Combination of Thymoquinone and Intermittent Fasting as a Treatment for Breast Cancer Implanted in Mice
Abstract
:1. Introduction
2. Results
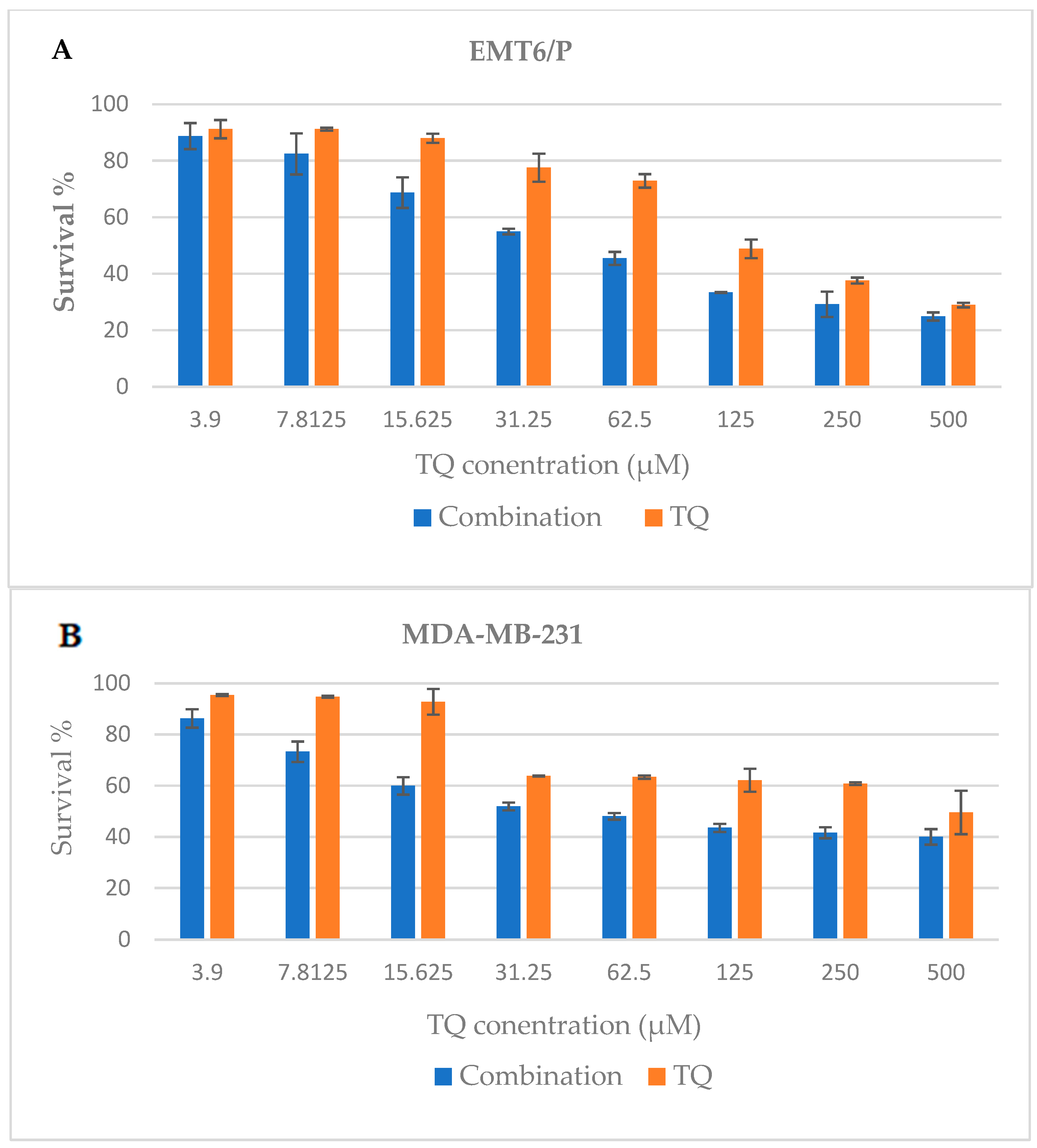
2.1. Anti-Proliferative Assay
2.2. Antitumor Effects of Single TQ, IF, and Their Combination on EMT6/P Cells Implanted in Mice
2.3. Effect of Single TQ, IF, and Their Combination on the Glucose and β-Hydroxybutyrate Levels
2.4. Effect of Single TQ, IF, and Their Combination on the Serum Levels of IGF-1
2.5. Effect of Single TQ, IF, and Their Combination on the Serum Leptin Concentration
2.6. Effects of Single TQ, IF, and Their Combination on the Serum Levels of AST, ALT and Creatinine
3. Discussion
4. Materials and Methods
4.1. In Vitro Experiments
4.1.1. Cell Culture
4.1.2. Preparation of the Thymoquinone Working Solutions and Glucose/Serum-Restricted Tissue Culture Media
4.1.3. Anti-Proliferative Assay
4.2. In Vivo Experiments
4.2.1. Establishing the TQ Dose for the In Vivo Experiment
4.2.2. Tumor Inoculation and Antitumor Activity Assay
4.2.3. Evaluation of the Serum Levels of Glucose and β-Hydroxybutyrate
4.2.4. Measuring Serum IGF-1
4.2.5. Measuring the Serum Leptin Concentration
4.2.6. Evaluation of Liver and Kidney Functions in the Treated Mice
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, H.-D.; Yu, Y.-W.; Li, N.; Chen, W.-Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A.; Lindsey-Boltz, L.A.; Gaddameedhi, S.; Selby, C.P.; Ye, R.; Chiou, Y.-Y.; Kemp, M.G.; Hu, J.; Lee, J.H.; Ozturk, N. Circadian clock, cancer, and chemotherapy. Biochemistry 2015, 54, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Mahalanobish, S.; Saha, S.; Ghosh, S.; Sil, P.C. Natural products: An upcoming therapeutic approach to cancer. Food Chem. Toxicol. 2019, 128, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Erustes, A.G.; Costa, A.J.; Nascimento, A.C.; Bincoletto, C.; Ureshino, R.P.; Pereira, G.J.S.; Smaili, S.S. Autophagy and intermittent fasting: The connection for cancer therapy? Clinics 2018, 73, e814s. [Google Scholar] [CrossRef] [PubMed]
- Clifton, K.K.; Ma, C.X.; Fontana, L.; Peterson, L.L. Intermittent fasting in the prevention and treatment of cancer. CA Cancer J. Clin. 2021, 71, 527–546. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, N.; Kim, K.-w.; Lee, M.; Lee, Y.-h.; Lee, Y.-h.; Kang, E.S.; Cha, B.-S.; Lee, B.-W. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef]
