The Systematic Assessment of the Membrane-Stabilizing and Antioxidant Activities of Several Kazakhstani Plants in the Asteraceae Family
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. The Preparation of Plant Extracts
2.3. The Estimation of Total Phenolic and Flavonoid Content
2.4. The Estimation of Lipid Peroxidation in Liver Microsomes
2.5. The Isolation of Rat Erythrocytes
2.6. The Estimation of the Osmotic Resistance of Erythrocytes
2.7. Statistical Data Analysis
3. Results
3.1. The Properties of Plant Extracts
3.2. The Influence of Herbal Extracts of the Family Asteracea
3.3. The Combination Extracts of Plants and Tea Provide Antioxidant and Membrane-Stabilizing Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Ydyrys, A.; Zhaparkulova, N.; Aralbaeva, A.; Mamataeva, A.; Seilkhan, A.; Syraiyl, S.; Murzakhmetova, M. Systematic Analysis of Combined Antioxidant and Membrane-Stabilizing Properties of Several Lamiaceae Family Kazakhstani Plants for Potential Production of Tea Beverages. Plants 2021, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Nurlybekova, A.; Kudaibergen, A.; Kazymbetova, A.; Amangeldi, M.; Baiseitova, A.; Ospanov, M.; Aisa, H.A.; Ye, Y.; Ibrahim, M.A.; Jenis, J. Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia. Molecules 2022, 27, 5128. [Google Scholar] [CrossRef] [PubMed]
- Vallès, J.; Garcia, S.; Hidalgo, O.; Martín, J.; Pellicer, J.; Sanz, M.; Garnatje, T. Biology, genome evolution, biotechnological issues and research including applied perspectives in Artemisia (Asteraceae). Adv. Bot. Res. 2011, 60, 349–419. [Google Scholar] [CrossRef]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D. Phytochemistry and pharmacological activity of the genus artemisia. Arch. Pharmacal Res. 2021, 44, 439–474. [Google Scholar] [CrossRef]
- Nigam, M.; Atanassova, M.; Mishra, A.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive Compounds and Health Benefits of Artemisia Species. Natl. Prod. Commun. 2019, 14, 1934578X19850354. [Google Scholar] [CrossRef]
- Ekiert, H.; Pajor, J.; Klin, P.; Rzepiela, A.; Ślesak, H.; Szopa, A. Significance of A. vulgaris L. (Common Mugwort) in the History of Medicine and Its Possible Contemporary Applications Substantiated by Phytochemical and Pharmacological Studies. Molecules 2020, 25, 4415. [Google Scholar] [CrossRef]
