Projected Impacts of Climate Change on the Range Expansion of the Invasive Straggler Daisy (Calyptocarpus vialis) in the Northwestern Indian Himalayan Region
Abstract
:1. Introduction
2. Materials and Methods
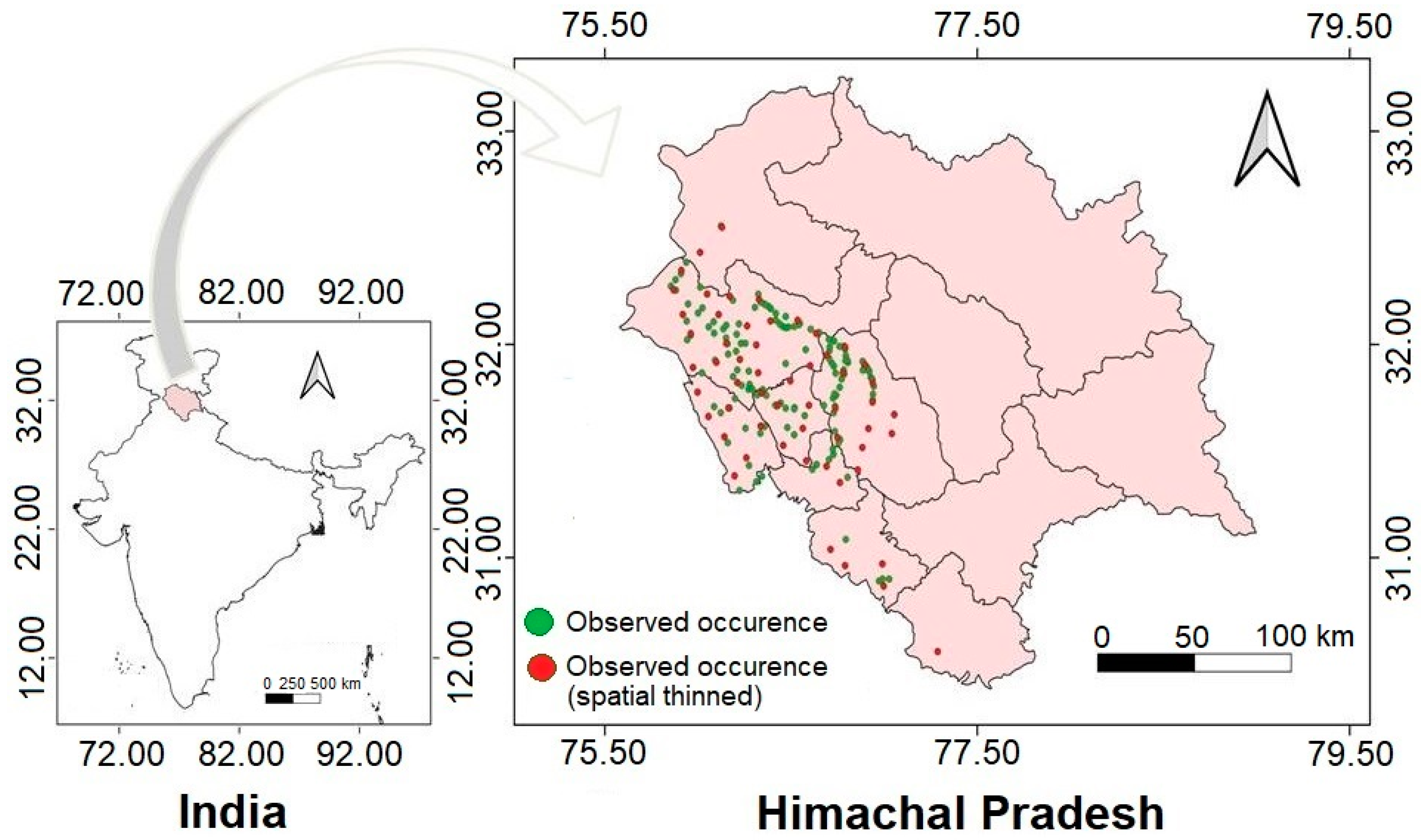
2.1. Study Area
2.2. Data Collection
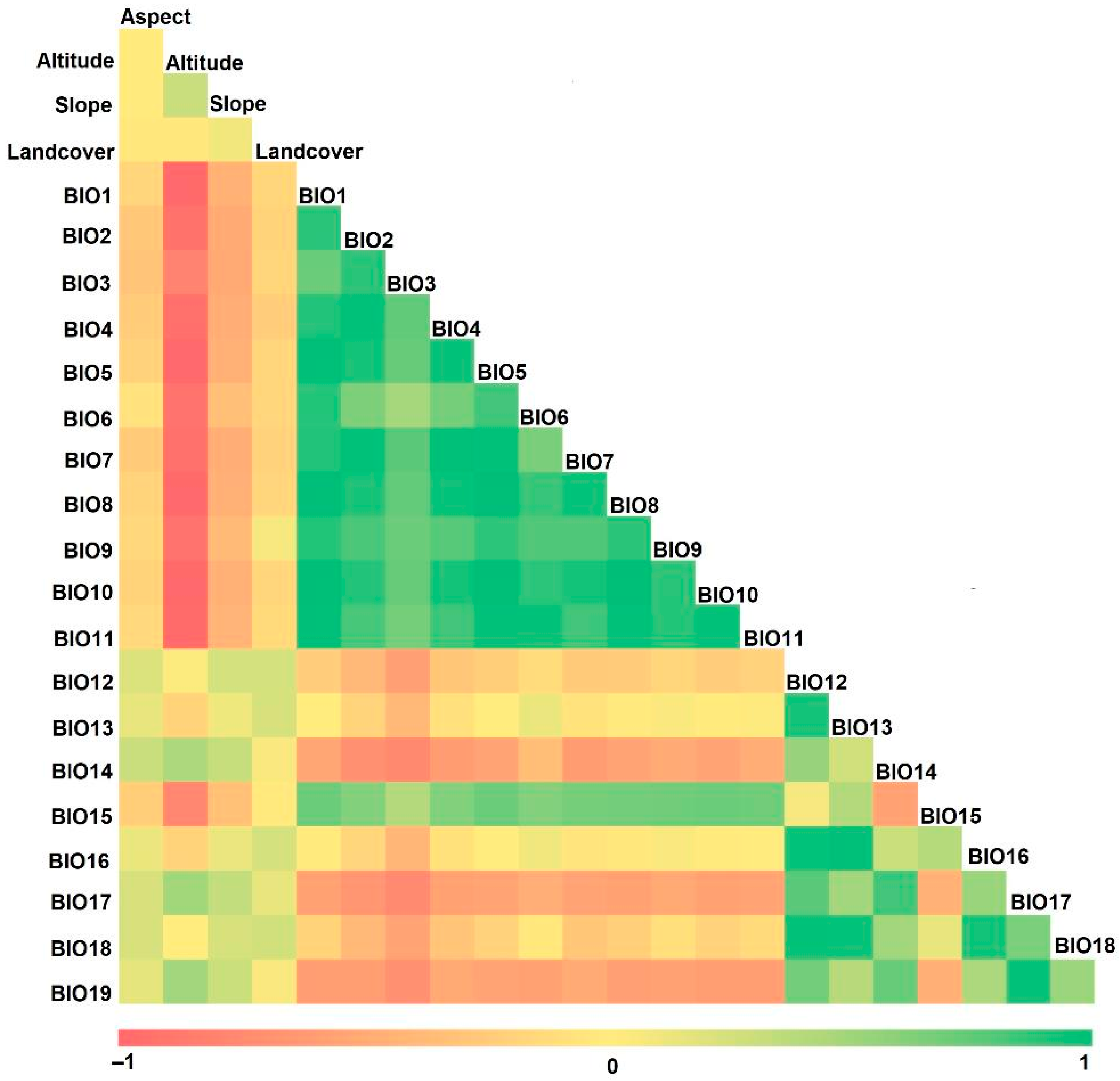
2.3. Data Processing and Predictor Variables
2.4. Ecological Niche Modeling Algorithm
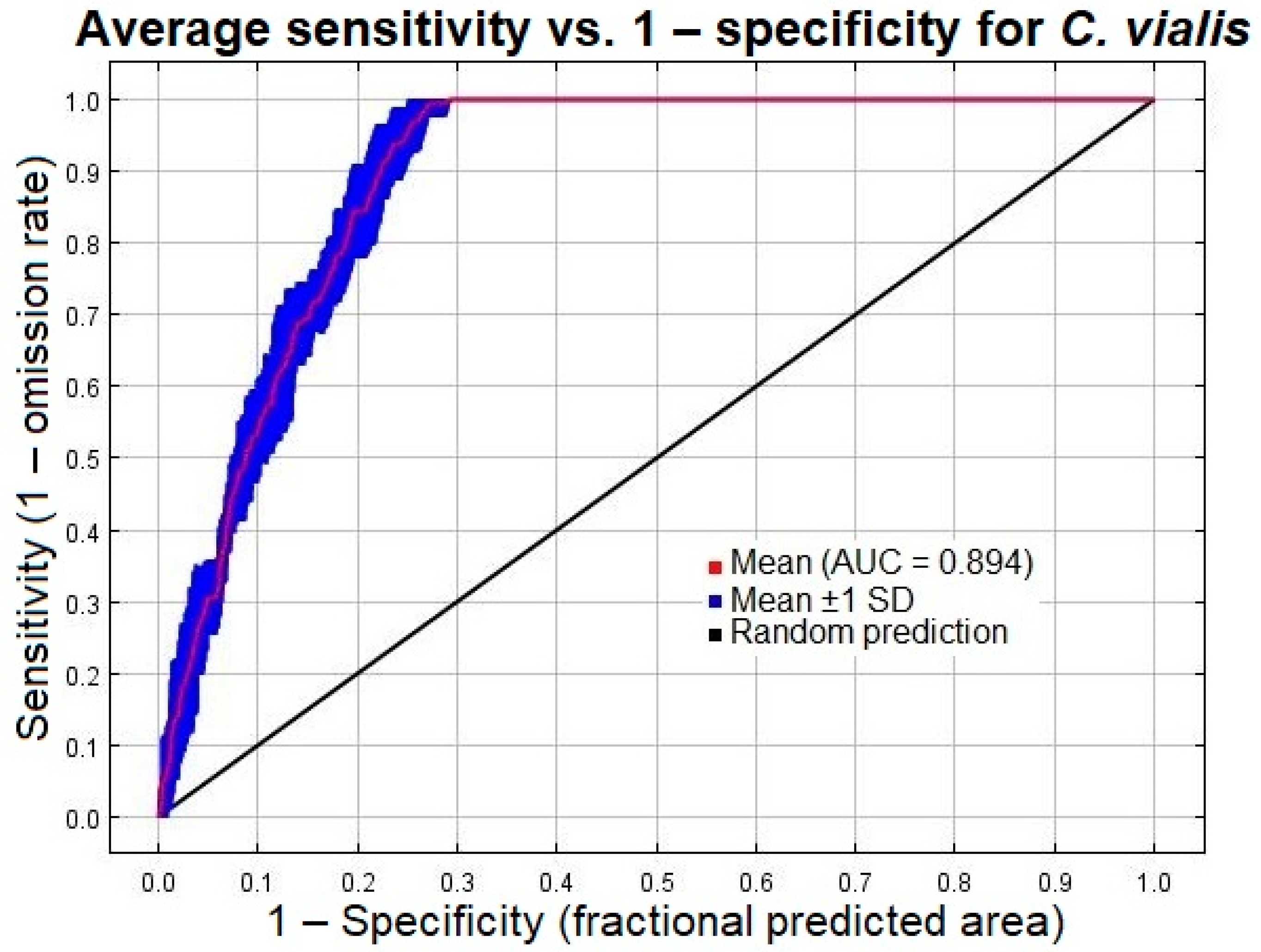
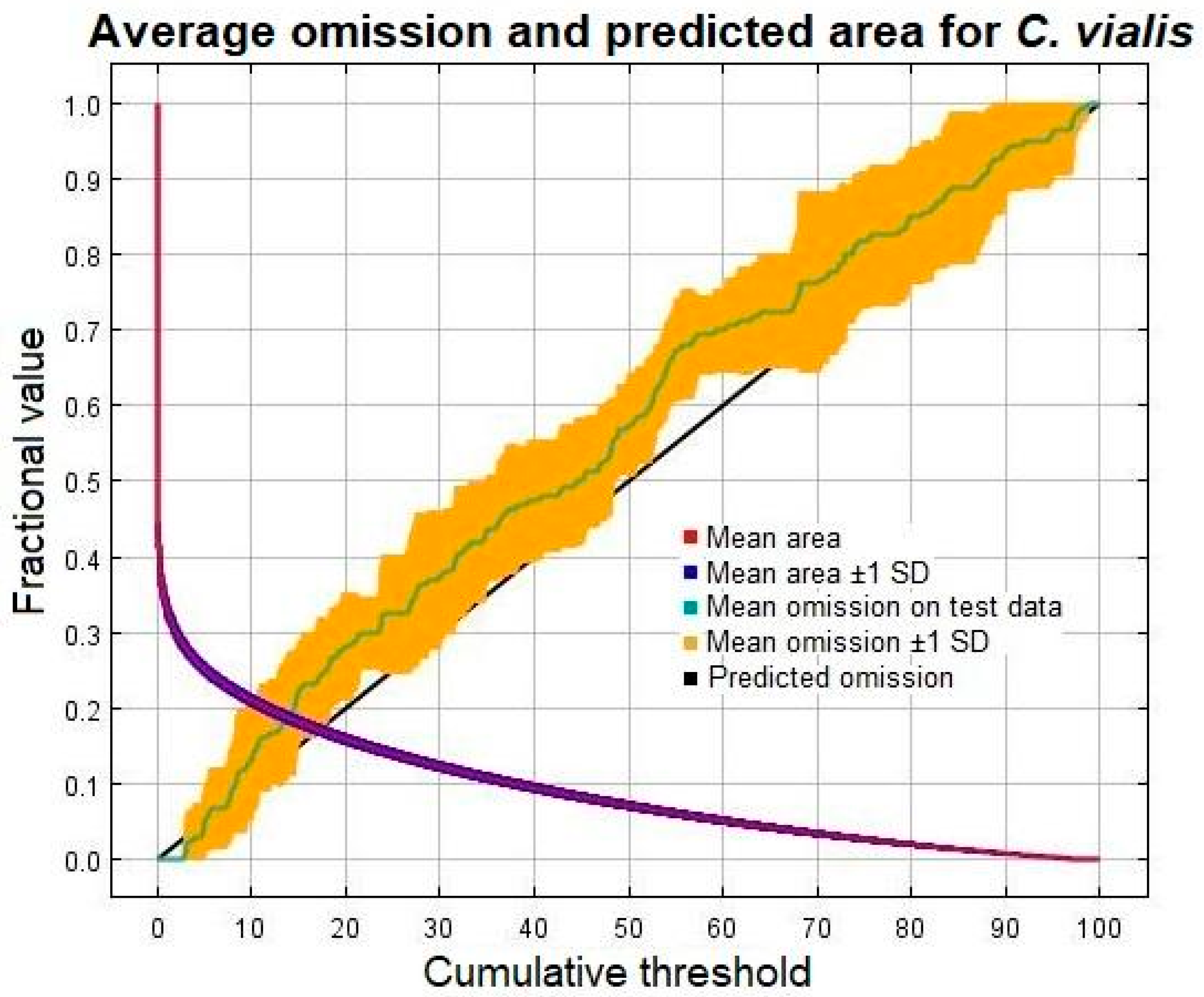
2.5. Model Evaluation
2.6. Map Projection and Data Presentation
3. Results
3.1. Model Performance and Accuracy
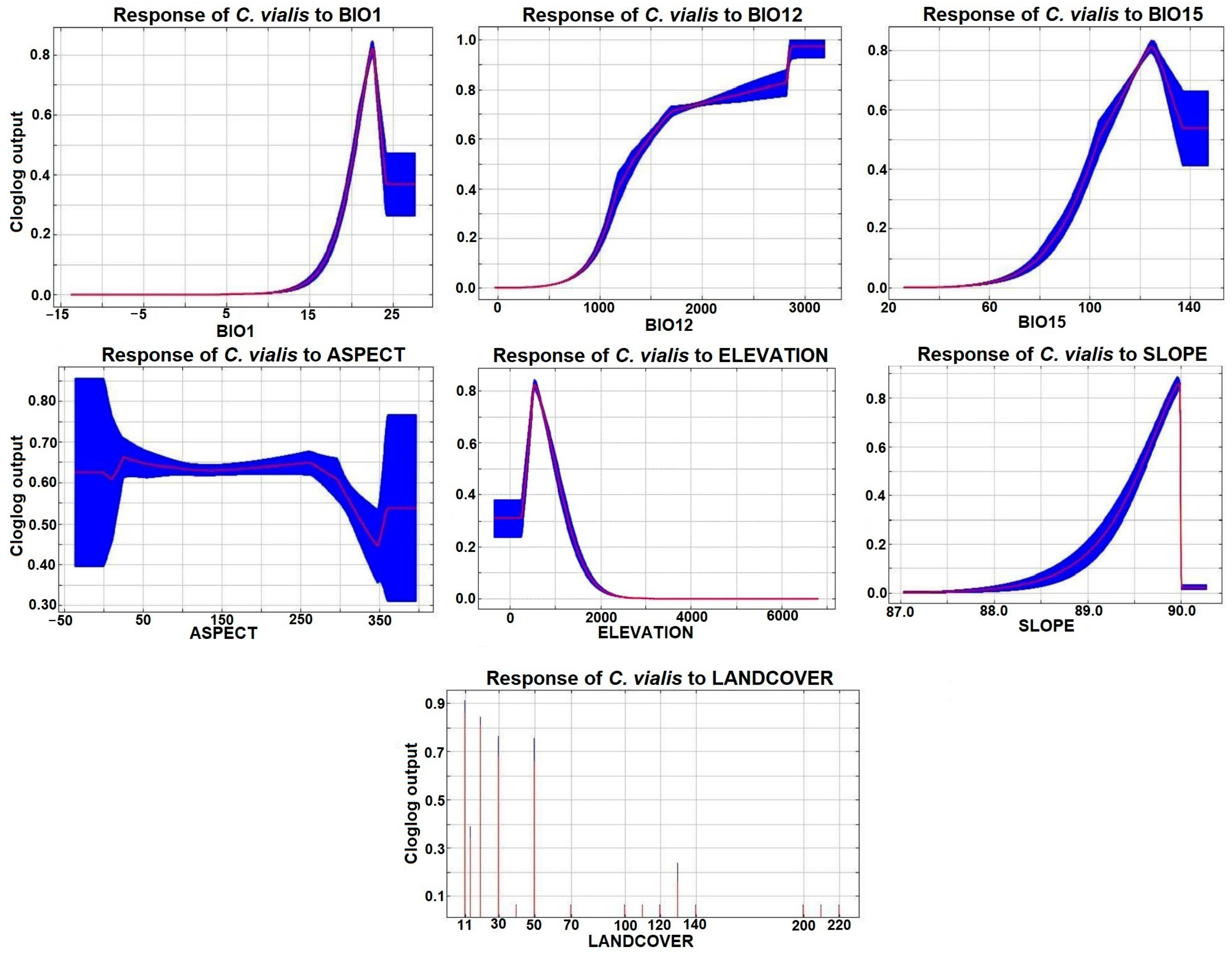
3.2. Key Environmental Variables
3.3. Distribution of Calyptocarpus vialis
4. Discussion
4.1. Significance of Predictor Variables
4.2. Expansion of Suitable Habitats for C. vialis under Future Climate Scenario
4.3. Niche expansion of C. vialis from Lower to Higher Elevations
4.4. Management of C. vialis in the Northwestern IHR Demands Meticulous Attention
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osland, M.J.; Feher, L.C. Winter climate change and the poleward range expansion of a tropical invasive tree (Brazilian pepper—Schinus terebinthifolius). Glob. Chang. Biol. 2020, 26, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Prevéy, J.S.; Rixen, C.; Rüger, N.; Høye, T.T.; Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Ashton, I.W.; Cannone, N.; Chisholm, C.L.; et al. Warming shortens flowering seasons of tundra plant communities. Nat. Ecol. Evol. 2019, 3, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Du, Y.; Yang, B.; Wang, J.; Cui, M.; Dai, Z.; Adomako, M.O.; Rutherford, S.; Du, D. Influence of precipitation dynamics on plant invasions: Response of alligator weed (Alternanthera philoxeroides) and co-occurring native species to varying water availability across plant communities. Biol. Invasions 2023, 25, 519–532. [Google Scholar] [CrossRef]
- Diez, J.M.; D’Antonio, C.M.; Dukes, J.S.; Grosholz, E.D.; Olden, J.D.; Sorte, C.J.B.; Blumenthal, D.M.; Bradley, B.A.; Early, R.; Ibáñez, I.; et al. Will extreme climatic events facilitate biological invasions? Front. Ecol. Environ. 2012, 10, 249–257. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phenotypic variations alter the ecological impact of invasive alien species: Lessons from Parthenium hysterophorus. J. Environ. Manag. 2019, 241, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Iacarella, J.C.; Aldridge, D.C.; Blackburn, T.M.; Carlton, J.T.; Catford, J.A.; Dick, J.T.A.; Hulme, P.E.; Jeschke, J.M.; Liebhold, A.M.; et al. Four priority areas to advance invasion science in the face of rapid environmental change. Environ. Rev. 2021, 29, 119–141. [Google Scholar] [CrossRef]
