Ion Changes and Signaling under Salt Stress in Wheat and Other Important Crops
Abstract
:1. Introduction
2. Na+ Uptake and Accumulation under Salt Stress
2.1. The Hydraulic Conductivity (Lp) Affects Ion Transport
2.2. Uptake of Na+ and Cl− at the Whole Plant Level
2.3. Ion Uptake across a Membrane
2.4. Cellular Uptake of Na+
2.4.1. Cytosolic Uptake of Na+ in Wheat and Rice by NSCCs, CCCs, HKTs, and AKT
2.4.2. Cytosolic Na+ Uptake in Arabidopsis
2.5. Long-Distance Translocation of Sodium
2.5.1. Long-Distance Translocation of Sodium in Rice by HKTs
2.5.2. Long-Distance Translocation of Sodium in Barley by HKTs
2.5.3. Long-Distance Translocation of Sodium in Arabidopsis by HKTs
2.5.4. The CCCs, Cation-Chloride Cotransporters
2.6. Salt Tolerance
2.6.1. Plants Tolerate Salt Stress by Different Mechanisms
2.6.2. Halophytes and Glycophytes
2.6.3. Regulation of Na+ Transport at the at Xylem/Parenchyma Cell Border
2.6.4. Different Barley Cultivars Differ in Salt Tolerance
2.6.5. Tolerant Rice Cultivars Have Different Salt-Tolerance Mechanisms
2.6.6. SOS1 Role in Salt Tolerance
2.6.7. Na+ and K+ Transport into the Vacuole
2.7. Measurements of Cytosolic Ion Changes in Different Species/Cultivars under Salinity
2.7.1. Cytosolic Na+ Influx and Efflux from Salt-Tolerant and -Sensitive Species of Quince, Sugar Beet, and Wheat Differ
2.7.2. Cytosolic Na+ and pH Changes Are Different in the Halophyte Quinoa and the Glycophyte Pea
2.7.3. Cytosolic Na+ Influx and Efflux in Tolerant and Sensitive Rice
3. Cl− Uptake and Transport under Salinity
3.1. Antagonism between Cl− and Other Anions in Wheat and Other Cereals, and in Tomatoes and Rose Plants
3.2. Wheat Leaves Might Accumulate Less Na+ and Cl− Than Leaves of Barley, Canola, and Chickpea
3.3. Chloride Channels and Transporters under Salinity
3.3.1. Voltage-Dependent Chloride Channels CLCs
Uptake of Na+ and Cl− and Their Translocation from Root to Shoot May Differ in Different Wheat Species and Cultivars
Cl− Influx Channels in Rice by CLCs
CLC-Channels in Tonoplast of Soybean and Cotton Decreases Cl−/NO3− Ratio
Voltage-Dependent Influx Channels in Barley and Maize
Voltage-Dependent SLAC/SLAH Channels: Arabidopsis
The Involvement of SLAC/SLAH in Chloride Efflux from Barley and Arabidopsis
Ca2+-Activated Cl− Efflux Channel in Sorghum
Intracellular Chloride-Channels in Arabidopsis
Chloride Efflux Channels in Guard Cells
3.3.2. Stretch-Activated Cl− Efflux Channels in Guard Cells and Pollen Tubes
3.3.3. Ion Channels in Xylem Parenchyma Cells of the Root
3.3.4. ALMT Channels
3.4. Ca2+, Boron, Malate, and Aluminum Affect the Accumulation of Cl− in Wheat
3.5. Cl− Transporters under Salinity
3.5.1. CLCs Transporting Cl− in Antiport with Protons
CLCs in Maize, Soybean and Arabidopsis
Subcellular CLCs Transporters in Arabidopsis
3.5.2. Nitrate Transporter 1/Peptide Transporter Family (NFP)
Accumulation of Cl− in Wheat Is Inhibited by Silicon
3.6. Cl− Compartmentalizing Transporters under Salinity
3.6.1. Cation Chloride Cotransporters (CCCs)
CCCs in Rice, Soybean, Grapevine, and Arabidopsis
4. K+ Concentrations and Signaling under Salinity
4.1. Cytosolic K+ Retention Is Higher in Salt-Tolerant Plants
4.2. K+ Channels under Salinity
4.2.1. Shaker K+ Channels under Salinity
Shaker K+ Channels in Arabidopsis
Shaker K+ Channels in Rice and Soybean
4.2.2. Tandem-Pore K+ Channels
4.2.3. Nonselective Cation Channels (NSCCs) under Salinity
4.2.4. Cyclic Nucleotide-Gated Channels (CNGCs) and Glutamate-Like Receptors (GLRs)
4.2.5. Two-Pore K+ Channels under Salinity
TPKs in Rice
4.3. K+ Transporters under Salinity
4.3.1. HAK Transport Systems under Salinity
HAKs in Rice, Maize, Medicago and Pepper
4.3.2. KUP-Transport Systems under Salinity
4.3.3. HKT-Transport Systems under Salinity
HKTs in Wheat, Rice, Barley, Soybean, and Tomato
4.4. Exchangers and Antiporters under Salinity
4.4.1. CPA1 Transporters in Transgenic Arabidopsis, Sugar Beet, Rice, and Cotton with Increased Salt Tolerance
K+ Efflux Antiporters, KEAs (CPA2) Mediate Both K+ Influx and Efflux
4.5. CHX Transporters in Arabidopsis
4.6. K+ Signaling under Salinity
5. Cytosolic Ca2+ Signaling
5.1. Ca2+ Transport System
5.1.1. Ca2+ Transport by Channels
5.1.2. Ca2+ Transport from the Cytosol and Chloroplast and into ER, Golgi, and Vacuole
5.2. Ca2+ Signals Depend on the Type of Stress, Transporter Location and Type, and Duration of Stress
Dynamics of the Cytosolic Ca2+ Signals
5.3. Possible Na+ Sensors
5.3.1. The Na+/H+ Antiporter SOS1 Is Critical for Cytosolic Ca2+ Elevation, as Shown in Arabidopsis, Soybean, and Rice
5.3.2. GIPC Can Bind Na+
5.3.3. A Proposed Structure and Function of SOS1
5.3.4. ANN1, KEAs and FERONIA and Other Ca2+ Transporters
6. Ca2+ Signal Transmission into Intracellular Downstream Reactions
7. pH Changes and Signaling under Salinity
7.1. PH Signaling under Salinity
7.2. Salinity Induces Different Cytosolic and Vacuolar pH Changes in Salt-Sensitive and Salt-Tolerant Species or Cultivars
7.3. Extra Addition of Calcium or Potassium under Cultivation of Wheat and Field Beans in Saline Medium Affects the Cellular pH and H+-ATPase Activity
8. Systemic Ca2+ Signaling
8.1. Electrical Signals Were First Proposed in Mimosa pudica
8.2. Translocation of Amino Acids by Diffusion and Bulk Flow Activates Ca2+ Channels
8.3. Electrical Signals and Glutamate May Be Involved
8.4. Ca2+-ROS Interactions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Sodium in plants: Perception, signaling and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Colmeneros-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peindo-Torrubia, P.; Rosales, M.A. Chloride as a beneficial macronutrient in higher plants: New Roles and regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef] [PubMed]
- Franco-Navarro, J.D.; Rosales, M.A.; Cubero-Font, P.; Calvo, P.; Álvarez, R.; Diaz-Espejo, A.; Colmenero-Flores, J.M. Chloride as a macronutrient increases water-use efficiency by anatomically driven reduced stomatal conductance and increased mesophyll diffusion to CO2. Plant J. 2019, 99, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentration of Na+ and Cl− ions in soil solution has simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integrat. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, X.; Pei, Z.M. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fricke, W. Changes in root hydraulic conductivity in wheat (Triticum aestivum L.) in response to salt stress and day/night can best be explained through altered activity of aquaporins. Plant Cell Environ. 2023, 46, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Res. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.; Yeo, A. The driving force for water and solute movement. In Plant Solute Transport; Yeo, A., Flowers, T., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 1992; pp. 29–45. [Google Scholar]
- Kronzucker, H.J.; Britto, D.T. Sodium transport in plants: A critical review. New Phytol. 2011, 189, 54–81. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Flowers, T.J.; Gong, H. Silicon decreases chloride transport in rice (Oryza sativa L.). J. Plant Physiol. 2013, 170, 847–853. [Google Scholar] [CrossRef]
- Amtmann, A.; Sanders, D. Mechanisms of Na+ uptake by plant roots. Adv. Bot. Res. 1999, 29, 75–112. [Google Scholar] [CrossRef]
- Zhang, J.L.; Flowers, T.J.; Wang, S.M. Mechanisms of sodium uptake by roots of higher plants. Plant Soil 2010, 326, 45–60. [Google Scholar] [CrossRef]
- Haro, R.; Banuelos, M.A.; Rodriguez-Navarro, A. High affinity uptake of sodium in land plants. Plant Cell Physiol. 2010, 51, 68–79. [Google Scholar] [CrossRef]
- Davenport, R.J.; Tester, M. A weakly voltage-dependent, nonselective cation channel mediates toxic sodium influx in wheat. Plant Physiol. 2000, 12, 823–834. [Google Scholar] [CrossRef]
- Demidschik, V.; Maathuis, F.J.M. Physiological roles of nonselective cation channels in plants: From salt stress to signaling and development. New Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef]
- Leng, Q.; Mercier, R.W.; Hua, B.; Fromm, H.; Berkowitz, G.A. Electrophysiological analysis of cloned cyclic nucleotide-gated ion channels. Plant Physiol. 2002, 128, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Keisham, M.; Mukherjee, S.; Bhatla, S. Mechanisms of sodium transport in plants—Progresses and challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanism of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Golldack, D.; Su, H.; Quigley, F.; Kamasani, U.R.; Munoz-Garay, C.; Balderas, E.; Popova, O.V.; Bennett, J.; Hans, J.; Bohnert, H.J.; et al. Characterization of an HKT-type transporter in rice as a general alkali cation transporter. Plant J. 2002, 31, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R.J. Na+ transport and Na+ tolerance in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Greger, M.; Ahmad, H.; Kabir, A.H.; Landberg, T.; Maity, P.J.; Lindberg, S. Silicate reduces cadmium uptake into cells of wheat. Environ. Poll. 2016, 211, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Mian, A.; Oomen, R.J.; Isayenkov, S.; Sentenac, H.; Maathuis, F.J.; Véry, A.A. Over-expression of a Na+ and K+ permeable HKT transporter in barley improves salt tolerance. Plant J. 2011, 68, 468–479. [Google Scholar] [CrossRef]
