Nanotechnology in Agriculture: Manganese Ferrite Nanoparticles as a Micronutrient Fertilizer for Wheat
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanocomposite Characterization of MnFe2O4 NPs
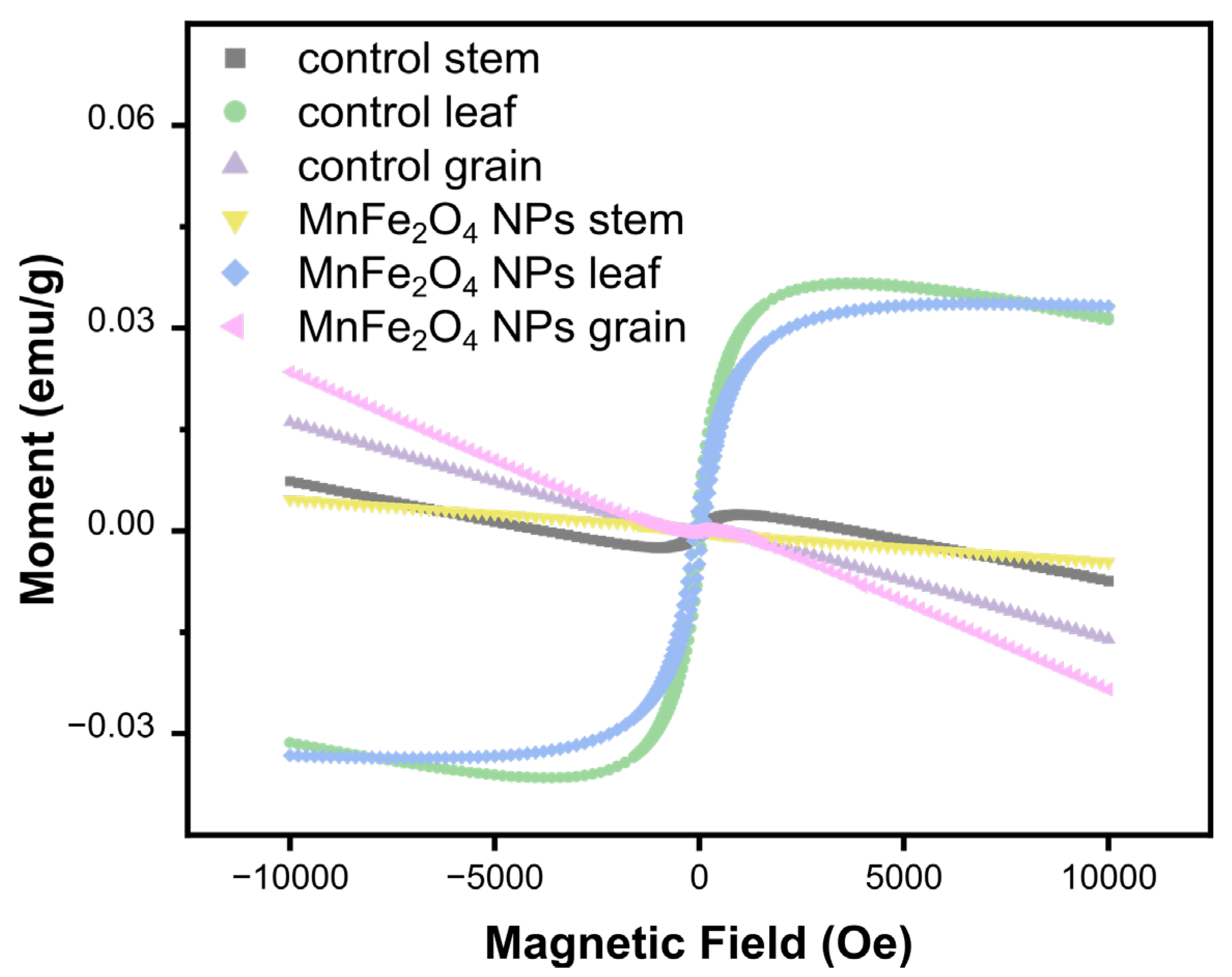
2.2. MnFe2O4 NP Distribution in Plant Tissues
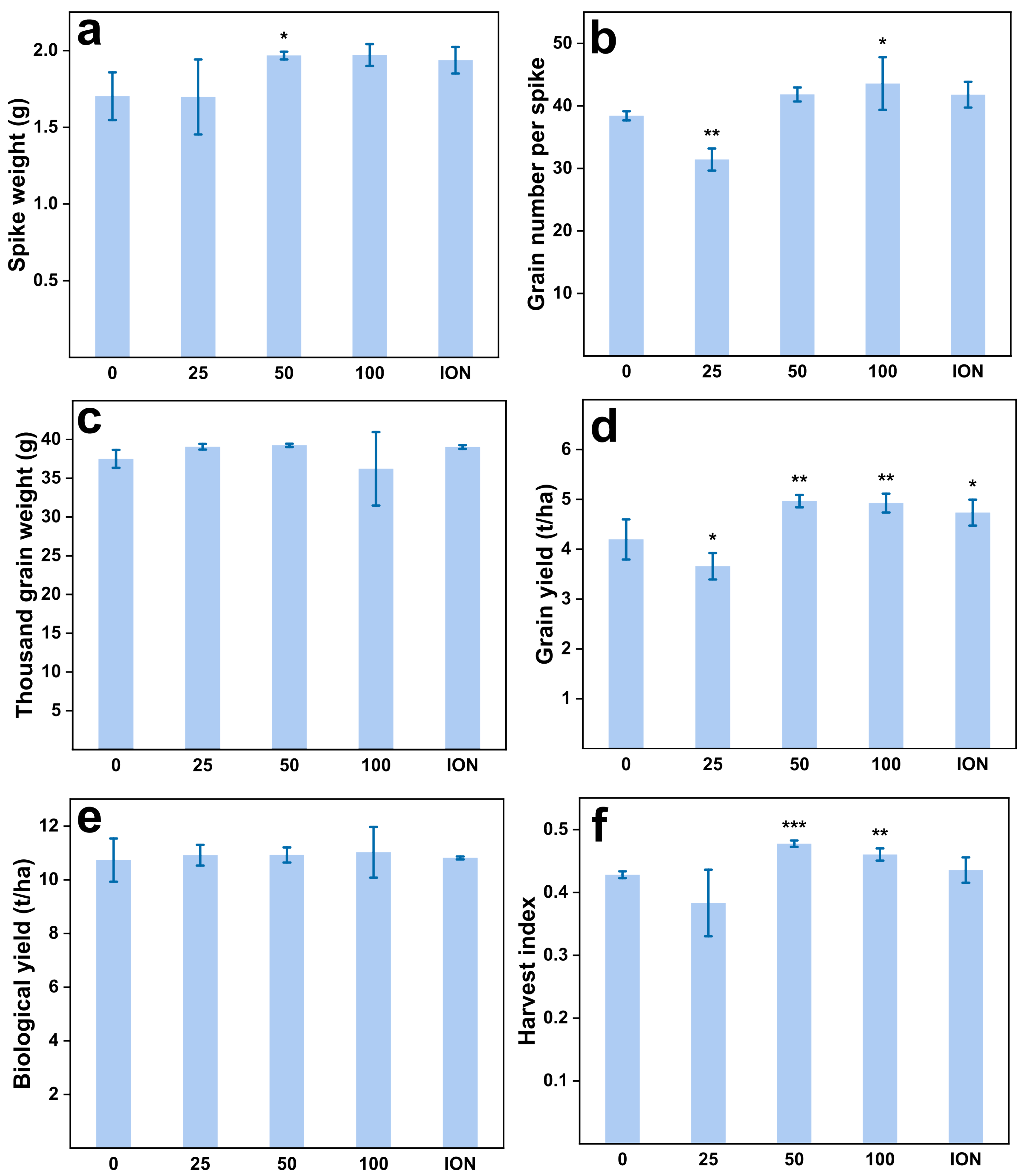
2.3. Increased Yield of Wheat Grain upon MnFe2O4 NP Exposure
