Abstract
Satureja bachtiarica is an endemic plant from the Lamiaceae family, growing in the Zagros mountain range in Iran. Even if S. bachtiarica is reported to possess many biological activities, little is known about its chemical composition. For this reason, in the present research, a phytochemical investigation of this species was carried out. To have a preliminary metabolite profile of S. bachtiarica, the n-BuOH extract was analyzed using LC-ESI/LTQOrbitrap/MS/MS in negative ion mode, allowing the identification of specialized metabolites belonging to flavonoid, monoterpene, indol, phenylpropanoid, phenolic, lignan, coumarin, biphenyl, and triterpene classes. The LC-MS/MS analysis guided the isolation of compounds, and their structures were characterized using spectroscopic methods including 1D- and 2D-NMR experiments and HRMSn analysis. In this way, a compound never reported before belonging to the biphenyl class was identified. Total flavonoid content of the extract along with the antioxidant activity were assessed. Based on the traditional uses of S. bachtiarica suggesting potential antibacterial properties, an evaluation of the biofilm inhibitory activity of the extract and isolated compounds against mature biofilms of Acinetobacter baumannii, Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa, and Staphylococcus aureus, as well as their influence on the metabolism of sessile bacterial cells, was conducted. The results evidenced that some compounds including parmentin B, biphenyls, and 1-(1H-indole-3-carboxylate)-β-D-glucopyranoside might inhibit some changes occurring in the bacterial cells, which increases their virulence. In particular, biphenyl derivatives at a concentration of 80 μg/mL were capable of limiting remarkably the mature biofilms of A. baumannii and L. monocytogenes remarkably at a percentage ranging between 52.76% and 75.02%, and they reached an inhibition percentage of 69.28 % against E. coli. Biphenyl derivatives were also effective in exerting an inhibitory action against the mature biofilm of P. aeruginosa (inhibition ranging from 59.38% to 81.08%) and Staphylococcus aureus (inhibition percentage reached 82.94%). Of note, the biphenyl derivatives resulted in being capable of acting on the metabolism of the cells within the biofilm of all five pathogens.
1. Introduction
The Lamiaceae family is one of the major sources of medicinal and food plants, highly valued mainly for their content in essential oils. Members of Lamiaceae find culinary applications as spices or are considered medicinal plants in Iranian folk medicine for their biological activities [1]. The essential oils of the Lamiaceae family have been studied extensively for several uses including antibacterial, antioxidant, and natural preservative properties [2].
The genus Satureja (Lamiaceae) was first named by the Roman writer Pliny. The name “Satureja” is derived from the Latin word “satureia”, and it means “herb of satyrs”. According to historical accounts, the cultivation of Satureja was banned in monasteries, possibly due to its association with pagan traditions or beliefs. Satureja comprises approximately 200 species of aromatic herbs and shrubs. These plants are known for their aromatic leaves and often have culinary and medicinal uses. More than 30 species of Satureja are found in the eastern parts of the Mediterranean area, where they are native [3]. Phytochemical investigations on Satureja spp. have revealed the occurrence of flavones, anthocyanins, phenolic acids, triterpenes, diterpenes, and sterols [4]. The essential oils derived from Satureja species have been found to exhibit a diverse array of biological and pharmacological properties, including antioxidant, anti-inflammatory, antispasmodic, analgesic, antiseptic, antibacterial, antiviral, and anti-nociceptive actions [1,5]. Moreover, the genus Satureja is receiving particular attention due to its antifungal and antimicrobial properties [6].
Bakhtiari savory (S. bachtiarica), also named “Marzeh e koohi” in Persian, has long been cultivated as a spice and as remedy in traditional medicine in Iran. The leaves are applied extensively as a flavoring agent for several type of foodstuffs due to their strong aroma and unique flavor [5,7]. The essential oil composition of the aerial parts of S. bachtiarica has been extensively investigated [5,8]. They represent a rich source of phenolic monoterpenes like carvacrol and thymol, along with other monoterpenes like γ-terpinene and p-cymene [9].
In the literature, few articles have reported on the biological properties of S. bachtiarica polar extracts. The ethanol extract from the Satureja species demonstrated an immunostimulant effect in Wistar rats [10], while the methanol extract was evaluated for its total flavonoid and phenolic content and antioxidant activity [7]. In a study by Alghooneh et al., the antibacterial and antioxidant capabilities of S. bachtiarica extract had a substantial impact on the characteristics of cheese [7]. In another study reported by Skendi et al., satureja, oregano, and thyme used in a bread recipe showed antimicrobial properties and could be used as antimicrobial ingredients in food products [6]. To the best of our knowledge, this is the first phytochemical investigation of the polar extract of the aerial parts of S. bachtiarica performed using HR-LC-ESI-Orbitrap-MS followed by isolation and structure elucidation of compounds via 1D- and 2D-NMR experiments. Moreover, to more deeply investigate the biological effects of S. bachtiarica extract, the total flavonoid content and the antioxidant activity of the extract were evaluated.
In recent years, there is an increasing interest in the identification of new antimicrobials to contrast the emergence of antibiotic resistance among pathogenic microorganisms, along with the formation of biofilm. Therefore, considering the reported activity of Satureja spp. including S. bachtiarica in foods, the antimicrobial activities of the extract and isolated compounds were evaluated against different Gram-positive and Gram-negative human bacteria. Their ability to inhibit the mature biofilms created by these bacteria and to affect the metabolism of microbial cells present within the biofilms was also tested.
2. Results and Discussion
2.1. LC-MS and NMR Analysis of Specialized Metabolites Occurring in S. bachtiarica n-BuOH Extract
To obtain a preliminary metabolite profile of n-BuOH extract of S. bachtiarica, an analytical approach based on LC-ESI-FT-MS and LC-ESI-FT-MS/MS analysis in negative ion mode was developed, with the detection of 39 compounds (Figure 1). Based on the accurate masses of the main peaks, their distinctive fragmentation patterns, and comparison of the results to information on the Satureja species found in the literature and database (“KNApSAcK”), some of the main peaks were putatively assigned (Table 1). To this extent, specialized metabolites of different classes, mainly corresponding to flavonoids (1, 6–14, 19, 20, 22–23, 25–26, 27, 30–34), monoterpenes (2, 3, 33), indol (4), phenylpropanoids (16, 28), phenolics (5, 15, 17, 18, 29), lignan (21), coumarin (24), biphenyls (35–38), and triterpene (39) were identified.
