluene: ethyl acetate | Wedelolactone | [53] | ||
| HPLC | C-18 column | UV detector at 351 nm | MP—methanol—acetic acid (0.5%) buffer, 0.5 mL/min at 40 °C | Wedelolactone | [64] | |
| Aerial parts | HSCCC | Separation column | UV detector at 254 nm | Petroleum ether–ethyl acetate–methanol–water (3:7:5:5), 20 mL/min for stationary phase (SP), 1.5 mL/min for MP | Wedelolactone, isodemethylwedelolactone, and luteolin | [63] |
| HPLC | A Thermo ODS2-Hypersil column | PDA detector at 350 nm | MP—acetonitrile (A) and 0.1% formic acid aqueous solution (B), 1 mL/min, IV—10 µL | Wedelolactone | [62] | |
| HPLC | C18 reversed-phase column | PDA detector at 352 nm | MP—methanol: 0.4% phosphoric acid, 1 mL/min, IV—10 μL at 30 °C | Luteolin | [61] | |
| HPLC | C18 column | UV detector at 249 nm | MP—0.5% aqueous glacial acetic acid; 1 mL/min at 30 °C, IV—20 µL | Wedelolactone | [60] | |
| CC, TLC | Silica gel CC | Methanol and chloroform | Wedelolactone | [65] | ||
| CC, HPLC | 1. Sephadex LH-20 CC; 2. Silica gel CC; 3. ODS; 4. Preparative HPLC | G1365D Multiple Wavelength Detector | 1. Dichloromethane–water; 2. Methanol; 3. Methanol; 4. Methanol | Ecliptasaponin A, 7-O-methylorobol-4′-O-β-D-glucopyranoside, 3-oxo-16α-hydroxy-olean-12-en-28-oic acid, 3′-hydroxybiochanin A, echinocystic acid, echinocystic acid 28-O-β-D-glucopyranoside, eclalbasaponin I and IV | [82] | |
| CC, TLC | 1.macroporous resin CC; 2. Silica gel CC for fractionation; 3,4. Sephadex LH-20 CC; 5. ODS CC | 1. Ethanol–water; 2. Chloroform–methanol–water; 3. Methanol; 4. Chloroform–methanol; 5. Methanol–water | Wedelolactone, Eclalbasaponin I and luteolin, Luteolin-7-O-glucoside | [10] | ||
| CC, HPLC | 1. D-101 macroporous resin CC; 2. Silica gel CC; 3. Sephadex LH-20 CC; 4. RP-18 CC for subfractions; 5. Silica gel CC; 6. Semipreparative HPLC—Agilent SB-C18 column, ODS-A column for isolation and purification | 1. Ethanol–water; 2. Petroleum ether–ethyl acetate; 3. Dichloromethane–methanol; 4. Methanol–water; 5. Dichloromethane–methanol; 6. 65% Acetonitrile–water | Ecliprostins A–C | [11] | ||
| HPLC | 5C18-AR-II analytical column | UV detector at 320 nm | MP—10 mm KH2PO4, pH 4.0 and acetonitrile: methanol: water, 0.8 mL/min, IV—20 µL | Chlorogenic acid | [58] | |
| CC, HPLC | 1. Silica gel CC; 2. ODS CC; 3. Sephadex LH-20 CC; 4. Semipreparative HPLC | 1. Dichloromethane–methanol–water; 2.,3., and 4. Methanol–water. | Three new triterpenes | [12] | ||
| Leaves | HPLC | C-18 column | DAD detector at 351 nm, | MP—Methanol–water, 0.3 mL/min, at 30 ± 2 °C | Wedelolactone | [54] |
| HPLC | C18 column | UV detector at 268 nm | MP—acetonitrile: 1% formic acid, 1 mL/min at 20 °C | Luteolin | [84] | |
| HPLC | C-18 column | DAD detector at 351 nm, | MP—Methanol–water acidified with 0.1% acetic acid, 1 mL/min | Wedelolactone | [55] | |
| Part of the Plant | Identification and Characterization | Operating Parameters | Bioactive Compounds | References |
|---|---|---|---|---|
| Whole plants | TLC | SP—silica gel 60, MP—chloroform–methanol, detecting agent—iron (III) chloride | Wedelolactone | [94] |
| GC–MS | A GC–MS with an elite column, IV—2 μL with temperature range from 40 to 280 °C, carrier gas—helium, 1 mL/min | Various phytochemical compounds | [95] | |
| Aerial parts | NMR | 1H–NMR at 400 MHz and 13C–NMR at 101 MHz using dimethyl sulfoxide as a solvent | Luteolin, luteolin-7-O-glucoside, wedelolactone, and eclalbasaponin I, | [10] |
| LC–MS | UHPLC–Q–TOF–MS UPLC—HSS T3 column, MP—acetonitrile and water (containing 0.1% formic acid), IV—5 μL, 0.3 mL/min at 30 °C. LC–QQQ–MS HPLC—C18 column, MP—acetonitrile and water (containing 0.1% formic acid), IV—1 μL, 0.5 mL/min at 35 °C. | Luteolin-7-O-β-D-glucopyranoside, luteolin, apigenin, ecliptasaponin A, C, and I, | [96] | |
| 28-O-β-D-glucopyranoside, echinocystic acid, and 3-oxo-16α-hydroxy-olean-12-en-28-oic acid | ||||
| GC–MS | HP–5 MS capillary column (5% diphenyl, 95% dimethyl polysiloxane) at 70 eV, 50–600 amu of mass scan range, 1 mL/min, IV—0.1 μL with temperature range from 60 °C to 215 °C for 45 min | Fifty-nine volatile compounds, including α-pinene, caryophyllene, α-humulene, α-pinene, camphene, allo-aromadendrene, α-amorphene | [97] | |