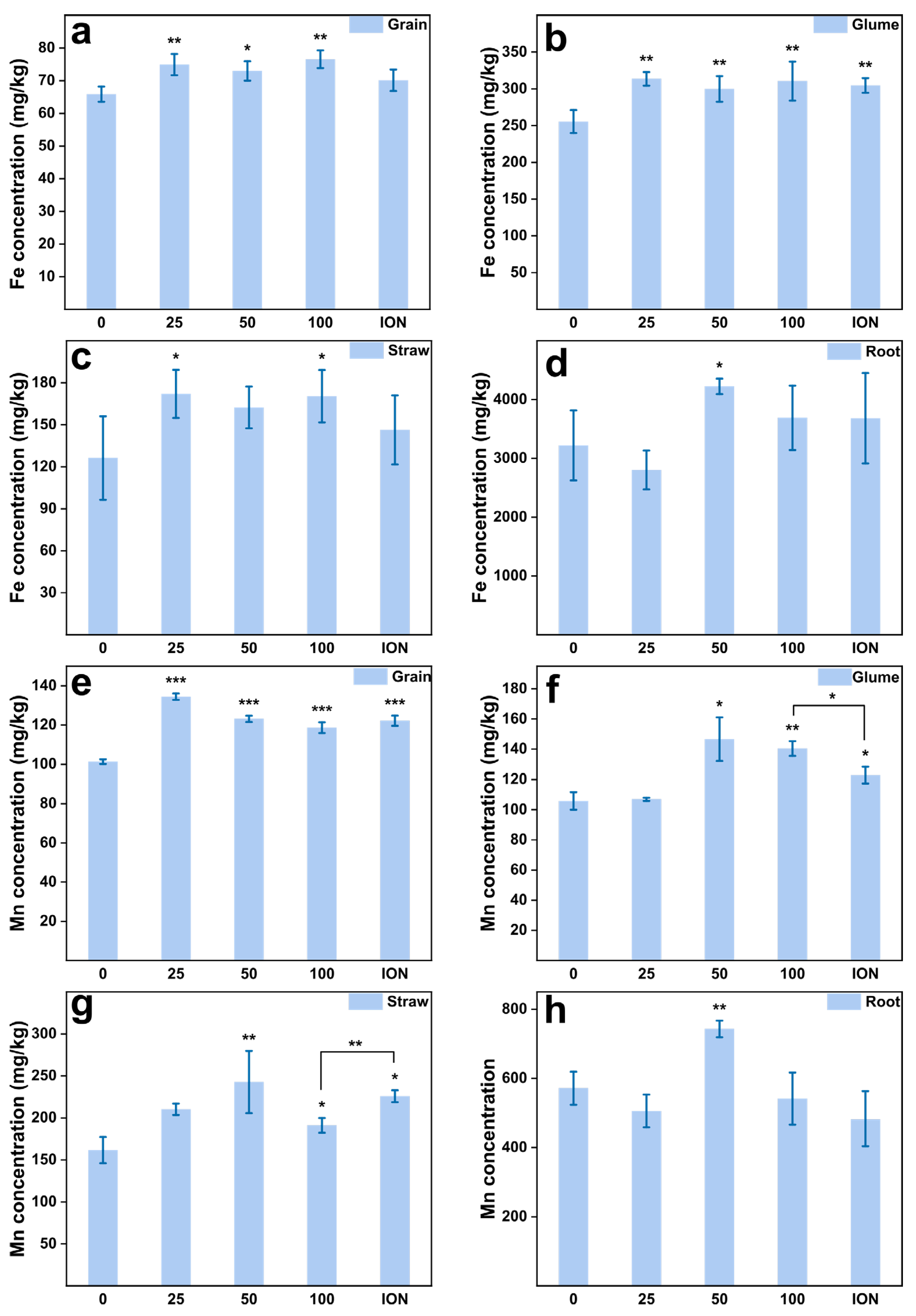
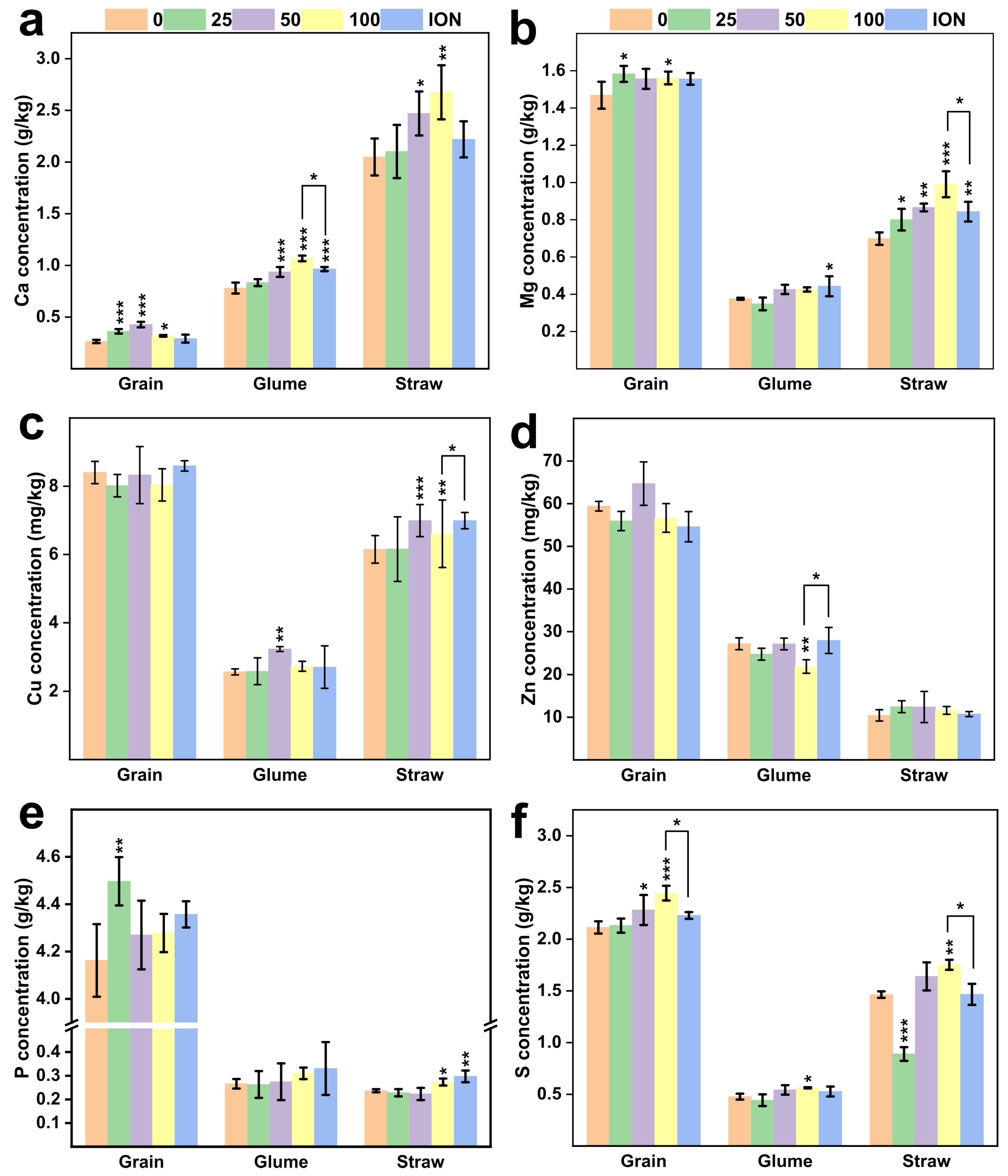
2.4. Enhanced Wheat Grain Quality by MnFe2O4 NPs
2.5. Decreased Phytic Acid by MnFe2O4 NPs
3. Materials and Methods
3.1. Synthesis and Properties of MnFe2O4 Nanoparticles
3.2. Field Experiment and Plant Growth
3.3. Measurement of Chlorophyll Concentrations
3.4. Plant Harvesting and Agronomic Traits
3.5. Assay of the Protein Quality
3.6. Elemental Content and Phytic Acid in Wheat
3.7. Fe Biochemical Stains in Wheat Grains
4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, X.-L.; Tian, J.-C.; Zhi, H.; Zhang, W.-D. Protein content and amino acid composition in grains of wheat-related species. Agric. Sci. China 2008, 7, 272–279. [Google Scholar] [CrossRef]
- Kong, D.; Khan, S.A.; Wu, H.; Liu, Y.; Ling, H.Q. Biofortification of iron and zinc in rice and wheat. J. Integr. Plant Biol. 2022, 64, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32, S31–S40. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Malnutrition: It’s about more than Hunger. 2017. Available online: https://www.who.int/news-room/commentaries/detail/malnutrition-it-s-about-more-than-hunger (accessed on 11 May 2024).