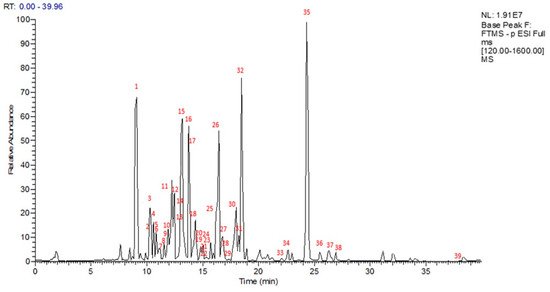
Figure 1.
LC-MS profile in negative ion mode of S. bachtiarica n–BuOH extract.

Table 1.
Identified compounds in aerial parts of S. bachtiarica using LC-ESI/LTQOrbitrap/MS/MSn analysis in negative ion mode and NMR analysis.
To obtain a comprehensive knowledge of the specialized metabolites of the aerial parts of S. bachtiarica, and to unambiguously attribute the peaks occurring in the LC-ESI-FT-MS/MS profile, a phytochemical investigation of the n-BuOH extract was performed. Thus, the n-BuOH extract was submitted to different chromatographic steps, using Sephadex LH-20 and HPLC-UV, affording compounds reported in Table 1. Their structures were elucidated using extensive spectroscopic methods including 1D- (1H, 13C, TOCSY) and 2D-NMR (DQF-COSY, HSQC and HMBC) experiments as well as ESI-MS analysis (Figure 2).
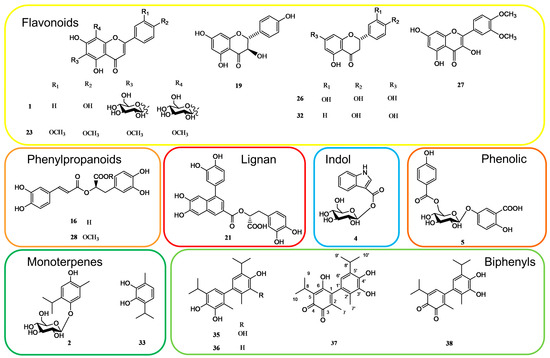
Figure 2.
Some of the specialized metabolites isolated from n-BuOH extract of S. bachtiarica.
From the LC-MS profile, flavonoids resulted as one of the main phenolic classes in this species. The fragmentation pattern of compounds 1, 6–14, 19–20, 22–23, 25–27, 30–32, and 34 allowed identification of the presence of sugar units linked via different combinations to flavone (apigenin, luteolin, and diosmetin), flavonol (kaempferol and quercetin), flavanone (hesperidin, eriodictyol, and isosakuranetin), and flavanonol (dihydrokaempferol) aglycones giving rise to neutral losses of 162 and 132 Da corresponding to the loss of hexose and pentose units, respectively; a neutral loss of 120 Da corresponding to a C-linked hexose unit; and 176 Da related to a glucuronic unit. Compounds 25 and 31 were already reported in S. bachtiarica [1], while the other flavonoids are reported here for the first time in this species.
Compounds 5, 15–18, 28, and 29 showed typical molecular formulae and fragmentation patterns observed for compounds belonging to the phenolic class. Compound 16 was one of the highest peaks in the LC-MS profile, and it was characterized as rosmarinic acid. Rosmarinic acid (16) and p-coumaric acid (17) were previously reported in S. bachtiarica [1]. Compounds 5, 15, 18, 28, and 29 were identified as parmentin B, 12-hydroxyjasmonic acid-(6-O-caffeoyl)-glucoside, (3R,7R)-tuberonic acid-12-O-[6′-O-(E)-feruloyl]-β-D-glucopyranoside, methylrosmarinic acid, and caffeic acid ethyl ester, respectively, reported here for the first time in S. bachtiarica.
Compounds 2, 3, and 33 were identified as monoterpenes. Compounds 2 and 3 showed the same [M-H]− ion at m/z 327.1442, as well as the same molecular formula and fragmentation pattern with a loss of 162 Da (corresponding to sugar unit) [11]. They were identified as thymoquinol 5-O-β-D-glucopyranoside and thanol 3-O-β-D-glucoside, respectively, previously reported in the Lamiaceae family [12]. Compound 33 showed a [M-H]− ion at m/z 165.0918 corresponding to the molecular formula C10H14O2 and two fragment ions at 151 and 138 Da; it was identified as thanol as previously reported in Thymus vulgaris leaves [13].
Compound 4 showed an ion at m/z 322.0925, supporting the molecular formula C15H17O7N, and it was identified as 1-(1H-Indole-3-carboxylate)-glucopyranose, reported in Saccocalyx satureioides [14]. Compound 21 was proposed as globoidnan A, which belongs to the lignan class; this was also confirmed by the fragment ion at m/z 311.06 which was similar to the fragmentation pattern already reported in the literature [15]. Compound 24 with a [M-H]− ion at m/z 535.0872 and molecular formula C27H20O12 was identified as sagecoumarin, isolated previously from Salvia officinalis [16]. Compound 39 showed a [M-H]− ion at m/z 455.3511 supporting a molecular formula C30H48O3, with fragment ions at m/z 411.33 [M-H-44]−, corresponding to the loss of CO2, and at m/z 409.34 and 343.29, corresponding to the fragmentation pattern reported in the literature for oleanolic acid, previously described in S. montana [17].
In the LC-MS profile, compounds 35–38 showed similar molecular formulae suggesting 20 carbon atoms. Their isolation and characterization via NMR analysis allowed us to identify these compounds as dimeric monoterpenes belonging to the biphenyl class.
In detail, compound 37 showed a [M-H]− ion at m/z 343.1541, supporting a molecular formula C20H24O5; its MS/MS spectrum was characterized by three principal fragment ions at m/z 325.14 [M-H-18]−, 315.16 [M-H-28]−, and 300.10 [M-H-43]−, suggesting the presence of hydroxyl groups.
The 1H NMR spectrum showed the presence of two doublets at δ 1.26 and 1.22 (each 6H, J = 7.20 Hz) each coupled with benzylic septets at δ 3.29 and 3.25, respectively, indicating the presence of two isopropyl groups. The signals at δ 1.80 and 1.91 were attributed to two methyl groups linked to aromatic rings. In addition, only one aromatic singlet at δ 6.37 was observed in the 1H NMR spectrum. This pattern required a pentasubstituted phenyl moiety and a totally substituted phenyl moiety.