- Mehal, K.K.; Sharma, A.; Kaur, A.; Kalia, N.; Kohli, R.K.; Singh, H.P.; Batish, D.R. Modelling the ecological impact of invasive weed Verbesina encelioides on vegetation composition across dryland ecosystems of Punjab, northwestern India. Environ. Monit. Assess. 2023, 195, 725. [Google Scholar] [CrossRef]
- Alexander, J.M.; Levine, J.M. Earlier phenology of a nonnative plant increases impacts on native competitors. Proc. Natl. Acad. Sci. USA 2019, 116, 6199–6204. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Shrestha, B.B. Climate change amplifies plant invasion hotspots in Nepal. Divers. Distrib. 2019, 25, 1599–1612. [Google Scholar] [CrossRef]
- Ni, M.; Deane, D.C.; Li, S.; Wu, Y.; Sui, X.; Xu, H.; Chu, C.; He, F.; Fang, S. Invasion success and impacts depend on different characteristics in non-native plants. Divers. Distrib. 2021, 27, 1194–1207. [Google Scholar] [CrossRef]
- Rathee, S.; Ahmad, M.; Sharma, P.; Singh, H.P.; Batish, D.R.; Kaur, S.; Kaur, A.; Yadav, S.S.; Kohli, R.K. Biomass allocation and phenotypic plasticity are key elements of successful invasion of Parthenium hysterophorus at high elevation. Environ. Exp. Bot. 2021, 184, 104392. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, M.; Lin, H.; Ma, X. Reproductive strategies of Xanthium italicum differ from those of native Xanthium sibiricum, and they are key to its invasiveness. Plant Ecol. 2022, 223, 453–463. [Google Scholar] [CrossRef]
- Mehal, K.K.; Kaur, A.; Singh, H.P.; Batish, D.R. Investigating the phytotoxic potential of Verbesina enceliodes: Effect on growth and performance of co-occurring weed species. Protoplasma 2023, 260, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kaur, S.; Singh, H.P.; Batish, D.R. Is intraspecific trait differentiation in Parthenium hysterophorus a consequence of hereditary factors and/or phenotypic plasticity? Plant Divers. 2023, 45, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, J.; Ren, G.; Zhao, K.; Wang, X. Global potential distribution prediction of Xanthium italicum based on Maxent model. Sci. Rep. 2021, 11, 16545. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Lee, Y.H.; Poudel, A.; Hong, S.H.; Park, Y.S. Global spatial distribution of Chromolaena odorata habitat under climate change: Random forest modeling of one of the 100 worst invasive alien species. Sci. Rep. 2023, 13, 9745. [Google Scholar] [CrossRef]
- Zurell, D.; Zimmermann, N.E.; Gross, H.; Baltensweiler, A.; Sattler, T.; Wüest, R.O. Testing species assemblage predictions from stacked and joint species distribution models. J. Biogeogr. 2020, 47, 101–113. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive performance of presence-only species distribution models: A benchmark study with reproducible code. Ecol. Monogr. 2022, 92, e01486. [Google Scholar] [CrossRef]