- World Health Organization. Levels and Trends in Child Malnutrition: UNICEF; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Lowe, N.M. The global challenge of hidden hunger: Perspectives from the field. Proc. Nutr. Soc. 2021, 80, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, L.M.; Pourzahedi, L.; Laughton, S.; Gao, X.; Zimmerman, J.B.; Theis, T.L.; Westerhoff, P.; Lowry, G.V. Guiding the design space for nanotechnology to advance sustainable crop production. Nat. Nanotechnol. 2020, 15, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Murmu, G.; Mukherjee, K.; Saha, S.; Maity, D. A comprehensive overview of nanotechnology in sustainable agriculture. J. Biotechnol. 2022, 355, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Sha, H.; Ye, Z.; Wang, Y.; Mao, B. Nanomaterials in plant management: Functions, mechanisms and prospects. Environ. Sci. Nano 2023, 10, 3232–3252. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Song, W.; Zhao, B.; Wang, C.; Ozaki, Y.; Lu, X. Functional nanomaterials with unique enzyme-like characteristics for sensing applications. J. Mater. Chem. B 2019, 7, 850–875. [Google Scholar] [CrossRef] [PubMed]
- Tombuloglu, H.; Tombuloglu, G.; Slimani, Y.; Ercan, I.; Sozeri, H.; Baykal, A. Impact of manganese ferrite (MnFe2O4) nanoparticles on growth and magnetic character of barley (Hordeum vulgare L.). Environ. Pollut. 2018, 243, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Giust, D.; Lucío, M.I.; El-Sagheer, A.H.; Brown, T.; Williams, L.E.; Muskens, O.L.; Kanaras, A.G. Graphene oxide–upconversion nanoparticle based portable sensors for assessing nutritional deficiencies in crops. ACS Nano 2018, 12, 6273–6279. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, A.; Du, W.; Mao, L.; Wei, Z.; Wang, S.; Yuan, H.; Ji, R.; Zhao, L. Insight into the interaction between Fe-based nanomaterials and maize (Zea mays) plants at metabolic level. Sci. Total Environ. 2020, 738, 139795. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Avellan, A.; Yun, J.; Zhang, Y.; Spielman-Sun, E.; Unrine, J.M.; Thieme, J.; Li, J.; Lombi, E.; Bland, G.; Lowry, G.V. Nanoparticle size and coating chemistry control foliar uptake pathways, translocation, and leaf-to-rhizosphere transport in wheat. ACS Nano 2019, 13, 5291–5305. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Dimkpa, C.O. Soil properties influence the response of terrestrial plants to metallic nanoparticles exposure. Curr. Opin. Environ. Sci. Health 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef]
- Marchiol, L.; Iafisco, M.; Fellet, G.; Adamiano, A. Nanotechnology support the next agricultural revolution: Perspectives to enhancement of nutrient use efficiency. Adv. Agron. 2020, 161, 27–116. [Google Scholar]
- Dimkpa, C.O.; Singh, U.; Adisa, I.O.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Effects of manganese nanoparticle exposure on nutrient acquisition in wheat (Triticum aestivum L.). Agronomy 2018, 8, 158. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Q.; Li, Y.; Pan, Z.; Liu, C.; Lin, D.; Gao, J.; Tang, Z.; Li, Z.; Wang, R. Role of Soil and Foliar-Applied Carbon Dots in Plant Iron Biofortification and Cadmium Mitigation by Triggering Opposite Iron Signaling in Roots. Small 2023, 2301137. [Google Scholar] [CrossRef]