These observations were further supported by the 13C NMR spectrum, which showed twelve aromatic carbons, three of which hydroxylated (δ 147.0, 142.0, 142.0), six of which were quaternary (δ 146.0, 137.0, 124.0, 126.0, 120.8, 132.5), one of which was protonated (δ 118.3), and there were two signals at δ 185.0 and 186.7, supporting the presence of an ortho-quinone function. Other signals were attributed to two methine (δ 27.6, and 25.0) and six methyl carbons (δ 19.9 and 22.7, each for 2C and 12.5 13.2), which indicated the presence of two isopropyl and two methyl groups. Via a comparison of the NMR data (Table 2), it was possible to deduce for 37 a dimeric monoterpene structure giving rise to a biphenyl skeleton made up of a thanol moiety (33) and an orto thymoquinone-related moiety [18,19]. A detailed investigation suggested how the NMR data were comparable with those of 3′,4′-dihydroxy-5,5′-diisopropyl-2,2′-dimethylbiphenyl-3,4-dione (38), except for the absence of an aromatic proton. The HMBC spectrum showed for the quinone derivative the correlations between the methyl signal at δ 1.80 (Me-7) with the carbon resonances at δ 185.0 (C-3), 146.0 (C-1), and 137.0 (C-2), and between the proton signal at δ 3.25 (H-8) with the carbon resonances at δ 186.7 (C-4) and 147.0 (C-6). For the thanol moiety, correlations between the methyl signal at δ 1.91 (Me-7′) with the carbon resonances at δ 142.0 (C-3′), 126.0 (C-1′), and 120.8 (C-2′) and between the proton signal at δ 3.29 (H-8′) with the carbon resonances at δ 142.0 (C-4′) and 118.3 (C-6′) were observed in the HMBC spectrum. Furthermore, correlations between the proton signal at δ 6.37 (H-6′) with the carbon resonances at δ 146.0 (C-1), 142.0 (C-3′), 120.8 (C-2′), and 27.6 (C-8′) allowed us to establish the linkage between the thanol moiety and the orto thymoquinone-related moiety. Based on this evidence, the structure of compound 37, never described previously, was assigned as reported in Figure 2 and named 6,3′,4′-trihydroxy-5,5′-diisopropyl-2,2′-dimethylbiphenyl-3,4-dione.

Table 2.
13C and 1H NMR (J in Hz) of compound 37 (600 MHz, δ ppm, in CD3OD).
Compounds 35 and 36 were identified as 3,4,3′,4′-tetrahydroxy-5,5′-diisopropyl-2,2′-dimethylbiphenyl and 3,4,4′-trihydroxy-5,5′-diisopropyl-2,2′-dimethylbiphenyl [19,20].
The supposed reaction pathway for thanol (33), also known as p-cymene-2,3-diol, leading to the formation of the oxidation products 35–38 was previously described by Rainis for the dimeric oxidation products of thanol. It follows a common reaction pathway of phenols, which starts with oxygen-catalyzed radical formation of the monoterpene molecules via loss of two hydrogens (one from each molecule) and then proceeds with electron migration to the unsubstituted para carbon atom, leading to a radical dimerization and subsequently to the biphenyl compounds [19]. Biphenyl compounds were isolated from the leaves of thyme and showed to have deodorizing, antioxidative, and human platelet aggregation-inhibiting activities [18,19].
2.2. Evaluation of the Flavonoid Content and Antioxidant Activity of S. bachtiarica Extract
The total flavonoid content and the antioxidant activity of S. bachtiarica BuOH extract have been evaluated using the allumine chloride colorimetric assay and DPPH assay, respectively (Table 3). The DPPH assay revealed that the antiradical activity of the extract was 85.31 μg/mL, using ascorbic acid as a reference compound (1.35 μg/mL). This result agrees with the high content of flavonoids (162.33 μg/g of plant extract), expressed as rutin equivalents. The results suggest that the S. bachtiarica extract has significant antioxidant activity, which is likely attributed to its high flavonoid content.

Table 3.
Antioxidant activity and flavonoid content of n-BuOH extract of S. bachtiarica evaluated via spectrophotometric assays.
2.3. Evaluation of the Antimicrobial Activity of Isolated Compounds
The minimal inhibitory concentration (MIC) exhibited by S. bachtiarica extract and isolated compounds was evaluated. The obtained results showed MIC equal or superior to 100 µg/mL. Two concentrations, 40 and 80 µg/mL, were used both in the crystal violet and in the MTT assays to have a scenario of the strength of each sample in inhibiting the mature biofilm and the metabolism of the sessile cells of five pathogenic strains. Results are shown in Table 4 and Table 5, respectively.

Table 4.
Inhibitory activity (expressed in terms of percentage) of Satureja bachtiarica extract and purified compounds on the bacterial mature biofilm.

Table 5.
Inhibitory activity (expressed in terms of percentage) of Satureja bachtiarica extract and its compounds on the bacterial sessile cell metabolism.
The inhibitory activity exhibited by samples against the mature biofilm was variegated depending on the strain (Table 4). A. baumannii was sensitive to the action of several compounds, particularly to some compounds like the flavonoid naringenin (32), to a monoterpene (2), and above all to biphenyl compounds (35–38), which caused an inhibition of the mature biofilm ranging between 52.76% and 74.21%. This confirms that, in addition to the presence of the most well-known molecules, such as flavonoids, phenolic acids, and lignans, biphenyls can play a significant inhibitory biofilm role against various pathogenic strains [21] including A. baumannii [22]. Moreover, globoidnan A (21) was determined to inhibit the mature biofilm of A. baumannii equal to 59.04%. To our knowledge, it is the first time that such a capacity has been observed for this lignan, which also exhibited a similar ability against all the other pathogenic strains used in our tests, Gram-positive and Gram-negative; therefore, although the results should be corroborated by further tests conducted on different pathogenic strains, we could hypothesize that this molecule potentially has a broad spectrum of action. The fact that the extract as a whole exhibited a biofilm inhibitory capacity that did not exceed 48%, compared to an inhibition % that even exceeded 74%, could be due to the presence of some compounds, in particular compounds 16, 19, 27, and 28 (and to a lesser extent to compounds 1 and 4), which not only proved ineffective against A. baumannii but also affected the inhibitory efficacy of the extract, somehow slowing down its inhibitory action.