- Harvey, A.E.; Lashinger, L.M.; Hays, D.; Harrison, L.M.; Lewis, K.; Fischer, S.M.; Hursting, S.D. Calorie restriction decreases murine and human pancreatic tumor cell growth, nuclear factor-κB activation, and inflammation-related gene expression in an insulin-like growth factor-1—Dependent manner. PLoS ONE 2014, 9, e94151. [Google Scholar] [CrossRef]
- Lee, J.S.; Yost, S.E.; Yuan, Y. Neoadjuvant treatment for triple negative breast cancer: Recent progresses and challenges. Cancers 2020, 12, 1404. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.N.; Prajapati, C.P.; Gore, P.R.; Patil, C.R.; Mahajan, U.B.; Sharma, C.; Talla, S.P.; Ojha, S.K. Therapeutic potential and pharmaceutical development of thymoquinone: A multitargeted molecule of natural origin. Front. Pharmacol. 2017, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Sutton, K.M.; Greenshields, A.L.; Hoskin, D.W. Thymoquinone, a bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr. Cancer 2014, 66, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, M.N.; Mehdiabady, E.M.; Iranpour, F.G.; Bahramian, H. Effect of thymoquinone on P53 gene expression and consequence apoptosis in breast cancer cell line. Int. J. Prev. Med. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, İ.H.; Azzawri, A.A.; Duran, T. Thymoquinone induces apoptosis via targeting the Bax/BAD and Bcl-2 pathway in breast cancer cells. Dicle Tıp Derg. 2019, 46, 411–417. [Google Scholar] [CrossRef]
- Alobaedi, O.H.; Talib, W.H.; Basheti, I.A. Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac. J. Trop. Med. 2017, 10, 400–408. [Google Scholar] [CrossRef]
- Banerjee, S.; Padhye, S.; Azmi, A.; Wang, Z.; Philip, P.A.; Kucuk, O.; Sarkar, F.H.; Mohammad, R.M. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr. Cancer 2010, 62, 938–946. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M.; Fu, J. Epigenetic role of thymoquinone: Impact on cellular mechanism and cancer therapeutics. Drug Discov. Today 2019, 24, 2315–2322. [Google Scholar] [CrossRef]
- Mostofa, A.G.M.; Hossain, M.K.; Basak, D.; Bin Sayeed, M.S. Thymoquinone as a potential adjuvant therapy for cancer treatment: Evidence from preclinical studies. Front. Pharmacol. 2017, 8, 295. [Google Scholar] [CrossRef]
- Woo, C.C.; Hsu, A.; Kumar, A.P.; Sethi, G.; Tan, K.H.B. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: The role of p38 MAPK and ROS. PLoS ONE 2013, 8, e75356. [Google Scholar] [CrossRef]