- Ivănescu, B.; Burlec, A.F.; Crivoi, F.; Roșu, C.; Corciovă, A. Secondary Metabolites from Artemisia Genus as Biopesticides and Innovative Nano-Based Application Strategies. Molecules 2021, 26, 3061. [Google Scholar] [CrossRef]
- Bordean, M.-E.; Ungur, R.A.; Toc, D.A.; Borda, I.M.; Marțiș, G.S.; Pop, C.R.; Filip, M.; Vlassa, M.; Nasui, B.A.; Pop, A.; et al. Antibacterial and Phytochemical Screening of Artemisia Species. Antioxidants 2023, 12, 596. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Woźniak, K.; Luca, S.V. Unveiling the Phytochemical Profile and Biological Potential of Five Artemisia Species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Yosri, N.; Alsharif, S.; Xiao, J.; Musharraf, S.G.; Zhao, C.; Saeed, A.; Gao, R.; Said, N.S.; Di Minno, A.; Daglia, M.; et al. Arctium lappa (Burdock): Insights from ethnopharmacology potential, chemical constituents, clinical studies, pharmacological utility and nanomedicine. Biomed. Pharmacother. 2023, 158, 114104. [Google Scholar] [CrossRef] [PubMed]
- Skowrońska, W.; Granica, S.; Dziedzic, M.; Kurkowiak, J.; Ziaja, M.; Bazylko, A. Arctium lappa and A. tomentosum, Sources of Arctii radix: Comparison of Anti-Lipoxygenase and Antioxidant Activity as well as the Chemical Composition of Extracts from Aerial Parts and from Roots. Plants 2021, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, W.; Piao, H.; Xu, W.; Shi, H.; Zhao, C. The Genus Gnaphalium L. (Compositae): Phytochemical and Pharmacological Characteristics. Molecules 2013, 18, 8298–8318. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Akhmetova, A.B.; Mukhitdinov, N.M.; Ydyrys, A.; Ametov, A.A.; Inelova, Z.; Öztürk, M. Studies on the root anatomy of rubber producing endemic of Kazakhstan, Taraxacum Kok-Saghyz L.E. Rodin. J. Pla Sci. 2018, 28, 1400–1404. [Google Scholar]
- Ajazuddin, A.A.; Qureshi, A.; Kumari, L.; Vaishnav, P.; Sharma, M.; Saraf, S.; Saraf, S. Role of herbal bioactives as a potential bioavailability enhancer for Active Pharmaceutical Ingredients. Fitoterapia 2014, 97, 1–14. [Google Scholar] [CrossRef]