The compounds 1-(1H-indole-3-carboxylate)-β-D-glucopyranoside (4) and parmentin B (5) were more effective in exerting an inhibitory action against the mature biofilm of E. coli (inhibition equal to 78.49% and 79.29%, respectively). For the first time, an excellent antimicrobial activity against E. coli has been highlighted for parmentin B. Of note, parmentin B (5) was isolated for the first time from Parmentina cereifera [23], a plant species whose extracts have shown various biological properties, including cardioprotective activity and antimicrobial activities (against E.coli, S. aureus, and P. aeruginosa), the latter determined using the inhibition zone test [24].
Furthermore, the biphenyl compounds (35–38) exhibited inhibitory biofilm activity, which reached an inhibition percentage of 69.28%.
Vicenin 2 (1), parmentin B (5), globoidnan A (21), and mainly biphenyl derivatives 35–38 proved to act effectively on the mature biofilm of L. monocytogenes, with an inhibition % ranging between 54.99% and 75.02%.
As regards the mature biofilm of P. aeruginosa, the 3,4,4′-trihydroxy-5,5′-diisopropyl-2,2′-dimethylbiphenyl (36) showed an excellent biofilm-inhibitory activity (above 80%). Also, the biphenyl derivatives 35 and 38 were able to exert a significant inhibitory action with values ranging from 64.54% to 68.94%. In the tests carried out on P. aeruginosa, the weakness of some compounds (1, 16, 19, and 27) in inhibiting the biofilm of this pathogen could negatively affect the biofilm inhibitory action exhibited by extract. This trend was more evident against S. aureus, despite the very high inhibition values recorded for compounds 32 and 36–38 (Table 4).
The excellent inhibitory activity exhibited by biphenyl derivatives against all five pathogens used as tester strains confirms the antimicrobial and antibiofilm properties of biphenyls, which could be used against several Gram-positive and Gram-negative bacteria, in agreement with the literature data [25]. Indeed, some biphenyls were synthesized to enhance biological properties as the antimicrobial activity [26].
The antibiofilm activity exhibited by naringenin (32) against S. aureus expands the field of action regarding the use of such flavonoids against some of the most dangerous pathogens. Its effect can even improve the antibiofilm capacity of conventional antibacterial drugs [27].
Considering the chemistry of the tested compounds, among the flavonoid derivatives, compounds 1 and 23 showed higher inhibitory activities, while among the lignans, compound 21 showed the highest inhibitory activity.
A recent investigation tested the antimicrobial activity of rosmarinic acid (16) and globoidnan A (21) against S. aureus and P. aeruginosa, reporting MIC values higher than 300 µL/mL [28]. Herein, even if rosmarinic acid (16) was found not active, globoidnan A (21) showed interesting activity on the mature bacterial biofilms of these two strains.
Based on the above results, biphenyls 35–38, dimeric oxidation products of thanol (33), showed a biofilm inhibitory activity higher than their precursor (33), especially against A. baumannii, P. aeruginosa, and S. aureus, while their effects against E. coli and L. monocytogenes were comparable to those of thanol.
The MTT test, performed to evaluate the capacity of the extract of S. bachtiarica and the individual compounds on the metabolism of the sessile cells, highlighted some interesting results (Table 5).
The data obtained against A. baumannii showed that the metabolism of its sessile cells could be inhibited by several compounds, compared to what was observed in the crystal violet test. The influence of the extract on the sessile metabolism of A. baumannii was undoubtedly more remarkable than in the CV test (inhibition = 67.35% and 47.99%, respectively). This data could imply that the inhibitory action would be very much expressed in its cellular metabolism and involved different compounds including flavonoids, monoterpenes, phenolic acids, and biphenyls; these last were not dominant, in contrast to the data from the violet crystal test. Most of the compounds determined an inhibitory action ranging between 49% and 68% with the highest activity shown by compounds 2, 5, and 32 (77.03%, 75.91%, and 70.71%, respectively). Once again, the inhibitory action on a pathogen could also be expressed by blocking or limiting the metabolic changes it undergoes that lead to an increase in virulence and greater resistance to conventional drugs.
The inhibitory influence on the metabolism of E. coli involved fewer compounds than what was observed against A. baumannii. Compound 19 showed that it inhibited the metabolism of E. coli (55.56%), along with compounds 27 (52.60%) and 28 (57.03). Compound 32, which was determined to have an inhibition of 45.93% on the mature biofilm, gave the impression of acting above all on its cellular metabolism, as seen from the inhibition percentage data (72.93%). The results of the MTT test also displayed that compound 36, which was determined to have an effective biofilm inhibitory action, also acted on the metabolism of E. coli, as demonstrated by the inhibition percentage (61.83%); similarly, compound 38 not only worked against the mature biofilm of E. coli but also gave higher inhibition values in the MTT test (61.47%). However, S. bachtiarica extract, overall, failed to act decisively on the metabolism of E. coli; in fact, while an inhibition percentage of 40.69% was recorded in the test with crystal violet (Table 4), in the MTT test we observed an inhibitory percentage of just 28.72%.
Compound 19 also inhibited the metabolism of S. aureus (inhibition = 42.99%). The results of the MTT test showed how the biphenyl derivatives 36–38, which were determined to have an effective biofilm inhibitory action, also acted on the metabolism of E. coli.
Compounds 4, 5, 19, 33, and 36–39 were the most effective in inhibiting the metabolism of sessile cells of L. monocytogenes. This effectiveness would be associated with an almost equally effective response of S. bachtiarica extract, which determined an inhibition of the metabolism of L. monocytogenes equal to 55.32%.
Compounds as 23, 32, and 36–38 influenced the sessile cell metabolism of P. aeruginosa in a percentage ranging from 42.39% to 58.92%, even if a weaker inhibitory action was observed for the extract.
In the test conducted against S. aureus, compounds 1, 4, 5, 19, 33, and 35–38 were the most active in counteracting the metabolism of the sessile cells in the range 46.49–68.93%.
The behavior exhibited by compounds regarding how they affected the metabolism of the cells present within the biofilm in some cases differed with respect to the inhibitory effect on the biofilm formation. Thus, compounds 1, 4, 5, 16, 19, 21, 27, and 28, although demonstrating less or no strength in inhibiting the formation of biofilm of A. baumannii, were more effective in inhibiting the metabolism of sessile cells once the biofilm was formed.