- Han, Y.; Yin, S.; Huang, l.; Wu, X.; Zeng, J.; Liu, X.; Qiu, L.; Munns, R.; Chen, Z.-H.; Zhang, G. A sodium transporter HvHKT1 confers salt tolerance in barley via regulating tissue cell ion homeostasis. Plant Cell Physiol. 2018, 59, 1976–1989. [Google Scholar] [CrossRef]
- Essah, P.A.; Davenport, R.; Tester, M. Sodium influx and accumulation in Arabidopsis. Plant Physiol. 2003, 133, 307–318. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Byrt, C.S.; Zhao, M.; Kourghi, M.; Bose, J.; Henderson, S.W.; Qiu, J.; Gilliham, M.; Schultz, C.; Schwarz, M.; Ramesh, S.A.; et al. Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca2+ and pH. Plant Cell Environ. 2017, 40, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.A.; Seidel, T.; Golldack, D.; Lindberg, S. Expressions of OsHKT1, OsHKT2 and OsVHA are differently regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. J. Exp. Bot. 2006, 57, 4257–4268. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Yoshida, K.; Nakayama, H.; Yamada, K.; Oiki, S.; Shinmyo, A. Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J. 2001, 27, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.; Nieves-Cordones, M.; Horie, T.; Shabala, S. Doing ‘business as usual’ comes with a cost: Evaluating energy cost of maintaining plant intracellular K+ homeostasis under saline conditions. New Phytol. 2020, 225, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Berthomieu, P.; Cone, G.; Nublat, A.; Brackenbury, W.J.; Lambert, C.; Savio, C.; Uozumi, N.; Oiki, S.; Yamada, K.; Cellier, F.; et al. Functional analysis of AtHKT1 in Arabidopsis shows that Na recirculation by the phloem is crucial for salt tolerance. EMBO J. 2003, 22, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gao, J.P.; Li, L.G. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Gen. 2005, 37, 1141–1146. [Google Scholar] [CrossRef]
- Brini, F.; Masmoudi, K. Ion transporters and abiotic stress tolerant plants. ISRN Mol. Biol. 2012, 2012, 927436. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kuang, L.; Wu, L.; Wu, D.; Zhang, G. Comparison in functions of HKT1;5 transporters between Hordeum marinum and Hordeum vulgare in responses to salt stress. Plant Growth Reg. 2019, 89, 309–319. [Google Scholar] [CrossRef]
- Møller, I.S.; Gilliham, M.; Jha, D.; Mayo, G.M.; Roy, S.J.; Coates, J.C.; Haseloff, J.; Tester, M. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell 2009, 21, 2163–2178. [Google Scholar] [CrossRef]
- Davenport, R.; Munoz-Mayer, A.; Jha, D.; Essah, P.A.; Rus, A.; Tester, M. The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ. 2007, 30, 497–507. [Google Scholar] [CrossRef]
- Colmeneros-Flores, J.M.; Martinez, G.; Gamba, G.; Vazquez, N.; Iglesias, D.J.; Brumos, J.; Talon, M. Identification and functional characterization of cation-chloride cotransporters in plants. Plant J. 2007, 50, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.W.; Wege, S.; Gilliham, M. Plant cation-chloride cotransporters (CCC): Evolutionary origin and functional insights. Int. J. Mol. Sci. 2018, 19, 492. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Dabravolski, S.A.; Pan, T.; Shabala, S. Phylogenetic diversity and physiological roles of plant monovalent cation/H+ antiporters. Front. Plant Sci. 2020, 11, 573564. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2014, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Zhang, J.L.; Liu, X.S.; Li, Z.; Wu, G.Q.; Cai, J.Y.; Wang, S.M. Puccinellia tenuiflora maintains a low Na+ level under salinity by limiting unidirectional Na+ influx resulting in a high selectivity for K+ over Na+. Plant Cell Environ. 2009, 32, 486–496. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Schroeder, J.I. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 1994, 370, 655–658. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Liu, W.H. Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci. 1999, 4, 281–287. [Google Scholar] [CrossRef]
- Wang, S.M.; Zhang, J.L.; Flowers, T.J. Low-affinity Na+ uptake in the halophyte Suaeda maritima. Plant Physiol. 2007, 145, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Jazo, I.; Shabala, S.; Chen, Z.; Pottosin, I.I. Na+-K+ transport. in roots under salt stress. Plant Signal. Behav. 2008, 3, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.K.; Tester, M. Permeation of Ca2+ and monovalent cations through an outwardly rectifying channel in maize root stelar cells. J. Exp. Bot. 1997, 48, 839–846. [Google Scholar] [CrossRef]
- Adem, G.D.; Chen, G.; Shabala, L.; Chen, Z.H. GORK channel: A master switch of plant metabolism. Trends Plant Sci. 2020, 25, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lindberg, S.; Shabala, L.; Morgan, S.; Shabala, S.; Jacobsen, S.E. A comparative analysis of cytosolic Na+ changes under salinity between halophyte quinoa (Chenopodium quinoa) and glycophyte pea (Pisum sativum). Environ. Exp. Bot. 2017, 141, 154–160. [Google Scholar] [CrossRef]
- Shabala, S.; Shabala, L.; Cuin, T.A.; Pang, J.; Percey, W.; Chen, Z.; Wegner, L.H. Xylem ionic relations and salinity tolerance in barley. Plant J. 2010, 61, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Signaling by potassium: Another second messenger to add to the list? J. Exp. Bot. 2017, 68, 4003–4007. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Hariadi, Y.; Jacobsen, S.E. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J. Plant Physiol. 2013, 170, 906–914. [Google Scholar] [CrossRef]
- Jia, W.; Wang, Y.; Zhang, S.; Zhang, J. Salt-stress-induced ABA accumulation is more sensitively triggered in roots than in shoots. J. Exp. Bot. 2002, 53, 2201–2206. [Google Scholar] [CrossRef]
- Shi, H.Z.; Quintero, F.J.; Pardo, J.M.; Zhu, J.K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, M.; Shabala, L.; Shabala, S. Physiological and molecular mechanisms mediating xylem Na+ loading in the context of salinity stress tolerance. Plant Cell Environ. 2016, 40, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kuang, L.; Li, X.; Wu, L.; Wu, D.; Zhang, G. Metabolomics and transcriptomic analyses reveal the reason why Hordeum marinum has higher salt tolerance than Hordeum vulgare. Env. Exp. Bot. 2018, 156, 48–61. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Tri, P.N.; Le Hong, T.; Quoc, C.D. Biomolecular evaluation of three contrasting rice cultivars (Oryza sativa L.) in salt stress response at seedling stage. Plant Sci. Today 2022, 9, 491–503. [Google Scholar] [CrossRef]
- Foster, K.J.; Miklavcic, S.J. A comprehensive biophysical model of ion and water transport in plant roots. II. Clarifying the rules of SOS1 in the salt-stress response Arabibopsis. Front. Plant Sci. 2019, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.J.; Lynch, J.P. Effect of salinity on cytosolic Na+ and K+ in root hairs of Arabidopsis thaliana: In vivo measurement using the fluorescent dyes SBFI and PBFI. J. Exp. Bot. 2003, 54, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.H.; Kader, M.A.; Lindberg, S. Cytosolic sodium influx in mesophyll protoplasts of Arabidopsis thaliana, Wt, sos1;1, and nhx1 differs and induces different calcium changes. Plants 2022, 11, 3439. [Google Scholar] [CrossRef] [PubMed]
- Bassil, E.B.; Zhang, S.; Gong, H.; Tajima, H.; Blumwald, E. Cation specificity of vacuolar NHX type Cation/H+ antiporters. Plant Physiol. 2019, 179, 616–629. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Di, L.; Shen, Y.; Wang, G. AtCCX1 transports Na+ and K+ in Pitch pastoris. Afr. J. Biotechnol. 2011, 10, 9743–9750. [Google Scholar] [CrossRef]
- Zhu, X.; Pan, T.; Zhang, X.; Fan, L.; Quintero, F.J.; Zhao, H.; Su, X.; Li, X.; Villalta, I.; Mendoza, I.; et al. K+ efflux antiporters 4, 5, and 6 mediate pH and K+ homeostasis in endomembrane compartments. Plant Physiol. 2018, 178, 1657–1678. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Guo, X.; Chen, P.; Cheng, Y.; Mao, K.; Ma, F. Cation/Ca2+ exchanger1 (MdCCX1), a plasma membrane-localized Na+ transporter, enhances plant salt tolerance by inhibiting excessive accumulation of Na+ and reactive oxygen species. Front. Plant Sci. 2021, 12, 746189. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Kader, A.; Lindberg, S. Uptake of sodium in quince, wheat and sugar beet protoplasts determined by the fluorescent sodium-binding benzofuran isophthalate dye. J. Plant Physiol. 2005, 162, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.; Lindberg, S. Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice, Oryza sativa L. J. Exp. Bot. 2005, 56, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Negulescu, P.A.; Harootunian, A.; Tsien, R.Y.; Machen, T.E. Fluorescence measurement of cytosolic free Na concentration influx and efflux in gastri cells. Cell Regul. 1990, 1, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Demidschik, V.; Tester, M. Sodium fluxes through nonselective cation channels in plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol. 2002, 128, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Rodrigo-Moreno, A.; Lai, D.; Xie, Y.; Shen, W.; Shabala, S. Rapid regulation of plasma membrane H+ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Ann. Bot. 2015, 115, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, K.B.; Biondi, S.; Martínez, E.A.; Orsini, F.; Antognoni, F.; Jacobsen, S.E. Quinoa—A model crop for understanding salt tolerance mechanisms in halophytes. Plant Biosyst. 2016, 150, 357–371. [Google Scholar] [CrossRef]