| GC–MS | GC–HP–5MS capillary column at 70 eV, maximum temperature at 350 °C, helium as carrier gas, 1 mL/min, IV—1 μL | Heptadecane, n-hexadecanoic acid, pentadecane, and other 52 volatile compounds | [66] | |
| LC–MS, NMR | ESI–MS analyses—Triple Quad LC–MS instrument, HR–ESIMS analysis—Q–TOF mass spectrometer, NMR—a Bruker Avance DRX600 spectrometer, 2D 1H-1H COSY and HMBC NMR | Ecliprostins A–C | [11] | |
| FT–IR, LC–MS, NMR, UV | Varian 640-IR FT–IR spectrophotometer, Negative-ion HRESI–TOF–MS, Bruker 500 MHz NMR at 500 and 125 MHz, using tetramethylsilane (TMS), UV–Vis spectrometer | Ecliptasaponin A, 7-O-methylorobol-4′-O-β-D-glucopyranoside, 3-oxo-16α-hydroxy-olean-12-en-28-oic acid, 3′-hydroxybiochanin A, echinocystic acid, echinocystic acid 28-O-β-D-glucopyranoside, eclalbasaponin I and IV | [82] | |
| NMR, MS, IR, UV, GC–MS | 1D, 2D NMR spectra and TOF LC–MS, FT–IR spectrometer, UV–Vis spectrophotometer, GC apparatus using an L-Chirasil-Val column | Three new triterpenes | [12] | |
| Leaves | GC–MS | Elite-5MS (5% diphenyl/95% dimethyl polysiloxane), a capillary column, at 70 eV, IV—2 μL with temperature range from 110 to 280 °C, and the carrier gas—helium, 1 mL/min for 36 min | Methyl ester, methyl, methyl ester, pentadecanic aciddiethyl phthalate, glycine, c-sitosterol, eicosyl ester, and 10-octadeconic acid | [98] |
| GC–MS | REX column, Temperature range 70–300 °C, helium—carrier gas, IV—2 μL | Glycine, hydrazine carboxyamide, garbamic acid, naphthoquinone, and other substances | [99] | |
| LC–MS | LC–ESI–QTOF–MS/MS—C18, 500 µL/min, IV—5 µL, MP—water + 0.1% formic acid and acetonitrile + 0.1% formic acid at 35 °C | Phenolic acids (gallic acid, protocatechuic acid, etc.), Flavonoids (luteolin, etc.), wedelolactone, triterpenoids, and phenolic aldehyde (protocatechualdehyde) | [22] | |
| HPTLC | Silica 60F 254 on aluminum sheet, 10 s/µL of syringe injection rate; IV—2 µL for plant extract and 1 µL for standards; MP—toluene–ethyl acetate–formic acid for 5 min at room temperature | Wedelolactone, luteolin, chlorophyll | [22] | |
| TLC, Q–TOF–MS, UV, NMR, FT–IR | Silica gel 60 F254 precoated plates, MP—ethyl acetate–toluene–formic acid–methanol, the spraying agent—NP-PEG, Micromass, Q–TOF–MS, UV–Vis spectrophotometer, Bruker AV-500 NMR spectrometer using DMSO as a solvent | Luteolin | [84] | |
| FT–IR | BRUKER IFS 66 model FT–IR spectrometer | Wedelolactone | [24] | |
| GC–MS | Elite-5MS (5% diphenyl/95% dimethyl polysiloxane), a capillary column, at 70 eV, IV—2 μL with temperature range from 110 to 280 °C, and the carrier gas—helium, 1 mL/min for 36 min | 2-ethyl-2-methyl, 1-Heptatriacotanol, butyl octyl ester, Dodecanoic acid, Oleic acid, eicosyl ester, 9,19- Cyclocholestan-3-ol-7-one,4a-dimethly-[20R], 10- Octadecenoic acid, c-Sitosterol, methyl ester, 1,2 Benzenedicarboxylic acid, 10 methyl, methyl ester, Tridecanol | [100] | |
| SEM–EDS | 20 kV, high vacuum mode | Elements including sodium, magnesium, potassium, calcium, copper, zinc, and iron | [24] | |
| Stems | FT–IR | BRUKER IFS 66 model FT–IR spectrometer | Wedelolactone | [24] |
| SEM–EDS | 20 kV, high vacuum mode | Elements including sodium, magnesium, potassium, calcium, copper, zinc, and iron | [24] | |
| Roots | SEM–EDS | 20 kV, high vacuum mode | Elements including sodium, magnesium, potassium, calcium, copper, zinc, and iron | [24] |
| FT–IR | BRUKER IFS 66 model FT–IR spectrometer | Wedelolactone | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myo, H.; Liana, D.; Phanumartwiwath, A. Unlocking Therapeutic Potential: Comprehensive Extraction, Profiling, and Pharmacological Evaluation of Bioactive Compounds from Eclipta alba (L.) Hassk. for Dermatological Applications. Plants 2024, 13, 33. https://doi.org/10.3390/plants13010033
Myo H, Liana D, Phanumartwiwath A. Unlocking Therapeutic Potential: Comprehensive Extraction, Profiling, and Pharmacological Evaluation of Bioactive Compounds from Eclipta alba (L.) Hassk. for Dermatological Applications. Plants. 2024; 13(1):33. https://doi.org/10.3390/plants13010033
Chicago/Turabian StyleMyo, Hla, Desy Liana, and Anuchit Phanumartwiwath. 2024. "Unlocking Therapeutic Potential: Comprehensive Extraction, Profiling, and Pharmacological Evaluation of Bioactive Compounds from Eclipta alba (L.) Hassk. for Dermatological Applications" Plants 13, no. 1: 33. https://doi.org/10.3390/plants13010033