3. Experimental Setup
3.1. General Methods
NMR experiments were performed in methanol-d4 (99.95%, Sigma-Aldrich, Milan, Italy) on a Bruker DRX600 spectrometer (Bruker BioSpin GmBH, Rheinstetten, Germany) equipped with a Bruker 5 mm PATXI Probe at 300 K. Topspin 3.2 software was used to process the data. Semi-preparative HPLC-UV was performed with Phenomenex C18 Synergy-Hydro-RP (25 cm × 10 mm, 10 μm) on an Agilent 1260 Infinity system (Agilent Technologies, Palo Alto, CA, USA), equipped with a binary pump (G-1312C), a UV detector (G-1314 B), and a Rheodyne injector with a loop of 20 µL. TLC (Thin Layer Chromatography) was performed on silica gel F254 (Macherey-Nagel Deltek, Naples, Italy) plates (20 cm × 10 cm), and mixtures of CHCl3–CH3OH–H2O (80:18:2), BuOH, acetic acid, and H2O (60:15:25) (VWR international PBI s.r.l., Milan, Italy) were used as mobile phases to obtain a separate ion distance of 80 mm. Detection was carried out by spraying cerium (IV) sulphate followed by heating at 100 °C for five minutes. HR/ESI-MS data were acquired on an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, San Josè, CA, USA) operating in negative ion mode.
3.2. Reagents
Ethanol and water for extraction and HPLC grade solvents were purchased from VWR International PBI (Milan, Italy). LC-MS grade solvents were procured by Merck (Darmstadt, Germany). DPPH (2,2-Diphenyl-1-picrylhydrazyl), NaOH (sodium hydroxide), NaNO2 (sodium nitrite), AlCl3 (aluminum chloride), and methanol-d4 were purchased from Sigma-Aldrich (Darmstadt, Germany).
3.3. Plant Material
The aerial parts of S. bachtiarica Bunge were collected in Chaharmahal va Bakhtiari, southern Iran, before flowering, 2400 m above sea level in July 2019 at 32°17′ N and 50°33′ E. The identification of S. bachtiarica Bunge was confirmed by Dr. Shirmardi, H.A., Ph.D. (Research Center of Agriculture and Natural Resources, Shahrekord, Iran), and voucher specimens were deposited in the Herbarium of this Research Center (No. 3621). The plants were consequently air dried for five days at 30 ± 2 °C in a shaded environment.
3.4. Extraction
Air-dried and powdered aerial parts of S. bachtiarica Bunge (50 g) were extracted with ethanol/water (70: 30, v/v) (3 × 3 L, 72 h) at room temperature. The dried ethanol/water extract (5.17 g) was obtained after filtration and solvent evaporation to dryness in a vacuum. The residue was dissolved in water and extracted with n-BuOH (40 mL) to remove free sugars. After filtration and solvent evaporation to dryness under reduced pressure, 3.16 g of the n-BuOH extract were obtained.
3.5. HR-LC-ESI-Orbitrap-MS and HR-LC-ESI-Orbitrap-MS/MS Analysis
S. bachtiarica n-BuOH extract was analyzed by LC-ESI/LTQOrbitrap/MS using a HPLC coupled to a hybrid mass spectrometer, which combines the linear trap quadrupole (LTQ) and Orbitrap mass analyzer in negative ion mode. All experiments were performed using a Thermo scientific liquid chromatography system constituted of a quaternary Accela 600 pump and an Accela autosampler, connected to an LTQ-Orbitrap XL mass spectrometer with electrospray ionization (ESI). An amount of 5 µL of the sample was injected with the mass spectrometer operating in negative ion mode with a mobile phase consisting of H2O + 0.1% formic acid (A) and CH3CN + 0.1% formic acid (B) and a stationary phase consisting of Luna C18 5 μm (150 × 2.1 mm) (Phenomenex, Aschaffenburg, Germany) column. A linear gradient held at a flow rate of 0.200 mL/min of solvent B was used: 0–35 min, from 5 to 95%; 35–40 min, isocratic at 95%; then, back to 5% for 10 min. ESI source parameters were as follows: capillary voltage—48 V; tube lens voltage—176.47; capillary temperature 280 °C; sheath gas flow at 15 (arbitrary units); auxiliary gas flow at 5 (arbitrary units); sweep gas 0; and spray voltage 5. Full range acquisition was set at m/z 120–1600, while for the MS/MS study, a data-dependent scan was carried out, selecting precursor ions corresponding to the most intensive peaks in the LC-MS analysis. Xcalibur software version 2.1 was used for instrument control, data acquisition, and data analysis.
3.6. Extraction and Isolation Procedure
An amount of 3 g of n-BuOH extract was fractionated on a Sephadex LH-20 (GE Healthcare, Sigma Aldrich, Milan, Italy) column (100 × 5 cm), using MeOH as the mobile phase, affording 82 fractions (10 mL), as monitored using TLC. Based on TLC analysis, fractions with similar compositions were combined to yield 14 main fractions. Further purifications were carried out using an RP-HPLC-UV system. Fractions were dissolved in MeOH in a concentration of 100 mg/mL with injections of 20 μL for a total of 14 chromatographic runs. The elution gradient was obtained using H2O + 0.1% formic acid (A) and CH3CN + 0.1% formic acid (B) as mobile phases at a flow rate of 2.0 mL/min. The detection wavelength was 254 nm, and the analysis was performed at room temperature. The peaks collected at HPLC-UV were evaporated to dryness in a vacuum and were dried before NMR analysis.
For fractions 5, 6, 7, and 11–14, the HPLC gradient started at 5% B; after 30 min % B was 100%, and it remained at 100% for 5 min. Fraction 5 (237.3 mg) afforded compounds 2 (3.9 mg, tR = 14.91 min), 15 (1.6 mg, tR = 16.55 min), and 18 (2.7 mg, tR = 17.67 min).