- Anil, V.S.; Krishnamurthy, H.; Mathew, M. Limiting cytosolic Na confers salt tolerance in rice cell in culture: A two-photon microscopy study of SBFI-loaded cells. Physiol. Plant. 2007, 129, 607–621. [Google Scholar] [CrossRef]
- Blumwald, E.; Poole, R.J. The Na+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol. 1985, 78, 163–167. [Google Scholar] [CrossRef]
- Hassidim, M.; Braun, Y.; Lerner, H.R.; Reinhold, L. Na+/H+ and K+/H+ antiport in root membrane vesicles isolated from the halophyte Atriplex and the glycophyte cotton. Plant Physiol. 1990, 94, 1795–1801. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Mahi, H.E.; Pérez-Hormaec, H.J.; Luca, A.D.; Villalta, I.; Espartero, J.; Gámez-Arjona, F.; Fernández, J.L.; Bundó, M.; Mendoza, I.; Mieulet, D.; et al. A critical role of sodium flux via the plasma membrane Na+/H+ exchanger SOS1 in the salt tolerance of rice. Plant Physiol. 2018, 180, 1046–1065. [Google Scholar] [CrossRef] [PubMed]
- Sze, H.; Schumacher, K.; Muller, M.; Padmanaban, S.; Taiz, L. A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+ATPase. Trends Plant Sci. 2002, 7, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Teakle, N.L.; Tyerman, S.D. Mechanisms of Cl− transport contributing to salt tolerance. Plant Cell Environ. 2010, 33, 566–589. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, I.; Aberle, T.; Plieth, C. Salt stress-induced chloride flux: A study using transgenic Arabidopsis expressing a fluorescent anion probe. Plant J. 2004, 38, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Geilfus, C.M. Review on the significance of chlorine for crop yield and quality. Plant Sci. 2018, 270, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhou, X.; Song, H.; Zhang, M.; Jiang, C. Advances in deciphering salt tolerance mechanism in maize. Crop J. 2023, 11, 1001–1010. [Google Scholar] [CrossRef]
- Rajendran, K.; Tester, M.; Roy, S.J. Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ. 2009, 32, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Chen, S.; Chen, F.; Liu, Z.; Fang, W.; Tang, J. Comparison of stress effect of NaCl, Na+ and Cl− on two Chrysanthemum species. Acta Hortic. 2012, 937, 369–375. [Google Scholar] [CrossRef]
- Abdelgadir, E.M.; Oka, M.; Fujiyama, H. Characteristics of nitrate uptake by plants under salinity. J. Plant Nutr. 2005, 28, 33–46. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Spatial distribution sand net deposition rates of mineral elements in the elongating wheat (Triticum aestivum L.) leaf under saline soil conditions. Planta 1998, 204, 212–219. [Google Scholar] [CrossRef]
- Massa, D.; Mattson, N.S.; Lieth, H.J. Effects of saline root environment (NaCl) on nitrate and potassium uptake kinetics for rose plants: A Michaelis–Menten modelling approach. Plant Soil 2009, 318, 101–115. [Google Scholar] [CrossRef]
- Grewal, H.S. Water uptake, water use efficiency, plant growth and ionic balance of wheat, barley, canola and chickpea plants on a sodic vertosol with variable subsoil NaCl salinity. Agric. Water Manag. 2010, 97, 148–156. [Google Scholar] [CrossRef]
- Tregeagle, J.M.; Tisdall, J.M.; Tester, M.; Walker, R.R. Cl- uptake, transport and accumulation in grapevine rootstocks of differing capacity for Cl- exclusion. Funct. Plant Biol. 2010, 37, 665–673. [Google Scholar] [CrossRef]
- Yamashita, K.; Kasai, M.; Yamamoto, Y.; Matsumoto, H. Stimulation of plasma membrane H+-transport activity in barley roots by salt stress. Possible role of increase in chloride permeability. Soil Sci. Plant Nutr. 1994, 40, 555–563. [Google Scholar] [CrossRef]
- Skerrett, M.; Tyerman, S.D. A channel that allows inwardly-directed fluxes of anions in protoplasts derived from wheat roots. Planta 1994, 192, 295–305. [Google Scholar] [CrossRef]
- Wegner, L.H.; Raschke, K. Ion channels in the xylem parenchyma of barley roots. Plant Physiol. 1994, 105, 799–813. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Separation of K+ and Cl−-selective ion channels from rye roots on a continuous sucrose density gradient. J. Exp. Bot. 1995, 46, 361–376. [Google Scholar] [CrossRef]
- Ryan, P.R.; Skerrett, M.; Findlay, G.P.; Tyerman, S.D. Aluminum activates an anion channel in the apical cells of wheat roots. Proc. Natl. Acad. Sci. USA 1997, 94, 6547–6552. [Google Scholar] [CrossRef]
- Kiegle, E.; Gilliham, M.; Haseloff, J.; Tester, M. Hyperpolarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant J. 2000, 21, 225–229. [Google Scholar] [CrossRef]
- Diatloff, E.; Roberts, M.; Sanders, D.; Roberts, S.K. Characterization of anion channels in the plasma membrane of Arabidopsis epidermal root cells and the identification of a citrate permeable channel induced by phosphate starvation. Plant Physiol. 2004, 136, 4136–4149. [Google Scholar] [CrossRef]
- Ouerghi, Z.; Cornic, G.; Roudani, M.; Ayadi, A.; Brulfert, J. Effect of NaCl on photosynthesis of two wheat species (Triticum durum and T. aestivum) differing in their sensitivity to salt stress. Plant Physiol. 2000, 156, 335–340. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A. Screening methods for salinity tolerance: A case study with tetraploid wheat. Plant Soil 2003, 253, 201–218. [Google Scholar] [CrossRef]
- Barbier-Brygoo, H.; Vinauger, M.; Colcombet, J.; Ephritikhine, G.; Frachisse, J.M.; Maurel, C. Anion channels in higher plants: Functional characterization, molecular structure and physiological role. Biochim. Biophys. Acta (BBA) Biomem. 2000, 1465, 199–218. [Google Scholar] [CrossRef]
- Tyerman, S.D.; Skerrett, M.; Garrill, A.; Findlay, G.P.; Leigh, R.A. Pathways for the permeation of Na+ and Cl− into protoplasts derived from the cortex of wheat roots. J. Exp. Bot. 1997, 48, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Diédhiou, C.J.; Golldack, D. Salt-dependent regulation of chloride channel transcripts in rice. Plant Sci. 2006, 170, 793–800. [Google Scholar] [CrossRef]
- Nakamura, A.; Fukuda, A.; Sakai, S.; Tanaka, Y. Molecular cloning, functional expression and subcellular localization of two putative vacuolar voltage-gated chloride channels in rice (Oryza sativa L.). Plant Cell Physiol. 2006, 47, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Che, B.; Shen, L.; Cui, Y.; Wu, S.; Cheng, C.; Liu, F.; Li, M.A.; Bingjun Yu, B.; Lam, H.M. Identification and functional characterization of the chloride channel gene, GsCLC-c2 from wild soybean. BMC Plant Biol. 2019, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Agorio, A.; Jossier, M.; Depré, S.; Thomine, S.; Filleur, S. Characterization of the chloride channel-like, AtCLCg, involved in chloride tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 764–775. [Google Scholar] [CrossRef]
- Liu, W.; Feng, J.; Ma, W.; Zhou, Y.; Ma, Z. GhCLCg-1, a vacuolar chloride channel, contributes to salt tolerance by regulating ion accumulation in upland cotton. Front. Plant Sci. 2021, 12, 765173. [Google Scholar] [CrossRef]
- Jossier, M.; Kroniewicz, L.; Dalmas, F.; Le Thiec, D.; Ephritikhine, G.; Thomine, S.; Barbier-Brygoo, H.; Vavasseur, A.; Filleur, S.; Leonhardt, N. The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J. 2010, 64, 563–576. [Google Scholar] [CrossRef]
- Kohler, B.; Raschke, K. The delivery of salts to the xylem. Three types of anion conductance in the plasmalemma of the xylem parenchyma of roots of barley. Plant Physiol. 2000, 122, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Gilliham, M.; Tester, M. The regulation of anion loading to the maize root xylem. Plant Physiol. 2005, 137, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.K. Plasma membrane anion channels in higher plants and their putative functions in roots. New Phytol. 2006, 169, 647–666. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R.; Becker, D. Green circuits—The potential of plant specific ion channels. Plant Mol. Biol. 1994, 26, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Hedrich, R. Anions permeate and gate GCAC1, a voltage-dependent guard cell anion channel. Plant J. 1998, 15, 479–487. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. The importance of Cl− exclusion and vacuolar Cl− sequestration: Revisiting the role of Cl− transport in plant salt tolerance. Front. Plant Sci. 2019, 10, 1418. [Google Scholar] [CrossRef] [PubMed]
- Negi, J.; Matsuda, O.; Nagasawa, T.; Oba, Y.; Takahashi, H.; Kawai-Yamada, M.; Uchimiya, H.; Hashimoto, M.; Iba, K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 2008, 452, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Vahisalu, T.; Kollist, H.; Wang, Y.F.; Nishimura, N.; Chan, W.Y.; Valerio, G.; Lamminmäki, A.; Brosché, M.; Moldau, H.; Desikan, R.; et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 2008, 452, 487–491. [Google Scholar] [CrossRef]