- Salehi, H.; Chehregani, A.; Lucini, L.; Majd, A.; Gholami, M. Morphological, proteomic and metabolomic insight into the effect of cerium dioxide nanoparticles to Phaseolus vulgaris L. under soil or foliar application. Sci. Total Environ. 2018, 616, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, G.B.; Miguel-Rojas, C.; Montanha, G.S.; Carmona, F.J.; Dal Sasso, G.; Sillero, J.C.; Skov Pedersen, J.; Masciocchi, N.; Guagliardi, A.; Pérez-de-Luque, A. Reducing nitrogen dosage in Triticum durum plants with urea-doped nanofertilizers. Nanomaterials 2020, 10, 1043. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-de-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering biomimetic calcium phosphate nanoparticles: A green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Avellan, A.; Laughton, S.; Vaidya, R.; Rodrigues, S.M.; Casman, E.A.; Lowry, G.V. CuO nanoparticle dissolution and toxicity to wheat (Triticum aestivum) in rhizosphere soil. Environ. Sci. Technol. 2018, 52, 2888–2897. [Google Scholar] [CrossRef]
- Hu, J.; Guo, H.; Li, J.; Gan, Q.; Wang, Y.; Xing, B. Comparative impacts of iron oxide nanoparticles and ferric ions on the growth of Citrus maxima. Environ. Pollut. 2017, 221, 199–208. [Google Scholar] [CrossRef]
- Elhaj Baddar, Z.; Unrine, J.M. Effects of Soil pH and Coatings on the Efficacy of Polymer coated ZnO Nanoparticulate fertilizers in Wheat (Triticum aestivum). Environ. Sci. Technol. 2021, 55, 13532–13540. [Google Scholar] [CrossRef]
- Yue, L.; Feng, Y.; Ma, C.; Wang, C.; Chen, F.; Cao, X.; Wang, J.; White, J.C.; Wang, Z.; Xing, B. Molecular mechanisms of early flowering in tomatoes induced by manganese ferrite (MnFe2O4) nanomaterials. ACS Nano 2022, 16, 5636–5646. [Google Scholar] [CrossRef]
- Parsons, J.G.; Lopez, M.L.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Determination of arsenic (III) and arsenic (V) binding to microwave assisted hydrothermal synthetically prepared Fe3O4, Mn3O4, and MnFe2O4 nanoadsorbents. Microchem. J. 2009, 91, 100–106. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. Manganese ferrite (MnFe2O4) Nanoparticles: From synthesis to application—A review. J. Ind. Eng. Chem. 2021, 103, 292–304. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.-H.; Kang, S.-H.; Choi, C.-J.; Rajesh, J.A.; Ahn, K.-S. Facile hydrothermal synthesis of cubic spinel AB2O4 type MnFe2O4 nanocrystallites and their electrochemical performance. Appl. Surf. Sci. 2017, 413, 83–91. [Google Scholar] [CrossRef]
- Noor, R.; Yasmin, H.; Ilyas, N.; Nosheen, A.; Hassan, M.N.; Mumtaz, S.; Khan, N.; Ahmad, A.; Ahmad, P. Comparative analysis of iron oxide nanoparticles synthesized from ginger (Zingiber officinale) and cumin seeds (Cuminum cyminum) to induce resistance in wheat against drought stress. Chemosphere 2022, 292, 133201. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Keller, A.A. Metabolomic response of early-stage wheat (Triticum aestivum) to surfactant-aided foliar application of copper hydroxide and molybdenum trioxide nanoparticles. Nanomaterials 2021, 11, 3073. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, P.; Adeel, M.; Guo, Z.; Chetwynd, A.J.; Ma, C.; Bai, T.; Hao, Y.; Rui, Y. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2021, 269, 116134. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S.K.; Sun, Y.; Hu, J.; Yin, H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2019, 126, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.A.; Reynolds, M.; Salvucci, M.E.; Raines, C.; Andralojc, P.J.; Zhu, X.G.; Price, G.D.; Condon, A.G.; Furbank, R.T. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 2011, 62, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.D.; Yoon, H.; Singh, J.P.; Chae, K.H.; Rho, S.-c.; Hwang, D.S.; Chang, Y.-S. Uptake, distribution, and transformation of zerovalent iron nanoparticles in the edible plant Cucumis sativus. Environ. Sci. Technol. 2018, 52, 10057–10066. [Google Scholar] [CrossRef]