Compounds 33 (1.2 mg, tR = 25.70 min), 35 (2.4 mg, tR = 26.58 min), and 36 (1.4 mg, tR = 28.31 min) were isolated from fraction 6 (182.3 mg). Compounds 1 (1 mg, tR = 13.07 min), 4 (1.2 mg, tR = 14.82 min), 29 (1.4 mg, tR = 21.35 min), and 38 (1.6 mg, tR = 26.61 min) were isolated from fraction 7 (95.8 mg); compounds 16 (3.7 mg, tR = 17.38 min), 19 (1.2 mg, tR = 18.88 min), 28 (1.2 mg, tR = 20.05 min), and 34 (1.2 mg, tR = 25.68 min) were isolated from fraction 10 (94.6 mg); compounds 9 (1.2 mg, tR = 11.5 min), 27 (1.2 mg, tR = 20.95 min), and 32 (4.3 mg, tR = 21.80 min) were isolated from fraction 11 (107.6 mg); compounds 11 (1.2 mg, tR = 14.80 min), 7 (1.2 mg, tR = 15.50 min), 5 (1.3 mg, tR = 16.10 min), 14 (1.2 mg, tR = 16.70 min), and 26 (1.3 mg, tR = 20.25 min) were isolated from fraction 12 (29.4 mg); compounds 10 (1.2 mg, tR = 15.35 min), 8 (1.1 mg, tR = 13.01 min), 13 (1.2 mg, tR = 16.60 min), 24 (1.6 mg, tR = 18.62 min), and 31 (1.2 mg, tR = 21.29 min) were isolated from fraction 13 (53.4 mg); and compounds 21 (1.8 mg, tR = 18.03 min) and 25 (2.6 mg, tR = 20.08 min) were isolated fraction 14 (58.5 mg).
Fraction 6 was further purified using a linear gradient starting from 5% to 20% of B over 5 min, and later from 20% to 60% of B over 20 min, and from 60% to 85% of B over 5 min; later, the linear gradient went from 85% to 100% of B in 10 min, yielding compounds 3 (1.2 mg, tR = 22.95 min), 12 (1.3 mg, tR = 17.46 min), 37 (1.8 mg, tR = 35.53 min), and 39 (1.2 mg, tR = 48.40 min).
Fraction 8 (44.5 mg) was purified using a linear gradient starting from 5% to 36% of B over 15 min, then from 36% to 40% of B over 5 min, from 40% to 55% of B over 15 min, and after 5 min it reached 100% of B. Compounds 17 (1.2 mg, tR = 16.75 min) and 20 (1.2 mg, tR = 18.05 min) were obtained. Finally, fraction 9 (96.5 mg) was purified using a HPLC gradient starting from 5% to 52% of B over 10 min, then from 52% to 60% of B over 5 min, and from 60% to 70% of B over 20 min, and in 5 min 100% of B was reached. Compounds 6 (1.3 mg, tR = 12.80 min), 22 (1.2 mg, tR = 21.54 min), 23 (1.0 mg, tR = 23.92 min), and 30 (1.2 mg, tR = 19.04 min) were isolated.
3.7. Total Flavonoid Content
The previously published method was followed to quantify the total flavonoid content using the allumine chloride colorimetric assay and rutin as a reference (Supplementary Materials) [29].
3.8. DPPH• Radical Scavenging Activity
For DPPH, the percentage of DPPH• radical scavenging activity (%) was plotted against the extract concentration (µg/mL) to determine the IC50 (Supplementary Materials) [30].
3.9. Antimicrobial Activity
3.9.1. Microorganisms and Culture Conditions
Acinetobacter baumannii (ATCC 19606), Escherichia coli (DSM 8579), Pseudomonas aeruginosa (DSM 50071), Listeria monocytogenes (ATCC 7644), and Staphylococcus aureus subsp. aureus Rosebach (ATCC 25923) were used as tester strains in the experiments. Before the tests, they were cultured in Luria Broth for at 37 °C or 35 °C (depending on the strain) for 18 h and 80 rpm (Corning LSE, Pisa, Italy).
3.9.2. Minimal Inhibitory Concentration (MIC)
The MIC was evaluated using the resazurin microtiter-plate assay [31], in flat-bottomed 96-well microtiter plates (Falcon, VWR International, Milan, Italy), which were incubated at 37 °C or 35 °C for 24 h. The MIC value was revealed by the visual color change from dark purple to colorless. Tetracycline (previously dissolved in DMSO, 1 mg/mL) and sterile DMSO were the positive and negative controls, respectively. Determinations were performed in triplicate.
3.9.3. Inhibition of Mature Biofilm
The capacity of S. bachtiarica extract and purified compounds to affect the mature bacterial biofilm was evaluated in flat-bottomed 96-well microtiter plates [32]. The overnight bacterial cultures were adjusted to 0.5 McFarland with fresh culture broth. Later, 10 µL of the bacterial cultures and sterile Luria–Bertani broth (LB, Sigma Aldrich Italia, Milan, Italy) were added to each well to reach a final volume of 250 µL. The microtiter plates were covered with parafilm tape to preclude the evaporation of material included in the wells and incubated for 24 h at 37 °C or 35 °C. Planktonic cells were removed; then, 40 or 80 μg/mL of the Satureja extract or singular compounds were added, and sterile Luria–Bertani broth was added to reach a final volume of 250 μL. After another 24 h of incubation at 37 °C or 35 °C, following the removal of the planktonic cells, the attached cells were lightly washed twice with sterile phosphate buffered saline (PBS), which was then discarded. The plates were kept for 10 min under a flow laminar hood before the addition of 200 µL of methanol in each well for 15 min to allow the fixation of the sessile cells. The methanol was then discarded, and each plate was left to dry; then, 200 µL of 2% w/v crystal violet solution were added to each well. After 20 min, the staining solution was removed, and the plates were lightly washed with sterile PBS and left to dry. The addition of 200 µL of glacial acetic acid 20% w/v allowed the release of the bound dye. The absorbance was measured at λ = 540 nm (Cary50Bio, Varian, Palo Alto, CA, USA). The percent value of adhesion was calculated with respect to the control (represented by the bacterial cells grown without the presence of the samples, for which the inhibition rate was assumed as 0%). Triplicate tests were performed, and results were expressed as the mean ± SD.
3.9.4. Inhibitory Activity of Metabolism of Sessile Bacterial Cells
The effect of S. bachtiarica extracts and their compounds on the metabolic activity of sessile cells was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric method using 96-well microtiter plates [32]. The overnight bacterial cultures were adjusted to 0.5 McFarland, and the plates, with Luria broth up to 250 μL, were prepared as previously described. After 24 h total of incubation, bacterial suspension, representing the planktonic cells, was removed, and as previously described, 40 and 80 μL/mL of samples were added, along with Luria–Bertani broth, to reach a final volume of 250 mL. Following another 24 h of incubation, planktonic cells were removed, and 150 µL of PBS and 30 µL of 0.3% of MTT (Sigma, Milan, Italy) were added to each well, keeping microplates at 37 °C or 35 °C. The MTT solution was removed after two h, and the microplates were twice washed with 200 µL sterile physiological solution. Next, 200 µL of DMSO were added, leading to the formazan crystals’ dissolution, measured at 570 nm after two h. Triplicate tests were done, taking the average results for reproducibility, and results were expressed as the mean ± SD.