- Planes, M.D.; Niñoles, R.; Rubio, L.; Bissoli, G.; Bueso, E.; García-Sánchez, M.J.; Alejandro, S.; Gonzalez-Guzmán, M.; Hedrich, R.; Rodriguez, P.L.; et al. A mechanism of growth inhibition by abscisic acid in germinating seeds of Arabidopsis thaliana based on inhibition of plasma membrane H+-ATPase and decreased cytosolic pH, K+, and anions. J. Exp. Bot. 2015, 66, 813–825. [Google Scholar] [CrossRef]
- Cubero-Font, P.; Maierhofer, T.; Jaslan, J.; Rosales, M.A.; Espartero, J.; Díaz-Rueda, P.; Müller, H.M.; Hürter, A.L.; Al-Rasheid, K.A.; Marten, I.; et al. Silent S-type anion channel subunit SLAH1 gates SLAH3 open for chloride root-to-shoot translocation. Curr. Biol. 2016, 26, 2213–2220. [Google Scholar] [CrossRef]
- Flowers, T.J.; Hajibagheri, M.A. Salinity tolerance in Hordeum vulgare: Ion concentrations in root cells of cultivars differing in salt tolerance. Plant Soil 2001, 231, 1–9. [Google Scholar] [CrossRef]
- Brumos, J.; Talon, M.; Bouhal, R.; Colmenero-Flores, J.M. Cl− homeostasis in includer and excluder citrus rootstocks: Transport mechanisms and identification of candidate genes. Plant Cell Environ. 2010, 33, 2012–2027. [Google Scholar] [CrossRef] [PubMed]
- Boursier, P.; Läuchli, A. Mechanisms of chloride partitioning in the leaves of salt-stressed Sorghum bicolor L. Physiol. Plant. 1989, 77, 537–544. [Google Scholar] [CrossRef]
- Thomine, S.; Zimmermann, S.; Guern, J.; Barbier-Brygoo, H. ATP-dependent regulation of an anion channel at the plasma membrane of epidermal cells of Arabidopsis hypocotyls. Plant Cell 1995, 7, 2091–2100. [Google Scholar] [CrossRef]
- Allen, G.J.; Sanders, D. The Plant Vacuole. In Advances in Botanical Research; Leigh, R.A., Sanders, D., Eds.; Academic Press: London, UK; New York, NY, USA, 1997; Volume 25, pp. 218–252. [Google Scholar] [CrossRef]
- Herdean, A.; Teardo, E.; Nilsson, A.; Pfeil, B.E.; Johansson, O.N.; Ünnep, R.; Nagy, G.; Zsiros, O.; Dana, S.; Solymosi, K.; et al. A voltage-dependent chloride channel fine-tunes photosynthesis in plants. Nat. Commun. 2016, 7, 11654. [Google Scholar] [CrossRef]
- Keller, B.; Hedrich, R.; Raschke, K. Voltage-dependent anion channels in the plasma membrane of guard cells. Nature 1989, 341, 450–453. [Google Scholar] [CrossRef]
- Speer, M.; Kaiser, W.M. Ionic relations of symplastic and apoplastic space in leaves from Spinacia oleracea L. and Pisum sativum L. under salinity. Plant Physiol. 1991, 97, 990–997. [Google Scholar] [CrossRef]
- Cosgrove, D.J.; Hedrich, R. Stretch-activated Cl−, K+ and Ca2+ channels coexisting in plasma membrane of guard cells of Vicia faba L. Planta 1991, 186, 143–153. [Google Scholar] [CrossRef]
- Ward, J.M.; Maser, P.; Schroeder, J.I. Plant ion channels: Gene families, physiology, and functional genomics analyses. Annu. Rev. Physiol. 2009, 71, 59–82. [Google Scholar] [CrossRef]
- Veley, K.M.; Haswell, E.S. Plastids and pathogens: Mechanosensitive channels and survival in a hypoosmotic world. Plant Signal Behav. 2012, 7, 668–671. [Google Scholar] [CrossRef]
- Maksaev, G.; Haswell, E.S. MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc. Natl. Acad. Sci. USA 2012, 109, 19015–19020. [Google Scholar] [CrossRef] [PubMed]
- Deger, A.G.; Scherzer, S.; Nuhkat, M.; Kedzierska, J.; Kollist, H.; Brosch, M.; Unyayar, S.; Boudsocq, M.; Hedrich, R.; Roelfsema, M.R.G. Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytol. 2015, 208, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Michard, E.; Simon, A.A.; Tavares, B.; Wudick, M.M.; Feijó, J.A. Signaling with ions: The keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol. 2017, 173, 91–111. [Google Scholar] [CrossRef] [PubMed]
- De Angeli, A.; Zhang, J.; Meyer, S.; Martinoia, E. AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun. 2013, 4, 1804. [Google Scholar] [CrossRef]
- Husain, S.; Susanne von Caemmerer, S.V.; Munns, R. Control of salt transport from roots to shoots of wheat in saline soil. Funct. Plant Biol. 2004, 31, 1115–1126. [Google Scholar] [CrossRef]
- Yermiyahu, U.; Ben-Gal, A.; Keren, R.; Reid, R.J. Combined effect of salinity and excess boron on plant growth and yield. Plant Soil 2008, 304, 73–87. [Google Scholar] [CrossRef]
- Masood, S.; Wimmer, M.A.; Witzel, K.; Zörb, C.; Mühling, K.H. Interactive effects of high boron and NaCl stresses on subcellular localization of chloride and boron in wheat leaves. J. Agron. Crop Sci. 2012, 198, 227–235. [Google Scholar] [CrossRef]
- Pineros, M.A.; Cancado, G.M.A.; Kochian, L.V. Novel properties of the wheat aluminum tolerance organic acid transporter (TaALMT1) revealed by electrophysiological characterization in Xenopus oocytes: Functional and structural implications. Plant Physiol. 2008, 147, 2131–2146. [Google Scholar] [CrossRef]
- Li, B.; Qiu, J.; Jayakannan, M.; Xu, B.; Li, Y.; Mayo, G.M.; Tester, M.; Gilliham, M.; Roy, S.J. AtNPF2.5 modulates chloride (Cl−) efflux from roots of Arabidopsis thaliana. Front. Plant Sci. 2017, 7, 02013. [Google Scholar] [CrossRef]
- Li, B.; Byrt, C.; Qiu, J.; Baumann, U.; Hrmova, M.; Evrard, A.; Johnson, A.A.T.; Birnbaum, K.D.; Mayo, G.M.; Jha, D.; et al. Identification of a stelar-localized transport protein that facilitates root-to-shoot transfer of chloride in Arabidopsis. Plant Physiol. 2016, 170, 1014–1029. [Google Scholar] [CrossRef]
- Baetz, U.; Eisenach, C.; Tohge, T.; Martinoia, E.; Angeli, A.D. Vacuolar chloride fluxes impact ion content and distribution during early salinity stress. Plant Physiol. 2016, 172, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tester, M.; Gilliham, M. Chloride on the Move. Trends Plant Sci. 2017, 22, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Wege, S.; Gilliham, M.; Henderson, S.W. Chloride: Not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J. Exp. Bot. 2017, 68, 3057–3069. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, S.Z.; Wu, Y.; Li, S.P.; Shan, X.H.; Liu, H.K.; Wang, S.; Yuan, Y.P. Overexpression of maize chloride channel gene ZmCLC-d in Arabidopsis thaliana improved its stress resistance. Biol. Plant. 2015, 59, 55–64. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, Y.; Li, J.; Zhang, P.; Chen, K.; Song, W.; Wang, X.; Yang, J.; Lu, X.; Lu, B.; et al. Molecular dissection of maize seedling salt tolerance using a genome-wide association analysis method. Plant Biotechnol. J. 2021, 19, 1937–1951. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.F.; Wong, F.L.; Tsai, S.N.; Phang, T.H.; Shao, G.H.; Lam, H.M. Tonoplast-located GmCLC1 and GmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (BY)-2 cells. Plant Cell Environ. 2006, 29, 1122–1137. [Google Scholar] [CrossRef]
- Wong, T.H.; Li, M.W.; Yao, X.Q.; Lam, H.M. The GmCLC1 protein from soybean functions as a chloride ion transporter. J. Plant Physiol. 2013, 170, 101–104. [Google Scholar] [CrossRef]
- Wei, P.; Wang, L.; Liu, A.; Yu, B.; Lam, H.M. GmCLC1 confers enhanced salt tolerance through regulating chloride accumulation in soybean. Front. Plant Sci. 2016, 7, 1082. [Google Scholar] [CrossRef]
- Guan, R.; Qu, Y.; Guo, Y.; Yu, L.; Liu, Y.; Jiang, J.; Chen, J.; Ren, Y.; Liu, G.; Tian, L.; et al. Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J. 2014, 80, 937–950. [Google Scholar] [CrossRef]
- Do, T.D.; Chen, H.; Hien, V.T.T.; Hamwieh, A.; Yamada, T.; Sato, T.; Yan, Y.; Cong, H.; Shono, M.; Suenaga, K.; et al. Ncl synchronously regulates Na+, K+ and Cl− in soybean and greatly increases the grain yield in saline field conditions. Sci. Rep. 2016, 6, 19147. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, L.; Qu, Y.; Chen, J.; Liu, X.; Hong, H.; Liu, Z.; Chang, R.; Gilliham, M.; Qiu, L.; et al. GmSALT3, which confers improved soybean salt tolerance in the field, increases leaf Cl− exclusion prior to Na+ exclusion but does not improve early vigor under salinity. Front. Plant Sci. 2016, 7, 1485. [Google Scholar] [CrossRef] [PubMed]
- Zifarelli, G.; Pusch, M. CLC transport proteins in plants. FEBS Lett. 2010, 584, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Tang, R.; Liu, H.; Gao, X.; Li, Y.; Zheng, H.; Zhang, H. Cloning and molecular analyses of the Arabidopsis thaliana chloride channel gene family. Plant Sci. 2009, 176, 650–661. [Google Scholar] [CrossRef]
- Flowers, T.J.; Hajibagheri, M.A.; Yeo, A.R. Ion accumulation in the cell-walls of rice plants growing under saline conditions: Evidence for the Oertli hypothesis. Plant Cell Environ. 1991, 14, 319–325. [Google Scholar] [CrossRef]
- Barbier-Brygoo, H.; De Angeli, A.; Filleur, S.; Frachisse, J.M.; Gambale, F.; Thomine, S.; Wege, S. Anion channels/transporters in plants: From molecular bases to regulatory networks. Annu. Rev. Plant Biol. 2011, 62, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Corratgé-Faillie, C.; Jabnoune, M.; Zimmermann, S.; Véry, A.A.; Fizames, C.; Sentenac, H. Potassium and sodium transport in non-animal cells: The Trk/Ktr/HKT transporter family. Cell Mol. Life Sci. 2010, 67, 2511–2532. [Google Scholar] [CrossRef]