- Blair, M.E.; Le, M.D.; Xu, M. Species distribution modeling to inform transboundary species conservation and management under climate change: Promise and pitfalls. Front. Biogeogr. 2022, 14, e54662. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hemami, M.R.; Kaboli, M.; Shabani, F. MaxEnt brings comparable results when the input data are being completed; Model parameterization of four species distribution models. Ecol. Evol. 2023, 13, e9827. [Google Scholar] [CrossRef] [PubMed]
- Sorbe, F.; Gränzig, T.; Förster, M. Evaluating sampling bias correction methods for invasive species distribution modeling in Maxent. Ecol. Inform. 2023, 76, 102124. [Google Scholar] [CrossRef]
- Anand, V.; Oinam, B.; Singh, I.H. Predicting the current and future potential spatial distribution of endangered Rucervus eldii eldii (Sangai) using MaxEnt model. Environ. Monit. Assess. 2021, 193, 147. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Amat, E.; Mateo, R.G.; Nieto-Lugilde, D.; Morueta-Holme, N.; Svenning, J.C.; García-Amorena, I. Impact of model complexity on cross-temporal transferability in Maxent species distribution models: An assessment using paleobotanical data. Ecol. Model. 2015, 312, 308–317. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, Y.H.; Lee, G.; Lee, D.H.; Adhikari, P. Predicting impacts of climate change on northward range expansion of invasive weeds in South Korea. Plants 2021, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Peng, P.; Wang, G.; Zhao, G.; Zhou, Y.; Tang, Z. Mapping the distribution and dispersal risks of the alien invasive plant Ageratina adenophora in China. Diversity 2022, 14, 915. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.H.; Poudel, A.; Lee, G.; Hong, S.H.; Park, Y.S. Predicting the impact of climate change on the habitat distribution of Parthenium hysterophorus around the world and in South Korea. Biology 2023, 12, 84. [Google Scholar] [CrossRef]
- Kariyawasam, C.S.; Kumar, L.; Ratnayake, S.S. Potential risks of invasive alien plant species on agriculture under climate change scenarios in Sri Lanka. Curr. Res. Environ. Sustain. 2021, 3, 100051. [Google Scholar] [CrossRef]
- Lal, R.; Kaur, A.; Kaur, S.; Batish, D.R.; Singh, H.P.; Sharma, M.; Kohli, R.K. Nature of phytotoxic interference of alien weed ‘Calyptocarpus vialis’ against some crop plants. Environ. Monit. Assess. 2021, 193, 334. [Google Scholar] [CrossRef]
- Peng, C.I.; Kao, M.T. Calyptocarpus vialis Less. (Asteraceae), a newly naturalized weed in Taiwan. Bot. Bull. Acad. Sin. 1984, 25, 171–176. [Google Scholar]
- Wu, S.H.; Hsieh, C.F.; Rejmánek, M. Catalogue of the naturalized flora of Taiwan. Taiwania 2004, 49, 16–31. [Google Scholar]
- Shen, J.H.; Hu, G.W.; Gao, T.G. Calyptocarpus (Asteraceae: Heliantheae: Ecliptinae), a newly naturalized genus in Tropical East Africa. Phytotaxa 2020, 441, 69–77. [Google Scholar] [CrossRef]