4. Conclusions
In the present study, a total of 39 compounds were detected in S. bachtiarica n-BuOH extract using a LC-ESI/LTQOrbitrap/MS/MS analysis. The LC-MS analysis guided the isolation of compounds, whose structures were elucidated using NMR analysis allowing us to identify specialized metabolites belonging to different classes. The phytochemical investigation revealed that some compounds such as rosmarinic acid (16), p-coumaric acid (17), luteolin (25), and apigenin (31) were already reported as present in the aerial parts of S. bachtiarica [3], while several other compounds (1–15, 18–19, 21–24, 26–30, and 32–39) are reported here for the first time in S. bachtiarica. Of note, the biphenyl derivative (37) is a molecule never reported before in the literature.
In this work, the antioxidant potential of S. bachtiarica extract was investigated, highlighting interesting antioxidant activity, related to phenolic and flavonoid content. Furthermore, interesting antimicrobial activity was observed for the tested compounds, which were able to inhibit the mature biofilms produced by different bacteria and to affect the metabolism of microbial cells present within the biofilms.
It is worth noting that some compounds exhibit excellent activity; these compounds include 1-(1H-indole-3-carboxylate)-β-D-glucopyranoside (4), parmentin B (5), dihydrokaempferol (19), and biphenyl derivatives (35–38), capable of remarkably limiting the mature biofilm and the metabolism of sessile cells of one or all five pathogens. Among the tested compounds, parmentin B (5) exerted an inhibitory action on the metabolism of the sessile cells of all five pathogenic strains, with a preference of action for the sessile cells of A. baumannii, L. monocytogenes, and S. aureus. Of note, the biphenyl derivatives 36–38 resulted in being capable of acting in a highly effective manner not only on the mature biofilm but also on the metabolism of the cells within the biofilm.
To our knowledge, this is the first time that such a high-performance efficacy of biphenyl compounds on the metabolism of sessile cells, which, therefore, as previously mentioned, are somehow “blocked” regarding the possibility of transforming thus becoming more virulent and more resistant to the actions of conventional antibiotics, has been demonstrated. The fact that these compounds act effectively against all five pathogenic strains assumes a particular relevance, considering the importance of these pathogens from a clinical and nutritional point of view.
Based on the obtained results, S. bachtiarica can be considered a rich source of bioactive compounds with antibacterial properties. These data reinforce the use of S. bachtiarica in human nutrition as a food rich in different classes of bioactive and healthy flavonoids and phenolics with beneficial effects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13010067/s1, Total Flavonoid Content and DPPH assays; Figures S1–S6: MS and NMR spectra of compound 37. Figures S7–S36: 1H NMR spectra of isolated compounds.
Author Contributions
Conceptualization, M.R.S., M.M. and S.P.; methodology, M.R.S., G.D., F.N. and F.F.; software, M.R.S. and G.D.; validation, M.R.S., G.D., F.N. and F.F.; formal analysis, M.R.S. and M.M.; investigation, M.R.S., G.D. and F.N.; resources, S.P.; data curation, M.M. and F.N.; writing—original draft preparation, M.R.S.; writing—review and editing, F.N., M.M. and S.P.; supervision, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rahimmalek, M.; Afshari, M.; Sarfaraz, D.; Miroliaei, M. Using HPLC and multivariate analyses to investigate variations in the polyphenolic compounds as well as antioxidant and antiglycative activities of some Lamiaceae species native to Iran. Ind. Crops Prod. 2020, 154, 112640. [Google Scholar] [CrossRef]
- Hlebová, M.; Hleba, L.; Tancinová, D.; Florková, M.; Foltinová, D.; CHarousová, I.; Vrbová, K.; Božik, M.; Kloucek, P. Inhibitory Effect of Essential Oils from Some Lamiaceae Species on Growth of Eurotium spp. Isolated from Bread. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 857–862. [Google Scholar]
- Tepe, B.; Cilkiz, M. A pharmacological and phytochemical overview on Satureja. Pharm. Biol. 2016, 54, 375–412. [Google Scholar] [CrossRef] [PubMed]
- Malmir, M.; Gohari, A.R.; Saeidnia, S.; Silva, O. A new bioactive monoterpene–flavonoid from Satureja khuzistanica. Fitoterapia 2015, 105, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, S.M.; Ghasemi Pirbalouti, A.; AdibNejad, M. Chemical composition and yield of essential oils from Bakhtiari savory (Satureja bachtiarica Bunge.) under different extraction methods. Ind. Crops Prod. 2015, 76, 809–816. [Google Scholar] [CrossRef]
- Skendi, A.; Katsantonis, D.; Chatzopoulou, P.; Irakli, M.; Papageorgiou, M. Antifungal Activity of Aromatic Plants of the Lamiaceae Family in Bread. Foods 2020, 9, 1642. [Google Scholar] [CrossRef] [PubMed]
- Alghooneh, A.; Alizadeh Behbahani, B.; Taghdir, M.; Sepandi, M.; Abbaszadeh, S. Understanding the Relationship between Microstructure and Physicochemical Properties of Ultrafiltered Feta-Type Cheese Containing Satureja bachtiarica Leaf Extract. Foods 2022, 11, 1728. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Arjmand, H.; Mazaheri, D.; Hadian, J.; Majnoon Hosseini, N.; Ghorbanpour, M. Essential Oils Composition, Antioxidant Activities and Phenolics Content of Wild and Cultivated Satureja bachtiarica Bunge Plants of Yazd Origin. J. Med. Plants 2014, 13, 6–14. [Google Scholar]
- Memarzadeh, S.M.; Gholami, A.; Pirbalouti, A.G.; Masoum, S. Bakhtiari savory (Satureja bachtiarica Bunge.) essential oil and its chemical profile, antioxidant activities, and leaf micromorphology under green and conventional extraction techniques. Ind. Crops Prod. 2020, 154, 112719. [Google Scholar] [CrossRef]