- Wen, Z.; Tyerman, S.D.; Dechorgnat, J.; Ovchinnikova, E.; Dhugga, K.S.; Kaiser, B.N. Maize NPF6 proteins are homologs of Arabidopsis CHL1 that are selective for both nitrate and chloride. Plant Cell 2017, 29, 2581–2596. [Google Scholar] [CrossRef]
- Premkumar, A.; Javed, M.T.; Pawlowski, K.; Lindberg, S.M. Silicate inhibits the cytosolic influx of chloride in protoplasts of wheat and affects the chloride transporters, TaCLC1 and TaNPF2.4/2.5. Plants 2022, 11, 1162. [Google Scholar] [CrossRef]
- Chen, Z.C.; Yamaji, N.; Fujii-Kashino, M.; Ma, J.F. A cation-chloride cotransporter gene is required for cell elongation and osmoregulation in rice. Plant Physiol. 2016, 171, 494–507. [Google Scholar] [CrossRef]
- Nikolovski, N.; Rubtsov, D.; Segura, M.P.; Miles, G.P.; Stevens, T.J.; Dunkley, T.P.J.; Munro, S.; Lilley, K.S.; Dupree, P. Putative glycosyltransferases and other plant Golgi apparatus proteins are revealed by LOPIT proteomics. Plant Physiol. 2012, 160, 1037–1051. [Google Scholar] [CrossRef]
- Groen, A.J.; Sancho-Andrés, G.; Breckels, L.M.; Gatto, L.; Aniento, F.; Lilley, K.S. Identification of trans-golgi network proteins in Arabidopsis thaliana root tissue. J. Prote. Res. 2014, 13, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.W.; Wege, S.; Qiu, J.; Blackmore, D.H.; Walker, A.R.; Tyerman, S.D.; Walker, R.R.; Gilliham, M. Grapevine and Arabidopsis cation-chloride cotransporters localise to the golgi and trans-golgi network and indirectly influence long-distance ion transport and plant salt tolerance. Plant Physiol. 2015, 169, 2215–2229. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Horie, T. A conserved primary salt tolerance mechanism mediated by HKT transporters: A mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ. 2010, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Smethurst, C.F.; Rix, K.; Garnett, T.; Auricht, G.; Bayart, A.; Lane, P.; Wilson, S.J.; Shabala, S. Multiple traits associated with salt tolerance in lucerne: Revealing the underlying cellular mechanisms. Funct. Plant Biol. 2008, 35, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, X.; Giraldo, J.P.; Shabala, S. It is not all about sodium: Revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 2018, 431, 1–17. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Shankar, A.; Chandran, A.K.N.; Sharma, M.; Jung, K.H.; Suprasanna, P.; Pandey, G.K. Emerging concepts of potassium homeostasis in plants. J. Exp. Bot. 2020, 71, 608–619. [Google Scholar] [CrossRef]
- Adhikari, S.; Anuragi, H.; Chandra, K.; Tarte, S.H.; Dhaka, S.R.; Jatav, H.S.; Hingonia, K. Molecular basis of plant nutrient use efficiency—Concepts and challenges for its improvement. In Sustainable Plant Nutrition: Molecular Interventions and Advancements for Crop Improvement; Academic Press: New York, NY, USA, 2023; pp. 107–151. [Google Scholar] [CrossRef]
- Britto, D.T.; Coskun, D.; Kronzucker, H.J. Potassium physiology from Archean to Holocene: A higher-plant perspective. J. Plant Physiol. 2021, 262, 153432. [Google Scholar] [CrossRef]
- Sharma, T.; Dreyer, I.; Riedelsberger, J. The role of K+ channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Front. Plant Sci. 2013, 4, 224. [Google Scholar] [CrossRef]
- Sánchez-Barrena, M.J.; Chaves-Sanjuan, A.; Raddatz, N.; Mendoza, I.; Cortés, Á.; Gago, F.; González-Rubio, J.M.; Benavente, J.L.; Quintero, F.J.; Pardo, J.M.; et al. Recognition and activation of the plant AKT1 potassium channel by the kinase CIPK23. Plant Physiol. 2020, 182, 2143–2153. [Google Scholar] [CrossRef]
- Durdagi, S.; Noskov, S.Y. Mechanism of K+/Na+ selectivity in potassium channels from the perspective of the non-selective bacterial channel NaK. Channels 2011, 5, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Kitamoto, H.K.; Nakamura, A.; Fukuda, A.; Tanaka, Y. Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant Physiol. 2007, 144, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Shen, L.; Luan, J.; Zhou, Z.; Guo, D.; Shen, Y.; Jing, W.; Zhang, B.; Zhang, A.; Zhang, W. Rice shaker potassium channel OsAKT2 positively regulates salt tolerance and grain yield by mediating K+ redistribution. Plant Cell Environ. 2021, 44, 2951–2965. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, J.; Fang, Q.; Chang, X.; Sun, M.; Li, W.; Li, Y. GmAKT1 is involved in K+ uptake and Na+/K+ homeostasis in Arabidopsis and soybean plants. Plant Sci. 2021, 304, 110736. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; He, C.; Wang, Y.; Xu, H.; Xu, K.; Zhao, Y.; Yao, B.; Zhang, Y.; Zhao, Y.; Carther, K.F.I.; et al. Genome-wide identification of soybean Shaker K+ channel gene family and functional characterization of GmAKT1 in transgenic Arabidopsis thaliana under salt and drought stress. J. Plant Physiol. 2021, 266, 153529. [Google Scholar] [CrossRef]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef]
- Wang, Q.; Ni, L.; Cui, Z.; Jiang, J.; Chen, C.; Jiang, M. The NADPH oxidase OsRbohA increases salt tolerance by modulating K+ homeostasis in rice. Crop J. 2022, 10, 1611–1622. [Google Scholar] [CrossRef]
- Gobert, A.; Isayenkov, S.; Voelker, C.; Maathuis, F.J.M. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Nat. Acad. Sci. USA 2007, 104, 10726–10731. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Flowers, T.J. Sodium chloride compartmentation leaf vacuoles of the halophyte Suaeda maritima (L.) Dum. and its relation to tonoplast permeability. J. Exp. Bot. 1992, 43, 1219–1223. [Google Scholar] [CrossRef]
- Bonales-Alatorre, E.; Pottosin, I.; Shabala, L.; Chen, Z.H.; Zeng, F.; Jacobsen, S.E.; Shabala, S. Differential activity of plasma and vacuolar membrane transporters contributes to genotypic differences in salinity tolerance in a halophyte species, Chenopodium quinoa. Int. J. Mol. Sci. 2013, 14, 9267–9285. [Google Scholar] [CrossRef]
- Choi, W.G.; Toyota, M.; Kim, S.H.; Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Nat. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Pottosin, I.; Dobrovinskaya, O. Non-selective cation channels in plasma and vacuolar membranes and their contribution to K+ transport. J. Plant Physiol. 2014, 171, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shabala, L.; Zhou, M.; Shabala, S. Durum and bread wheat differ in their ability to retain potassium in leaf mesophyll: Implications for salinity stress tolerance. Plant Cell Physiol. 2014, 55, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Apsea, M.P.; Blumwaldb, E. Na+ transport in plants. FEBS Lett. 2007, 581, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Hamam, A.M.; Coskun, D.; Britto, D.; Plett, D.T.; Kronzucker, H.J. Plasma-membrane electrical responses to salt and osmotic gradients contradict radiotracer kinetics, and reveal Na+-transport dynamics in rice (Oryza sativa L.). Planta 2019, 249, 1037–1051. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.; Chen, J.; Shabala, L.; Mithöfer, A.; Wang, H.; Qu, M.; Yu, M.; Cui, J.; Shabala, S. GABA operates upstream of H+-ATPase and improves salinity tolerance in Arabidopsis by enabling cytosolic K+ retention and Na+ exclusion. J. Exp. Bot. 2019, 70, 6349–6361. [Google Scholar] [CrossRef]
- Bose, J.; Shabala, L.; Pottosin, I.; Zeng, F.; Velarde-Buendi, A.; Massart, A.; Poschenrieder, C.; Haridi, Y.; Shabala, S. Kinetics of xylem loading, membrane potential maintenance, and sensitivity of K+-permeable channels to reactive oxygen species: Physiological traits that differentiate salinity tolerance between pea and barley. Plant Cell Environ. 2014, 37, 589–600. [Google Scholar] [CrossRef]
- Wang, H.; Shabala, L.; Zhou, M.; Shabala, S. Hydrogen peroxide-induced root Ca2+ and K+ fluxes correlate with salt tolerance in cereals: Towards the cell-based phenotyping. Int. J. Mol. Sci. 2018, 19, 702. [Google Scholar] [CrossRef]
- Bridges, D.; Fraser, M.E.; Moorhead, G.B. Cyclic nucleotide binding proteins in the Arabidopsis thaliana and Oryza sativa genomes. BMC Bioinform. 2005, 6, 6. [Google Scholar] [CrossRef]
- Gobert, A.; Park, G.; Amtmann, A.; Sanders, D.; Maathuis, F.J.M. Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a non-selective ion transporter involved in germination and cation transport. J. Exp. Bot. 2006, 57, 791–800. [Google Scholar] [CrossRef]