- McGrath, J.C.; Drummond, G.B.; McLachlan, E.M.; Kilkenny, C.; Wainwright, C.L. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Zhamanbaeva, G.T.; Murzakhmetova, M.K.; Tuleukhanov, S.T.; Danilenko, M.P. Antitumor Activity of Ethanol Extract from Hippophae rhamnoides L. Leaves towards Human Acute Myeloid Leukemia Cells In Vitro. Bull. Exp. Biol. Med. 2014, 158, 252–255. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.; Lester, P. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Murzakhmetova, M.; Moldakarimov, S.; Tancheva, L.; Abarova, S.; Serkedjieva, J. Antioxidant and prooxidant properties of a polyphenol-rich extract from Geranium sanguineum L. in vitro and in vivo. Phytother. Res. 2008, 22, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M. A Review of the Science of Colorful, Plant-Based Food and Practical Strategies for “Eating the Rainbow”. J. Nutr. Metab. 2019, 2019, 2125070. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.B.; Hennig, B. Protective influence of healthful nutrition on mechanisms of environmental pollutant toxicity and disease risks. Ann. N. Y. Acad. Sci. 2017, 1398, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Chandra, S.; Dey, P.; Bhattacharya, S. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study. J. Adv. Pharm. Technol. Res. 2012, 3, 136–138. [Google Scholar] [CrossRef]
- Tang, G.; Meng, X.; Gan, R.; Zhao, C.-N.; Liu, Q.; Feng, Y.-B.; Li, S.; Wei, X.-L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef]
- Fujimura, Y.; Miura, D.; Tachibana, H. A Phytochemical-Sensing Strategy Based on Mass Spectrometry Imaging and Metabolic Profiling for Understanding the Functionality of the Medicinal Herb Green Tea. Molecules 2017, 22, 1621. [Google Scholar] [CrossRef]
- Deka, A.; Vita, J. Tea and cardiovascular disease. Pharmacol. Res. 2011, 64, 136–145. [Google Scholar] [CrossRef]
- Chen, Z.M.; Lin, Z. Tea and human health: Biomedical functions of tea active components and current issues. J. Zhejiang Univ. Sci. B 2015, 16, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bădărau, A.; Swamy, M.; Shaw, S.; Maggi, F.; da Silva, L.E.; López, V.; Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Arctium Species Secondary Metabolites Chemodiversity and Bioactivities. Front Plant Sci. 2019, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Alibek, Y. An Overview of Medical Uses and Chemical Composition of Arctium tomentosum mill. Eng. Sci. 2023, 26, 984. [Google Scholar] [CrossRef]