- Naithani, H.B.; Chandra, S. Synedrella vialis (Less.) A. Gray: A new record for Uttar Pradesh, India. J. Bombay Nat. Hist. Soc. 1988, 86, 272. [Google Scholar]
- Hebbar, S.S.; Harsha, V.H.; Shripathi, V.; Hegde, G.R. Record of Synedrella vialis (Asteraceae) and Passiflora suberosa (Passifloraceae) from Dharwad, Karnataka. Indian For. 2002, 128, 461–464. [Google Scholar]
- Lal, B.; Parkash, O.M.; Sharma, V.; Singh, R.D.; Uniyal, S.K. Synedrella vialis (Less.) A. Gray—A new record to the flora of Himachal Pradesh. Indian For. 2009, 135, 89. [Google Scholar]
- Vaishya, J.K.; Ansari, A.A.; Dubey, N.K. Synedrella vialis (Less.) A. Gray (Asteraceae)—A new record for Punjab. J. Econ. Taxon. Bot. 2014, 38, 352–354. [Google Scholar]
- Prasad, K.S.; Raveendran, K. Calyptocarpus vialis Less. (Asteraceae)—A new record for Kerala, India. Zoos’ Print 2013, 28, 23–24. [Google Scholar]
- Zou, F.; Li, H.; Hu, Q. Responses of vegetation greening and land surface temperature variations to global warming on the Qinghai-Tibetan Plateau, 2001–2016. Ecol. Indic. 2020, 119, 106867. [Google Scholar] [CrossRef]
- Jaryan, V.; Uniyal, S.K.; Gupta, R.C.; Singh, R.D. Alien flora of Indian Himalayan state of Himachal Pradesh. Environ. Monit. Assess. 2013, 185, 6129–6153. [Google Scholar] [CrossRef]
- Ahmad, M.; Uniyal, S.K.; Singh, R.D. Patterns of alien plant species richness across gradients of altitude: Analyses from the Himalayan state of Himachal Pradesh. Trop. Ecol. 2018, 59, 35–43. [Google Scholar]
- Sekar, K.C.; Thapliyal, N.; Pandey, A.; Joshi, B.; Mukherjee, S.; Bhojak, P.; Bisht, M.; Bhatt, D.; Singh, S.; Bahukhandi, A. Plant species diversity and density patterns along altitude gradient covering high-altitude alpine regions of west Himalaya, India. Geol. Ecol. Landsc. 2023. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, A.; Kohli, R.K.; Singh, H.P.; Batish, D.R. Bidens pilosa (Asteraceae) invasion reshapes the pattern of plant communities and edaphic properties across the north-western Himalayan landscape. Ecol. Inform. 2023, 77, 102281. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Arino, O.; Ramos Perez, J.J.; Kalogirou, V.; Bontemps, S.; Defourny, P.; Van Bogaert, E. Global Land Cover Map for 2009 (GlobCover 2009); European Space Agency (ESA) & Université Catholique de Louvain (UCL), Pangaea: Ottignies-Louvain-la-Neuve, Belgium, 2012. [Google Scholar]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, T.; Lamarque, J.-F.; et al. The representative concentration pathways: An overview. Clim. Change 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Chauhan, S.; Ghoshal, S.; Kanwal, K.S.; Sharma, V.; Ravikanth, G. Ecological niche modelling for predicting the habitat suitability of endangered tree species Taxus contorta Griff. in Himachal Pradesh (Western Himalayas, India). Trop. Ecol. 2022, 63, 300–313. [Google Scholar] [CrossRef]