- Senadheera, P.; Singh, R.K.; Maathuis, F.J.M. Differentially expressed membrane transporters in rice roots may contribute to cultivar dependent salt tolerance. J. Exp. Bot. 2009, 60, 2553–2563. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.K.; Jing, W.; Zhang, Q.; Zhang, W.H. Cyclic nucleotide gated channel 10 negatively regulates salt tolerance by mediating Na+ transport in Arabidopsis. J. Plant Res. 2015, 128, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Lecourieux, D.; Mazars, C.; Pauly, N.; Ranjeva, R.; Pugin, A. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 2002, 14, 2627–2641. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.C.; Brenner, E.D.; DeSalle, R.; Nitabach, M.N.; Holmes, T.C.; Coruzzi, G.M. Phylogenetic and expression analysis of the glutamate-receptor-like gene family in Arabidopsis thaliana. Mol. Biol. Evol. 2002, 19, 1066–1082. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Lee, C.E.; Lin, Y.S.; Lee, M.H.; Chen, P.Y.; Chang, H.C.; Chang, I.F. The glutamate receptor-like protein GLR3.7 interacts with 14-3-3ω and participates in salt stress response in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, X.; Sun, T.; Tian, Q.; Zhang, W.H. Glutamate receptor homolog 3.4 is involved in regulation of Seed germination under salt stress in Arabidopsis. Plant Cell Physiol. 2018, 59, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Vacuolar two-pore K+ channels act as vacuolar osmosensors. New Phytol. 2011, 191, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Pottosin, I.I.; Martínez-Estévez, M.; Dobrovinskaya, O.R.; Muñiz, J. Potassium-selective channel in the red beet vacuolar membrane. J. Exp. Bot. 2003, 54, 663–667. [Google Scholar] [CrossRef]
- Isner, J.C.; Begum, A.; Nuehse, T.; Hetherington, A.M.; Maathuis, F.J.M. KIN7 kinase regulates the vacuolar TPK1 K+ channel during stomatal closure. Curr. Biol. 2018, 28, 466–472. [Google Scholar] [CrossRef]
- Hamamoto, S.; Marui, J.; Matsuoka, K.; Maeshima, M.; Yabe, I.; Uozumi, N. Characterization of a tobacco TPK-type K+ channel as a novel tonoplast K+ channel using yeast tonoplasts. J. Biol. Chem. 2008, 283, 1911–1920. [Google Scholar] [CrossRef]
- Latz, A.; Mehlmer, N.; Zapf, S.; Mueller, T.D.; Wurzinger, B.; Pfister, B.; Csaszar, E.; Hedrich, R.; Teige, M.; Becker, D. Salt stress triggers phosphorylation of the Arabidopsis vacuolar K+ channel TPK1 by calcium-dependent protein kinases (CDPKs). Mol. Plant. 2013, 6, 274–1289. [Google Scholar] [CrossRef]
- Isayenkov, S.; Isner, J.C.; Maathuis, F.J. Membrane localisation diversity of TPK channels and their physiological role. Plant Signal Behav. 2011, 6, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Devonshire, J.; Mohamed, R.; Schultze, M.; Maathuis, F.J.M. Overexpression of the potassium channel TPKb in small vacuoles confers osmotic and drought tolerance to rice. New Phytol. 2016, 209, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Grabov, A. Plant KT/KUP/HAK potassium transporters: Single family—Multiple functions. Ann. Bot. 2007, 99, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Chen, Y.; Li, R.; Wang, H.; Weim, J. Genome-wide analysis and identification of HAK potassium transporter gene family in maize (Zea mays L.). Mol. Biol. Rep. 2012, 39, 8465–8473. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, A.J.; Peralta, M.L.; Santa-Maria, G.E. Expression of potassium-transporter coding genes, and kinetics of rubidium uptake, along a longitudinal root axis. Plant Cell Environ. 2005, 28, 850–886. [Google Scholar] [CrossRef]
- Bañuelos, M.A.; Garciadeblas, B.; Cubero, B.; Rodriguez-Navarro, A. Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol. 2002, 130, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; Rim, Y.; Kim, E.; Kim, J.S. Genome-wide and molecular evolution analyses of the KT/HAK/KUP family in tomato (Solanum lycopersicum L.). Genes Genom. 2014, 36, 365–374. [Google Scholar] [CrossRef]
- Song, Z.Z.; Ma, R.J.; Yu, M.L. Genome-wide analysis and identification of KT/HAK/KUP potassium transporter gene family in peach (Prunus persica). Genet. Mol. Res. 2015, 14, 774–787. [Google Scholar] [CrossRef]
- Li, Y.; Peng, L.; Xie, C.; Shi, X.; Dong, C.; Shen, Q.; Xu, Y. Genome-wide identification, characterization, and expression analyses of the HAK/KUP/KT potassium transporter gene family reveals their involvement in K+ deficient and abiotic stress responses in pear rootstock seedlings. Plant Growth Regul. 2018, 85, 187–198. [Google Scholar] [CrossRef]
- Jin, R.; Jiang, W.; Yan, M.; Zhang, A.; Liu, M.; Zhao, P.; Chen, X.; Tang, Z. Genome-wide characterization and expression analysis of HAK K+ transport family in Ipomoea. Biotech 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lu, X.; Wang, Y.; Xie, Y.; Ma, J.; Cheng, X.; Xia, E.; Wan, X.; Zhang, Z. HAK/KUP/KT family potassium transporter genes are involved in potassium deficiency and stress responses in tea plants (Camellia sinensis L.): Expression and functional analysis. BMC Genom. 2020, 21, 556. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Sugawara, M.; Okada, T.; Taira, T.; Kaothien-Nakayama, P.; Katsuhara, M.; Shinmyo, A.; Nakayama, H. Rice sodium-insensitive potassium transporter, OsHAK5, confers increased salt tolerance in tobacco BY2 cells. J. Biosci. Bioeng. 2011, 111, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, L.; Shen, Z.; Jing, W.; Ge, H.; Zhao, J.; Zhang, W. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Environ. 2015, 38, 2766–2779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, L.; Chen, B.; Qin, Z.; Xiao, Y.; Zhang, Y.; Yao, R.; Liu, H.; Yang, H. Progress in understanding the physiological and molecular responses of Populus to salt stress. Int. J. Mol. Sci. 2019, 20, 1312. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Shi, Y.; Shen, L.; Cao, C.; Shen, Y.; Jing, W.; Tian, Q.; Lin, F.; Li, W.; Zhang, W. An endoplasmic reticulum-localized cytochrome b5 regulates high-affinity K+ transport in response to salt stress in rice. Proc. Nat. Acad. Sci. USA 2021, 118, e2114347118. [Google Scholar] [CrossRef]
- Li, A.; Liu, A.; Wu, S.; Qu, K.; Hu, H.; Yang, J.; Shrestha, N.; Liu, J.; Ren, G. Comparison of structural variants in the whole genome sequences of two Medicago truncatula ecotypes: Jemalong A17 and R108. BMC Plant Biol. 2022, 22, 77. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Acharya, B.R.; Sandhu, D.; Dueñas, C.; Dueñas, M.; Pudussery, M.; Kaundal, A.; Ferreira, J.F.S.; Suarez, D.L.; Skaggs, T.H. Morphological, physiological, biochemical, and transcriptome studies reveal the importance of transporters and stress signaling pathways during salinity stress in Prunus. Sci. Rep. 2022, 12, 274. [Google Scholar] [CrossRef]
- Santa-María, G.E.; Rubio, F.; Dubcovsky, J.; Rodríguez-Navarro, A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 1997, 9, 2281–2289. [Google Scholar] [CrossRef]
- Su, H.; Golldack, D.; Katsuhara, M.; Zhao, C.S.; Bohnert, H.J. Expression and stress-dependent induction of potassium channel transcripts in the common ice plant. Plant Physiol. 2001, 125, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, R.P.; Nagpal, P.; Reed, J. A mutation in the Arabidopsis KT2/KUP2 potassium transporter gene affects shoot cell expansion. Plant Cell. 2002, 14, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Frans, J.M.; Maathuis, F.J.M. The role of monovalent cation transporters in plant responses to salinity. J. Exp. Bot. 2006, 57, 1137–1147. [Google Scholar] [CrossRef]
- Han, M.; Wu, W.; Wu, W.H.; Wang, Y. Potassium transporter KUP7 is involved in K+ acquisition and translocation in Arabidopsis root under K+-limited conditions. Mol. Plant 2016, 9, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Ródenas, R.; García-Legaz, M.F.; López-Gómez, E.; Martínez, V.; Rubio, F.; Botella, M.A. NO3−, PO43− and SO42− deprivation reduced LKT1-mediated low-affinity K+ uptake and SKOR-mediated K+ translocation in tomato and Arabidopsis plants. Physiol. Plant. 2017, 160, 410–424. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Horie, Y.; Hauser, F.; Schroeder, J.I. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009, 14, 660–668. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, Y.; Zhou, H.; Hong, Y.; Zhao, C.; Shabalak, S.; Lv, C.; Guo, B.; Zhou, M.; Xu, Z. Genome wide association study and haplotype analysis reveals the role of HvHKT1;5 in potassium retention but not Na+ exclusion in barley (Hordeum vulgare L.). Environ. Exp. Bot. 2022, 201, 104973. [Google Scholar] [CrossRef]
- Asins, M.J.; Villalta, I.; Aly, M.M.; Olías, R.; Morales, P.A.; Huertas, R.; Belver, A. Two closely linked tomato HKT coding genes are positional candidates for the major tomato QTL involved in Na+/K+ homeostasis. Plant Cell Environ. 2013, 36, 1171–1191. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Gu, H.; Wu, B.; Zhang, H.; Yuan, X.; Cui, X. GmHKT1;4, a novel soybean gene regulating Na+/K+ ratio in roots enhances salt tolerance in transgenic plants. Plant Growth Regul. 2014, 73, 299–308. [Google Scholar] [CrossRef]
- Venkataraman, V.; Shabala, S.; Véry, A.A.; Hariharan, G.N.; Somasundaram, S.; Pulipati, S.; Sellamuthu, G.; Harikrishnan, M.; Kumari, K.; Shabala, L.; et al. To exclude or to accumulate? Revealing the role of the sodium HKT1;5 transporter in plant adaptive responses to varying soil salinity. Plant Physiol. Biochem. 2021, 169, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Costa, A.; Nakayama, H.; Katsuhara, M.; Shinmyo, A.; Horie, T. OsHKT2;2/1-mediated Na+ influx over K+ uptake in roots potentially increases toxic Na+ accumulation in a salt-tolerant landrace of rice Nona Bokra upon salinity stress. J. Plant Res. 2016, 129, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.I.; Yamaji, N.; Yamamoto, H.; Okubo, K.; Ueno, H.; Costa, A.; Tanoi, K.; Matsumura, H.; Fujii-Kashino, M.; Horiuchi, T.; et al. OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Physiol. Plant. 2017, 91, 657–670. [Google Scholar] [CrossRef]