- Mondal, S.C.; Eun, J.B. Mechanistic insights on burdock (Arctium lappa L.) extract effects on diabetes mellitus. Food Sci. Biotechnol. 2022, 31, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. The medical benefit of Gnaphalium luteoalbum—A review. IOSR J. Phar. 2019, 9, 40–44. [Google Scholar]
- Boyadzhieva, S.; Coelho, J.A.P.; Errico, M.; Reynel-Avilla, H.E.; Yankov, D.S.; Bonilla-Petriciolet, A.; Stateva, R.P. Assessment of Gnaphalium viscosum (Kunth) Valorization Prospects: Sustainable Recovery of Antioxidants by Different Techniques. Antioxidants 2022, 11, 2495. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Semwal, P.; Painuli, S.; Badoni, H.; Ezzat, S.M.; Farid, M.M.; Merghany, R.M.; Aborehab, N.M.; Salem, M.A.; et al. Artemisia spp.: An Update on Its Chemical Composition, Pharmacological and Toxicological Profiles. Oxidative Med. Cell. Longev. 2022, 2022, 5628601. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Giordo, R.; Pintus, G.; Mohammed, S.A.; Orhan, I.E.; Fokou, P.V.T.; Sharopov, F.; Adetunji, C.O.; Gulsunoglu-Konuskan, Z.; Ydyrys, A.; et al. Medicinal and mechanistic overview of artemisinin in the treatment of human diseases. Biomed. Pharmacother. 2023, 163, 114866. [Google Scholar] [CrossRef]
- Taheri, Y.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Ezzat, S.M.; Merghany, R.M.; Shaheen, S.; Azmi, L.; Mishra, A.P.; Sener, B.; et al. Urtica dioica-Derived Phytochemicals for Pharmacological and Therapeutic Applications. Evid.-Based Complement. Altern. Med. 2022, 2022, 4024331. [Google Scholar] [CrossRef]
- Pezzani, R.; Jiménez-Garcia, M.; Capó, X.; Gürer, E.S.; Sharopov, F.; Rachel, T.Y.L.; Woutouoba, D.N.; Rescigno, A.; Peddio, S.; Zucca, P.; et al. Anticancer properties of bromelain: State-of-the-art and recent trends. Front. Oncol. 2023, 12, 1068778. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Seidel, V.; Izabela, M.; Monserrat-Mequida, M.; Sureda, A.; Ormazabal, V.; Zuniga, F.A.; Mangalpady, S.S.; Pezzani, R.; Ydyrys, A.; et al. Phenolic compounds as Nrf2 inhibitors: Potential applications in cancer therapy. Cell Commun. Signal. 2023, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sameen, A.; Aadil, R.M.; Shahid, M.; Sezen, S.; Zarrabi, A.; Ozdemir, B.; Sevindik, M.; Kaplan, D.N.; Selamoglu, Z.; et al. Citrus Genus and Its Waste Utilization: A Review on Health-Promoting Activities and Industrial Application. Evid.-Based Complement. Altern. Med. 2021, 2021, 2488804. [Google Scholar] [CrossRef] [PubMed]