- Wan, G.Z.; Wang, L.; Jin, L.; Chen, J. Evaluation of environmental factors affecting the quality of Codonopsis pilosula based on chromatographic fingerprint and MaxEnt model. Ind. Crops Prod. 2021, 170, 113783. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- Paul, S.; Samant, S.S.; Lal, M.; Ram, J. Population assessment and habitat distribution modelling of high value Corylus jacquemontii for in-situ conservation in the state of Himachal Pradesh, India. Proc. Indian Natl. Sci. Acad. 2019, 85, 275–289. [Google Scholar]
- Yan, H.; Feng, L.; Zhao, Y.; Feng, L.; Zhu, C.; Qu, Y.; Wang, H. Predicting the potential distribution of an invasive species, Erigeron canadensis L., in China with a maximum entropy model. Glob. Ecol. Conserv. 2020, 21, e00822. [Google Scholar] [CrossRef]
- Boral, D.; Moktan, S. Mapping the spatial distribution of the invasive Mexican Sunflower Tithonia diversifolia (Asteraceae) in South East Asia. J. Asia-Pac. Biodivers. 2022, 15, 425–434. [Google Scholar] [CrossRef]
- Qin, X.; Li, M. Predicting the potential distribution of Oxalis debilis Kunth, an invasive species in China with a maximum entropy model. Plants 2023, 12, 3999. [Google Scholar] [CrossRef] [PubMed]
- Boisvert-Marsh, L.; de Blois, S. Unravelling potential northward migration pathways for tree species under climate change. J. Biogeogr. 2021, 48, 1088–1100. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, J.S. Plant invasion in protected areas, the Indian Himalayan region, and the North East India: Progress and prospects. Proc. Indian Natl. Sci. Acad. 2021, 87, 19–35. [Google Scholar] [CrossRef]
- McMahon, D.E.; Urza, A.K.; Brown, J.L.; Phelan, C.; Chambers, J.C. Modelling species distributions and environmental suitability highlights risk of plant invasions in western United States. Divers. Distrib. 2021, 27, 710–728. [Google Scholar] [CrossRef]
- Fandohan, A.B.; Oduor, A.M.; Sodé, A.I.; Wu, L.; Cuni-Sanchez, A.; Assédé, E.; Gouwakinnou, G.N. Modeling vulnerability of protected areas to invasion by Chromolaena odorata under current and future climates. Ecosyst. Health Sust. 2015, 1, 1–12. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Heydari, M.; Ahmadi, K.; Khwarahm, N.R.; Karami, O.; Almasieh, K.; Naderi, B.; Bernard, P.; Mosavi, A. The current and future potential geographical distribution of Nepeta crispa Willd., an endemic, rare and threatened aromatic plant of Iran: Implications for ecological conservation and restoration. Ecol. Indic. 2022, 137, 108752. [Google Scholar] [CrossRef]
- Amiri, M.; Tarkesh, M.; Shafiezadeh, M. Modelling the biological invasion of Prosopis juliflora using geostatistical-based bioclimatic variables under climate change in arid zones of southwestern Iran. J. Arid Land 2022, 14, 203–224. [Google Scholar] [CrossRef]