- Kabil, N.; Bayraktar, R.; Kahraman, N.; Mokhlis, H.A.; Calin, G.A.; Lopez-Berestein, G.; Ozpolat, B. Thymoquinone inhibits cell proliferation, migration, and invasion by regulating the elongation factor 2 kinase (eEF-2K) signaling axis in triple-negative breast cancer. Breast Cancer Res. Treat. 2018, 171, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Ahmed, S.; Jan, B.; Bender, O.; Al Hagbani, T.; Alqarni, A.; Anwar, S. Drugs repurposed: An advanced step towards the treatment of breast cancer and associated challenges. Biomed. Pharmacother. 2022, 145, 112375. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, M.; Upadhyay, P.; Sarker, S.; Basu, A.; Das, S.; Ghosh, A.; Ghosh, S.; Adhikary, A. Combinatorial therapy of Thymoquinone and Emodin synergistically enhances apoptosis, attenuates cell migration and reduces stemness efficiently in breast cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2020, 1864, 129695. [Google Scholar] [CrossRef] [PubMed]
- Odeh, L.H.; Talib, W.H.; Basheti, I.A. Synergistic effect of thymoquinone and melatonin against breast cancer implanted in mice. J. Cancer Res. Ther. 2018, 14, 324. [Google Scholar] [CrossRef]
- Fu, C.; Lu, Y.; Zhang, Y.; Yu, M.; Ma, S.; Lyu, S. Intermittent fasting suppressed splenic CD205+ G-MDSC accumulation in a murine breast cancer model by attenuating cell trafficking and inducing apoptosis. Food Sci. Nutr. 2021, 9, 5517–5526. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Krueger, J.; Chow, A.; Xu, P.; Man, S.; Sundaravadanam, Y.; Miller, J.K.; Krzyzanowski, P.M.; Kerbel, R.S. Impact of chemical-induced mutational load increase on immune checkpoint therapy in poorly responsive murine tumors. Mol. Cancer Ther. 2018, 17, 869–882. [Google Scholar] [CrossRef]
- Chernicky, C.; Tan, H.; Yi, L.; de Mola, J.L.; Ilan, J. Treatment of murine breast cancer cells with antisense RNA to the type I insulin-like growth factor receptor decreases the level of plasminogen activator transcripts, inhibits cell growth in vitro, and reduces tumorigenesis in vivo. Mol. Pathol. 2002, 55, 102. [Google Scholar] [CrossRef]
- Cincotta, J. Intermittent Fasting: A Simple, yet Powerful Technique to Promote Health and Wellness. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4516560/ (accessed on 12 November 2023).