- Sakipova, Z.; Wong, N.S.; Bekezhanova, T.; Sadykova; Shukirbekova, A.; Boylan, F. Quantification of santonin in eight species of Artemisia from Kazakhstan by means of HPLC-UV: Method development and validation. PLoS ONE 2017, 12, e0173714. [Google Scholar] [CrossRef] [PubMed]
- Alibek, Y.; Abdolla, N.; Masimzhan, M.; Abdrasulova, Z.; Syraiyl, S. Cultivation and resource of A. schrenkiana (L.) for increased pharmaceutical perspective. Res. Crops 2023, 24, 171–178. [Google Scholar] [CrossRef]
- Syraiyl, S.; Ydyrys, A.; Ahmet, A.; Aitbekov, R.; Imanaliyeva, M. Phytochemical composition and antioxidant activity of three medicinal plants from southeastern Kazakhstan. Int. J. Biol. Chem. 2022, 15, 73–78. [Google Scholar] [CrossRef]
- Findura, P.; Kocira, S.; Hara, P.; Pawłowska, A.; Szparaga, A.; Kangalov, P. Extracts from A. vulgaris L. in Potato Cultivation—Preliminary Research on Biostimulating Effect. Agriculture 2020, 10, 356. [Google Scholar] [CrossRef]
- Dylenova, E.P.; Zhigzhitzhapova, S.V.; Emelyanova, E.A.; Tykheev, Z.A.; Chimitov, D.G.; Goncharova, D.B.; Taraskin, V.V. Chemical Diversity of A. rutifolia Essential Oil, Antimicrobial and Antiradical Activity. Plants 2023, 12, 1289. [Google Scholar] [CrossRef]
- Watson, H. Biological membranes. Essays Biochem. 2015, 59, 43–69. [Google Scholar] [CrossRef]
- Aralbaeva, A.N.; Mamataeva, A.T.; Zhaparkulova, N.I.; Utegalieva, R.S.; Khanin, M.; Danilenko, M.; Murzakhmetova, M.K. A composition of medicinal plants with an enhanced ability to suppress microsomal lipid peroxidation and a protective activity against carbon tetrachloride-induced hepatotoxicity. Biomed. Pharmacother. 2017, 96, 1283–1291. [Google Scholar] [CrossRef]
- Sivonová, M.; Waczulíková, I.; Kilanczyk, E.; Hrnciarová, M.; Bryszewska, M.; Klajnert, B.; Duracková, Z. The effect of Pycnogenol on the erythrocyte membrane fluidity. Gen. Physiol. Biophys. 2004, 23, 39–51. [Google Scholar]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