- Liang, W.; Tran, L.; Washington-Allen, R.; Wiggins, G.; Stewart, S.; Vogt, J.; Grant, J. Predicting the potential invasion of kudzu bug, Megacopta cribraria (Heteroptera: Plataspidae), in North and South America and determining its climatic preference. Biol. Invasions 2018, 20, 2899–2913. [Google Scholar] [CrossRef]
- Panda, R.M.; Behera, M.D.; Roy, P.S. Assessing distributions of two invasive species of contrasting habits in future climate. J. Environ. Manag. 2018, 213, 478–488. [Google Scholar] [CrossRef]
- Tu, W.; Xiong, Q.; Qiu, X.; Zhang, Y. Dynamics of invasive alien plant species in China under climate change scenarios. Ecol. Indic. 2021, 129, 107919. [Google Scholar] [CrossRef]
- Waheed, M.; Arshad, F.; Majeed, M.; Haq, S.M.; Aziz, R.; Bussmann, R.W.; Ali, K.; Subhan, F.; Jones, D.A.; Zaitouny, A. Potential distribution of a noxious weed (Solanum viarum Dunal), current status, and future invasion risk based on MaxEnt modeling. Geol. Ecol. Landsc. 2023. [Google Scholar] [CrossRef]
- Becerra López, J.L.; Esparza Estrada, C.E.; Romero Méndez, U.; Sigala Rodríguez, J.J.; Mayer Goyenechea, I.G.; Castillo Cerón, J.M. Evidence of niche shift and invasion potential of Lithobates catesbeianus in the habitat of Mexican endemic frogs. PLoS ONE 2017, 12, e0185086. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Jeschke, J.M.; Leroy, B.; Mace, G.M. Insights from modeling studies on how climate change affects invasive alien species geography. Ecol. Evol. 2018, 8, 5688–5700. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Kushwaha, S.P.S.; Saran, S.; Xu, J.; Roy, P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Chauvier, Y.; Thuiller, W.; Brun, P.; Lavergne, S.; Descombes, P.; Karger, D.N.; Renaud, J.; Zimmermann, N.E. Influence of climate, soil, and land cover on plant species distribution in the European Alps. Ecol. Monogr. 2021, 91, e01433. [Google Scholar] [CrossRef]
- Sintayehu, D.W.; Cherenet, E.; Ebrahim, A.S.; Woldeyes, F. Modeling invasion potential of Lantana camara under the changing climate and land use/land cover change in Ethiopia: Its implication for management of the species. Plant Biosyst. 2021, 155, 1189–1197. [Google Scholar] [CrossRef]
- Mainali, K.P.; Warren, D.L.; Dhileepan, K.; McConnachie, A.; Strathie, L.; Hassan, G.; Karki, D.; Shrestha, B.B.; Parmesan, C. Projecting future expansion of invasive species: Comparing and improving methodologies for species distribution modeling. Glob. Change Biol. 2015, 21, 4464–4480. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Sharma, K.P.; Devkota, A.; Siwakoti, M.; Shrestha, B.B. Potential impact of climate change on the distribution of six invasive alien plants in Nepal. Ecol. Indic. 2018, 95, 99–107. [Google Scholar] [CrossRef]