- Mohanty, A.; Chakraborty, K.; Mondal, S.; Jena, P.; Panda, R.K.; Samal, K.C.; Chattopadhyay, K. Relative contribution of ion exclusion and tissue tolerance traits govern the differential response of rice towards salt stress at seedling and reproductive stages. Environ. Exp. Bot. 2023, 206, 105131. [Google Scholar] [CrossRef]
- Wang, J.; Nan, N.; Li, N.; Liu, Y.; Wang, T.J.; Hwang, I.; Liu, B.; Xu, Z.Y. A DNA methylation reader-chaperone regulator-transcription factor complex activates OsHKT1;5 expression during salinity stress. Plant Cell 2020, 32, 3535–3558. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Yang, Z.; Li, C.; Yan, Y.; Liu, Z.; Nazir, M.M.; Xu, J. Loss-of-function mutations of OsbHLH044 transcription factor lead to salinity sensitivity and a greater chalkiness in rice (Oryza sativa L.). Plant Physiol. Biochem. 2022, 193, 110–123. [Google Scholar] [CrossRef]
- Hazzouri, K.M.; Khraiwesh, B.; Amiri, K.M.A.; Pauli, D.; Blake, T.; Shahid, M.; Mullath, S.K.; Nelson, D.; Mansour, A.L.; Salehi-Ashtiani, K.; et al. Mapping of HKT1;5 Gene in barley using GWAS approach and its implication in salt tolerance mechanism. Front. Plant Sci. 2018, 9, 156. [Google Scholar] [CrossRef]
- Hmidi, D.; Messedi, D.; Corratgé-Faillie, C.; Marhuenda, T.; Fizames, C.; Zorrig, W.; Abdelly, C.; Sentenac, H.; Véry, A. Investigation of Na+ and K+ transport in halophytes: Functional analysis of the HmHKT2;1 transporter from Hordeum maritimum and expression under saline conditions. Plant Cell Physiol. 2019, 60, 2423–2435. [Google Scholar] [CrossRef]
- Cao, Y.; Liang, X.; Yin, P.; Zhang, M.; Jiang, C. A domestication-associated reduction in K+-preferring HKT transporter activity underlies maize shoot K+ accumulation and salt tolerance. New Phytol. 2019, 222, 301–317. [Google Scholar] [CrossRef]
- Leidi, E.O.; Barragánm, V.; Rubio, L.; El-Hamdaoui, A.; Ruiz, M.T.; Cubero, B.; Fernández, J.A.; Bressan, R.A.; Hasegawa, P.M.; Quintero, F.J.; et al. The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J. 2010, 61, 495–506. [Google Scholar] [CrossRef]
- Kumari, P.H.; Kumar, S.A.; Sivan, P.; Katam, R.; Suravajhala, P.; Rao, K.S.; Varshney, R.K.; Kishor, P.B.K. Overexpression of a plasma membrane bound Na+/H+ antiporter-like protein (SbNHXLP) confers salt tolerance and improves fruit yield in tomato by maintaining ion homeostasis. Front. Plant Sci. 2017, 7, 2016. [Google Scholar] [CrossRef] [PubMed]
- Al-Harrasi, I.; Jana, G.A.; Patankar, H.V.; Al-Yahyai, R.; Rajappa, S.; Kumar, P.P.; Yaish, M.W. A novel tonoplast Na+/H+ antiporter gene from date palm (PdNHX6) confers enhanced salt tolerance response in Arabidopsis. Plant Cell Rep. 2020, 39, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, D.; Jin, T.; Chang, Q.; Yin, D.; Xu, S.; Liu, B.; Liu, L. The vacuolar Na+/H+ antiporter gene SsNHX1 from the halophyte Salsola soda confers salt tolerance in transgenic alfalfa (Medicago sativa L.). Plant Mol. Biol. Rep. 2011, 29, 278–290. [Google Scholar] [CrossRef]
- Joshi, S.; Kaur, K.; Khare, T.; Srivastava, A.K.; Suprasanna, P.; Kumar, V. Genome-wide identification, characterization and transcriptional profiling of NHX-type (Na+/H+) antiporters under salinity stress in soybean. 3 Biotech 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.E.; Kim, D.; Ali, S.; Fedoroff, N.V.; Al-Babili, S. The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci. 2017, 263, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Nakamura, A.; Hara, N.; Toki, S.; Tanaka, Y. Molecular and functional analyses of rice NHX-type Na+/H+ antiporter genes. Planta 2011, 233, 175–188. [Google Scholar] [CrossRef]
- Stephan, A.B.; Kunz, H.; Yang, E.; Schroeder, J.I. Rapid hyperosmotic-induced Ca2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters. Proc. Nat. Acad. Sci. USA 2018, 113, E5242–E5249. [Google Scholar] [CrossRef]
- Tsujii, M.; Kera, K.; Hamamoto, S.; Kuromori, T.; Shikanai, T.; Uozumi, N. Evidence for potassium transport activity of Arabidopsis KEA1-KEA6. Sci. Rep. 2019, 9, 10040. [Google Scholar] [CrossRef]
- Cellier, F.; Conéjéro, G.; Ricaud, L.; Luu, D.T.; Lepetit, M.; Gosti, F.; Casse, F. Characterization of AtCHX17, a member of the cation/H+ exchangers, CHX family, from Arabidopsis thaliana suggests a role in K+ homeostasis. Plant J. 2004, 39, 834–846. [Google Scholar] [CrossRef]
- Jia, B.; Sun, M.; Duan-Mu, H.; Ding, X.; Liu, B.; Zhu, Y.; Sun, X. GsCHX19.3, a member of cation/H+ exchanger superfamily from wild soybean contributes to high salinity and carbonate alkaline tolerance. Sci. Rep. 2017, 7, 9423. [Google Scholar] [CrossRef]
- Cai, X.; Jia, B.; Sun, M.; Sun, X. Insights into the regulation of wild soybean tolerance to salt-alkaline stress. Front. Plant Sci. 2022, 13, 1002302. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Day, D.A.; Fricke, W.; Watt, M.; Arsova, B.; Barkla, B.J.; Bose, J.; Byrt, C.S.; Chen, Z.; Foster, K.J.; et al. Energy costs of salt tolerance in crop plants. New Phytol. 2019, 225, 1072–1090. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Ramiske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhou, X.; Zhang, X.; Liu, A.; Xiang, Y.; Yan, M. The Arabidopsis AtUNC-93 acts as a positive regulator of abiotic stress tolerance and plant growth via modulation of ABA signaling and K+ homeostasis. Front Plant Sci. 2018, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, M.; Zhao, Y.; Wen, X.; Guo, Z.; Lu, S. CBL4-CIPK5 pathway confers salt but not drought and chilling tolerance by regulating ion homeostasis. Environ. Exp. Bot. 2020, 179, 104230. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, F.; Han, X.; Xia, X.; Yin, W. The calcium sensor PeCBL1, interacting with PeCIPK24/25 and PeCIPK26, regulates Na+/K+ homeostasis in Populus euphratica. Plant Cell Rep. 2013, 32, 611–621. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Yang, X.X.; Zhang, L.; Zhang, J.; Du, B.; Yao, L.; Li, X.T.; Guo, C. Alfalfa MsCBL4 enhances calcium metabolism but not sodium transport in transgenic tobacco under salt and saline-alkali stress. Plant Cell Rep. 2020, 39, 997–1011. [Google Scholar] [CrossRef]
- Amjad, M.; Akhtar, J.; Murtaza, B.; Abbas, G.; Jawad, H. Differential accumulation of potassium results in varied salt-tolerance response in tomato (Solanum lycopersicum L.) cultivars. Hortic. Environ. Biotechnol. 2016, 57, 248–258. [Google Scholar] [CrossRef]
- Che, Y.; Yao, T.; Wang, H.; Wang, Z.; Zhang, H.; Sun, G.; Zhang, H. Potassium ion regulates hormone, Ca2+ and H2O2 signal transduction and antioxidant activities to improve salt stress resistance in tobacco. Plant Physiol. Biochem. 2022, 186, 40–51. [Google Scholar] [CrossRef]
- Luo, Q.; Wei, Q.; Wang, R.; Zhang, Y.; Zhang, F.; He, Y.; Zhou, S.; Feng, J.; Yang, G.; He, G. BdCIPK31, a calcineurin B-like protein-interacting protein kinase, regulates plant response to drought and salt stress. Front Plant Sci. 2017, 8, 1184. [Google Scholar] [CrossRef]
- Khan, Y.; Xiong, Z.; Zhang, H.; Liu, S.; Yaseen, T.; Hui, T. Expression and roles of GRAS gene family in plant growth, signal transduction, biotic and abiotic stress resistance and symbiosis formation. Plant Biol. 2021, 24, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Q.-H.; Yu, Y.N.; Qiao, Y.-M.; ul Haq, S.; Gong, Z.-H. The CBL-CIPK pathway in plant response to stress signals. Int. J. Mol. Sci. 2020, 21, 5668. [Google Scholar] [CrossRef] [PubMed]
- Tomar, R.S.; Katarina, S.; Jajoo, A. Behind the scene: Critical role of reactive oxygen species and reactive nitrogen species in salt stress tolerance. J. Agron. Crop Sci. 2021, 207, 577–588. [Google Scholar] [CrossRef]
- Bose, J.; Pottosin, I.I.; Shabala, S.S.; Palmgren, M.G.; Shabala, S. Calcium efflux system in signaling and adaptation in plants. Front. Plant Sci. 2011, 2, 85. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Park, C.J.; Shin, R. Calcium channels and transporters: Roles in response to biotic and abiotic stresses. Front. Plant Sci. Sec. Plant Membr. Traffic Transp. 2022, 13, 964059. [Google Scholar] [CrossRef]
- Pirayesh, N.; Giridhar, M.; Khedher, A.B.; Vothknecht, U.C.; Chigri, F. Organellar calcium signaling in plants: An update. BBA Mol. Cell Res. 2021, 4, 118948. [Google Scholar] [CrossRef]
- Jiang, W.; Tong, T.; Chen, X.; Deng, F.; Zeng, F.; Pan, R.; Zhang, W.; Chen, G.; Chen, Z.H. Molecular response and evolution of plant anion transport systems to abiotic stress. Plant Mol. Biol. 2022, 110, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Shigaki, T.; Hirschi, K.D. Diverse functions and molecular properties emerging for CAX cation/H+ exchangers in plants. Plant Biol. 2006, 8, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shigaki, T.; Mei, H.; Guo, X.G.; Cheng, N.-H.; Hirschi, K.D. Interaction between Arabidopsis Ca2+/H+ exchanger CAX1 and CAX3. J. Biol. Chem. 2009, 284, 4605–4615. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Herde, M.; Romeis, T. Calcium-dependent protein kinases: Hubs in plant stress signaling and development. Plant Physiol. 2013, 163, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Yemelyanov, V.; Shishova, M.; Chirkova, T.V.; Lindberg, S. Anoxia-induced elevation of cytosolic Ca2+ concentration in rice and wheat protoplasts. Planta 2011, 234, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.H.; Lindberg, S.; Maity, P.J.; Geilfus, C.M.; Plieth, C.; Mühling, K.H. Apoplastic and cytosolic Ca2+ and pH dynamics in salt-stressed Vicia faba leaves change in response to calcium. Func. Plant Biol. 2017, 44, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Calcium signaling in Arabidopsis thaliana responsing to drought and salinity. Plant J. 1997, 12, 1067–1078. [Google Scholar] [CrossRef]