| Species | Total Polyphenols (μg GAE/mg) | Total Flavonoids (μg RE/mg) | Lipid Peroxidation IC50 (µg/mg Protein) | Membrane-Stabilizing Properties IC50 (µg/mL of RBC) |
|---|---|---|---|---|
| A. tomentosum | 348.4 ± 11.9 | 214.3 ± 19.2 | 4.6 ± 0.8 | 64.2 ± 7.3 |
| G. kasachstanicum | 312.4 ± 22.3 | 284.2 ± 38.3 | 9.7 ± 3.2 | 183 ± 9.4 |
| A. schrenkiana | 342.1 ± 2.1 * | 265.1 ± 1.2 * | 3.4 ± 0.07 ** | 195.1 ± 7.0 * |
| A. rutifolia | 236.4 ± 8.1 * | 71.2 ± 6.7 * | 4.7 ± 0.6 ** | >200 |
| A. cina | 253.6 ± 8.5 * | 131.2 ± 13.1 ** | 2.6 ± 0.5 *** | 183 ± 6.2 * |
| A. vulgaris | 240.3 ± 13.7 ** | 111.1 ± 7.6 * | 8.1 ± 2.3 * | 64.6 ± 3.6 * |
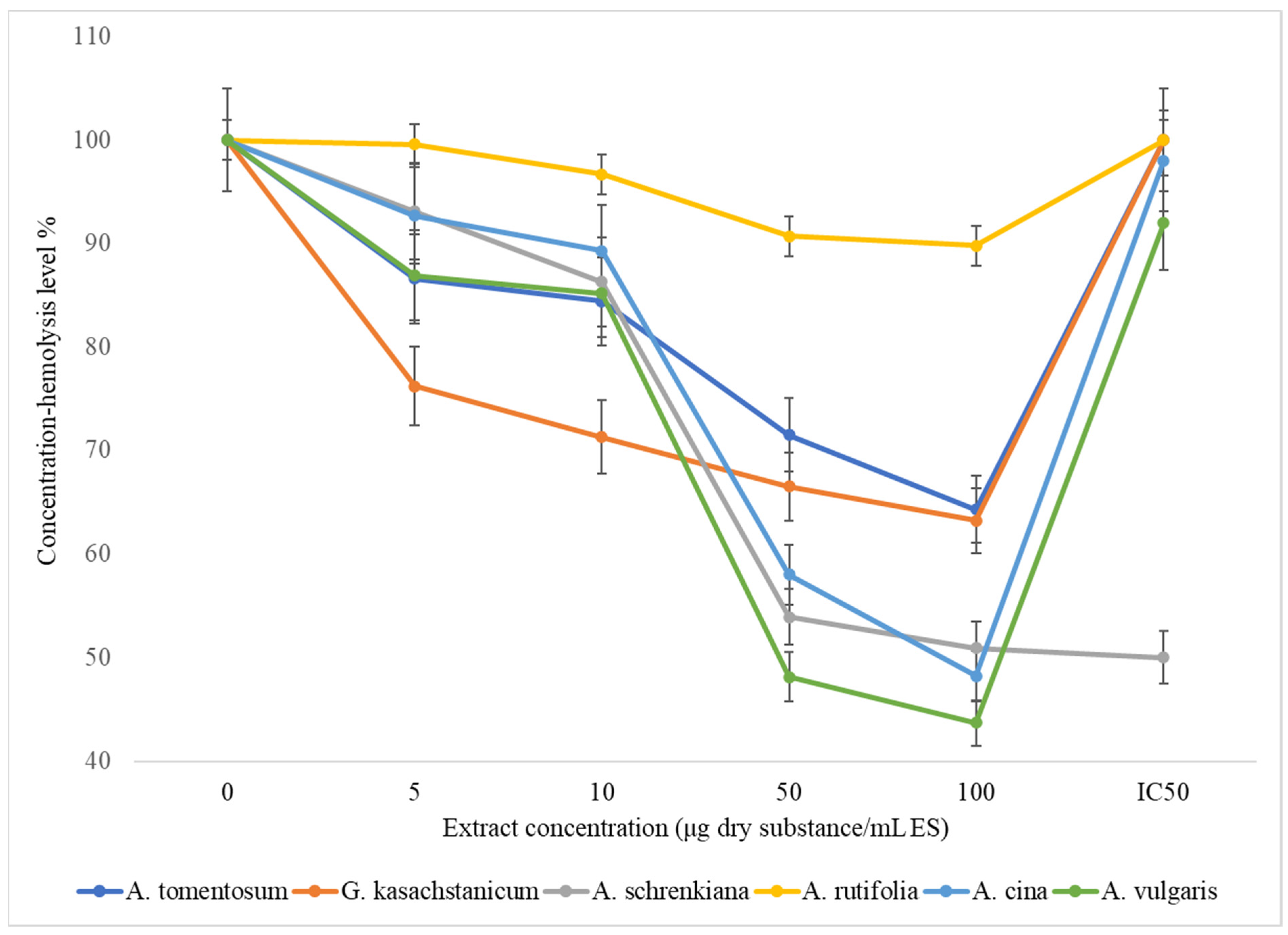
| Species | Extract Concentration (μg Dry Substance/mL ES) | |||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 50 | 100 | IC50 | |
| A. tomentosum | 100 | 86.6 ± 4.33 | 84.4 ± 4.2 | 71.5 ± 3.55 | 64.3 ± 3.21 | 100 μg < |
| G. kasachstanicum | 100 | 76.2 ± 2.81 | 71.3 ± 3.4 | 66.5 ± 2.32 | 63.2 ± 3.21 | 100 μg < |
| A. schrenkiana | 100 | 93.1 ± 1.2 * | 86.3 ± 3.4 ** | 53.9 ± 5.7 *** | 50.9 ± 0.9 * | 50 μg < |
| A. rutifolia | 100 | 99.6 ± 4.92 ** | 96.7 ± 4.8 *** | 90.7 ± 4.51 ** | 89.8 ± 4.47 *** | 100 μg < |
| A. cina | 100 | 92.7 ± 4.63 ** | 89.3 ± 4.46 * | 58.0 ± 2.85 * | 48.2 ± 2.4 * | 98 μg < |
| A. vulgaris | 100 | 86.9 ± 4.33 ** | 85.2 ± 4.25 * | 48.1 ± 2.4 | 43.7 ± 2.18 ** | 92 μg < |
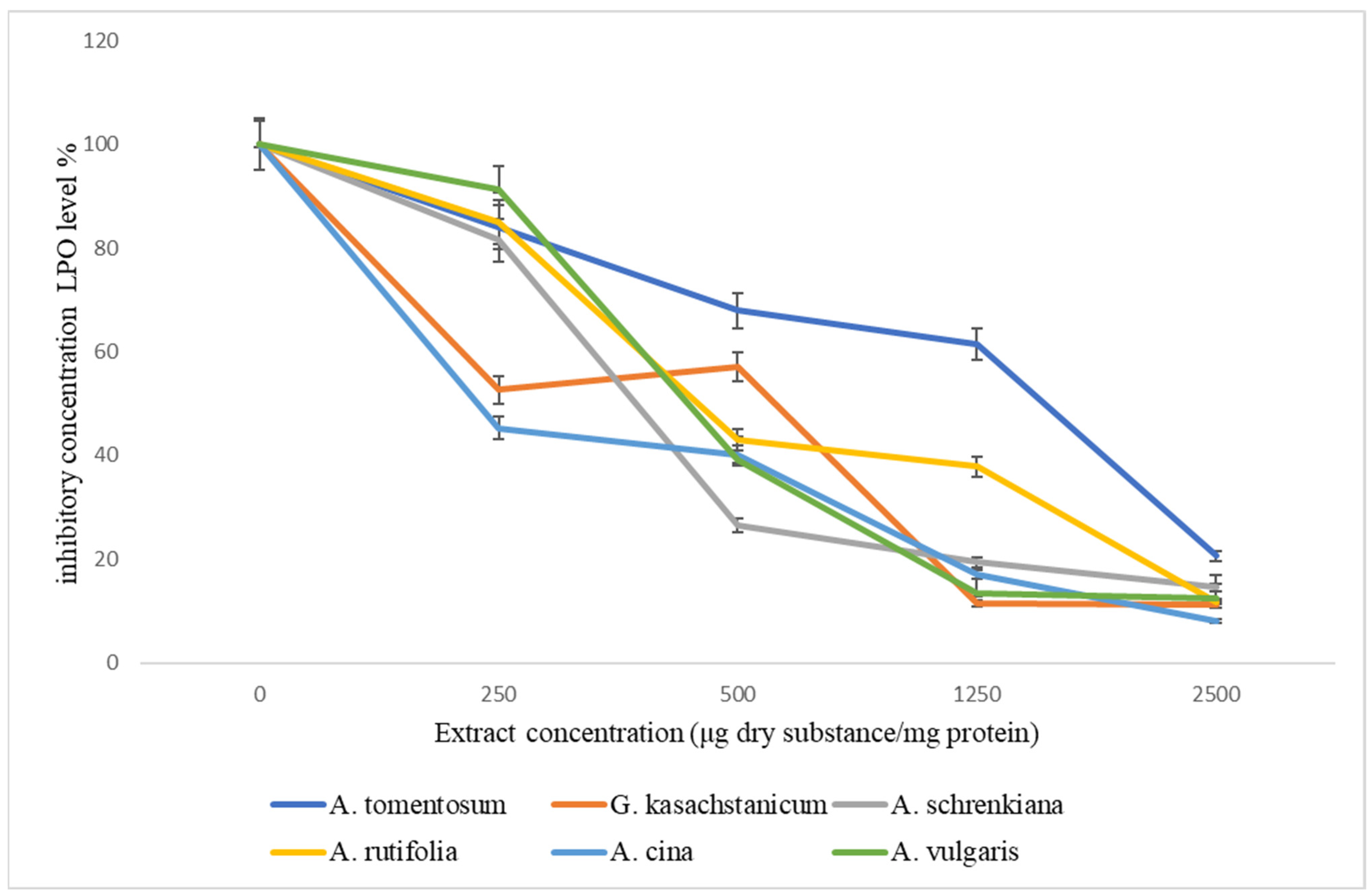
| Species by Common Name | Extract Concentration (μg Dry Substance/mg Protein) | |||||
|---|---|---|---|---|---|---|
| 0 | 250 | 500 | 1250 | 2500 | IC50 | |
| A. tomentosum | 100 | 84.0 ± 4.2 | 68.0 ± 3.4 | 61.5 ± 3.1 | 20.6 ± 1.01 | 1559 μg |
| G. kasachstanicum | 100 | 52.6 ± 2.96 | 57.1 ± 2.57 | 11.5 ± 5.7 | 11.2 ± 0.3 | 552 μg |
| A. schrenkiana | 100 | 81.5 ± 3.2 * | 26.6 ± 3.7 ** | 19.4 ± 2.5 ** | 14.5 ± 2.9 * | 392 μg |
| A. rutifolia | 100 | 85.0 ± 4.25 ** | 43.0 ± 2.1 *** | 37.8 ± 1.94 *** | 11.8 ± 0.59 *** | 444 μg |
| A. cina | 100 | 45.3 ± 2.7 *** | 40.0 ± 2.0 *** | 17.0 ± 0.85 *** | 8.0 ± 0.39 *** | 232 μg |
| A. vulgaris | 100 | 91.2 ± 4.5 *** | 39.1 ± 1.98 *** | 13.3 ± 0.65 *** | 12.4 ± 0.62 * | 432 μg |
| No. | Sample | IC50 (µg/mg Protein, Mean + SD) | ||
|---|---|---|---|---|