- Shabani, F.; Ahmadi, M.; Kumar, L.; Solhjouy-fard, S.; Tehrany, M.S.; Shabani, F.; Kalantar, B.; Esmaeili, A. Invasive weed species’ threats to global biodiversity: Future scenarios of changes in the number of invasive species in a changing climate. Ecol. Indic. 2020, 116, 106436. [Google Scholar] [CrossRef]
- Shrestha, B.B.; Shrestha, K.K. 2021.Invasions of alien plant species in Nepal: Patterns and process. In Invasive Alien Species: Observations and Issues from Around the World; Pullaiah, T., Lelmini, M.R., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2021; Volume 2, pp. 168–183. [Google Scholar]
- Park, J.S.; Lee, H. Predicting the spatio-temporal distribution of the invasive alien plant Andropogon virginicus, in the South Korean peninsula considering long-distance dispersal capacities. PLoS ONE 2023, 18, e0291365. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, C.S.; Kumar, L.; Ratnayake, S.S. Invasive plant species establishment and range dynamics in Sri Lanka under climate change. Entropy 2019, 21, 571. [Google Scholar] [CrossRef] [PubMed]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Zettlemoyer, M.A.; Schultheis, E.H.; Lau, J.A. Phenology in a warming world: Differences between native and non-native plant species. Ecol. Lett. 2019, 22, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Uniyal, S.K.; Sharma, P.; Rathee, S.; Batish, D.R.; Singh, H.P. Enhanced plasticity and reproductive fitness of floral and seed traits facilitate non-native species spread in mountain ecosystems. J. Environ. Manag. 2023, 348, 119222. [Google Scholar] [CrossRef] [PubMed]
- Clements, D.R.; Ditommaso, A. Climate change and weed adaptation: Can evolution of invasive plants lead to greater range expansion than forecasted? Weed Res. 2011, 51, 227–240. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef]
- Stroud, J.T. Island species experience higher niche expansion and lower niche conservatism during invasion. Proc. Natl. Acad. Sci. USA 2021, 118, e2018949118. [Google Scholar] [CrossRef]
- Pauchard, A.; Alaback, P.B. Influence of elevation, land use, and landscape context on patterns of alien plant invasions along roadsides in protected areas of South-Central Chile. Conserv. Biol. 2004, 18, 238–248. [Google Scholar] [CrossRef]
- Gardner, J.; Sinclair, J.; Berkes, F.; Singh, R.B. Accelerated tourism development and its impacts in Kullu-Manali, HP, India. Tour. Recreat. Res. 2002, 27, 9–20. [Google Scholar]
- Datta, A.; Schweiger, O.; Kühn, I. Niche expansion of the invasive plant species Ageratina adenophora despite evolutionary constraints. J. Biogeogr. 2019, 46, 1306–1315. [Google Scholar] [CrossRef]
- Thiney, U.; Banterng, P.; Gonkhamdee, S.; Katawatin, R. Distributions of alien invasive weeds under climate change scenarios in mountainous Bhutan. Agronomy 2019, 9, 442. [Google Scholar] [CrossRef]