- Gao, D.; Knight, M.R.; Trewavas, A.J.; Sattelmacher, B.; Plieth, C. Self-reporting Arabidopsis expressing pH and Ca2+ indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol. 2004, 134, 898–908. [Google Scholar] [CrossRef]
- Premkumar, A.; Lindberg, S.; Lager, I.; Rasmussen, U.; Schulz, A. Phospholipases D with C2-domains are involved in hypoxia signal transduction in Arabidopsis. Physiol. Plant. 2018, 167, 90–110. [Google Scholar] [CrossRef]
- Sebastiani, L.; Lindberg, S.; Vitagliano, C. Cytoplasmic free Ca2+ dynamics in single tomato (Lycopersicon esculentum) protoplasts subjected to chilling temperatures. Physiol. Plant. 1999, 105, 239–245. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, L.; Nie, J.; Zhang, C.; Han, X.; Li, Q.; Qin, L.; Wang, M.; Huang, X.; Yu, F.; et al. Structure and activation mechanism of the rice Salt Overly Sensitive 1 (SOS1) Na+/H+ antiporter. Nat. Plants 2023, 9, 1924–1936. [Google Scholar] [CrossRef] [PubMed]
- Lamers, J.; Van Der Meer, T.; Testerink, C. How plants sense and respond to stressful environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhou, Y.; Jiang, X. Structure, function, and regulation of the plasma membrane Na+/H+ antiporter Salt Overly Sensitive 1 in plants. Front. Plant Sci. 2022, 13, 866265. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.A.; Lindberg, S.; Seidel, T.; Golldack, D.; Yemelyanov, V. Sodium sensing induces different changes in free cytosolic calcium concentration and pH in salt-tolerant and -sensitive rice (Oryza sativa) cultivars. Physiol. Plant. 2007, 130, 99–111. [Google Scholar] [CrossRef]
- Laohavisit, A.; Richards, S.L.; Shabala, L.; Chen, C.; Colaço, R.D.D.A.; Swarbreck, S.M.; Shaw, E.; Dark, A.; Shabala, S.; Shang, Z.; et al. Salinity-induced calcium signaling and root adaptation in Arabidopsis require the calcium regulatory protein annexin1. Plant Physiol. 2013, 163, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Konopka-Postupolka, D.; Clark, G. Annexins overlooked regulators of membrane trafficking in plant cells. Int. J. Mol. Sci. 2017, 18, 863. [Google Scholar] [CrossRef] [PubMed]
- Demidschik, V.; Shabala, S. Mechanisms of cytosolic calcium elevation in plants: The role of ion channels, calcium extrusion systems and NADPH oxidase-mediated ‘ROS-Ca2+ Hub’. Func. Plant Biol. 2018, 45, 9–27. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Sig. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef]
- McCarty, D.R.; Chory, J. Conservation and innovation in plant signaling pathways. Cell 2000, 3, 201–209. [Google Scholar] [CrossRef]
- Rubio, F.; Fon, M.; Ródenas, R.; Nieves-Cordones, M.; Alemán, F.; Rivero, R.M.; Martínez, V. A low K+ signal is required for functional high-affinity K+ uptake through HAK5 transporters. Physiol Plant. 2014, 152, 558–570. [Google Scholar] [CrossRef]
- Pandey, G.K. Emergence of a novel calcium-signaling pathway in plants: CBL-CIPK signaling network. Phys. Mol. Biol. Plants 2008, 14, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Xu, Z.; Luoni, L.; Bonza, M.G.; Doccula, F.G.; De Michelis, M.I.; Morris, R.J.; Schwarzländer, M. Cellular Ca2+ signals generate defined pH signatures in plants. Plant Cell 2018, 30, 2704–2719. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Schroeder, J.I. Roles of ion channels in initiation of signal transduction in higher plants. In Signal Transduction in Plants; Aducci, P., Ed.; MCBU Molecular and Cell Biology Updates; Birkhäuser Basel: Basel, Switzerland, 1997. [Google Scholar] [CrossRef]
- Okazaki, Y.; Kikuyama, M.; Hiramoto, Y.; Iwasaki, N. Short-term regulation of cytosolic Ca2+, cytosolic pH and vacuolar pH under NaCl stress in the charophyte alga Nitellopsis obtuse. Plant Cell Environ. 1996, 19, 569–576. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiol, 4th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2006; p. 694. [Google Scholar]
- McKay, D.W.; McFarlane, H.E.; Qu, Y.; Situmorang, A.; Gilliham, M.; Wege, S. Plant trans-Golgi network/early endosome pH regulation requires cation chloride cotransporter (CCC1). Life 2022, 11, e70701. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.A.; Lindberg, S. Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal. Behav. 2010, 3, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Geros, H. Regulation by salt of vacuolar H+ATPase and H+pyrophosphatase activities and Na+/H+ exchange. Plant Signal. Behav. 2009, 4, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Pecherina, A.; Grinberg, M.; Ageyeva, M.; Zanegina, D.; Akinchits, E.; Brilkina, A.; Vodeneev, V. Salt-induced changes in cytosolic pH and photosynthesis in tobacco and potato leaves. Int. J. Mol. Sci. 2022, 24, 491. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Mader, C.; Schmitz, J.; Atladottir, J.; Fitchev, P.; Cornwell, M.L.; Koleske, A.J.; Crawford, S.E.; Gorelick, F. The vacuolar-ATPase modulates matrix metalloproteinase isoforms in human pancreatic cancer. Lab. Investig. 2011, 91, 732–743. [Google Scholar] [CrossRef]
- Hager, A. Role of plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J. Plant Res. 2003, 116, 4483–4505. [Google Scholar] [CrossRef]
- Pitann, B.; Schubert, S.; Mühling, K.H. Decline in leaf growth under salt stress due to an inhibition of H+-pumping activity and increase in apoplastic pH of maize leaves. J. Plant Nutr. Soil Sci. 2009, 172, 535–543. [Google Scholar] [CrossRef]
- Buch-Pedersen, M.J.; Rudashevskaya, E.L.; Berner, T.S.; Venema, K.; Palmgren, M.G. Potassium as an intrinsic uncoupler of the plasma membrane H+-ATPase. J. Biol. Chem. 2006, 15, 38285–38292. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, K.; Pedersen, B.P.; Sørensen, D.M.; Pedersen, M.J. Structural identification of cation binding pockets in the plasma membrane proton pump. Proc. Nat. Acad. Sci. USA 2012, 107, 21400–21405. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses. Role of calcium and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef]
- Hauwink, A.L. The conduction of excitation in Mimosa pudica. Recl. Trav. Bot. Neerl. 1935, 32, 51–91. [Google Scholar]
- Ricca, U. Transmission of stimuli in plants. Nature 1926, 117, 654–655. [Google Scholar] [CrossRef]
- Choi, W.G.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J. 2017, 90, 698–707. [Google Scholar] [CrossRef]
- Fichman, Y.; Mittler, R. Integration of electric, calcium, reactive oxygen species and hydraulic signals during rapid systemic signaling in plants. Plant J. 2021, 107, 7–20. [Google Scholar] [CrossRef]
- Felle, H.H.; Zimmermann, M.R. Systemic signalling in barley through action potentials. Planta 2007, 226, 203–214. [Google Scholar] [CrossRef]
- Bellandi, A.; Papp, D.; Breakspear, A.; Joyce, A.J.; Johnston, M.G.; de Keljzer, J.; Raven, E.C.; Ohtsu, M.; Vincent, T.R.; Miller, A.J.; et al. Diffusion and bulk flow of amino acids mediate calcium waves in plants. Sci. Adv. 2022, 8, eabo6693. [Google Scholar] [CrossRef]
- Shao, Q.; Gao, Q.; Lhamo, D.; Zhang, H.; Luan, S. The glutamate- and pH-regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis. Sci. Signal. 2020, 14, 453. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dang, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Choi, W.G.; Gilroy, S.; Morris, R.J. A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Wang, C.; Li, K.; Luan, S. The CBL–CIPK calcium signaling network: Unified paradigm from 20 years of discoveries. Trends Plant Sci. 2020, 25, 604–617. [Google Scholar] [CrossRef] [PubMed]
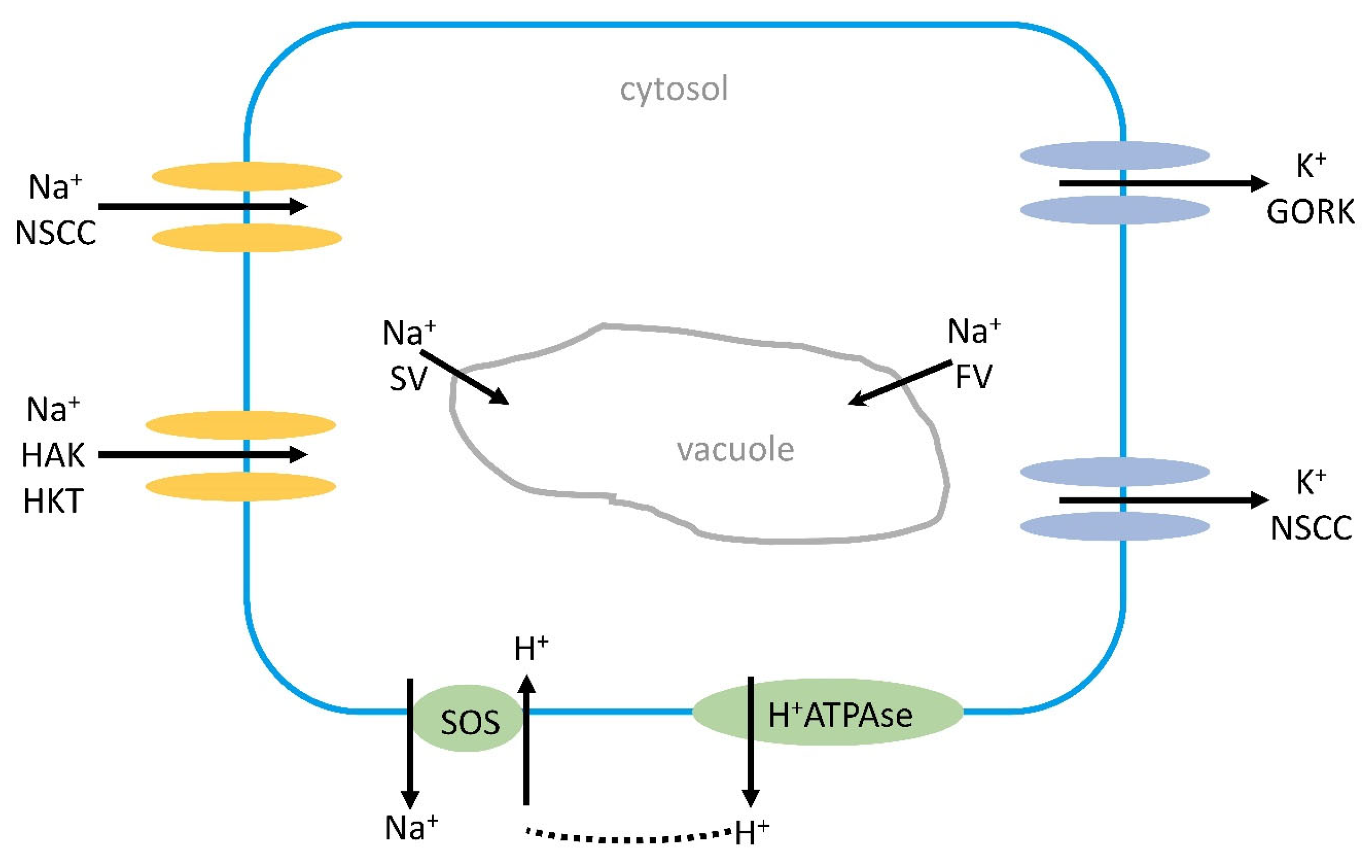

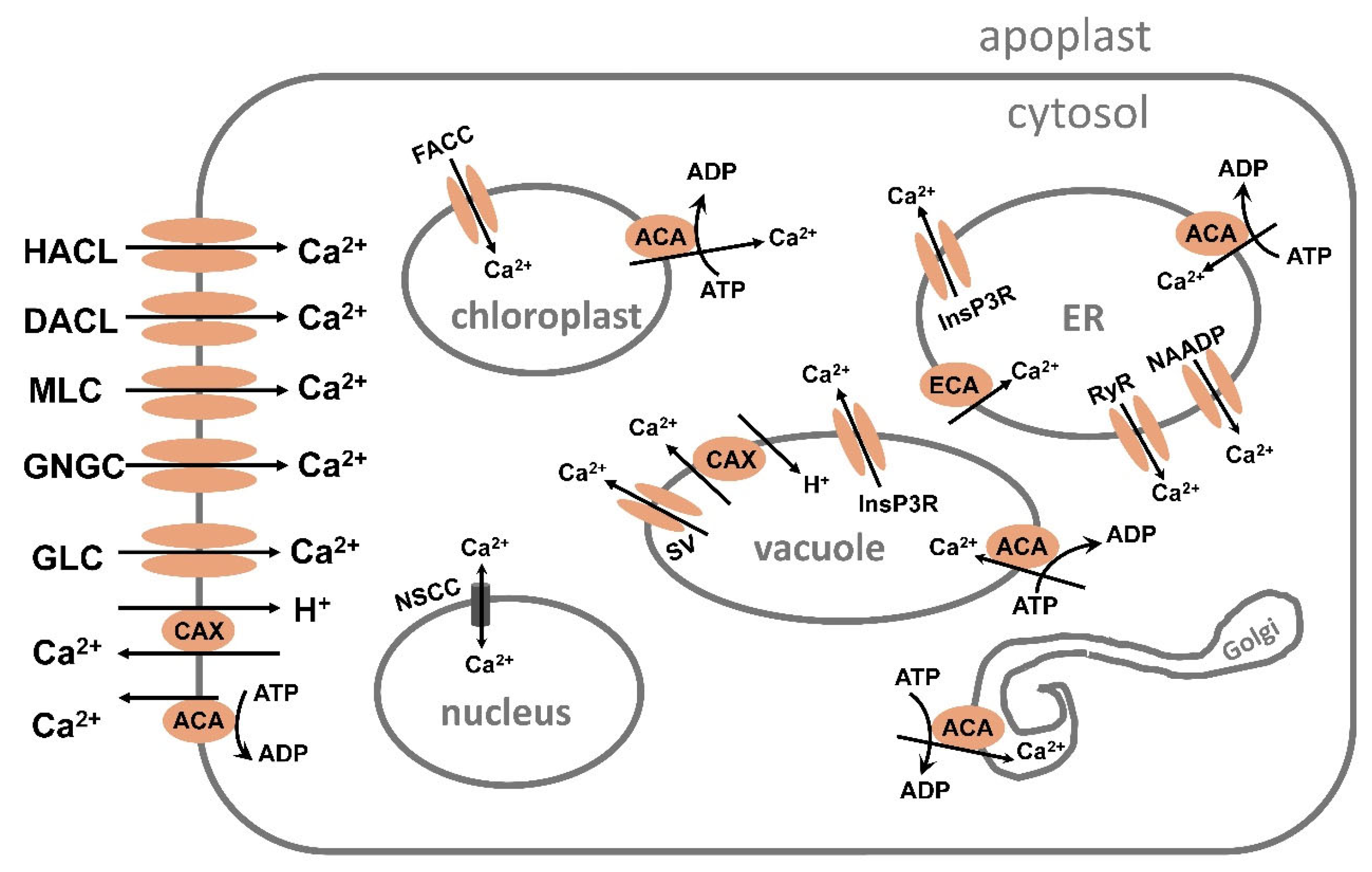
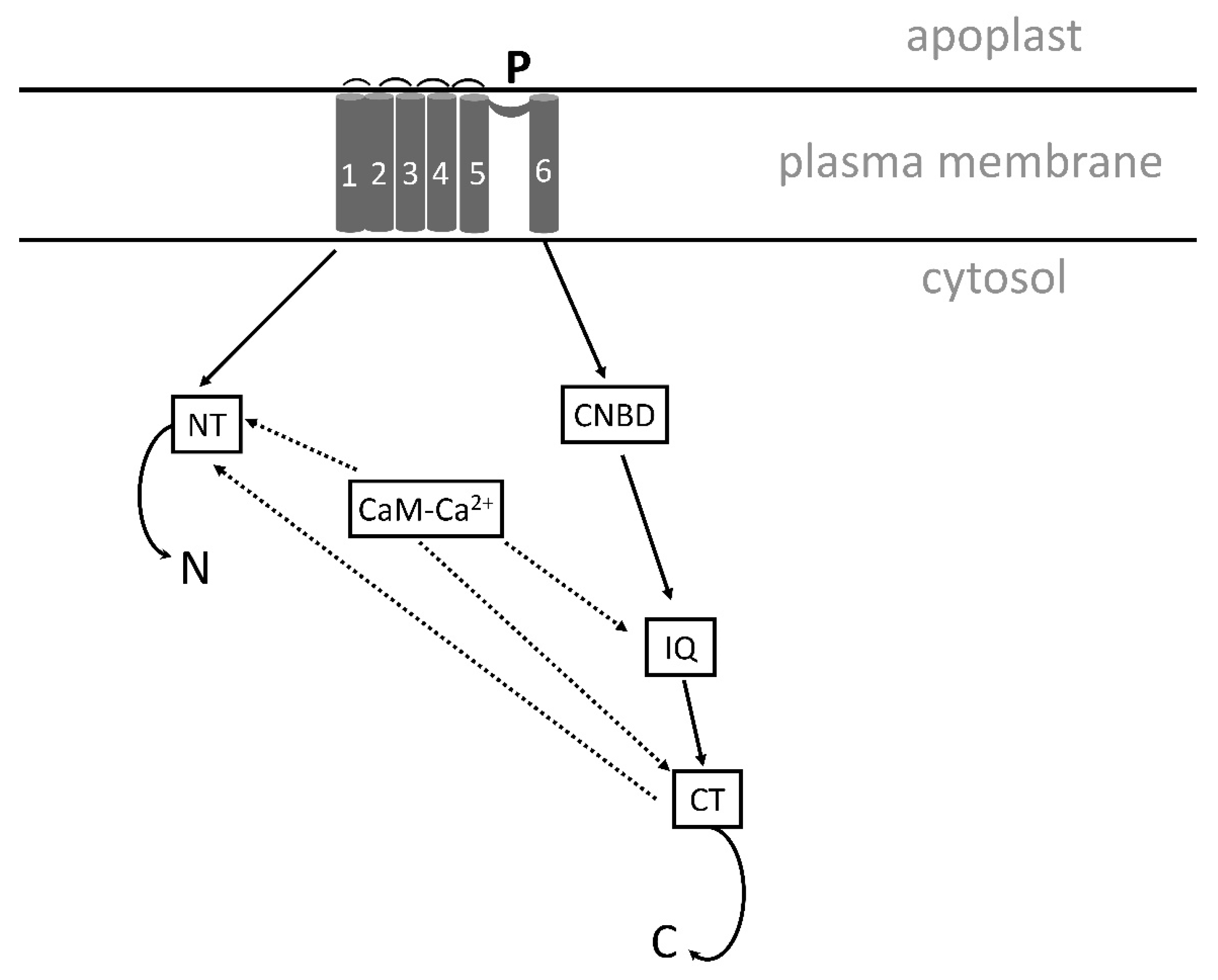
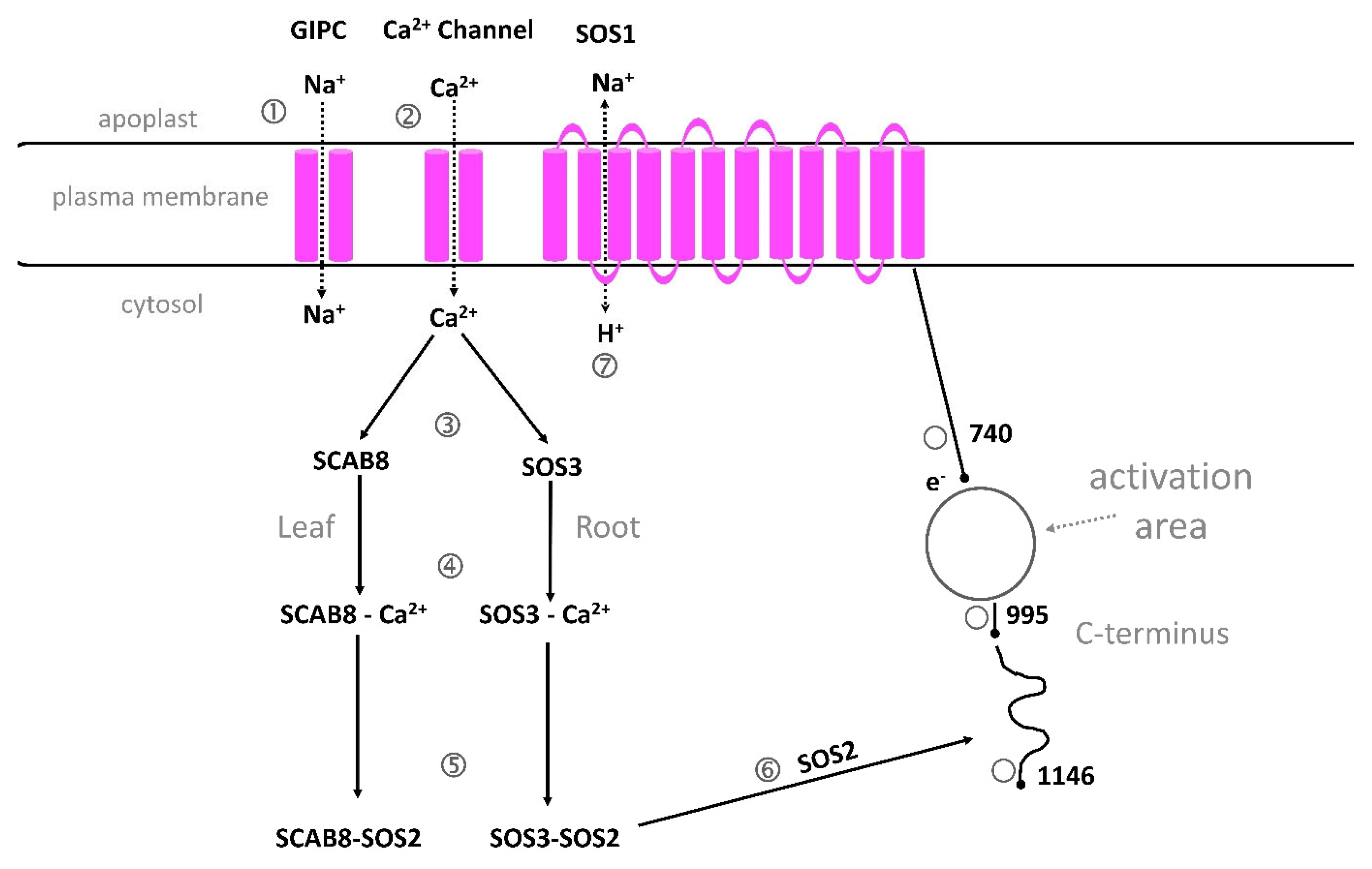

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindberg, S.; Premkumar, A. Ion Changes and Signaling under Salt Stress in Wheat and Other Important Crops. Plants 2024, 13, 46. https://doi.org/10.3390/plants13010046
Lindberg S, Premkumar A. Ion Changes and Signaling under Salt Stress in Wheat and Other Important Crops. Plants. 2024; 13(1):46. https://doi.org/10.3390/plants13010046
Chicago/Turabian StyleLindberg, Sylvia, and Albert Premkumar. 2024. "Ion Changes and Signaling under Salt Stress in Wheat and Other Important Crops" Plants 13, no. 1: 46. https://doi.org/10.3390/plants13010046
APA StyleLindberg, S., & Premkumar, A. (2024). Ion Changes and Signaling under Salt Stress in Wheat and Other Important Crops. Plants, 13(1), 46. https://doi.org/10.3390/plants13010046