- Dehkordi, H.S.; Dehkordi, M.J.; Chaleshtori, M.R.; Khamesipour, F.; Katsande, S. Effect of Alcohol Extract of Zataria multiflora (Boiss), Satureja bachtiarica (Bunge) and Zaravschanica membranacea (Boiss) on Immuno-Hematologic Factors in Rats. Trop. J. Pharm. Res. 2015, 14, 1999–2004. [Google Scholar] [CrossRef]
- Piacente, S.; Masullo, M.; De Neve, N.; Dewelle, J.; Hamed, A.; Kiss, R.; Mijatovic, T. Cardenolides from Pergularia tomentosa display cytotoxic activity resulting from their potent inhibition of Na+/K+-ATPase. J. Nat. Prod. 2009, 72, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Luo, Y.; Lai, C.-J.-S.; Zhang, J.; Feng, Y.; Wen, Q.; Tan, T. Comprehensive metabolic profile of phenolic acids and flavonoids in Glechomae Herba using ultra-high-performance liquid chromatography coupled to quadrupole-time-of-flight tandem mass spectrometry with diagnostic ion filtering strategy. J. Pharm. Biomed. Anal. 2019, 164, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Lu, Z.-G.; Fujita, T. New Monoterpene Glucoside from the Aerial Parts of Thyme (Thymus vulgaris L.). Biosci. Biotechnol. Biochem. 2004, 68, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Masullo, M.; Seigner, J.; Holper-Schichl, Y.M.; de Martin, R.; Plaza, A.; Piacente, S. LC-ESI-FT-MS(n) Metabolite Profiling of Symphytum officinale L. Roots Leads to Isolation of Comfreyn A, an Unusual Arylnaphthalene Lignan. Int. J. Mol. Sci. 2020, 21, 4671. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, L.Y.; Wong, H. Sagecoumarin, a novel caffeic acid trimer from Salvia officinalis. Phytochemistry 1999, 52, 1149–1152. [Google Scholar] [CrossRef]
- Gulluce, M.; Karadayi, M.; Guvenalp, Z.; Ozbek, H.; Arasoglu, T.; Baris, O. Isolation of some active compounds from Origanum vulgare L. ssp. vulgare and determination of their genotoxic potentials. Food Chem. 2012, 130, 248–253. [Google Scholar] [CrossRef]
- Miura, K.; Nakatani, N. Antioxidative Activity of Flavonoids from Thyme (Thymus vulgaris L.). Agric. Biol. Chem. 1989, 53, 3043–3045. [Google Scholar] [CrossRef]
- Rainis, G.; Ternes, W. Identification and characterization of dimeric oxidation products of p-cymene-2,3-diol isolated from Thymus vulgaris L. J. Agric. Food Chem. 2014, 62, 235–243. [Google Scholar] [CrossRef]
- Paloukopoulou, C.; Govari, S.; Soulioti, A.; Stefanis, I.; Angeli, A.; Matheeussen, A.; Capasso, C.; Cos, P.; Supuran, C.T.; Karioti, A. Phenols from Origanum dictamnus L. and Thymus vulgaris L. and their activity against Malassezia globosa carbonic anhydrase. Nat. Prod. Res. 2022, 36, 1558–1564. [Google Scholar] [CrossRef]
- Mehta, D.; Saini, V.; Aggarwal, B.; Khan, A.; Bajaj, A. Unlocking the bacterial membrane as a therapeutic target for next-generation antimicrobial amphiphiles. Mol. Asp. Med. 2021, 81, 100999. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, H.-Y.; He, W.; Xiang, Y.-T.; Yang, Z.-C.; Kuang, Y.; Yang, S.-X. Synthesis and Antibacterial Activity Evaluation of Biphenyl and Dibenzofuran Derivatives as Potential Antimicrobial Agents against Antibiotic-Resistant Bacteria. Curr. Issues Mol. Biol. 2022, 44, 4087–4099. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, N.M.; Hamed, A.N.E.; Khalil, H.E.; Samy, M.N.; Wanas, A.S.; Fouad, M.A.; Kamel, M.S. Phenolic acid glycosides from Parmentiera cereifera Seem. (Candle tree). Phytochem. Lett. 2014, 9, 74–77. [Google Scholar] [CrossRef]
- Reyad-ul-Ferdous, M.; Rashid, R.B.; Sikder, M.A.A.; Aktar, F.; Rashid, M.A. Preliminary in vitro biological and phytochemical screenings of Parmentiera cereifera Seem. Bangladesh J. Pharmacol. 2012, 15, 103–106. [Google Scholar] [CrossRef]
- Ghosh, C.; Sarkar, P.; Samaddar, S.; Uppu, D.; Haldar, J. l-Lysine based lipidated biphenyls as agents with anti-biofilm and anti-inflammatory properties that also inhibit intracellular bacteria. Chem. Commun. 2017, 53, 8427–8430. [Google Scholar] [CrossRef] [PubMed]
- Malani, A.; Makwana, A.; Monapara, J.; Ahmad, I.; Patel, H.; Desai, N. Synthesis, molecular docking, DFT study, and in vitro antimicrobial activity of some 4-(biphenyl-4-yl)-1,4-dihydropyridine and 4-(biphenyl-4-yl)pyridine derivatives. J. Biochem. Mol. Toxicol. 2021, 35, e22903. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Parai, D.; Banerjee, M.; Hossain, S.T.; Mukherjee, S.K. Naringin sensitizes the antibiofilm effect of ciprofloxacin and tetracycline against Pseudomonas aeruginosa biofilm. Int. J. Med. Microbiol. 2020, 310, 151410. [Google Scholar] [CrossRef] [PubMed]
- Paloukopoulou, C.; Tsadila, C.; Govari, S.; Soulioti, A.; Mossialos, D.; Karioti, A. Extensive analysis of the cultivated medicinal herbal drug Origanum dictamnus L. and antimicrobial activity of its constituents. Phytochemistry 2023, 208, 113591. [Google Scholar] [CrossRef]
- Cerulli, A.; Napolitano, A.; Hošek, J.; Masullo, M.; Pizza, C.; Piacente, S. Antioxidant and In Vitro Preliminary Anti-Inflammatory Activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) Burs, Leaves, and Chestnuts Extracts and Their Metabolite Profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants 2021, 10, 278. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Montoro, P.; Hošek, J.; Pizza, C.; Piacente, S. Metabolite profiling of “green” extracts of Corylus avellana leaves by 1H NMR spectroscopy and multivariate statistical analysis. J. Pharm. Biomed. Anal. 2018, 160, 168–178. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Amato, G.; Fratianni, F.; Coppola, R.; Candido, V.; De Feo, V.; Nazzaro, F. Chemical Characterization and Antibiofilm Activities of Bulbs and Leaves of Two Aglione (Allium ampeloprasum var. holmense Asch. et Graebn.) Landraces Grown in Southern Italy. Molecules 2020, 25, 5486. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).