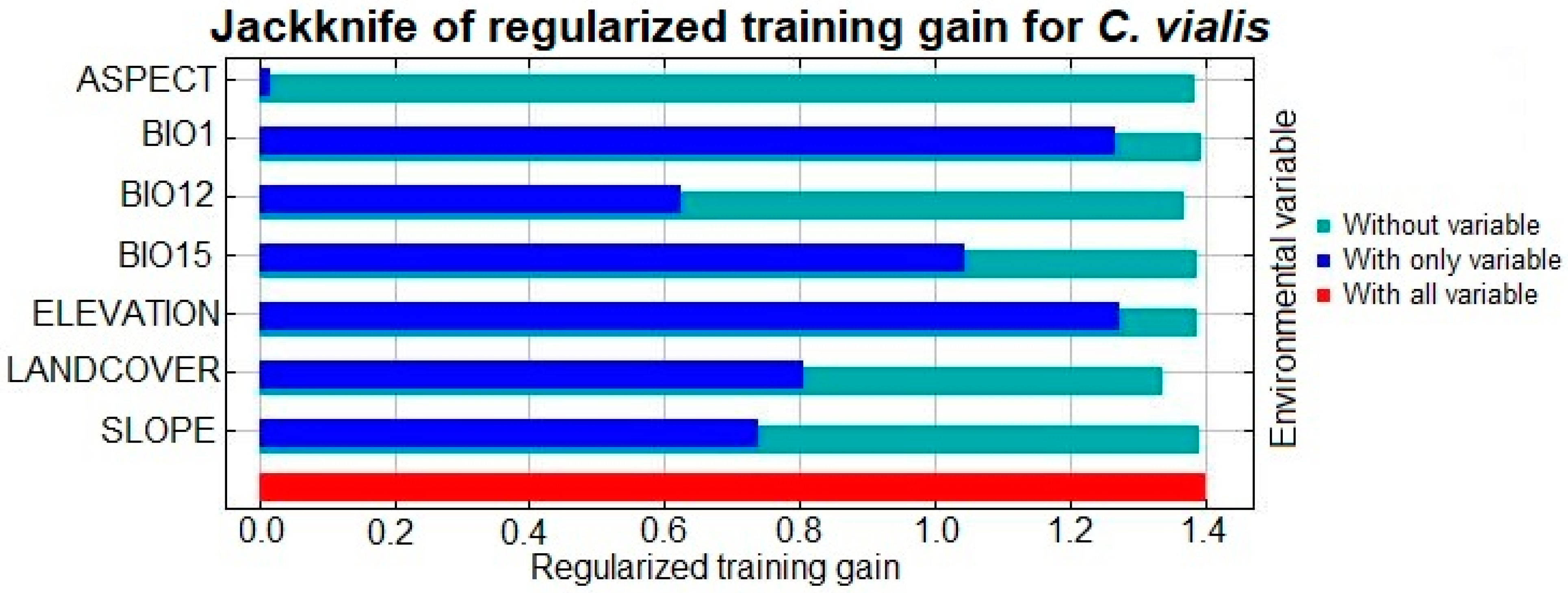


| Predictor Variables (Codes; Unit) | Percent Contribution | Permutation Importance |
|---|---|---|
| Annual mean temperature (BIO1; °C) | 35.7 | 57.1 |
| Annual precipitation (BIO12; mm) | 1.6 | 4.7 |
| Precipitation seasonality (BIO15) | 38.8 | 1.9 |
| Landcover | 6.3 | 4.9 |
| Elevation (m; asl) | 11.7 | 29.5 |
| Slope gradient (slope; degree) | 5 | 1.1 |
| Slope aspect (aspect; degree) | 1 | 0.7 |
| Climatic Scenario | Habitat Suitability | |||||
|---|---|---|---|---|---|---|
| Very Low | Low | Medium | High | Very High | ||
| Current | Area (km2) | 46,129.26 | 5330.18 | 4871.96 | 5605.29 | 3693.30 |
| Percent of total study area (%) | 70.29 | 8.12 | 7.42 | 8.54 | 5.63 | |
| RCP 2.6 | Area (km2) | 43,713.49 | 5457.42 | 5240.77 | 5667.18 | 5551.12 |
| Percent of total study area (%) | 66.61 | 8.32 | 7.99 | 8.64 | 8.46 | |
| RCP 4.5 | Area (km2) | 42,007.83 | 6077.26 | 6281.87 | 7723.60 | 3539.41 |
| Percent of total study area (%) | 64.01 | 9.26 | 9.57 | 11.77 | 5.39 | |
| RCP 6.0 | Area (km2) | 41,902.95 | 4853.04 | 4225.46 | 5991.29 | 8657.24 |
| Percent of total study area (%) | 63.85 | 7.39 | 6.44 | 9.13 | 13.19 | |
| RCP 8.5 | Area (km2) | 39,714.13 | 5123.85 | 5262.26 | 8576.43 | 6953.30 |
| Percent of total study area (%) | 60.51 | 7.81 | 8.02 | 13.07 | 10.59 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lal, R.; Chauhan, S.; Kaur, A.; Jaryan, V.; Kohli, R.K.; Singh, R.; Singh, H.P.; Kaur, S.; Batish, D.R. Projected Impacts of Climate Change on the Range Expansion of the Invasive Straggler Daisy (Calyptocarpus vialis) in the Northwestern Indian Himalayan Region. Plants 2024, 13, 68. https://doi.org/10.3390/plants13010068
Lal R, Chauhan S, Kaur A, Jaryan V, Kohli RK, Singh R, Singh HP, Kaur S, Batish DR. Projected Impacts of Climate Change on the Range Expansion of the Invasive Straggler Daisy (Calyptocarpus vialis) in the Northwestern Indian Himalayan Region. Plants. 2024; 13(1):68. https://doi.org/10.3390/plants13010068
Chicago/Turabian StyleLal, Roop, Saurav Chauhan, Amarpreet Kaur, Vikrant Jaryan, Ravinder K. Kohli, Rishikesh Singh, Harminder P. Singh, Shalinder Kaur, and Daizy R. Batish. 2024. "Projected Impacts of Climate Change on the Range Expansion of the Invasive Straggler Daisy (Calyptocarpus vialis) in the Northwestern Indian Himalayan Region" Plants 13, no. 1: 68. https://doi.org/10.3390/plants13010068
APA StyleLal, R., Chauhan, S., Kaur, A., Jaryan, V., Kohli, R. K., Singh, R., Singh, H. P., Kaur, S., & Batish, D. R. (2024). Projected Impacts of Climate Change on the Range Expansion of the Invasive Straggler Daisy (Calyptocarpus vialis) in the Northwestern Indian Himalayan Region. Plants, 13(1), 68. https://doi.org/10.3390/plants13010068