- Tembhurne, S.; Feroz, S.; More, B.; Sakarkar, D. A review on therapeutic potential of Nigella sativa (kalonji) seeds. J. Med. Plants Res. 2014, 8, 167–177. [Google Scholar]
- Tahtouh, R.; Wardi, L.; Sarkis, R.; Hachem, R.; Raad, I.; El Zein, N.; Hilal, G. Glucose restriction reverses the Warburg effect and modulates PKM2 and mTOR expression in breast cancer cell lines. Cell. Mol. Biol. 2019, 65, 26–33. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Alsalamat, H.A.; Aleidi, S.M.; Bashatwah, R.M. Glucose deprivation enhances the antiproliferative effects of oral hypoglycemic biguanides in different molecular subtypes of breast cancer: An in vitro study. Acta Pharm. 2018, 68, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Raut, G.K.; Chakrabarti, M.; Pamarthy, D.; Bhadra, M.P. Glucose starvation-induced oxidative stress causes mitochondrial dysfunction and apoptosis via Prohibitin 1 upregulation in human breast cancer cells. Free Radic. Biol. Med. 2019, 145, 428–441. [Google Scholar] [CrossRef]
- de Groot, S.; Vreeswijk, M.P.; Welters, M.J.; Gravesteijn, G.; Boei, J.J.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, J.J.; Nortier, J.W. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer 2015, 15, 652. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Cheng, Y.; Su, Q.; Ai, W.; Gong, L.; Wang, Y.; Li, L.; Ma, Z.; Pan, Q.; Qiao, Z. Effects of intermittent fasting on liver physiology and metabolism in mice. Exp. Ther. Med. 2021, 22, 950. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Antonelli, J.; Lloyd, J.; Masko, E.; Poulton, S.; Phillips, T.; Pollak, M.; Freedland, S. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate Cancer Prostatic Dis. 2010, 13, 350–355. [Google Scholar] [CrossRef]
- Caffa, I.; D’Agostino, V.; Damonte, P.; Soncini, D.; Cea, M.; Monacelli, F.; Odetti, P.; Ballestrero, A.; Provenzani, A.; Longo, V.D. Fasting potentiates the anticancer activity of tyrosine kinase inhibitors by strengthening MAPK signaling inhibition. Oncotarget 2015, 6, 11820. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhu, X.; Wang, H.; Wang, F.; Guan, W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: A systematic review and meta-analysis. PLoS ONE 2014, 9, e115147. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous III, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the metabolic switch: Understanding and applying the health benefits of fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Buettner, G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem.-Anti-Cancer Agents 2011, 11, 341–346. [Google Scholar] [CrossRef]
- Rossi, A.P.; Woolf, E.C.; Brooks, K.S.; Fairres, M.J.; Scheck, A.C. The ketone body β-hydroxybutyrate increases radiosensitivity in glioma cell lines in vitro. Cancer Res. 2015, 75 (Suppl. S15), 3346. [Google Scholar] [CrossRef]
- Silva-Nichols, H.B.; Woolf, E.C.; Deleyrolle, L.P.; Reynolds, B.A.; Scheck, A.C. ATPS-77 the ketone body β-hydroxybutyrate radiosensitizes glioblastoma multiforme stem cells. Neuro Oncol. 2015, 17 (Suppl. S5), v35. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, B. Intermittent Fasting and Metabolic Switching: A Brief Overview. Biomed. Pharmacol. J. 2020, 13, 1555–1562. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A signaling metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, M.M.; Yuen, J.S.; Protheroe, A.S.; Pollak, M.; Macaulay, V.M. The type 1 insulin-like growth factor receptor pathway. Clin. Cancer Res. 2008, 14, 6364–6370. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.M.; Lavigne, J.A.; Chandramouli, G.V.; Lui, H.; Barrett, J.C.; Hursting, S.D. Dose-dependent effects of calorie restriction on gene expression, metabolism, and tumor progression are partially mediated by insulin-like growth factor-1. Cancer Med. 2012, 1, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Caffa, I.; Spagnolo, V.; Vernieri, C.; Valdemarin, F.; Becherini, P.; Wei, M.; Brandhorst, S.; Zucal, C.; Driehuis, E.; Ferrando, L. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020, 583, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Newman, G.; Gonzalez-Perez, R.R. Leptin–cytokine crosstalk in breast cancer. Mol. Cell. Endocrinol. 2014, 382, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Huang, Y.; Wang, L.; Wang, H.; Pang, X.; Li, K.; Dang, W.; Tang, H.; Wei, L.; Su, M. Leptin promotes migration and invasion of breast cancer cells by stimulating IL-8 production in M2 macrophages. Oncotarget 2016, 7, 65441. [Google Scholar] [CrossRef]
- Mahbouli, S.; Der Vartanian, A.; Ortega, S.; Rougé, S.; Vasson, M.P.; Rossary, A. Leptin induces ROS via NOX5 in healthy and neoplastic mammary epithelial cells. Oncol. Rep. 2017, 38, 3254–3264. [Google Scholar] [CrossRef]
- Giordano, C.; Chemi, F.; Panza, S.; Barone, I.; Bonofiglio, D.; Lanzino, M.; Cordella, A.; Campana, A.; Hashim, A.; Rizza, P. Leptin as a mediator of tumor-stromal interactions promotes breast cancer stem cell activity. Oncotarget 2016, 7, 1262. [Google Scholar] [CrossRef]
- Zheng, Q.; Dunlap, S.M.; Zhu, J.; Downs-Kelly, E.; Rich, J.; Hursting, S.D.; Berger, N.A.; Reizes, O. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr.-Relat. Cancer 2011, 18, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Levêque, D.; Becker, G. The role of therapeutic drug monitoring in the management of safety of anticancer agents: A focus on 3 cytotoxics. Expert Opin. Drug Saf. 2019, 18, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Martella, R.; Ravera, S.; Marini, C.; Capitanio, S.; Orengo, A.; Emionite, L.; Lavarello, C.; Amaro, A.; Petretto, A. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget 2015, 6, 11806. [Google Scholar] [CrossRef] [PubMed]







| Cell Line | EMT-6 IC50 (μM) ± SEM | MDA-MB-231 IC50 (μM) ± SEM | T47D IC50 (μM) ± SEM | Vero IC50 (μM) ± SEM |
|---|---|---|---|---|
| Single TQ | 131 ± 5.0 | 100 ± 5.5 | 106 ± 5.7 | 497.7 ± 1.6 |
| Combination | 55 ± 4.0 | 59 ± 3.2 | 48.65 ± 0.29 | 499.35 ± 8.9 |
| Av. Initial Tumor Size (mm3) ± SEM | Av. Final Tumor Size (mm3) ± SEM | % Change in Tumor Size | % of Mice with No Detectable Tumor | Average Tumor Weight (g) | |
|---|---|---|---|---|---|
| TQ | 327.7 ± 24.7 | 204 ± 12.6 | −37.74 | 42.85714 | 0.362 ± 0.05 |
| IF | 391.4 ± 20 | 215.24 ± 22.4 | −45.01 | 42.85714 | 0.316 ± 0.12 |
| Combination of TQ and IF | 363 ± 17 | 153.28 ± 5.23 | −57.78 | 42.85714 | 0.298 ± 0.088 |
| Control | 306.6 ± 17.5 | 578.04 ± 62.43 | 88.54 | 0 | 0.45 ± 0.63 |
| Treatment Groups | ALT (IU/L) | AST (IU/L) | Cr (µmol/L) |
|---|---|---|---|
| TQ | 37.774 ± 4.44 | 82.21 ± 9.44 | 14.82 ± 0.0866 |
| IF | 38.32 ± 2.77 | 117.76 ± 4.44 | 13.26 ± 0.0866 |
| Combination | 45.55 ± 2.22 | 107.21 ± 10.55 | 8.84 ± 0.0866 |
| Control | 75.33 ± 1.29 | 101.65 ± 10.55 | 15.91 ± 0.0866 |
| Healthy mice | 47.77 ± 4.713 | 117.76 ± 4.44 | 8.84 ± 0.0866 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haif, S.K.; Al Kury, L.T.; Talib, W.H. Combination of Thymoquinone and Intermittent Fasting as a Treatment for Breast Cancer Implanted in Mice. Plants 2024, 13, 35. https://doi.org/10.3390/plants13010035
Haif SK, Al Kury LT, Talib WH. Combination of Thymoquinone and Intermittent Fasting as a Treatment for Breast Cancer Implanted in Mice. Plants. 2024; 13(1):35. https://doi.org/10.3390/plants13010035
Chicago/Turabian StyleHaif, Shatha Khaled, Lina T. Al Kury, and Wamidh H. Talib. 2024. "Combination of Thymoquinone and Intermittent Fasting as a Treatment for Breast Cancer Implanted in Mice" Plants 13, no. 1: 35. https://doi.org/10.3390/plants13010035