- Iannone, M.F.; Groppa, M.D.; Zawoznik, M.S.; Coral, D.F.; van Raap, M.B.F.; Benavides, M.P. Magnetite nanoparticles coated with citric acid are not phytotoxic and stimulate soybean and alfalfa growth. Ecotoxicol. Environ. Saf. 2021, 211, 111942. [Google Scholar] [CrossRef]
- Lian, J.; Cheng, L.; Zhai, X.; Wu, R.; Liu, W.; Pan, J.; Shohag, M.; Xin, X.; He, Z.; Yang, X. Foliar spray of combined metal-oxide nanoparticles alters the accumulation, translocation and health risk of Cd in wheat (Triticum aestivum L.). J. Hazard. Mater. 2022, 440, 129857. [Google Scholar] [CrossRef]
- Raboy, V.; Gerbasi, P.F.; Young, K.A.; Stoneberg, S.D.; Pickett, S.G.; Bauman, A.T.; Murthy, P.P.; Sheridan, W.F.; Ertl, D.S. Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1. Plant Physiol. 2000, 124, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Angel, R.; Tamim, N.; Applegate, T.; Dhandu, A.; Ellestad, L. Phytic acid chemistry: Influence on phytin-phosphorus availability and phytase efficacy. J. Appl. Poult. Res. 2002, 11, 471–480. [Google Scholar] [CrossRef]
- Lian, J.; Cheng, L.; Wang, X.; Chen, Y.; Deng, C.; Wang, Y.; Pan, J.; Shohag, M.J.I.; Xin, X.; He, Z. Bespoke ZnO NPs Synthesis Platform to Optimize Their Performance for Improving the Grain Yield, Zinc Biofortification, and Cd Mitigation in Wheat. ACS Sustain. Chem. Eng. 2024, 12, 716–727. [Google Scholar] [CrossRef]
- Kirk, P.L. Kjeldahl method for total nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Lian, J.; Cheng, L.; Zhai, X.; Wu, R.; Huang, X.; Chen, D.; Pan, J.; Shohag, M.; Xin, X.; Ren, X. Zinc glycerolate (Glyzinc): A novel foliar fertilizer for zinc biofortification and cadmium reduction in wheat (Triticum aestivum L.). Food Chem. 2023, 402, 134290. [Google Scholar] [CrossRef]
- Velu, G.; Bhattacharjee, R.; Rai, K.N.; Sahrawat, K.; Longvah, T. A simple and rapid screening method for grain zinc content in pearl millet. J. SAT Agric. Res. 2008, 6, 1–4. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Wang, X.; Liu, X.; Cheng, L.; Pan, J.; Yang, X. Nanotechnology in Agriculture: Manganese Ferrite Nanoparticles as a Micronutrient Fertilizer for Wheat. Plants 2024, 13, 1395. https://doi.org/10.3390/plants13101395
Huang X, Wang X, Liu X, Cheng L, Pan J, Yang X. Nanotechnology in Agriculture: Manganese Ferrite Nanoparticles as a Micronutrient Fertilizer for Wheat. Plants. 2024; 13(10):1395. https://doi.org/10.3390/plants13101395
Chicago/Turabian StyleHuang, Xiwei, Xin Wang, Xingxing Liu, Liping Cheng, Jianqing Pan, and Xiaoe Yang. 2024. "Nanotechnology in Agriculture: Manganese Ferrite Nanoparticles as a Micronutrient Fertilizer for Wheat" Plants 13, no. 10: 1395. https://doi.org/10.3390/plants13101395
APA StyleHuang, X., Wang, X., Liu, X., Cheng, L., Pan, J., & Yang, X. (2024). Nanotechnology in Agriculture: Manganese Ferrite Nanoparticles as a Micronutrient Fertilizer for Wheat. Plants, 13(10), 1395. https://doi.org/10.3390/plants13101395