| Individual Extract Mean | In Combination with Black Tea | In Combination with Green Tea | ||
| 1 | A. tomentosum | 4.3 ± 0.6 | 7.8 ± 2.6 | 5.0 ± 0.7 |
| 2 | G. kasachstanicum | 3.2 ± 0.8 | 2.8 ± 2.1 | 4.3 ± 1.4 |
| 3 | A. schrenkiana | 3.4 ± 0.1 | 2.85 ± 0.12 | 4.4 ± 1.3 |
| 4 | Green tea | 9.7 ± 3.1 | - | - |
| 5 | Black tea | 14.8 ± 4.5 | - | - |
| No. | Sample | IC50 (µg/mL of RBC, Mean + SD) | ||
|---|---|---|---|---|
| Individual Extract Mean | In Combination with Black Tea | In Combination with Green Tea | ||
| 1 | A. tomentosum | 86.1 ± 7.1 | 124.0 ± 6.3 | 89.1 ± 7.2 |
| 2 | A. schrenkiana | 195.1 ± 1.5 | 177.9 ± 9.2 | 143.22 ± 7.5 |
| 3 | G. kasachstanicum | 84.3 ± 4.2 | 81.0 ± 3.7 | 76.5 ± 4.5 |
| 4 | Green tea | 114.3 ± 9.5 | - | - |
| 5 | Black tea | >200 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ydyrys, A.; Zhamanbayeva, G.; Zhaparkulova, N.; Aralbaeva, A.; Askerbay, G.; Kenzheyeva, Z.; Tussupbekova, G.; Syraiyl, S.; Kaparbay, R.; Murzakhmetova, M. The Systematic Assessment of the Membrane-Stabilizing and Antioxidant Activities of Several Kazakhstani Plants in the Asteraceae Family. Plants 2024, 13, 96. https://doi.org/10.3390/plants13010096
Ydyrys A, Zhamanbayeva G, Zhaparkulova N, Aralbaeva A, Askerbay G, Kenzheyeva Z, Tussupbekova G, Syraiyl S, Kaparbay R, Murzakhmetova M. The Systematic Assessment of the Membrane-Stabilizing and Antioxidant Activities of Several Kazakhstani Plants in the Asteraceae Family. Plants. 2024; 13(1):96. https://doi.org/10.3390/plants13010096
Chicago/Turabian StyleYdyrys, Alibek, Gulzhan Zhamanbayeva, Nazgul Zhaparkulova, Arailym Aralbaeva, Gulnaz Askerbay, Zhanar Kenzheyeva, Gulmira Tussupbekova, Sayagul Syraiyl, Raushan Kaparbay, and Maira Murzakhmetova. 2024. "The Systematic Assessment of the Membrane-Stabilizing and Antioxidant Activities of Several Kazakhstani Plants in the Asteraceae Family" Plants 13, no. 1: 96. https://doi.org/10.3390/plants13010096
APA StyleYdyrys, A., Zhamanbayeva, G., Zhaparkulova, N., Aralbaeva, A., Askerbay, G., Kenzheyeva, Z., Tussupbekova, G., Syraiyl, S., Kaparbay, R., & Murzakhmetova, M. (2024). The Systematic Assessment of the Membrane-Stabilizing and Antioxidant Activities of Several Kazakhstani Plants in the Asteraceae Family. Plants, 13(1), 96. https://doi.org/10.3390/plants13010096







